1. Introduction
Cocaine, crack, and methamphetamine are sympathomimetic stimulants that can acutely precipitate both physical and psychological changes such as hyperthermia, elevated blood pressure, decreased appetite, insomnia, enhanced sex drive, and feelings of euphoria (Makisumi et al., 1998; Irwin et al., 2007). Despite the potent effects of stimulants, relatively few investigations have examined the extent to which these substances are immunomodulatory (Kopnisky et al., 2007). In vitro data indicate that methamphetamine up-regulates human immunodeficiency virus (HIV) reverse transcriptase activity, promotes the expression of CC chemokine receptor 5 (CCR5), and inhibits the production of interferon–alpha in monocyte/macrophage cultures (Liang et al., 2008). In addition, a number of investigations have observed that administration of cocaine to rodents decreases the number of circulating lymphocytes (Pellegrino & Bayer, 1998). In one study that examined a murine model of acquired immune deficiency syndrome, retrovirus-infected mice that received cocaine injections displayed increases in thymus weight compared to uninfected mice that received saline injections. Retrovirus-infected mice that received cocaine also displayed greater reductions in T-helper (CD4+) count in the thymus compared to other retrovirus-infected mice that did not receive cocaine (Lopez et al., 1992). Bearing in mind the fact that the sympathetic nervous system innervates lymphoid organs such as the thymus (Nance & Sanders, 2007), these effects may reflect the influence of cocaine-induced sympathetic nervous system activation. These findings are further supported by investigations of the effects of cocaine infusion among HIV-negative individuals with cocaine dependence. Irwin and colleagues (2007) observed decrements in both resting and stimulated monocyte expression of tumor necrosis factor–alpha (TNF-α) as well as decreased circulating levels of its soluble receptor (sTNF-R75) among men that received cocaine versus a placebo. Interestingly, cocaine-dependent participants displayed enhanced autonomic nervous system activation which was, in turn, associated with lower resting and stimulated monocyte expression of TNF-α. In another study where individuals were administered cocaine, stimulated peripheral blood mononuclear cells (PBMCs) demonstrated in vitro changes that favored an enhanced cellular-immune response. These cocaine-induced changes in stimulated cytokine production included increases in interferon-gamma (IFN-γ) and concurrent decreases in interleukin-10 (Gan et al., 1998). Although the exact nature and clinical relevance of the acute immunologic effects of stimulants remains unclear, findings provide some preliminary indication that these substances may directly impair the capacity of the immune system to effectively manage chronic viral infections such as HIV.
Because stimulants activate the autonomic nervous system (ANS), it may represent an important pathway for any observed immunomodulatory effects. The physiologic effects of stimulants appear to be mediated by ANS activation (Irwin et al., 2007; Makisumi et al., 1998), resulting in the release of norepinephrine at nerve terminals. By binding with β2 receptors on the lymphocyte membrane, norepinephrine activates the G protein linked adenyl cyclase-cAMP-protein kinase A signaling cascade (Kobilka, 1992). Cellular changes of this nature are associated with in vitro decrements in IFN-γ and interleukin-10 during the eight days following HIV infection of PBMCs. This suppression of IFN-γ and interleukin-10 production, in turn, predicts elevations in HIV viral load over time (Cole et al., 1998). Bearing in mind that the lymphoid organs have been shown to be a primary site for HIV replication, sympathetic innervation of both primary and secondary lymphoid tissue may provide an ideal microenvironment for ANS activation to accelerate HIV replication. This is supported by data indicating that simian immunodeficiency virus replication is enhanced by 3.9-fold near catecholaminergic varicosities (Sloan et al., 2006). Lending further support to the role of ANS activation, another study observed that Individuals who displayed higher ANS activity at rest prior to beginning anti-retroviral therapy (ART) subsequently demonstrated poorer suppression of HIV viral load and decreased CD4+ cell reconstitution over a 3 to 11 month period (Cole et al., 2001). Taken together, there is burgeoning evidence for the role of ANS activation in hastened HIV disease progression (Cole, 2008).
Greater output of norepinephrine may partially explain observations that stimulant users on ART display a markedly elevated HIV viral load (Ellis et al., 2003). Even after accounting for higher rates of self-reported ART non-adherence, regular stimulant use (2–3 times per week or more) is independently associated with 50% higher HIV viral load (Carrico et al., 2007). In the context of chronic HIV infection, elevated viral load may lead to sustained activation of the cellular-immune response (Hunt et al., 2006) that could be further exacerbated by the capacity of stimulants to increase IFN-γ production in the periphery (Gan et al., 1998). This may result in both direct and indirect effects of stimulants on enhanced immune activation. The potential direct effects of stimulants on immune activation are further supported by an investigation with injecting heroin users where cocaine use was independently associated with higher levels of neopterin, even after accounting for HIV serostatus (Fuchs et al., 1987). Among HIV-positive persons, this may have important clinical implications because markers of immune activation such as neopterin have been shown to independently predict more rapid HIV disease progression (Mildvan et al., 2005).
Immune activation among stimulant users may also promote degradation of L-trypothphan, an essential amino acid that serves as the precursor for several important compounds such as serotonin (Schroecksnadel et al., 2006). IFN-γ directly increases neopterin production by activating monocytes/macrophages and it stimulates indoleamine-(2,3)-dioxygenase (IDO) to catabolize tryptophan via the kynurenine pathway. Thus, the kynurenine/tryptophan (kyn/trp) ratio provides an estimate of IDO activity that can be accelerated by IFN-γ (Schroecksnadel et al., 2006). This is supported by findings that tryptophan degradation is partially reversed following initiation of ART (Zangerle et al., 2002), possibly via decreased immune activation. Among HIV-positive persons, the clinical relevance of tryptophan degradation is supported by findings indicating that a higher kyn/trp ratio is associated with depression and impaired quality of life (Schroecksnadel et al., 2008). Taken together, stimulant use and ART non-adherence are important behavioral factors that may be independently associated with HIV disease markers. The goal of the present study was to examine if stimulant use and ART non-adherence are independently associated with immune activation and indices of tryptophan degradation.
2. Methods
2.1. Procedures
HIV-positive individuals in four U.S. cities (San Francisco, Los Angeles, Milwaukee, and New York City) were recruited from community agencies and medical clinics between July 2000 and January 2002 for a randomized behavioral prevention trial of an intervention designed to reduce HIV transmission risk. Detailed information regarding the methods and results of this trial have been published elsewhere (Carrico et al., in press; Healthy Living Project Team, 2007; Johnson et al., 2007). In total, 858 participants who were enrolled in this trial provided baseline peripheral venous blood samples to measure CD4+ count and HIV viral load. Previous analyses of these data have observed that individuals on ART who reported using stimulants 2–3 times per week or more had a five-fold higher HIV viral load than ART-treated peers who reported using stimulants once a week or less (Carrico et al., 2007). The present study utilized stored plasma samples from a sub-set of these participants (n = 127) who were taking ART. All samples were collected within 60 days (median = 10 days) of the baseline assessment. We identified 44 available plasma samples for participants who reported using stimulants (cocaine, crack, or methamphetamine) 2–3 times a month or more at the New York (n = 16) and San Francisco (n = 28) study sites. Plasma samples from the Los Angeles and Milwaukee sites were not available. Then, using plasma samples from the San Francisco site, all available samples from ART-treated participants who reported no stimulant use in the past three months were included as a comparison group (n = 83). All plasma samples were stored at −80 °C.
2.2. Measures
2.2.1. Demographics
Age, ethnicity, gender, sexual orientation, education and time since HIV diagnosis were assessed by questionnaire.
2.2.2. Depressive Symptoms
The 21-item Beck Depression Inventory I (BDI) assesses the severity of somatic, affective, cognitive, and behavioral symptoms of depression during the past week (Beck et al., 1996). Using the BDI total score (Cronbach’s Alpha = .88), we classified participants based on the severity of depressive symptoms: no depression (0 – 9), mild-moderate (10–18), and moderate-severe (19 – 63).
2.2.3. Stimulant Use
Participants rated the frequency of cocaine, crack, and methamphetamine use during the past three months using the following responses: never, less than once a month, once a month, 2–3 times a month, once a week, 2–3 times per week, 4–6 times per week, once a day, and more than once a day. Forty-four participants who reported using stimulants 2–3 times a month or more (i.e., monthly stimulant use) were selected for the study. In addition, we examined a sub-sample of 27 participants who reported using stimulants at least weekly (i.e., weekly stimulant use) to test for possible “dose” effects.
2.2.4. Adherence to ART
Self-reported ART adherence was assessed over the prior three days to calculate percent adherence by dividing the number of pills taken by the total number of pills prescribed (Chesney et al., 2000). Previous investigations lend support to the validity of brief self-report measures of ART adherence in relation to immune status (Simoni et al., 2006). However, because measures are often negatively skewed, it is difficult to examine ART adherence as a continuous predictor. Consistent with prior investigations of ART adherence (Johnson et al., 2003), participants who reported less than 90% adherence were considered non-adherent.
2.2.5. Biological Assays
HIV-1 viral load was determined using the AMPLICOR ultrasensitive method for the in vitro reverse transcriptase polymerase chain reaction assay (Roche Laboratories, US # 83088), which has a valid range of 50 to 750,000 copies/ml. CD4+ cell count was determined by whole blood using direct immunoflourescence. Plasma neopterin was measured using ELISA (BRAHMS, Berlin, Germany). Tryptophan and kynurenine concentrations were determined by high performance liquid chromatography using a method described elsewhere (Widner et al., 1997).
2.3. Statistical Analyses
We began by examining the distributions for measures of immune status and indicators of tryptophan degradation. Data were transformed where skewness (> 2.0) and kurtosis (> 5.0) values indicated a non-normal distribution. For kynurenine a square root transformation was utilized. A log10 transformation was used for HIV viral load and neopterin. We conducted independent samples t-tests to examine differences in indices of immune status and tryptophan degradation as a function of ART non-adherence and stimulant use categories. Informed by these results, we conducted multiple linear regression analyses to examine whether stimulant use was independently associated with measures of immune status and indices of tryptophan degradation while simultaneously controlling for ART non-adherence. Because age, time since HIV diagnosis, and ART regimen type may influence biological outcomes, these were included as covariates in the multiple regression analyses. Bearing in mind that the nature of the relationship between adherence and HIV viral suppression appears to be variable across different classes of ART medications (Bangsberg et al., 2006), we also conducted exploratory analyses to examine if weekly stimulant use was independently associated with neopterin and tryptophan after simultaneously controlling for HIV viral load in a single regression block. Next, we utilized partial correlations to examine if neopterin was associated with indices of tryptophan degradation after controlling for HIV viral load. Finally, using a partial correlation and an analysis of covariance we examined whether the severity of depressive symptoms was associated with a higher kyn/trp ratio. Time since HIV diagnosis was selected as the covariate in these analyses to account for confounds between HIV-related symptoms and somatic symptoms of depression.
3. Results
3.1. Participant Characteristics
The mean age of participants was 43 (range: 20 – 64) years. The majority of participants were gay or bisexual men (74%). Other participants were women (13%) and heterosexual men (13%). The sample was relatively diverse with respect to ethnicity: 47% Caucasian, 33% African American, 9% Hispanic/Latino, 2% Asian/Pacific Islander, 1% Native American/Alaskan Native, and 8% of multi-cultural heritage. Most participants (65%) had completed at least some college. Individuals had been diagnosed with HIV for an average of 10 (range: 0.08 – 21) years. In total, 20 (16%) participants were taking an ART regimen that included both a protease inhibitor (PI) and non-nucleoside reverse transcriptase inhibitor (NNRTI). The majority of these participants (16/20) were also taking a nucleoside reverse transcriptase inhibitor (NRTI). Fifty (39%) participants were taking regimens that included both PI and NRTI medications, 36 (28%) were taking NNRTI-based regimens, 12 (9%) were taking triple NRTI regimens, and 9 (7%) were taking regimens that did not qualify as highly active anti-retroviral therapy (HAART). The average CD4+ count was 475 (SD = 311) cells/mm3 and the mean HIV viral load was 3.03 (SD = 1.29) log10 copies/ml. Approximately 34% of participants had an undetectable HIV viral load.
3.2. Weekly Stimulant use is Independently Associated with Immune Status and Tryptophan
Participants who were classified as non-adherent to their ART regimen had significantly higher (0.73 log10) HIV viral load (t (124) = −3.13, p < .01) and significantly higher (0.12 log10) neopterin (t (123) = −2.58, p < .05). Individuals who reported monthly stimulant use had significantly higher (0.11 log10) neopterin (t (124) = −2.32, p < .05). Finally, participants who reported weekly stimulant use had significantly higher (.73 log10) HIV viral load (t (108) = −2.61, p = .01), higher (0.17 log10) neopterin (t (107) = −3.07, p < .01), and lower (8.46 µmol/l) tryptophan (t (107) = 3.18, p < .01). Means and standard deviations for all dependent variables are presented in Table 1.
Table 1.
Differences in Indices of Immune Status and Tryptophan Degradation as a Function of ART Non-Adherence and Stimulant Use Categories
| ≥ 90% ART Adherence (N = 80) |
< 90 % ART Adherence (N = 46) |
No Stimulant Use (N = 83) |
Monthly Stimulant Use (N = 44) |
Weekly Stimulant Use (n =27) |
|
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| HIV Viral Load (log10) | 2.76 (1.23)a | 3.49 (1.27)a | 2.92 (1.25)c | 3.25 (1.34) | 3.65 (1.31)c |
| CD4+ Count | 498.78 (308.71) | 438.57 (316.03) | 494.81 (297.38) | 436.07 (335.64) | 435.31 (365.12) |
| Neopterin (log10) | 1.00 (.24)a | 1.12 (.26)a | 1.01 (.25)b,c | 1.12 (.23)b | 1.18 (.22)c |
| Tryptophan | 49.07 (11.20) | 45.99 (13.23) | 49.12 (12.70)c | 45.31 (10.38) | 40.66 (8.25)c |
| Kynurenine (square root) | 1.47 (.30) | 1.53 (.39) | 1.53 (.36) | 1.43 (.28) | 1.41 (.25) |
| Kynurenine/ Tryptophan | 47.64 (20.42) | 53.06 (28.16) | 50.65 (25.08) | 48.85 (21.75) | 52.59 (22.12) |
significant independent samples t-test (p < .05)
After controlling for age, time since HIV diagnosis, ART regimen type, and ART non-adherence, monthly stimulant use was not independently associated with neopterin (β = .14, p > .10). As summarized in Table 2, weekly stimulant use was independently associated with elevated HIV viral load, higher neopterin, and lower tryptophan. Findings were unchanged when ART adherence was examined as a continuous variable or in quartiles. Finally, in an exploratory analysis we observed that even after controlling for HIV viral load, weekly stimulant use continued to be significantly associated with higher neopterin (β = .18, p < .05) and lower tryptophan (β = −.26, p < .01).
Table 2.
Multiple Linear Regression Analyses Examining the Independent Effects of ART Non-Adherence and Weekly Stimulant Use on HIV Viral Load, Neopterin, and Tryptophan
| HIV Viral Load | Neopterin | Tryptophan | |
|---|---|---|---|
| β | β | β | |
| Age | .03 | −.07 | .06 |
| Time Since HIV Diagnosis | .11 | .06 | .12 |
| PI and NNRTI (Ref) | - | - | - |
| PI and NRTI | .20 | .24† | .05 |
| NNRTI-Based | −.14 | .14 | .15 |
| Triple NRTI | .09 | .03 | .09 |
| Not HAART | .11 | .34** | −.05 |
| < 90% Adherence | .23* | .19* | −.08 |
| Weekly Stimulant Use | .23* | .22* | −.26** |
| Model R2 | .23 | .23 | .13 |
p = .057
p < .05
p ≤ .01
3.3. Neopterin is Independently Associated with Indices of Tryptophan Degradation
HIV viral load was significantly associated with higher neopterin (r = .51, p < .001), lower tryptophan (r = −.24, p < .01) and a higher kyn/trp ratio (r = .29, p < .01). HIV viral load was not associated with kynurenine (r = .13, p > .10). After controlling for HIV viral load, neopterin remained significantly associated with lower tryptophan (r = −.22, p <.05), higher kynurenine (r = .36, p <.001), and a higher kyn/trp ratio (r = .50, p <.001).
3.4. The Kyn/Trp Ratio is Associated with Depressive Symptoms
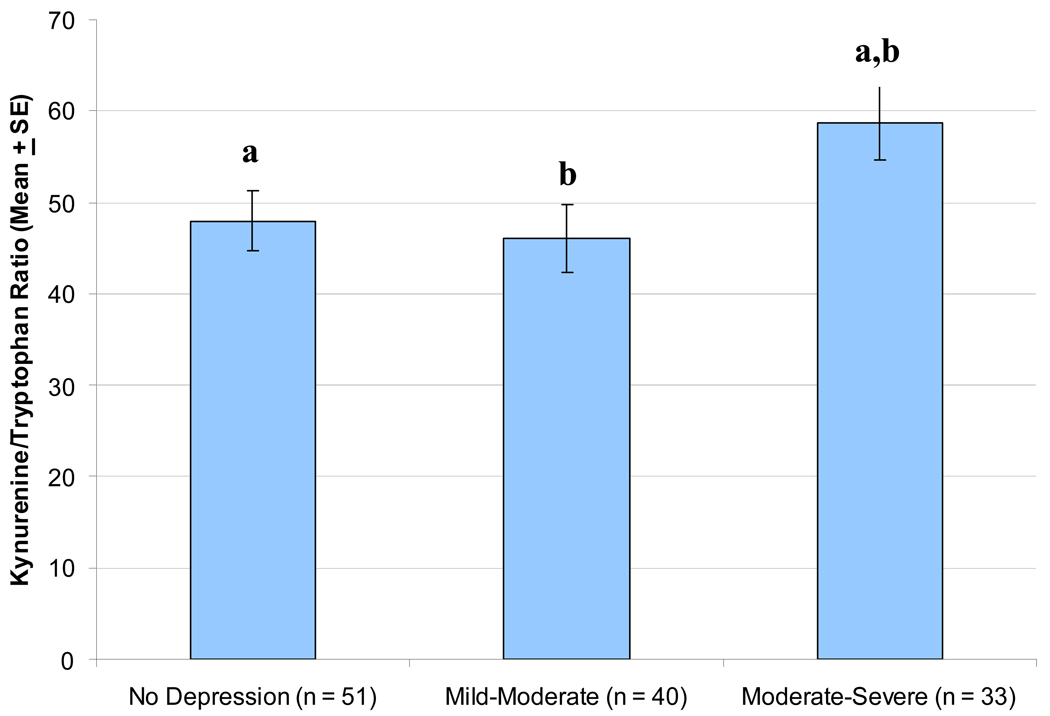
After controlling for time since HIV diagnosis, the kyn/trp ratio was significantly associated with greater depressive symptom severity (r = .19, p < .05). Similarly, significant mean differences in the kyn/trp ratio were observed between depressive symptom severity categories (F (2, 120) = 3.11, p < .05) after controlling for time since HIV diagnosis. As shown in Figure 1, pair-wise comparisons (using Tukey’s LSD) indicated that individuals with moderate-severe depressive symptoms had a significantly higher kyn/trp ratio compared to either individuals with no depression or those with mild-moderate depression (p < .05).
Figure 1. Mean Differences in the Kynurenine/Tryptophan Ratio as a Function of Depressive Symptom Severity Category.
After controlling for time since HIV diagnosis, individuals with moderate-severe depression had a significantly higher kynurenine/tryptophan ratio (Mean = 58.76, SE = 4.07) compared to either: a) those with no depression (Mean = 47.97, SE = 3.32; p < .05) or b) those mild-moderate depression (Mean = 45.97, SE = 3.71; p < .05).
4. Discussion
Results of the present investigation lend support to a previous in vitro study which reported that stimulants may accelerate HIV viral replication in monocytes/macrophages (Liang et al., 2008). We have previously demonstrated that individuals who report using stimulants 2–3 times per week or more display a 50% higher HIV viral load, independent of self-reported ART non-adherence (Carrico et al., 2007). The present study replicated this finding utilizing a less stringent criterion for regular stimulant use (i.e., at least once a week) in a sub-sample of participants included in our prior investigation. Furthermore, to our knowledge this investigation is the first to report that weekly stimulant use is independently associated with higher levels of neopterin among HIV-positive persons on ART. This clinically relevant marker of immune activation has been shown to independently predict more rapid HIV disease progression (Mildvan et al., 2005). Although the bio-behavioral pathways remain unclear, these data provide some preliminary evidence that stimulant use may directly increase HIV viral load and promote immune activation among ART-treated HIV-positive individuals.
By inducing cellular-immune activation, stimulants may promote tryptophan degradation. Even after accounting for viral load, neopterin remained a significant correlate of all indices of tryptophan degradation. This indicates that enhanced immune activation may be at least partially responsible for depleted tryptophan levels that were observed among weekly stimulant users in the current study. It is also plausible that lower tryptophan levels among weekly stimulant users are partially due to the effects of poor nutrition, which is common in this population (Quach et al., 2008). However, we did not observe lower kynurenine levels among weekly stimulant users, which would provide more definitive evidence for the influence of poor nutrition (Schroecksnadel et al., 2006). The role of nutritional deficits should be carefully examined in future investigations with stimulant users. Irrespective of the mechanism for the effect of stimulant use on lower tryptophan, findings from the present study lend further support to the clinical relevance of tryptophan degradation in HIV-positive persons. Consistent with a prior investigation (Schroecksnadel et al., 2008), we observed that a higher kyn/trp ratio was associated with increased severity of depressive symptoms. Those with moderate to severe depression displayed an elevated kyn/trp ratio compared to either individuals with no depression or those with mild-moderate depression. Finally, there is evidence that peripheral serotonin modulates immune function (Mosser & Lesch, 1998). Future investigations should directly measure serotonin levels among stimulant users and examine the extent to which depleted serotonin is independently associated with immune impairments.
Although findings from the present investigation are provocative, future studies should examine the bio-behavioral pathways that may account for the effects of stimulant use on HIV disease markers and depleted tryptophan. These plausible bio-behavioral pathways include: HIV genotypic resistance, sexually transmitted infections, sleep dysregulation, poor nutrition, and ANS activation. In addition, because the present investigation included only HIV-positive individuals on ART, it is difficult to determine whether the effects of stimulant use on immune activation and depleted tryptophan would be observed among untreated HIV-positive or HIV-negative populations. Although weekly stimulant use was associated with immune activation and lower tryptophan after controlling for HIV viral load, this is far from a definitive analysis. Given the low rates of undetectable viral load in this cohort, we were unable to examine the effect weekly stimulant use on immune activation and tryptophan depletion in the sub-set with undetectable viral load. Thus, it remains a possibility that weekly stimulant use is indirectly associated with immune activation via elevated viral load. Further research is necessary to determine whether the effects of stimulant use vary as a function of HIV serostatus and/or a current prescription for ART medications. It is noteworthy that the present study utilized self-report measures of stimulant use and ART adherence. Future investigations should include toxicology screening and electronic medication monitoring to better assess the influence of recent stimulant use and patterns of ART non-adherence (Hinkin et al., 2006). It is also important to measure other potential confounding variables such as pre-treatment nadir CD4+ count and hepatitis C co-infection that were not assessed in the present investigation. Finally, findings should be confirmed in future longitudinal investigations with more representative samples of HIV-positive persons. Individuals were selected for the present study because they reported HIV transmission risk behavior and chose to participate in a randomized controlled trial of a behavioral intervention. As a result, it is likely that individuals in the present investigation may have reported increased rates of a variety of risk taking behaviors than would have been observed in more representative samples of HIV-positive persons.
Acknowledgements
Funding for this research was provided by the Until There’s A Cure Foundation (560 Mountain Home Road Redwood City, CA 94062-2515). Additional support was provided by a Ruth L. Kirschstein National Research Service Award (T32-MH019391).
References
- Bangsberg DR, Acosta EP, Gupta R, Guzman D, Riley ED, Harrigan PR, Parkin N, Deeks SG. Adherence-resistance relationships for protease inhibitors and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20:223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of the Beck Depression Inventories –IA and –II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Carrico AW, Chesney MA, Johnson MO, Neilands TB, Remien RH, Rotheram-Borus MJ, Wong FL Healthy Living Project Team. Randomized controlled trial of a cognitive-behavioral intervention for HIV-positive persons: An investigation of treatment effects on psychosocial adjustment. AIDS Behav. doi: 10.1007/s10461-008-9429-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Johnson MO, Moskowitz JT, Neilands TB, Morin SF, Charlebois ED, Steward WT, Remien RH, Lightfoot MA, Rotheram-Borus MJ, Wong FL, Chesney MA. Affect regulation, stimulant use, and viral load among HIV-positive persons on anti-retroviral therapy. Psychosom. Med. 2007;69:785–792. doi: 10.1097/PSY.0b013e318157b142. [DOI] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. J. Immunol. 1998;161:610–616. [PubMed] [Google Scholar]
- Cole SW. Psychosocial influences on HIV-1 disease progression: Neural, endocrine, and virologic mechanisms. Psychosom. Med. 2008;70:562–568. doi: 10.1097/PSY.0b013e3181773bbd. [DOI] [PubMed] [Google Scholar]
- Cole SW, Naliboff BD, Kemeny ME, Griswold MP, Fahey JL, Zack JA. Impaired response to HAART in HIV-infected individuals with high autonomic nervous system activity. Proc. Natl. Acad. Sci. U S A. 2001;98:12695–12700. doi: 10.1073/pnas.221134198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J. Infect. Dis. 2003;188:1820–1826. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- Fuchs D, Hausen A, Reibnegger G, Schoenitzer D, Unterweger B, Blecha HG, Hengster P, Roessler H, Schulz T, Werner ER, Dierich MP, Hinterhuber H, Schauenstein K, Traill K, Wachter H. Immune status of drug abusers. Cancer Detect. Prev. Suppl. 1987;1:535–541. [PubMed] [Google Scholar]
- Gan X, Zhang L, Newton T, Chang SL, Ling W, Kermani V, Berger O, Graves MC, Fiala M. Cocaine infusion increases interferon-gamma and decreases interleukin-10 in cocaine-dependent subjects. Clinical Iimmunology and Immunopathology. 1998;89:181–190. doi: 10.1006/clin.1998.4607. [DOI] [PubMed] [Google Scholar]
- Healthy Living Project Team. Effects of a behavioral intervention to reduce risk of transmission among people living with HIV: the healthy living project randomized controlled study. J. Acquir. Immune Defic. Syndr. 2007;44:213–221. doi: 10.1097/QAI.0b013e31802c0cae. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, Myers HF, Longshore D. Drug Use and Medication Adherence among HIV-1 Infected Individuals. AIDS Behav. 2006;11:185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PW, Deeks SG, Bangsberg DR, Moss A, Sinclair E, Liegler T, Bates M, Tsao G, Lampiris H, Hoh R, Martin JN. The independent effect of drug resistance on T cell activation in HIV infection. AIDS. 2006;20:691–699. doi: 10.1097/01.aids.0000216369.30948.18. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmos L, Wang M, Valladares EM, Motivala SJ, Fong T, Newton T, Butch A, Olmstead R, Cole SW. Cocaine dependence and acute cocaine induce decreases of monocyte proinflammatory cytokine expression across the diurnal period: autonomic mechanisms. J. Pharmacol. Exp. Ther. 2007;320:507–515. doi: 10.1124/jpet.106.112797. [DOI] [PubMed] [Google Scholar]
- Johnson MO, Catz SL, Remien RH, Rotheram-Borus MJ, Morin SF, Charlebois E, Gore-Felton C, Goldsten RB, Wolfe H, Lightfoot M, Chesney MA. Theory-guided, empirically supported avenues for intervention on HIV medication nonadherence: findings from the Healthy Living Project. AIDS Patient Care STDS. 2003;17:645–656. doi: 10.1089/108729103771928708. [DOI] [PubMed] [Google Scholar]
- Johnson MO, Charlebois E, Morin SF, Remien RH, Chesney MA. Effects of a behavioral intervention on antiretroviral medication adherence among people living with HIV: the healthy living project randomized controlled study. J. Acquir. Immune Defic. Syndr. 2007;46:574–580. doi: 10.1097/qai.0b013e318158a474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka B. Adrenergic receptors as models for G protein-coupled receptors. Annu. Rev. Neurosci. 1992;15:87–114. doi: 10.1146/annurev.ne.15.030192.000511. [DOI] [PubMed] [Google Scholar]
- Kopnisky KL, Bao J, Lin YW. Neurobiology of HIV, psychiatric and substance abuse comorbidity research: workshop report. Brain Behav. Immun. 2007;21:428–441. doi: 10.1016/j.bbi.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Liang H, Wang X, Chen H, Song L, Ye L, Wang SH, Wang YJ, Zhou L, Ho WZ. Methamphetamine Enhances HIV Infection of Macrophages. American Journal of Pathology. 2008;172:1617–1624. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MC, Lucas LC, Huang DS, Yuejian W, Watson RR. Modification of thymic cell subsets induced by long-term cocaine administration during murine retroviral infection producing AIDS. Clin. Immunol Immunopathol. 1992;65:45–52. doi: 10.1016/0090-1229(92)90246-k. [DOI] [PubMed] [Google Scholar]
- Makisumi T, Yoshida K, Watanabe T, Tan N, Murakami N, Morimoto A. Sympatho-adrenal involvement in methamphetamine-induced hyperthermia through skeletal muscle hypermetabolism. Eur. J. Pharmacol. 1998;363:107–112. doi: 10.1016/s0014-2999(98)00758-4. [DOI] [PubMed] [Google Scholar]
- Mildvan D, Spritzler J, Grossberg SE, Fahey JL, Johnston DM, Schock BR, Kagan J. Serum neopterin, an immune activation marker, independently predicts disease progression in advanced HIV-1 infection. Clin. Infect. Dis. 2005;40:853–858. doi: 10.1086/427877. [DOI] [PubMed] [Google Scholar]
- Mosser R, Lesch KP. Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav. Immun. 1998;12:249–271. doi: 10.1006/brbi.1998.0532. [DOI] [PubMed] [Google Scholar]
- Pellegrino T, Bayer BM. In vivo effects of cocaine on immune cell function. J. Neuroimmunol. 1998;83:139–147. doi: 10.1016/s0165-5728(97)00230-0. [DOI] [PubMed] [Google Scholar]
- Quach LA, Wanke CA, Schmid CH, Gorbach SL, Mkaya Mwamburi D, Mayer KH, Spiegelman D, Tang AM. Drug use and other risk factors related to lower body mass index among HIV-infected individuals. Drug Alcohol Depend. 2008;95:30–36. doi: 10.1016/j.drugalcdep.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroecksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim. Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Schroecksnadel K, Sarcletti M, Winkler C, Mumelter B, Weiss G, Fuchs D, Kemmler G, Zangerle R. Quality of life and immune activation in patients with HIV-infection. Brain Behav. Immun. 2008 doi: 10.1016/j.bbi.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10:227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Tarara RP, Capitanio JP, Cole SW. Enhanced replication of simian immunodeficiency virus adjacent to catecholaminergic varicosities in primate lymph nodes. J. Virol. 2006;80:4326–4335. doi: 10.1128/JVI.80.9.4326-4335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin. Chem. 1997;43:2424–2426. [PubMed] [Google Scholar]
- Zangerle R, Widner B, Quirchmair G, Neurauter G, Sarcletti M, Fuchs D. Effective antiretroviral therapy reduces degradation of tryptophan in patients with HIV-1 infection. Clin. Immunol. 2002;104:242–247. doi: 10.1006/clim.2002.5231. [DOI] [PubMed] [Google Scholar]