Abstract
Fear extinction is impaired in psychiatric disorders such as posttraumatic stress disorder and schizophrenia, which have a major genetic component. However, the genetic factors underlying individual variability in fear extinction remain to be determined. By comparing a panel of inbred mouse strains, we recently identified a strain, 129S1/SvImJ (129S1), that exhibits a profound and selective deficit in Pavlovian fear extinction, and associated abnormalities in functional activation of a key prefrontal-amygdala circuit, as compared to C57BL/6J. The first aim of the present study was to assess fear extinction across multiple 129 substrains representing the strain’s four different genetic lineages (Parental, Steel, Teratoma, Contaminated). Results showed that 129P1/ReJ, 129P3/J, 129T2/SvEmsJ, and 129X1/SvJ exhibited poor fear extinction, relative to C57BL/6J, while 129S1 showed evidence of fear incubation. Based on these results, the second aim was to further characterize the nature and specificity of the extinction phenotype in 129S1, as an exemplar of the 129 substrains. Results showed that the extinction deficit in 129S1 was neither the result of a failure to habituate to a sensitized fear response, nor an artifact of a fear response to (unconditioned) tone per se. A stronger conditioning protocol (i.e., five × higher intensity shocks) produced an increase in fear expression in 129S1, relative to C57BL/6J, due to rapid rise in freezing during tone presentation. Taken together, these data demonstrate that impaired fear extinction is a phenotypic feature common across 129 substrains, and provide preliminary evidence that impaired fear extinction in 129S1 may be reflect a pro-fear incubation-like process.
Keywords: gene, behavior, PTSD, prefrontal cortex, amygdala, anxiety, rodent, genetics, sensitization, incubation
Introduction
Fear extinction, the learned inhibition of a conditioned fear response (Pavlov 1927), is impaired in anxiety disorders such as phobias and post-traumatic stress disorder (PTSD), as well as schizophrenia (Holt et al. 2008). This process is readily measurable in rodents and has emerged as a promising translational tool for studying the pathophysiology and treatment of anxiety disorders (Cryan & Holmes 2005; Kamprath & Wotjak 2004; Myers & Davis 2007; Quirk & Mueller 2008). However, while there is considerable genetic contribution to individual variability in risk for anxiety disorders (Kendler 2001), the specific genetic factors underlying impaired fear extinction in these disorders are still poorly understood.
As an initial step towards the long-term goal of identifying genes contributing to variation in fear extinction, we recently surveyed a panel of inbred mouse strains and identified a common inbred mouse strain, 129S1/SvImJ (129S1), that exhibits impaired fear extinction learning coupled with functional abnormalities in a cortico-amygdala circuit mediating extinction (Hefner et al. 2008) (for commentary, see Sotres-Bayon et al. 2008). These neural and behavioral abnormalities were demonstrable by comparison with another inbred strain, C57BL/6J (B6), which shows good fear extinction. However, although the degree of genetic variation between the 129S1 and B6 strain is lesser than between two unrelated human individuals, variation is still likely to be extensive; hampering the identification of candidate genes underlying strain differences in fear extinction.
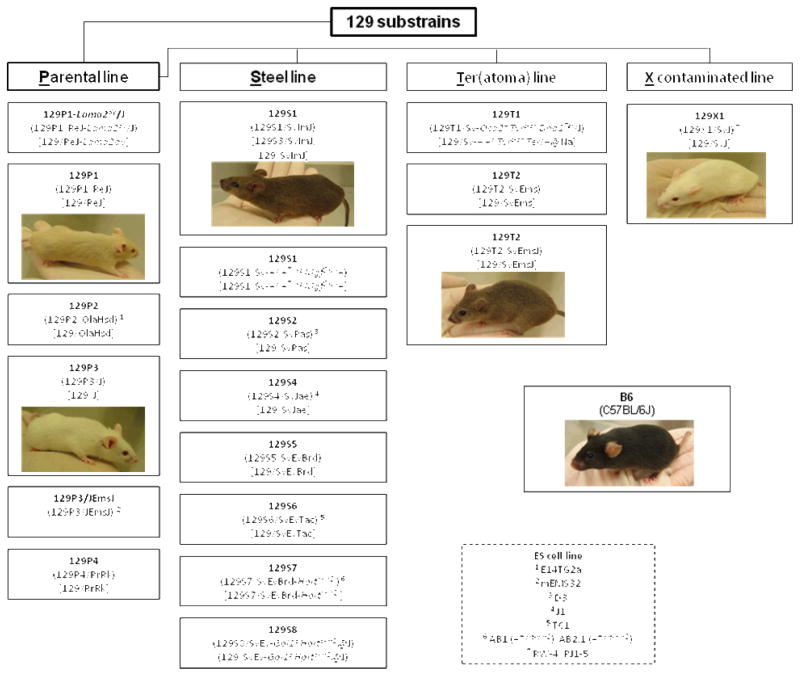
An extended family of 129 substrains has been produced by deliberate outcrossing of a single progenitor strain. Based upon an analysis of breeding history and current genetic composition, Simpson and colleagues categorized three major 129 substrain lineages, each containing multiple substrains: Parental (P), Teratoma (T), Steel (S) (Festing et al. 1999; Simpson et al. 1997). A fourth line [Contaminated (X)] consists of a single strain, and was produced by genetic contamination by an unknown donor strain (Adams et al. 2005; Threadgill et al. 1997; Wade & Daly 2005) (Figure 1). Because the degree of variation between 129 substrains, while still significant, is substantially lesser than between 129S1 and B6 (e.g., see Bothe et al. 2004), the discovery of fear extinction differences between 129 substrains could significantly expedite the discovery of the underlying genes.
Figure 1.
The four genetic lineages of the 129 substrain. Full designations are in round parenthesis. Abbreviated designations are emboldened. Designations prior to Festing et al.’s 1999 revised nomenclature are in square parenthesis. The substrains tested in the current study are depicted with a photograph. Commonly used ES cell lines derived from 129 substrains are shown with superscript number and in the key. For further information see (Festing et al. 1999; Simpson et al. 1997) and http://jaxmice.jax.org/faq/bulletin/bulletin01.html
Previous studies have compared various 129 substrains for fear conditioning and anxiety-related behaviors (e.g., Bothe et al. 2004, 2005; Cook et al. 2002), but a comparison of fear extinction across 129 substrains has not been reported. Therefore, the first aim of the present study was to assess fear extinction in a panel of 129 substrains representing each of the four genetic lineages.
The results of this strain survey showed impaired extinction across multiple 129 substrains tested, including a replication of our previous observation of this phenotype in 129S1 (Hefner et al. 2008). Therefore, the second aim of the current study was to further characterize the behavioral nature of the fear extinction deficit in 129S1, as an exemplar of the other 129 substrains.
Materials and Methods
Subjects
Subjects were male mice chosen on the basis of both genetic lineage (Figure 1) and availability from a single supplier (The Jackson Laboratory, Bar Harbor, ME). The 129 mouse substrains chosen were 129S1/SvImJ (129S1, JAX stock #002448), 129P1/ReJ (129P1, JAX stock #001137), 129P3/J (129P3, JAX stock #000690), 129T2/SvEmsJ (129T2, JAX stock #002065), and 129X1/SvJ (129X1, JAX stock #000691). The C57BL/6J strain (B6, JAX stock #000664) exhibits good fear extinction (Hefner et al. 2008; Herry & Mons 2004; Ponnusamy et al. 2005; Siegmund et al. 2005) and was included in the survey as a ‘positive control.’ Mice were obtained at ~8 weeks of age and tested between 10–20 weeks of age. All mice were housed (2/cage) side-by-side in a temperature (72 ± 5°F) and humidity (45 ± 15%) controlled vivarium under a 12 hr light/dark cycle (lights on 0600 h). The number of mice used in each experiment is given in the figure legends. All experimental procedures were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use and Austrian Ethical Committees on Animal Care and Use (Bundesministerium fur Wissenschaft und Forschung) and followed the National Institute of Health guidelines outlined in ‘Using Animals in Intramural Research’ and the local Animal Care and Use Committees.
Strain survey of fear conditioning and extinction
The conditioning apparatus was a 27 × 27 × 11 cm chamber with transparent walls and a metal rod floor, cleaned with a 79.5% water/19.5% ethanol/1% vanilla-extract solution to provide a distinctive olfactory cue. For conditioning, after a 180 sec acclimation period, mice received 3 pairings (60–120 sec variable inter-pairing interval) between a 30 sec, 80 dB, white noise (conditioned stimulus, CS) and a 2 sec, 0.6 mA scrambled footshock (unconditioned stimulus, US), in which the shock was presented during the last 2 sec of the CS. There was a 120 sec no-stimulus consolidation period after the final pairing before mice were returned to the home cage (Hefner & Holmes 2007). Twenty-four hr later, expression of the tone memory and within-session extinction of the memory was tested. Mice were placed in a novel context: Plexiglas cylinder with black/white-checkered walls, solid-Plexiglas opaque floor, cleaned with a 99% water/1% acetic acid solution, housed in a different room from conditioning. After 180 sec, mice received 50 × 30 sec CS presentations (5 sec no-stimulus interval). Twenty-four hr later, extinction retrieval was tested in the same context as extinction. After 180 sec, mice received 3 × 30 sec CS presentations (5 sec no-stimulus interval). Freezing scores during extinction and extinction retrieval were binned into 5- and 3-trial averages, respectively, for analysis. Stimulus presentation was controlled by the Med Associates VideoFreeze system (Med Associates, Burlington, VT). Freezing, manually scored every 5 sec as no visible movement except that required for respiration, was measured as an index of fear (Blanchard & Blanchard 1969), and converted to a percentage [(number of freezing observations/total numberof observations) × 100].
The effects of strain and trial/trial-block on freezing during conditioning and extinction were analyzed using 2-factor analysis of variance (ANOVA), with repeated measures for trial/trial-block, followed by Fisher’s LSD post hoc tests. As a measure of within-session extinction learning, paired t-tests compared freezing during the first and last trial-blocks of the extinction training session. Strain differences in freezing during extinction retrieval were analyzed using 1-factor ANOVA followed by Newman-Keuls post hoc tests. As a measure of between-session extinction retrieval, paired t-tests compared freezing during extinction retrieval with freezing during the first trial-block of extinction training. The threshold for statistical significance for this and all other analysis was set at p<.05. All data were analyzed using Statview (SAS Institute, Cary, NC).
Hot plate test for nociception
To test for possible differences in pain sensitivity that could contribute to strain differences in freezing, mice were assessed for nociception (>1 week after fear extinction) using the hot plate test as described previously (Karlsson et al. 2005). Mice were placed on a flat plate (Columbus Instruments, Columbus, OH) heated to 55°C, and the latency to show a hindpaw shake or lick was manually timed (maximum response latency: 30 sec). Strain differences were analyzed using 1-factor ANOVA followed by Fisher’s LSD post hoc tests.
Freezing to unconditioned tone
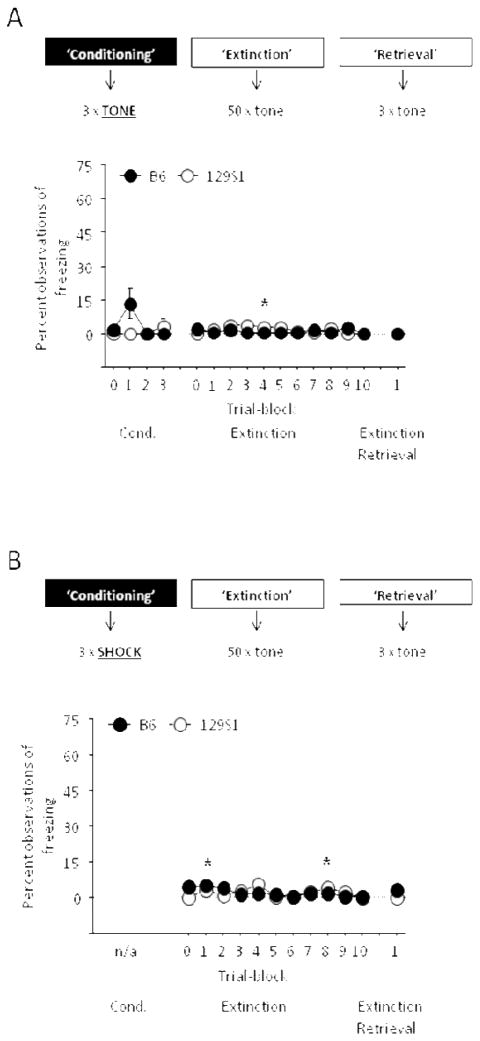
We conducted a control experiment to confirm that elevated freezing to tone during extinction training in 129S1 was not due to a freezing response to the tone per se (i.e., no prior pairing with shock). B6 and 129S1 mice underwent a sham conditioning and extinction procedure identical to the fear conditioning and extinction procedure as above, with the exception that the CS but not US was presented during conditioning (for schematic, see Figure 3A). The effects of strain and trial/trial-block on freezing during ‘conditioning’ and ‘extinction’ were analyzed using 2-factor ANOVA with repeated measures for trial/trial-block. Strain differences in freezing during ‘extinction retrieval’ were analyzed using Student’s t-test. Freezing during each phase was also compared to a hypothesized baseline of zero.
Figure 3.
Comparison of 129S1 and B6 for freezing to unconditioned tone and for possible fear sensitization. (A) Freezing was negligible in 129S1 and B6 mice when tone but not shock was presented during ‘conditioning’ (n=6/strain) (*p<.05 vs. zero baseline in 129S1). (B) Freezing was negligible in 129S1 and B6 mice when shock but not tone was presented during ‘conditioning’ (n=6–8/strain) (*p<.05 vs. zero baseline in B6). Data are Means ± SEM.
Fear sensitization and habituation
We next tested for possible fear sensitization under the same conditions that we examined fear extinction, we subjected B6 and 129S1 to a ‘sham’ conditioning and extinction procedure identical to the fear conditioning and extinction procedure as above, with the exception that the US but not CS was presented during conditioning (for schematic, see Figure 3B). The effects of strain and trial/trial-block on freezing during ‘extinction’ were analyzed using 2-factor ANOVA with repeated measures for trial-block. Strain differences in freezing during ‘extinction retrieval’ were analyzed using Student’s t-test. Freezing during each phase was also compared to a hypothesized baseline of zero.
Stronger fear conditioning procedure
Here, we tested whether 129S1 would exhibit greater expression of the tone memory than B6 under a stronger conditioning protocol. To this end, mice underwent conditioning in the same manner as in the strain survey, but with the exception that there were 5 (as opposed to 3) pairings of white noise and footshock, and footshock was increased to 0.7 (as opposed to 0.6) mA. The conditioning apparatus was a 26 × 30 × 32 cm chamber with transparent walls and a metal rod floor cleaned with water. For conditioning, after a 180 sec acclimation period, mice received 5 pairings between a 120 sec, 80 dB, white noise CS and a 2 sec, 0.7 mA scrambled footshock US, in which the shock was presented during the last 2 sec of the CS. A fixed 120 sec inter-pairing interval was used here given prior evidence that a fixed interval strengthens fear conditioning (Hymowitz 1973). There was a 120 sec no-stimulus consolidation period after the final pairing before mice were returned to the home cage. Twenty-four hr later, the expression of memory for the tone and within-session fear extinction was tested. Mice were placed in a novel context: 26 × 20 × 13 cm cage, illuminated to 10 lux cleaned with a 100% ethanol, and housed in a different room from conditioning. After 180 sec, mice received 15 × 120 sec CS presentations (5 sec no-stimulus interval). Twenty-four hr later, extinction retrieval was tested in the same context as extinction. After 180 sec, mice received 3 × 30 sec CS presentations (5 sec no-stimulus interval). Stimulus presentation was controlled by the Habitest operant system (Coulbourn Instruments, Allentown, PA, USA). Freezing was scored and analyzed as above.
Results
Strain survey of fear conditioning and extinction
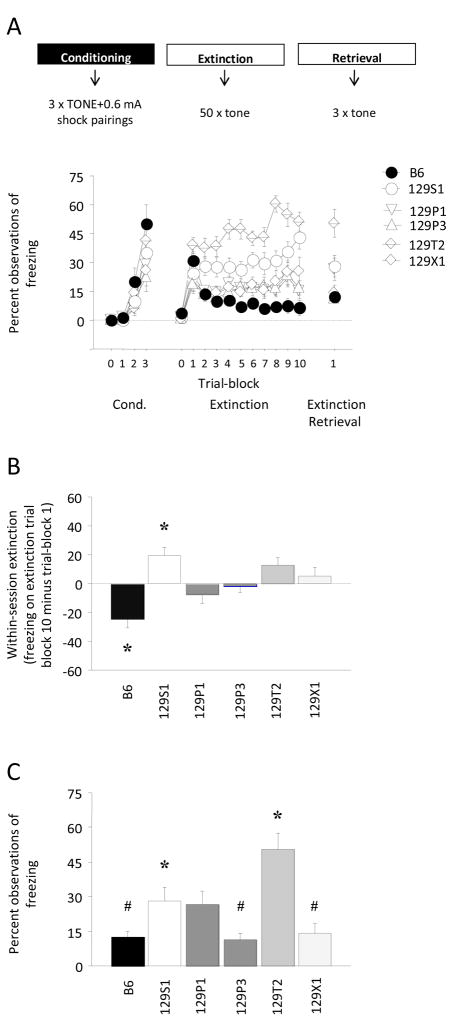
There was a significant effect of trial (F2,172=65.74, p<.01) but not strain and no trial × strain interaction for freezing during conditioning, reflecting an increase in freezing across conditioning trials in all strains (Figure 2A, left). There was a significant trial-block × strain interaction for freezing during the extinction session (F45,774=2.56, p<.01). Post hoc tests found no significant differences in freezing between B6 and any 129 substrain during the first trial-block (i.e., during initial expression of the tone memory (Figure 2A, middle, and Supplemental Table 1)). As compared to B6, freezing was significantly higher in 129T2 on extinction trial-blocks 2–10, higher in 129S1 on trial-blocks 3–10, and higher in 129X1 on trial-block 10. In addition, in comparison to 129S1, there was significantly higher freezing in 129T2 on trial-blocks 4–9, and significantly lower freezing in 129P1 on trial-blocks 2, 6 and 10, in 129P3 on trial blocks 3, 6 and 10, and in 129X1 during trial-blocks 2, 3, 6, and 10. Finally, 129T2 froze significantly more than 129P1, 129P3 and 129X1 during extinction trial-blocks 2–10. Comparing freezing on the first and final extinction trial-blocks as a measure of within-session extinction learning, only B6 showed a significant decrease in freezing and 129S1 showed a significant increase in freezing over the session (Figure 2B).
Figure 2.
Comparison of 129 substrains for fear conditioning, extinction and extinction retrieval. (A) There was an increase in freezing across conditioning trials in all strains but not significant differences between strains. During extinction training, there were no significant differences between strains in freezing during the first trial-block. As compared to B6, freezing was significantly higher in 129T2 on extinction trial-blocks 2–10, higher in 129S1 on trial-blocks 3–10, and higher in 129X1 on extinction trial-block 10. (B) B6, but no other strain, showed within-session extinction, as measured by a significant decrease in freezing from the first to the final extinction trial-block. 129S1 showed a significant increase in freezing during extinction training (*p<.05 vs. extinction trial-block 1 in same strain). (C) During extinction retrieval (see last data point in panel A also), there was significantly higher freezing in the 129S1 and 129T2 substrains than B6 (*p<.05 vs. B6). B6, 129P3 and 129X1 showed a significant decrease in freezing on extinction retrieval relative to the first trial-block of extinction training (#p<.05 vs. extinction trial-block 1). For clarity, significant differences between strains are denoted in Supplemental Table 1. n=14–16/strain. Data are Means ± SEM.
There was a significant effect of strain for freezing during extinction retrieval (F5,86=8.60, p<.01). Post hoc tests showed that freezing was significantly higher in 129S1 and 129T2 as compared to B6 (Figure 2A and, for clarity, Figure 2C). Comparison of freezing during extinction retrieval with freezing during the first trial-block of extinction training demonstrated a significant decrease in freezing in B6 (t=3.62, df=13, p<.01), 129P3 (t=2.21, df=15, p<.05) and 129X1 (t=2.41, df=13, p<.05) while 129S1, 129P1 and 129T2 showed no change.
Hot plate test for nociception
There was a significant effect of strain for latency to respond (F5,49=3.40, p<.01). Post hoc tests showed that response latencies were significantly higher in 129P1 and 129T2 than B6 (Table 1) indicating that lower pain sensitivity could have confounded fear conditioning in these 2 strains.
Table 1.
Comparison of 129 substrains for nociception in the hot plate test. Hot plate response latencies were significantly higher in the 129P1 and 129T2 substrains than B6, but were no different from B6 in the other 3 129 substrains. n=8–10/strain. Data are Means ± SEM seconds to show a response.
| B6 | 129S1 | 129P1 | 129P3 | 129T2 | 129X1 | |
| Latency | 9.7 ± 0.8 | 12.5 ± 2.2 | 15.2 ± 1.9* | 8.5 ± 1.1 | 14.6 ± 6* | 13.0 ± 1.2 |
p<.05 vs. B6.
Freezing to unconditioned tone
Freezing to tone in mice previously exposed to the tone in the absence the US was negligible and no different between strains during any phase of testing (Figure 3A). One exception was significantly more freezing than a hypothesized baseline of zero on trial-block 4 in 129S1 (t=2.71, df=5, p<.05).
Fear sensitization and habituation
Freezing to the CS was negligible and not significantly different between 129S1 or B6 (Figure 3B). Two exceptions were significantly more freezing than a hypothesized baseline of zero on trial-blocks 1 (t=3.97, df=7, p<.05) and 8 (t=2.38, df=7, p<.05) in B6.
Stronger fear conditioning procedure
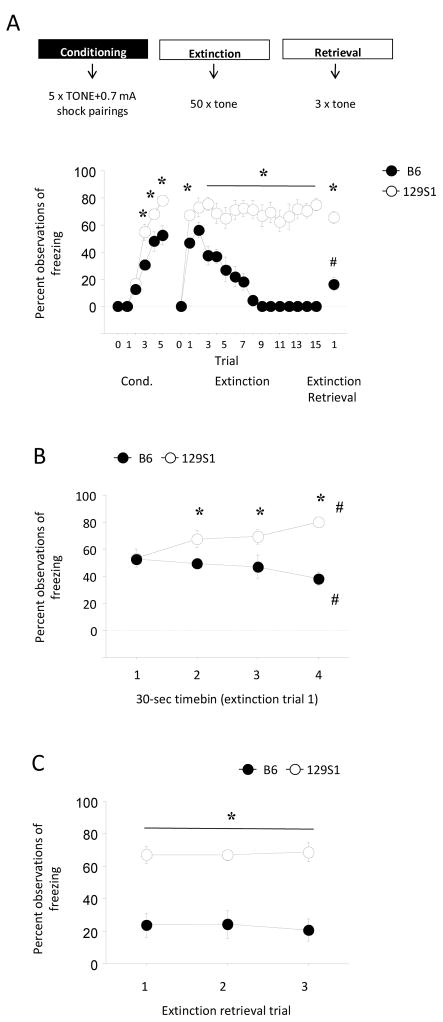
There was a significant trial × strain interaction for freezing during conditioning (F4,44=4.85, p<.01). Post hoc tests showed that freezing was significantly higher in 129S1 than B6 during the 3rd, 4th and 5th trials (Figure 4A). There was also a significant effect of trial/block × strain interaction for freezing during extinction training (F14,182=8.36, p<.01). Post hoc tests found that freezing was higher in 129S1 than B6 on all trials except the 2nd (all p<.05) (Figure 4A). During extinction retrieval, freezing was higher in 129S1 than B6 (t=6.15, df=11, p<.01) (Figure 4A).
Figure 4.
Comparison of 129S1 and B6 for fear conditioning, extinction and extinction retrieval using a stronger conditioning protocol. (A) Freezing was significantly higher in 129S1 than B6 on the 3rd, 4th and 5th conditioning trials, on all trials except the 2nd during extinction training, and on all trials during extinction retrieval (*p<.05 vs. B6/same trial(s), #p<.05 vs. extinction trial-block 1 in B6). (B) Temporal analysis of the first extinction trial revealed that freezing was no different between 129S1 and B6 during the first 30-sec timebin, but significantly differed between strains on subsequent timebins (*p<.05 vs. B6/same timebin). Freezing significant increased over timebins in 129S1 and significantly decreased in B6 (#p<.05 vs. timebin 1). n=5–8/strain. Data are Means ± SEM.
These data show that 129S1 exhibit increased freezing following a relatively strong conditioning protocol. However, the CS duration was longer in this experiment (i.e., 120 sec as opposed to 30 sec) than in our experiments in which freezing was equivalent between B6 and 129S1. Therefore, we examined freezing during the first 30 sec of the first 120-sec extinction CS. Analyses of freezing in discrete 30-sec timebins during this trial revealed a significant strain × bin interaction for freezing (F3,33=5.68, p<.01). Post hoc tests showed that freezing was significantly higher in 129S1 than B6 during the 2nd, 3rd and 4th, but importantly, not the 1st, timebin (all p<.05) (Figure 4B). In addition, paired t-test comparisons of freezing during the first and last timebins indicated a significant decrease in B6 (t=3.20, df=4, p<.05) but a significant increase in 129S1 (t=4.63, df=7, p<.01). We also analyzed the extinction retrieval trial-block as 3 individual trials. This showed a significant effect of strain (F1,11=41.90, p<.01) but no trial and no strain × trial interaction; demonstrating higher freezing in 129S1 than B6 across all 3 retrieval trials, and no change across trials in either strain.
The finding that B6 showed a rapid decrease, and 129S1 a rapid increase, in freezing during a single but relatively lengthy CS, led us to reanalyze the data from our strain survey experiment by breaking down the first extinction trial-block and the extinction retrieval trial-block into individual trials. This analysis revealed a significant effect of trial (F4,344=3.40, p<.01) but not strain and no trial × strain interaction for freezing during extinction (Supplemental Table 2). This indicates that, statistically, freezing changed across the first 5 extinction trial-blocks but not in a manner significantly affected by strain. Analysis of the individual trials of extinction retrieval revealed a significant effect of strain (F5,86=8.60, p<.01) but not trial and no strain × trial interaction for freezing during extinction (Supplemental Table 2). This indicates that the strain differences described above for the data averaged across the 3 trials did not statistically differ as a function of individual trial.
Discussion
We recently identified a common inbred mouse strain, 129S1, that exhibits impaired fear extinction and functional abnormalities in a cortico-amygdala circuit mediating extinction (Hefner et al. 2008; Sotres-Bayon et al. 2008). The major novel finding of the current study was that impaired fear extinction extends to four other 129 substrains. Similar to 129S1, these substrains displayed normal fear (as measured by freezing) as compared to B6, but failed to show significant within-session extinction learning. Consistent with poor extinction learning, 129S1, 129P1 and 129T2 showed no evidence of extinction retrieval. Although 129P3 and 129X1 did display a reduction in freezing during the retrieval test relative to pre-extinction, this between-session reduction is most parsimoniously explained by decay of the original fear response unrelated to extinction because these two substrains showed no extinction learning.
Our data suggest that impaired fear extinction is a phenotypic abnormality common to the four separate genetic lineages of the 129 strains. This conclusion must be qualified by the fact that we only examined one representative substrain from the Steel and Teratoma lines and two substrains from the Parental line. The other dozen or so substrains have yet to be tested for this behavior and we cannot exclude the possibility that some will exhibit intact extinction. Notwithstanding, the current data do not identify a 129 substrain that shows good fear extinction. Such a strain would be very useful to compare with the poor extinguishing 129S1 substrain as a means to expedite the discovery of the genes underlying the differences in extinction efficacy.
The finding that impaired fear extinction appears to be a common phenotypic feature of the 129 strain could have implications for the use of this strain as a source of embryonic stem (ES) cells in the generation of targeted gene mutant mice. The popularity of these strains for this purpose largely stems from the relative success with which 129 ES cells can be derived and then incorporated into the germline of host blastocysts (‘germline competency’) (Evans & Kaufman 1981; Thomas & Capecchi 1987). Due to various abnormal phenotypic features at the molecular (Koike et al. 2006), neuroanatomical (Wahlsten 1982) and behavioral level (Wolfer et al. 1997), 129 strains have not been favored as a genetic background for mutant mice (Crawley et al. 1997). Nonetheless, even with repeated backcrossing onto a more suitable strain, such as B6, a mutant line generated with 129 ES cells will still harbor 129 genes in the region flanking the target mutation (Crusio et al. 2008; Wolfer et al. 2002). Thus, this raises the possibility that impairments in fear extinction attributed to a targeted gene mutation could in fact be a false positive caused by extant 129 flanking genes. It would seem prudent to at least bear this caveat in mind in future studies of fear extinction in mutant mice.
The current study replicates our recent observation (Hefner et al. 2008) of impaired fear extinction in the 129S1 substrain, relative to B6, and extends the finding in a number of ways. To ensure that elevated freezing in 129S1 during extinction training was not due to a fear response to the tone per se, we tested mice under conditions in which they received tone presentations, but not shocks, during conditioning, and found that freezing was negligible in both strains under these conditions. We also tested the possibility that impaired extinction in 129S1 was due to abnormalities in non-associative processes known as fear sensitization and habituation. Fear sensitization produces an increased fear response to a stimulus (e.g., tone) after exposure to an aversive event (e.g., footshocks), even though the tone has not been previously experienced (Kamprath et al. 2006; Kamprath & Wotjak 2004; McSweeney & Swindell 2002). A failure to habituate to this sensitized response with repeated tone presentations would manifest in the same way as impaired fear extinction. Indeed, impaired habitation has been found to contribute to apparent deficits in fear extinction in, for example, cannabinoid CB1 receptor knockout mice (Kamprath et al. 2006). Current results indicate that the deficient extinction in these mice does not appear to reflect impaired habituation of a sensitized fear response, as exposure to the same number and intensity of footshocks (without pairing to tone) was not sufficient to produce sensitized fear in either 129S1 or B6. Taken together, these findings provide further support for the specificity of the fear extinction deficit in the 129S1 strain.
The extinction deficit in 129S1 was observed in the current study using a testing paradigm modified from that used in the Hefner et al., 2008 study which produces more modest levels of fear (e.g., see Norcross et al. 2008). This reduces the potential for ‘ceiling’ levels of fear to confound the assessment of extinction. It was interesting to note that against this modest level of fear, 129S1 showed not only a failure to reduce fear, but demonstrated a modest increase in fear during extinction training. This profile is reminiscent of fear incubation; defined by Eysenck as ‘an increment in conditioned responding over a period of time when the CS is applied once or a number of times, but without reinforcement’ (Eysenck 1968). The mechanisms underlying fear incubation have not yet been as thoroughly described as for those mediating fear extinction (Quirk & Mueller 2008). Previous studies have shown that various experimental manipulations, such as spaced extinction training, administration of the β-adrenergic receptor agonist propranolol or extensive tone-shock conditioning, can produce fear incubation in rats and C57BL/6Tac mice (Cain et al. 2003; Pickens et al. 2009). It will be of great interest to apply these manipulations in future studies to more directly test for a potential fear incubation phenotype in 129S1. Some initial insights into this issue, however, were made in the current study.
We have previously shown that 129S1 and B6 do not differ in fear expression using relatively mild conditioning protocols involving 1 or 3 pairings between tone and 0.6 mA footshock (Hefner et al. 2008). This is consistent with most previous studies that have also found either minimal differences or lesser fear in 129 substrains (including 129P1, 129P3, 129S1, 129S2/SvHsd, 129/SvevTacfBr, 129S6/SvEvTac, 129T2, 129X1) as compared to B6 (Balogh & Wehner 2003; Bolivar et al. 2001; Bothe et al. 2004; Cook et al. 2002; Holmes et al. 2002; Nguyen et al. 2000; Owen et al. 1997; Schimanski & Nguyen 2005). These data do not, however, rule out the possibility that 129 substrains could exhibit increased fear under stronger fear conditioning protocols. Here, we found that 129S1 can exhibit increased freezing during fear conditioning and expression, relative to B6, under a stronger (i.e., more pairings, higher shock intensity) conditioning protocol. Importantly, however, close inspection of the increased freezing during the first extinction trial indicated that immediate fear during the first 30 seconds of the CS was normal (i.e., B6-like) in 129S1, but that fear in these mice rapidly increased above B6 levels over the next 90 seconds of the CS. Thus, elevated 129S1 freezing during the first CS presentation cannot simply be explained by an increase in fear, but instead reflects a more complex response involving a rapid rise in fear during exposure to a long continuous CS. This is again suggestive of an incubation of fear, similar to the effects seen across multiple short-CS exposures discussed above. Alternative interpretations (e.g., timing of shock onset at tone termination) cannot be ruled out at this point, and further studies will be needed to more fully explore fear incubation and possible mechanisms underlying it (e.g., abnormal prelimbicamygdala circuitry, see Vidal-Gonzalez et al. 2006).
In summary, we extend our recent finding that the 129S1 inbred mouse strain exhibits impaired Pavlovian fear extinction by showing that deficiency in this behavior is common across different genetic lineages of the 129 strain. Current results also demonstrate that the extinction deficit in 129S1 was not likely due to non-associative fear sensitization or a fear response to the auditory tone per se. Our data do, however, raise the intriguing possibility that impaired extinction in this strain may reflect (or unmask) a pro-fear incubation-like process. Further studies using this mouse model of impaired extinction could provide insight into the pathophysiology and improved treatment of extinction impairment in neuropsychiatric disorders such as PTSD (Holmes & Wellman 2009).
Supplementary Material
Acknowledgments
Work supported by the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program, the Fonds zur Forderung der Wissenschaftlichen Forschung, NFN-S102 and the German Academic Exchange Service (DAAD).
References
- Adams DJ, Dermitzakis ET, Cox T, Smith J, Davies R, Banerjee R, Bonfield J, Mullikin JC, Chung YJ, Rogers J, Bradley A. Complex haplotypes, copy number polymorphisms and coding variation in two recently divergent mouse strains. Nat Genet. 2005;37:532–536. doi: 10.1038/ng1551. [DOI] [PubMed] [Google Scholar]
- Balogh SA, Wehner JM. Inbred mouse strain differences in the establishment of long-term fear memory. Behav Brain Res. 2003;140:97–106. doi: 10.1016/s0166-4328(02)00279-6. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Pooler O, Flaherty L. Inbred strain variation in contextual and cued fear conditioning behavior. Mamm Genome. 2001;12:651–656. doi: 10.1007/s003350020039. [DOI] [PubMed] [Google Scholar]
- Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav. 2004;3:149–157. doi: 10.1111/j.1601-183x.2004.00064.x. [DOI] [PubMed] [Google Scholar]
- Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comp Med. 2005;55:326–334. [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Temporally massed CS presentations generate more fear extinction than spaced presentations. J Exp Psychol Anim Behav Process. 2003;29:323–333. doi: 10.1037/0097-7403.29.4.323. [DOI] [PubMed] [Google Scholar]
- Cook MN, Bolivar VJ, McFadyen MP, Flaherty L. Behavioral differences among 129 substrains: implications for knockout and transgenic mice. Behav Neurosci. 2002;116:600–611. [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Crusio WE, Goldowitz D, Holmes A, Wolfer D. Standards for the publication of mouse mutant studies. Genes Brain Behav. 2008 doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. A theory of the incubation of anxiety-fear responses. Behav Res Ther. 1968;6:309–321. doi: 10.1016/0005-7967(68)90064-8. [DOI] [PubMed] [Google Scholar]
- Festing MF, Simpson EM, Davisson MT, Mobraaten LE. Revised nomenclature for strain 129 mice. Mamm Genome. 1999;10:836. doi: 10.1007/s003359901099. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur J Neurosci. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav. 2002;1:55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, Cassidy BS, Walsh JP, Goff DC. Extinction Memory Is Impaired in Schizophrenia. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymowitz N. Comparisons between variable-interval and fixed-interval schedules of electric shock delivery. J Exp Anal Behav. 1973;19:101–111. doi: 10.1901/jeab.1973.19-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V, Lutz B, Wotjak CT. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–6686. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Wotjak CT. Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learn Mem. 2004;11:770–786. doi: 10.1101/lm.86104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Holmes A, Heilig M, Crawley JN. Anxiolytic-like actions of centrally-administered neuropeptide Y, but not galanin, in C57BL/6J mice. Pharmacol Biochem Behav. 2005;80:427–436. doi: 10.1016/j.pbb.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Twin studies of psychiatric illness: an update. Arch Gen Psychiatry. 2001;58:1005–1014. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc Natl Acad Sci U S A. 2006;103:3693–3697. doi: 10.1073/pnas.0511189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney FK, Swindell S. Common processes may contribute to extinction and habituation. J Gen Psychol. 2002;129:364–400. doi: 10.1080/00221300209602103. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000;7:170–179. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcross M, Mathur P, Enoch AJ, Karlsson RM, Brigman JL, Cameron HA, Harvey-White J, Holmes A. Effects of adolescent fluoxetine treatment on fear-, anxiety- or stress-related behaviors in C57BL/6J or BALB/cJ mice. Psychopharmacology (Berl) 2008;200:413–424. doi: 10.1007/s00213-008-1215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80:1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. Oxford University Press; London: 1927. [Google Scholar]
- Pickens CL, Golden SA, Adams-Deutsch T, Nair SG, Shaham Y. Long-Lasting Incubation of Conditioned Fear in Rats. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy R, Nissim HA, Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn Mem. 2005;12:399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimanski LA, Nguyen PV. Mouse models of impaired fear memory exhibit deficits in amygdalar LTP. Hippocampus. 2005;15:502–517. doi: 10.1002/hipo.20075. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Langnaese K, Wotjak CT. Differences in extinction of conditioned fear in C57BL/6 substrains are unrelated to expression of alpha-synuclein. Behav Brain Res. 2005;157:291–298. doi: 10.1016/j.bbr.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Corcoran KA, Peters J, Sierra-Mercado D. Neural correlates of individual variability in fear extinction. J Neurosci. 2008;28:12147–12149. doi: 10.1523/JNEUROSCI.4373-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Yee D, Matin A, Nadeau JH, Magnuson T. Genealogy of the 129 inbred strains: 129/SvJ is a contaminated inbred strain. Mamm Genome. 1997;8:390–393. doi: 10.1007/s003359900453. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CM, Daly MJ. Genetic variation in laboratory mice. Nat Genet. 2005;37:1175–1180. doi: 10.1038/ng1666. [DOI] [PubMed] [Google Scholar]
- Wahlsten D. Deficiency of corpus callosum varies with strain and supplier of the mice. Brain Res. 1982;239:329–347. doi: 10.1016/0006-8993(82)90513-3. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Crusio WE, Lipp HP. Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 2002;25:336–340. doi: 10.1016/s0166-2236(02)02192-6. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Muller U, Stagliar M, Lipp HP. Assessing the effects of the 129/Sv genetic background on swimming navigation learning in transgenic mutants: a study using mice with a modified beta-amyloid precursor protein gene. Brain Res. 1997;771:1–13. doi: 10.1016/s0006-8993(97)00673-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.