Abstract
Background
Whether outcome of central nervous system (CNS) cryptococcosis in solid organ transplant (SOT) recipients treated with lipid formulations of amphotericin B is different from that with amphotericin B deoxycholate (AmBd) is not known.
Methods
A cohort comprising consecutive SOT recipients with CNS cryptococcosis in a multicenter study.
Results
Of 75 patients treated with the polyenes as induction regimens, 55 (73.3%) received lipid formulations of amphotericin B and 20 (26.7%) received AmBd. Similar proportions of patients in two groups had renal failure at baseline (p=0.94). Overall mortality at 90 days was 10.9% in the lipid formulations of amphotericin B and 40.0% in the AmBd group. In univariate analysis, non-receipt of calcineurin inhibitors (p=0.034), renal failure at baseline (p=0.016) and fungemia (p=0.003) were significantly associated with mortality. Compared with AmBd, lipid formulations of amphotericin B were associated with a lower mortality (p=0.007). Mortality did not differ in patients receiving lipid formulations of amphotericin B with or without flucytosine (p=0.349). In stepwise logistic regression analysis, renal failure at baseline [odds ratio (OR) 4.61, 95% confidence interval (CI) 1.02-20.80, p=0.047] and fungemia (OR 10.66, 95% CI 2.08-54.55, p=0.004) were associated with an increased mortality while lipid formulations of amphotericin B were associated with a lower mortality (OR 0.11, 95% CI 0.02-0.57, p=0.008).
Conclusion
Lipid formulations of amphotericin B were independently associated with better outcome and may be considered the first-line treatment for CNS cryptococcosis in these patients.
Keywords: cryptococcosis, central nervous system infection, transplants, lipid formulations of amphotericin B
Introduction
Invasive fungal disease is a significant post-transplant complication in solid organ transplant (SOT) recipients [1-3]. Whereas the overall incidence of opportunistic mycoses, particularly those caused by Candida and Aspergillus species appears to have decreased with improvements in transplantation practices and wider use of antifungal prophylaxis, that of cryptococcosis has remained unchanged over last two decades [1, 4-6]. Currently, cryptococcosis is the third most common invasive fungal infection in SOT recipients with an overall incidence of ∼2.8% (range 0.3%-5%) [7]. Central nervous system (CNS) involvement has been documented in 54%-68% of patients with cryptococcal disease, and mortality is approximately 30%-51% despite our expanding antifungal armamentarium [6-9].
Our previous study demonstrated that renal failure at baseline is associated with higher mortality in SOT recipients with cryptococcosis while the receipt of a calcineurin inhibitor agent was independently associated with lower mortality [10]. Whether these observations can also be applied to SOT recipients with CNS cryptococcosis is unknown. Although amphotericin B deoxycholate is active against a wide range of fungi, its use is limited by significant toxicity. Despite lack of nephrotoxocity, echinocandins, a potent antifungal class against yeasts, are not active against cryptococci [11]. On the other hand, lipid formulations of amphotericin B not only maintain the antifunal spectrum of amphotericin B deoxycholate, but also have the advantages of less nephrotoxicity and less infusion-related pro-inflammatory toxicities, and have emerged as the preferred treatment option for SOT recipients with cryptococcosis in the current era [6]. However, little is known about their efficacy for the treatment of CNS cryptococcosis in the SOT population. Flucytosine is a critical component of the treatment of CNS cryptococcosis in HIV-infected patients, but its use as induction therapy in SOT recipients appears to be declining, especially when induction therapy comprises lipid formulations of amphotericin B [6]. Whether such practice affects outcome deserves further investigation.
To date, no studies have probed these issues exclusively in the setting of SOT population. Thus, the goal of the present study was to prospectively assess the variables influencing the mortality of SOT recipients with CNS cryptococcosis, including the effects of induction antifungal regimens.
Patients and Methods
Consecutive SOT recipients with cryptococcosis were prospectively enrolled at participating centers from 2001 through 2007, and patients with documented CNS cryptococcosis comprised the present study population. Patient management was per the standard of care at the participating centers. A detailed description of this cohort has been reported elsewhere [9, 10]. None of the patients were HIV-positive. Cryptococcosis was defined per criteria proposed by the European Organization for Research and Treatment in Cancer and the Mycoses Study Group [12]. Patients were considered to have CNS cryptococcosis if they had positive cerebrospinal fluid (CSF) culture for Cryptococcus or positive CSF cryptococcal antigen. CSF or serum cryptococcal antigen level >1:512 was considered to represent high fungal load as previously reported [13, 14]. Data collected included demographic characteristics, type of organ transplant, immunosuppressive regimen at the time of diagnosis, renal failure at baseline (defined as serum creatinine >2 mg/dL at the time of diagnosis), prior rejection, retransplantation, cytomegalovirus infection or disease, other sites of infection, antifungal therapy employed, and mortality.
Data collected with regards to antifungal therapy included antifungal agents employed and duration of therapy. Lipid formulations of amphotericin B comprised liposomal amphotericin B and amphotericin B lipid complex. Primary therapy (or induction therapy) was defined as receipt of an antifungal agent as initial therapy for cryptococcosis as previously reported [15]. An antifungal regimen to which the patient was switched following induction and continued thereafter was considered maintenance therapy [15]. Mortality was assessed at 90 days after the treatment of CNS cryptococcosis.
Statistical Analyses
Statistical analyses were performed using Intercooled Stata version 9.2 (College Station, TX). Categorical data were compared using the Chi-square test or Fisher exact test. Continuous variables were compared using the ranksum test. A maximum-likelihood logistic model was used to estimate odd ratios and 95% confidence intervals. A multivariable logistic model was used to estimate the effects of multiple variables on a dichotomous endpoint. A backward step wise selection was used. All variables significant at the <0.20 level in the univariate analysis were entered into the model, and then removed in a stepwise design if p>0.20. Interactions among the main effects were examined and the final model was checked with the Hosmer-Lemeshow goodness of fit test. A Cox proportional hazards regression model was used to estimate the survival curves for lipid formulations of amphotericin B and amphotericin B deoxycholate. The end point was date of death prior to day 90 or day 91 in survivors. A log-log plot was constructed to check for violations of the proportional hazards assumption.
Results
CNS cryptococcosis was documented in a total of 80 patients based on positive CSF culture in 77.5% (62/80) and positive CSF antigen in 22.5% (18/80). The demographic and clinical characteristics of these 80 patients are presented in Table 1. CNS cryptococcosis developed a median of 25 months (interquartile range 9-67 months) after transplantation with 68.7% (55/80) of the cases occurring after 1 year post-transplantation. In addition to the CNS, cryptococcal disease involved the lung in 41.2% (33/80) of the patients, skin in 18.7% (15/80), and other sites in 3.7% (3/80) (Table 2). Fungemia was documented in 38.2% (29/80) of the cases, serum cryptococcal antigen >1:512 in 50.9% (28/55), and CSF cryptococcal antigen >1:512 in 36.5% (27/74).
Table 1. Demographic and clinical characteristics of solid organ transplant recipients with central nervous system cryptococcosis (n=80).
| Variables | |
|---|---|
| Age, mean (interquartile range), year | 51.5 (43-60) |
| Male, % (n) | 73.8 (59/80) |
| Type of transplant, % (n) | |
| Kidney | 55.0 (44) |
| Liver | 21.2 (17) |
| Lung | 5 (4) |
| Heart | 5 (4) |
| Pancreas | 2.5 (2) |
| Multi-organ | 11.3 (9) |
| Kidney-pancreas | 6.2 (5) |
| Kidney-heart | 2.5 (2) |
| Kidney-liver | 2.5 (2) |
| Primary immunosuppressive agents, % (n) | |
| Tacrolimus | 68.7 (55) |
| Cyclosporine A | 15.0 (12) |
| Others1 | 16.2 (13) |
| Other immunosuppression, % (n) | |
| Prednisone | 96.2 (77) |
| Dose, median (interquartile range), mg/day | 10 (5-10) |
| Mycophenolate mofetil | 53.7 (43) |
| Anti-T cell antibody | 7.5 (6) |
| Renal failure at baseline | 30.0 (24) |
| Retransplantation | 11.2 (9) |
| Rejection | 22.5 (18) |
| Cytomegalovirus infection | 20.0 (16) |
| Cytomegalovirus disease | 8.7 (7) |
Others included azothioprine (9), mycophenolate mofetil and prednisone (2), rapamycin, mycophenolate mofetil and prednisone (1), and prednisone only (1)
Table 2. Characteristics of central nervous system cryptococcosis in 80 solid organ transplant recipients.
| Variables | % (n) |
|---|---|
| Time to onset of central nervous system cryptococcosis | |
| Median (interquartile range), month | 25.0 (9-67) |
| 0-30 days | 2.5% (2) |
| 31-90 days | 8.7% (7) |
| 91 days – 1 year | 20% (16) |
| >1 year | 68.7% (55) |
| Involved sites other than the central nervous system | |
| Lung | 41.2% (33) |
| Skin | 18.7% (15) |
| Other sites1 | 3.7% (3) |
| Fungemia | 38.2% (29) |
| Serum cryptococcal antigen level, median | 512 |
| Level ≥1:512 | 50.9% (28/55) |
| Cerebrospinal fluid analysis | |
| White blood count, median (interquartile range) | 67 (13-160) |
| Glucose level, median (interquartile range) | 47 (31-69) |
| Cryptococcal antigen level, median (interquartile range) | 64 (8-1024) |
| Level ≥1:512 | 36.5% (27/74) |
| Positive culture | 77.5% (62) |
| Initial antifungal agents2 | |
| Lipid formulations of amphotericin B | 68.8% (55) |
| Duration, median (interquartile range), days | 21 (14-32) |
| Amphotericin B lipid complex | 18 (14-31) |
| Liposomal amphotericin B | 25 (20-31) |
| Dosage3 | |
| Total daily dose, median (interquartile range), mg/day | 350 (300-400) |
| Amphotericin B lipid complex | 350 (300-400) |
| Liposomal amphotericin B | 375 (337.5-400) |
| Daily dose, median (interquartile range), mg/kg/day | 4.5 (3.75-5) |
| Amphotericin B lipid complex | 5 (4.5-5) |
| Liposomal amphotericin B | 4 (3.75-5) |
| Concurrent use of 5-FC4 | 67.3% (37/55) |
| Duration of 5-FC, median (interquartile range), days | 14 (9-23) |
| Amphotericin B deoxycholate | 25.0% (20) |
| Duration, median (interquartile range), days | 20 (14-49) |
| Dosage5 | |
| Total daily dose, median (interquartile range), mg/day | 50 (47.5-62.5) |
| Daily dose, median (interquartile range), mg/kg/day | 1 (0.93-1.19) |
| Concurrent use of 5-FC | 40.0% (8/20) |
| Duration of 5-FC, median (interquartile range), days | 37 (25-49) |
Other sites included peritoneal fluid, bile duct, groin mass, and urine;
5 patients treated with fluconazole alone were excluded from these analyses;
Dosage was available as mg/day in 41 patients and as mg/kg/day in 12 patients;
5-FC= flucytosine;
Dosage was available as mg/day in 7 patients and as mg/kg/day in 8 patients.
Outcome
In all, 75 of 80 patients received a polyene as primary therapy for CNS cryptococcosis; 5 patients treated with fluconazole were excluded from further analyses. The polyenes used were lipid formulations of amphotericin B in 68.8% (55/80) that included liposomal amphotericin B in 32.5% (26/80) and amphotericin B lipid complex in 36.3% (29/80), and amphotericin B deoxycholate in 25% (20/80) (Table 2). Renal failure at baseline was documented in 29% (6/55) of patients receiving lipid formulations of amphotericin B and 30% (6/20) of patients receiving amphotericin B deoxycholate (p=0.94; regression model for this interaction, p=0.482). Thirty-seven (67.3%) of the 55 SOT recipients in lipid formulations of amphotericin B group had concurrent flucytosine use; these included 16 patients in the liposomal amphotericin B group and 21 in the amphotericin B lipid complex group. Of 20 patients receiving amphotericin B deoxycholate, 8 (40%) had concurrent use of flucytosine. Fourteen patients (18.7%,14/75) died while receiving primary therapy. Of 61 patients who survived after the induction period, 49 patients (80.3%, 49/61) received fluconazole as maintenance therapy. Nine patients (14.7%, 9/61) continued the initial therapy without changes, including amphotericin B deoxycholate in 3, amphotericin B lipid complex in 3, and liposomal amphotericin B in 3. The remaining 2 patients (3.3%, 2/61) had no information available. Graft loss was observed in 8% (6/75) of the patients; these included 6/55 patients in the lipid formulations of amphotericin B and 0/20 patients in the amphotericin B deoxycholate group (p=0.18).
CSF analysis was repeated at 2 weeks in 31.2% (25/80) of the patients. Of 23 initially culture positive, the repeat CSF culture became sterile in 56.5% (13/23). Overall 64% of the patients who received a lipid formulation of amphotericin B were culture negative at 2 weeks versus 37% of those who received amphotericin B deoxycholate (p=0.22).
Overall, the mortality rate at 90 days after the treatment of CNS cryptococcosis was 18.7% (14/75). The mortality was 40% (8/20) in patients receiving amphotericin B deoxycholate, 10.3% (3/29) in amphotericin B lipid complex, and 11.5% (3/26) in liposomal amphotericin B. Mortality rate was 20% in patients with CSF culture positive at 2 weeks and 8% in those with culture negative (p=0.57). In amphotericin B deoxycholate group, deaths were considered to be due to cryptococcosis in 3 cases, due to unrelated events in 2 (cardiopulmonary arrest and myocardial infarction, respectively), and of undetermined or unknown etiology in 3. Of 6 patients who died in the lipid formulations of amphotericin B group, 2 died of cryptococcosis, 1 death each was related to myocardial infarction, intraabdominal sepsis with acute respiratory distress syndrome and multiorgan failure, intracranial bleeding and progressive liver failure, and cardiomyopathy.
Candidate variables analyzed as predictors of mortality at 90 days after the treatment of cryptococcosis are outlined in Table 3. In univariate analysis, no receipt of calcineurin inhibitors [odds ratio (OR) 4.28, 95% confidence interval (CI) 1.11-16.49, p=0.034], renal failure at baseline (OR 4.48, 95% CI 1.32-15.09, p=0.016) and fungemia (OR 8.627, 95% CI 2.13-34.88, p=0.003) were significantly associated with mortality. Compared with amphotericin B deoxycholate, lipid formulations of amphotericin B were associated with lower mortality (OR 0.184, 95% CI 0.05-0.62, p=0.007). Additionally, compared with amphotericin B deoxycholate, both liposomal amphotericin B (OR 0.261, 95% CI 0.06-1.04, p=0.058) and amphotericin B lipid complex (OR 0.115, 95% CI 0.02-0.63, p=0.012) were associated with lower mortality, respectively. Mortality did not differ in patients receiving lipid formulations of amphotericin B with or without flucytosine (OR 0.441, 95% CI 0.08-2.44, p=0.349). Age, gender, type of organ transplant, receipt of anti-T cell antibody, prior rejection, and retransplantation, year of cryptococcal diagnosis, time to the onset of CNS cryptococcosis post-transplantation, abnormal mental status, CSF opening pressure >20 cm, positive CSF culture, interval from the onset of CNS cryptococcosis to the initiation of treatment, and flucytosine use were not associated with mortality (Table 3).
Table 3. Variables associated with mortality at 90 days after the treatment of central nervous system cryptococcosis.
| Factor | Reference group | Univariate analysis | Multivariate analysis1 | ||
|---|---|---|---|---|---|
| OR2 (95% CI3) | p value | OR (95% CI) | p value | ||
| Age | Continuous variable | 1.00(0.955-1.05) | .941 | ||
| Female (n=20) | Male (n=55) | 0.398(0.08-1.96) | .258 | ||
| Liver transplant (n=13) | Renal transplant (n=43) | 0.687 (0.13-3.67) | .661 | ||
| Lung transplant (n=4) | Renal transplant | 1.26 (0.12-13.60) | .849 | ||
| Heart transplant (n=4) | Renal transplant | 1.26 (0.12-13.60) | .849 | ||
| Pancreas transplant (n=2) | Renal transplant | UC4 | .325 | ||
| Multiorgan transplant (n=4) | Renal transplant | 1.26 (0.12-13.60) | .849 | ||
| No CNI5 (n=12) | CNI (n=63) | 4.28 (1.11-16.49) | .034 | ||
| Anti-T cell antibody (n=6) | No anti-T cell antibody (n=69) | UC | .586 | ||
| Renal failure at baseline (n=22) | No renal failure at baseline (n=53) | 4.48 (1.32-15.09) | .016 | 4.61(1.02-20.80) | .047 |
| Prior rejection (n=16) | No prior rejection (n=59) | 0.560 (0.11-2.80) | .480 | ||
| Retransplantation (n=9) | No retransplant (n=66) | 1.286 (0.23-6.98) | .771 | ||
| Year of cryptococcal diagnosis | Continuous variable | 0.849 (0.66-1.09) | .209 | ||
| Time to onset post-transplant | Continuous variable | 1.01 (0.99-1.02) | .216 | ||
| Abnormal mental status (n=36) | Normal mental status (n=39) | 2.267 (0.68-7.56) | .183 | ||
| Fungemia (n=28) | No fungemia (n=43) | 8.627 (2.13-34.88) | .003 | 10.66 (2.08-54.55) | .004 |
| OP6 ≥20 cm (n=33) | OP <20 cm (n=10) | 0.55 (0.08-3.58) | .533 | ||
| Positive CSF7 culture (n=60) | Negative CSF culture (n=15) | 3.872 (0.46-32.25) | .211 | ||
| CSF antigen ≥1:512 (n=26) | CSF antigen <1:512 (n=44) | 1.071 (0.31-3.70) | .913 | ||
| Interval from onset to treatment | Continuous variable | 0.996 (0.97-1.02) | .789 | ||
| Lipid formulations of amphotericin B (n=55) | AmBd8 (n=20) | 0.184 (0.05-0.62) | .007 | 0.11 (0.02-0.57) | .008 |
| L-AmB9 (n=26) | AmBd (n=20) | 0.261 (0.06-1.04) | .058 | ||
| ABLC10 (n=29) | AmBd | 0.115 (0.02-0.63) | .012 | ||
| Flucytosine use (n=45) | No flucytosine use (n=30) | 0.865 (0.27-2.80) | .809 | ||
| Lipid formulations of amphotericin B with flucytosine (n=37) | Lipid formulations of amphotericin B without flucytosine (n=18) | 0.441 (0.08-2.44) | .349 | ||
Variables significant at the <0.20 level in the univariate analysis, that is, receipt of calcineurin inhibitors, renal failure at baseline, abnormal mental status, fungemia, and lipid formulations of amphotericin B, were entered into the model and then removed in a stepwise design if p>0.20. Receipt of calcineurin inhibitor agents and abnormal mental status were removed from multivariate model because of p>0.20;
OR= odds ratio;
CI= confidence interval;
UC= unable to calculate; odds ratio was not calculable because of zero value [mortality in pancreas transplant recipients and renal transplant recipients was 0/2 vs. 9/43 (20.9%); mortality in cases with and without anti-T cell antibody was 0/6 vs. 14/69 (20.3%)];
CNI= calcineurin inhibitors;
OP= opening pressure [57.3% (43/75) of the cases had opening pressure recorded];
CSF= cerebrospinal fluid;
AmBd= amphotericin B deoxycholate;
L-AmB= liposomal amphotericin B;
ABLC= amphotericin B lipid complex
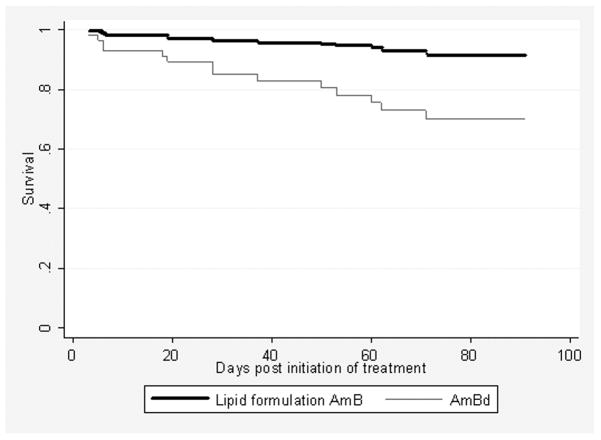
Using stepwise logistic regression analysis with variable significant at the <0.2 level in the model, that is, receipt of calcineurin inhibitors, renal failure at baseline, abnormal mental status, fungemia, and the use of lipid formulations of amphotericin B, only renal failure at baseline (OR 4.61, 95% CI 1.02-20.80, p=0.047) and fungemia (OR 10.66, 95% CI 2.08-54.55, p=0.004) were independently associated with an increased mortality while lipid formulations of amphotericin B were associated with a lower mortality (OR 0.11, 95% CI 0.02-0.57, p=0.008) (Table 3). The Hosmer-Lemeshow goodness of fit for this model showed an overall good fit (p=0.627), and receiver operator curve value was 0.857. The outcome in study patients was also analyzed with Cox proportional hazards regression model (Figure 1). When adjusted for fungemia and renal failure at baseline, the 90-day survival probability with the receipt of lipid formulations of amphotericin B was significantly higher than that with the receipt of amphotericin B deoxycholate (p=0.008). We also evaluated Cox model by creating log-log plots, and the lines were parallel, indicating the proportion-hazards assumption was not violated.
Figure 1.
Cox proportional hazards regression survival curves with the receipt of lipid formulations of amphotericin B (lipid formulation AmB) and amphotericin B deoxycholate (AmBd). Receipt of lipid formulations of amphotericin B remained associated with a significantly improved 90-day survival after adjusted for two other factors associated with increased mortality, renal failure at baseline and fungemia (p=0.008).
Discussion
Despite substantial attributable nephrotoxicity and infusion-related reactions, amphotericin B deoxycholate has been the mainstay of therapy for CNS cryptococcosis for decades. Lipid formulations of amphotericin B retain the antifungal spectrum of amphotericin B deoxycholate with improved toxicity profile and offer an attractive alternative therapeutic option for cryptococcosis. Current guidelines for the management of cryptococcal disease in SOT recipients recommend amphotericin B deoxycholate in combination with flucytosine as the first-line therapy for CNS cryptococcosis and suggest that lipid formulations of amphotericin B may be useful for patients with cryptococcal meningitis and renal insufficiency [16]. The upcoming updated guidelines, on the other hand, preferentially recommend lipid formulations of amphotericin B as induction therapy for CNS and severe non-CNS disease in SOT recipients [17]. The basis of this recommendation is largely the lower incidence of drug associated nephrotoxicity as evidence-based data on improved outcomes with these agents are largely unavailable at present [17]. To our knowledge, our study is the first to systematically evaluate the efficacy of lipid formulations of amphotericin B for CNS cryptococcosis exclusively in SOT recipients.
To date, published reports comparing the efficacy of lipid formulations of amphotericin B with amphotericin B deoxycholate in patients with cryptococcal meningitis remain limited [18, 19]. In a randomized study of 55 patients with HIV-associated cryptococcal meningitis, those treated with amphotericin B lipid complex had a clinical response rate of 86% compared to 65% for patients treated with amphotericin B deoxycholate [18]. In another small randomized trial of 28 HIV-positive patients with cryptococcal meningitis, liposomal amphotericin B sterilized CSF cultures significantly more rapidly than amphotericin B deoxycholate (7-14 days vs. 21 days) although the clinical response rates (86% vs. 80%) were similar [19]. An unpublished randomized, double-blind trial of 267 HIV-infected patients with cryptococcal meningitis showed no difference in the efficacy of liposomal amphotericin B, 3 mg/kg/day or 6 mg/kg/day, and amphotericin B deoxycholate regarding CSF culture conversion at 2 weeks (58.3% or 48% vs. 47.5%) and success at 10 weeks (37% or 49% vs. 53%) however, more nephrotoxicity was observed with the higher dose of liposomal amphotericin B [20, 21]. In a prospective observational study, no differences in outcome existed in patients with CNS cryptococcosis when the combination of amphotericin B plus flucytosine included deoxycholate or one of its lipid formulations [22].
Nevertheless, a meta-analysis showed that lipid formulations of amphotericin B significantly reduced all-cause mortality risk of systemic fungal infections, including cryptococcosis, by an estimated 28% compared with amphotericin B deoxycholate (OR 0.72, 95% CI 0.54-0.97) [23]. Our data show that the use of lipid formulations of amphotericin B was independently associated with a lower mortality in SOT recipients with CNS cryptococcosis (Table 3). The precise reason why lipid formulations of amphotericin B have better efficacy than amphotericin B deoxycholate is not known although animal model data support some hypotheses. Lipid formulations of amphotericin B reduced the fungal burden to a significantly greater degree and achieved higher survival rates than amphotericin B deoxycholate in animal models [24, 25]. In murine cryptococcal meningitis, 20 or 30 mg/kg of liposomal amphotericin B completely cleared the yeast from the brains in 44% and 78% of mice, respectively, whereas 3 mg/kg of amphotericin B deoxycholate did not [24]. In another study, mice with cryptococcal meningitis treated with 10 mg/kg of liposomal amphotericin B had a better survival rate on day 5 than those treated with 1 mg/kg of amphotericin B deoxycholate (50%-60% vs. 12.5%-30%) [25].
Additionally, lipid formulations of amphotericin B have unique immunomodulatory characteristics compared with amphotericin B deoxycholate that may be beneficial during the treatment of cryptococcosis [26]. Liposomal amphotericin B and amphotericin B lipid complex either down-regulate or have no effect on inflammatory cytokine gene expression [26-28]. Liposomal amphotericin B induces Toll-like receptor4 (TLR4) dependent signaling in neutrophils, and amphotericin B deoxycholate induces TLR2-mediated inflammatory cascade in human monocytes and macrophages [29, 30]. TLR4 activation is associated with a more anti-inflammatory pattern of cytokine production and subsequently lesser degrees of inflammatory tissue damage during fungal infection [30]. These anti-inflammatory properties might be derived from the ability of liposomes to lessen the extravasation of neutrophils into sites of inflammation by modifying the intracellular signaling [26, 31, 32]. In addition, empty liposomes improve fungal clearance and survival of corticosteroid-treated mice with invasive pulmonary aspergillosis by attenuating the immunopathology [33]. It has been increasingly recognized that surviving an infection requires a tightly controlled immune system that straddles a fine line between successful eradication of the invading pathogen and limiting the damage to tissues from a dysregulated immune response [34]. It is probable that liposomes not only facilitate delivery of amphotericin B to the site of infection but also limit the subsequent immune-related tissue damaged caused by the antifungal agent.
Flucytosine plays a pivotal role in the treatment of cryptococcal meningitis. It has been well documented that amphotericin B deoxycholate plus flucytosine has significantly greater early fungicidal activity than amphotericin B deoxycholate alone for cryptococcal meningitis in HIV-positive patients not receiving highly active antiretroviral therapy [35, 36]. In addition, lack of flucytosine for induction therapy is independently associated with lack of CSF sterilization at week 2 in HIV-positive patients with cryptococcal meningitis (OR 24.4, 95% CI 4.8-123.5, p<0.001) and with mycological failure at week 2 in HIV-positive or HIV-negative patients with cryptococcosis (OR 3.8, 95% CI 1.9-7.8, p<0.001) [14]. In patients with meningoencephalitis, lack of induction therapy with amphotericin B formulations and flucytosine compared with any other induction therapies was an independent factor of treatment failures at week 2 (OR 51.25, 95% CI 9.67-271.52, p<0.0001) [22]. Furthermore, prescription of flucytosine for less than 14 days was independently associated with treatment failure of cryptococcosis at month 3 [22]. It is not clear why flucytosine did not have such profound effects on SOT recipients with CNS cryptococcosis in our study. The patient populations in the aforementioned studies comprised primarily HIV-infected persons (77%) who typically have higher fungal burden (CSF antigen level >1:512) [14, 22]. Since flucytosine expedites the CSF sterilization in the early stage of treatment, this effect might not be prominent or clinically relevant in the setting of patients with low fungal burden, such as SOT recipients [9]. In addition, most studies used CSF sterilization at week 2 as measure of the efficacy of treatment with flucytosine while the current study employed the mortality at 90 days after the treatment. The small number of our patients receiving flucytosine may also have precluded meaningful analysis.
Several weaknesses of our study deserve to be acknowledged. The data regarding outcomes were systematically assessed using standardized criteria however, our results should be interpreted with caution since this was not a randomized trial evaluating therapeutic efficacy of antifungal regimens. Additionally, while the data analyses controlled for all potential confounders, it is plausible that unknown or unmeasured factors could have influenced outcomes, such as management of immunosuppression after diagnosis of cryptococcosis, development of renal failure during various therapeutic agents, or contribution of other opportunistic infections. We note, however, that the comparison group in our study was concurrent and contemporaneous, thus rendering our result more relevant than if a historic comparator was utilized.
In summary, the lipid formulations of amphotericin B as primary therapy, regardless of flucytosine use were independently associated with improved survival in SOT recipients with CNS cryptococcosis. Given significant implications of these data for the management of CNS cryptococcal disease not only in SOT recipients but in other hosts as well, future studies to validate our findings are warranted.
Acknowledgments
Financial support: National Institutes of Health, National Institute of Allergy and Infectious Diseases (R01 AI 054719-01 to Nina Singh)
Footnotes
Conflict of interest: Barbara D. Alexander has received grant/research support from Astellas, Pfizer, Enzon, and has served on consultant/advisory board for Enzon, Pfizer, Schering-Plough, Basilea, and Abbott Diagnostics. Oliver Lortholary has received research support from Astellas. Graeme Forrest has received research support from Astellas. G. Marshall Lyon has received research support, honoraria, and/or consulted for Astellas, Merck, Pfizer, and Schering-Plough. Leonard Johnson has served on the speaker's bureau of Pfizer. Nina Singh has received grant support from Schering-Plough and Pfizer. Other authors have no conflicts.
References
- 1.Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev. 2005;18:44–69. doi: 10.1128/CMR.18.1.44-69.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paya CV. Fungal infections in solid-organ transplantation. Clin Infect Dis. 1993;16:677–88. doi: 10.1093/clind/16.5.677. [DOI] [PubMed] [Google Scholar]
- 3.Kanj SS, Welty-Wolf K, Madden J, et al. Fungal infections in lung and heart-lung transplant recipients. Report of 9 cases and review of the literature. Medicine (Baltimore) 1996;75:142–56. doi: 10.1097/00005792-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Fortun J, Martin-Davila P, Moreno S, et al. Prevention of invasive fungal infections in liver transplant recipients: the role of prophylaxis with lipid formulations of amphotericin B in high-risk patients. J Antimicrob Chemother. 2003;52:813–9. doi: 10.1093/jac/dkg450. [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Paterson DL, Gayowski T, Wagener MM, Marino IR. Preemptive prophylaxis with a lipid preparation of amphotericin B for invasive fungal infections in liver transplant recipients requiring renal replacement therapy. Transplantation. 2001;71:910–3. doi: 10.1097/00007890-200104150-00016. [DOI] [PubMed] [Google Scholar]
- 6.Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566–76. doi: 10.1086/598936. [DOI] [PubMed] [Google Scholar]
- 7.Singh N, Dromer F, Perfect JR, Lortholary O. Cryptococcosis in solid organ transplant recipients: current state of the science. Clin Infect Dis. 2008;47:1321–7. doi: 10.1086/592690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375–81. doi: 10.3201/eid0703.010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N, Lortholary O, Dromer F, et al. Central nervous system cryptococcosis in solid organ transplant recipients: clinical relevance of abnormal neuroimaging findings. Transplantation. 2008;86:647–51. doi: 10.1097/TP.0b013e3181814e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh N, Alexander BD, Lortholary O, et al. Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortality. J Infect Dis. 2007;195:756–64. doi: 10.1086/511438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denning DW. Echinocandin antifungal drugs. Lancet. 2003;362:1142–51. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 12.Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 13.Lortholary O, Poizat G, Zeller V, et al. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS. 2006;20:2183–91. doi: 10.1097/01.aids.0000252060.80704.68. [DOI] [PubMed] [Google Scholar]
- 14.Dromer F, Mathoulin-Pelissier S, Launay O, Lortholary O. Determinants of disease presentation and outcome during cryptococcosis: the CryptoA/D study. PLoS Med. 2007;4:e21. doi: 10.1371/journal.pmed.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh N, Lortholary O, Alexander BD, et al. Antifungal management practices and evolution of infection in organ transplant recipients with cryptococcus neoformans infection. Transplantation. 2005;80:1033–9. doi: 10.1097/01.tp.0000173774.74388.49. [DOI] [PubMed] [Google Scholar]
- 16.Saag MS, Graybill RJ, Larsen RA, et al. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:710–8. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- 17.Perfect JR, et al. Clinical practice guidelines for the management of cryptococcal disease: 2009 update by the Infectious Disease Society of America. Clin Infect Dis. 2009 doi: 10.1086/649858. in development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharkey PK, Graybill JR, Johnson ES, et al. Amphotericin B lipid complex compared with amphotericin B in the treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1996;22:315–21. [PubMed] [Google Scholar]
- 19.Leenders AC, Reiss P, Portegies P, et al. Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS-associated cryptococcal meningitis. AIDS. 1997;11:1463–71. doi: 10.1097/00002030-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Astellas Pharma US I. Treatment of Cryptococcal Meningitis in HIV-Infected Patients (Study 94-0-013) [cited 2009 March 08]; Available from: http://www.ambisome.com/index2.php?section=about&page=trials.
- 21.Hamill R, Sobel J, El-Sadr W, Johnson P, Graybill JR. Randomized double-blind trial of AmBisome (liposomal amphotericin B) and amphotericin B in acute cryptococcal meningitis in AIDS patients. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA. 1999. [Google Scholar]
- 22.Dromer F, Bernede-Bauduin C, Guillemot D, Lortholary O. Major role for amphotericin B-flucytosine combination in severe cryptococcosis. PLoS ONE. 2008;3:e2870. doi: 10.1371/journal.pone.0002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett JP, Vardulaki KA, Conlon C, et al. A systematic review of the antifungal effectiveness and tolerability of amphotericin B formulations. Clin Ther. 2003;25:1295–320. doi: 10.1016/s0149-2918(03)80125-x. [DOI] [PubMed] [Google Scholar]
- 24.Adler-Moore JP, Proffitt RT. Amphotericin B lipid preparations: what are the differences? Clin Microbiol Infect. 2008;14 4:25–36. doi: 10.1111/j.1469-0691.2008.01979.x. [DOI] [PubMed] [Google Scholar]
- 25.Takemoto K, Yamamoto Y, Ueda Y. Influence of the progression of cryptococcal meningitis on brain penetration and efficacy of AmBisome in a murine model. Chemotherapy. 2006;52:271–8. doi: 10.1159/000095820. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Ami R, Lewis RE, Kontoyiannis DP. Immunocompromised hosts: immunopharmacology of modern antifungals. Clin Infect Dis. 2008;47:226–35. doi: 10.1086/589290. [DOI] [PubMed] [Google Scholar]
- 27.Arning M, Kliche KO, Heer-Sonderhoff AH, Wehmeier A. Infusion-related toxicity of three different amphotericin B formulations and its relation to cytokine plasma levels. Mycoses. 1995;38:459–65. doi: 10.1111/j.1439-0507.1995.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 28.Simitsopoulou M, Roilides E, Dotis J, et al. Differential expression of cytokines and chemokines in human monocytes induced by lipid formulations of amphotericin B. Antimicrob Agents Chemother. 2005;49:1397–403. doi: 10.1128/AAC.49.4.1397-1403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sau K, Mambula SS, Latz E, Henneke P, Golenbock DT, Levitz SM. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. J Biol Chem. 2003;278:37561–8. doi: 10.1074/jbc.M306137200. [DOI] [PubMed] [Google Scholar]
- 30.Bellocchio S, Gaziano R, Bozza S, et al. Liposomal amphotericin B activates antifungal resistance with reduced toxicity by diverting Toll-like receptor signalling from TLR-2 to TLR-4. J Antimicrob Chemother. 2005;55:214–22. doi: 10.1093/jac/dkh542. [DOI] [PubMed] [Google Scholar]
- 31.Devine DV, Wong K, Serrano K, Chonn A, Cullis PR. Liposome-complement interactions in rat serum: implications for liposome survival studies. Biochim Biophys Acta. 1994;1191:43–51. doi: 10.1016/0005-2736(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 32.Eierman DF, Yagami M, Erme SM, et al. Endogenously opsonized particles divert prostanoid action from lethal to protective in models of experimental endotoxemia. Proc Natl Acad Sci U S A. 1995;92:2815–9. doi: 10.1073/pnas.92.7.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis RE, Chamilos G, Prince RA, Kontoyiannis DP. Pretreatment with empty liposomes attenuates the immunopathology of invasive pulmonary aspergillosis in corticosteroid-immunosuppressed mice. Antimicrob Agents Chemother. 2007;51:1078–81. doi: 10.1128/AAC.01268-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casadevall A, Pirofski LA. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Horst CM, Saag MS, Cloud GA, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. N Engl J Med. 1997;337:15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 36.Brouwer AE, Rajanuwong A, Chierakul W, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004;363:1764–7. doi: 10.1016/S0140-6736(04)16301-0. [DOI] [PubMed] [Google Scholar]