Summary
The bacterial tmRNA•SmpB system recycles stalled translation complexes in a process termed ‘ribosome rescue’. tmRNA•SmpB specifically recognizes ribosomes that are paused at or near the 3′ end of truncated mRNA, and therefore nucleolytic mRNA processing is required before paused ribosomes can be rescued from full-length transcripts. Here, we examine the recycling of ribosomes paused on both full-length and truncated mRNAs. Peptidyl-tRNAs corresponding to each paused translation complex were identified, and their turnover kinetics used to estimate the half-lives of paused ribosomes in vivo. Ribosomes were paused at stop codons on full-length mRNA using a nascent peptide motif that interferes with translation termination and elicits tmRNA•SmpB activity. Peptidyl-tRNA turnover from these termination-paused ribosomes was slightly more rapid in tmRNA+ cells (T1/2 = 22 ± 2.2 s), compared to ΔtmRNA cells (T1/2 = 32 ± 1.6 s). Overexpression of release factor-1 (RF-1) greatly accelerated peptidyl-tRNA turnover from termination-paused ribosomes in both tmRNA+ and ΔtmRNA cells, whereas other termination factors had little or no effect on recycling. In contrast to inefficient translation termination, ribosome recycling from truncated transcripts lacking in-frame stop codons was dramatically accelerated by tmRNA•SmpB. However, peptidyl-tRNA still turned over from nonstop-paused ribosomes at a significant rate (t1/2 = 61 ± 7.3 s) in ΔtmRNA cells. Overexpression of RF-1, RF-3, and ribosome recycling factor (RRF) in ΔtmRNA cells failed to accelerate ribosome recycling from nonstop mRNA. These results indicate that tmRNA•SmpB activity is rate-limited by mRNA cleavage, and that RF-3 and RRF do not constitute a tmRNA-independent rescue pathway as previously suggested. Peptidyl-tRNA turnover from nonstop-paused ribosomes in ΔtmRNA cells suggests the existence of another uncharacterized ribosome rescue pathway.
Keywords: peptidyl-tRNA, release factor, ribosome pausing, tmRNA, translation termination
Introduction
Protein synthesis is terminated when the ribosome encounters a stop codon in the A site. In eubacteria, the three “universal” stop codons are recognized by two distinct protein release factors (RF). RF-1 decodes UAA and UAG, whereas RF-2 decodes UAA and UGA stop codons. Upon binding the A-site stop codon, RF-1 and RF-2 coordinate hydrolysis of the nascent protein chain from the P-site tRNA 1; 2, releasing the newly synthesized protein from the ribosome. Additional protein factors are required to disassemble and recycle the post-termination ribosome. Release factor-3 (RF-3) is a non-essential GTP-binding protein that facilitates dissociation of both RF-1 and RF-2 from the post-termination ribosome 3; 4, thereby enhancing translation termination by increasing the available pool of RF-1 and RF-2. After RF-1 or RF-2 are removed (or dissociate) from the post-termination ribosomal A site, ribosome recycling factor (RRF) and elongation factor G (EF-G) collaborate to separate the 70S ribosome into individual 50S and 30S subunits 5. The free ribosomal subunits are then able to initiate protein synthesis on other messages.
It has long been recognized that nascent peptide sequences influence translation termination in Escherichia coli. Isaksson and colleagues extensively characterized the effect of C-terminal dipeptide sequences on translation termination, and found that nascent chains with Asp, Gly, and especially Pro residues significantly increased stop codon readthrough 6; 7. Subsequently, C-terminal Asp-Pro and Pro-Pro nascent peptide sequences were found to induce tmRNA•SmpB activity and A-site mRNA cleavage during translation termination in E. coli 8; 9. A-site cleavage activity truncates the message, producing a nonstop mRNA, which lacks an in-frame stop codon. Ribosomes stalled on nonstop mRNA are unable to undergo canonical translation termination, and are instead “rescued” by the tmRNA•SmpB system in eubacteria 10; 11. tmRNA (transfer-messenger RNA) is a stable RNA that functions both as a tRNA and an mRNA during ribosome rescue. tmRNA binds the A site of stalled ribosomes using its aminoacylated tRNA-like domain, and the nascent chain is rapidly transferred to tmRNA via peptidyl transferase activity. The truncated message is then released, and translation resumes using a small open reading frame within tmRNA 10; 12. This process results in the co-translational addition of the tmRNA-encoded SsrA peptide tag to the nascent protein, followed by translation termination and ribosome recycling. The SsrA peptide is a degradation tag recognized by several bacterial proteases 13; 14; 15. SmpB is a tmRNA-binding protein that is required for delivery of tmRNA to the ribosome, and translation of the SsrA peptide tag 16; 17. Thus, tmRNA•SmpB is a quality control system that recycles stalled ribosomes and targets incompletely synthesized proteins for degradation. Taken together, these observations suggest that specific nascent peptides inhibit RF-mediated translation termination, while still allowing suppressor tRNA and/or tmRNA•SmpB activity on paused ribosomes.
Heretofore, nascent peptide-induced ribosome pausing during translation termination has been inferred from A-site mRNA cleavage and SsrA-peptide tagging activities 8; 9; 18; 19. Although A-site mRNA cleavage and SsrA tagging are generally correlated with ribosome pausing, there are instances where strong translational arrest does not elicit either activity in E. coli 20; 21. Therefore, to more directly examine the kinetics of translational pauses, we used pulse-chase experiments to monitor peptidyl-tRNA turnover from paused ribosomes. During nascent peptide-mediated translational pausing at stop codons, peptidyl-tRNA turnover occurred at similar rates in ΔtmRNA and tmRNA+ cells. In contrast, ribosomes arrested at the ends of nonstop mRNA turned over much more rapidly in tmRNA+ cells than in ΔtmRNA cells. These results are consistent with data showing that tmRNA•SmpB-mediated ribosome rescue requires truncated mRNA 22.
However, paused ribosomes were still recycled from nonstop mRNA in the absence of tmRNA. We demonstrate that RF-3 and RRF do not play a significant role in this tmRNA-independent ribosome recycling. These findings suggest the existence of another, uncharacterized ribosome-recycling pathway.
Results
Peptidyl prolyl-tRNAPro is a marker of termination-paused ribosomes
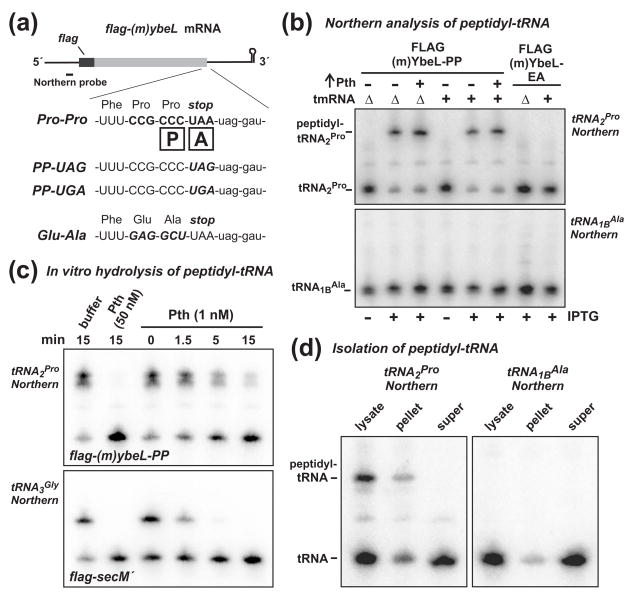
C-terminal Pro-Pro residues in the nascent peptide interfere with translation termination and induce a variety of alternative translation reactions in Escherichia coli 6; 8; 9. We reasoned that peptidyl prolyl-tRNAPro bound to the ribosomal P site should accumulate when ribosomes pause during translation termination. Peptidyl-tRNA has reduced mobility during gel electrophoresis, and can be readily distinguished from aminoacyl-tRNA by Northern blot. To facilitate peptidyl prolyl-tRNA analysis, we generated a mini-gene expression construct based on the previously characterized ybeL-PP gene from E. coli 8; 9. The flag-(m)ybeL-PP mini-gene encodes a 60-residue peptide composed of an N-terminal FLAG epitope fused to the C-terminal 49 residues of YbeL-PP (Fig. 1a). The C-terminal Pro residue of FLAG-(m)YbeL-PP was coded as CCC (Fig. 1a), which is decoded exclusively by tRNA2Pro in E. coli 23. The other five Pro residues in FLAG-(m)YbeL-PP were coded as CCG, which is decoded by either tRNA1Pro or tRNA3Pro 23. Therefore, upon overexpression of FLAG-(m)YbeL-PP, the accumulation of peptidyl prolyl-tRNA2Pro should be indicative of termination-paused ribosomes. Indeed, Northern blot analysis showed that the majority of tRNA2Pro was mobility shifted in cells expressing FLAG-(m)YbeL-PP, but not in uninduced cells (Fig. 1b). Peptidyl prolyl-tRNA2Pro did not accumulate in cells expressing the FLAG-(m)YbeL-EA (Figs. 1a & 1b), whose mRNA does not contain a CCC Pro codon, and should undergo efficient translation termination based on previous results with full-length YbeL-EA protein 8; 9. Moreover, peptidyl alanyl-tRNA1BAla was not detected in cells expressing FLAG-(m)YbeL-EA, indicating that peptidyl-tRNA does not typically accumulate as a result of translation termination (Fig. 1b).
Figure 1. Peptidyl prolyl-tRNA2Pro accumulates during inefficient translation termination.
(a) FLAG-(m)YbeL expression constructs. The flag-(m)ybeL message is shown schematically along with the Northern probe binding site. The 3′ coding sequences of flag-(m)ybeL-PP, -PP(uag), -PP(uga), and -EA are shown. The boxed P and A indicate the positions of the ribosomal P and A sites during translation termination. (b) Northern blot analysis of peptidyl-tRNA. Total RNA was isolated from tmRNA+ and ΔtmRNA cells, resolved on acid-urea polyacrylamide gels, and analyzed by Northern blot hybridization using probes specific for tRNA2Pro and tRNA1BAla. Expression of FLAG-(m)YbeL-PP (+ IPTG) resulted in the accumulation of peptidyl prolyl-tRNA2Pro in both tmRNA+ and ΔtmRNA cells, whereas no peptidyl-tRNA was detected in cells expressing FLAG-(m)YbeL-EA. Overexpression of Pth had no significant effect on peptidyl prolyl-tRNA2Pro levels. (c) Treatment of peptidyl-tRNA with Pth-His6 in vitro. Total RNA from cells expressing FLAG-(m)YbeL-PP or FLAG-SecM′ was treated with purified Pth-His6 for the indicated time, and analyzed by Northern blot hybridization for tRNA2Pro or tRNA3Gly, respectively. (d) Fractionation of peptidyl-tRNA. Cells expressing FLAG-(m)YbeL-PP were broken by French press and fractionated by sucrose density ultracentrifugation. RNA was extracted from the lysate, pellet, and supernatant fractions, and analyzed by Northern blot for tRNA2Pro and tRNA1BAla. Peptidyl prolyl-tRNA2Pro was only found in the high-speed pellet fraction.
Although the observed peptidyl prolyl-tRNA2Pro is likely to be ribosome-associated, peptidyl-tRNA can “drop-off” of paused ribosomes under some circumstances. Drop-off products are rapidly hydrolyzed by peptidyl-tRNA hydrolase (Pth), whereas ribosome-bound peptidyl-tRNA is protected from Pth activity 24; 25. However, because the prolyl-tRNAPro ester bond is apparently resistant to hydrolysis during translation termination, it is possible that Pth hydrolyzes this product slowly, allowing peptidyl prolyl-tRNA2Pro to accumulate off of the ribosome. To test this hypothesis, we used purified His6-tagged Pth (Pth-His6) to hydrolyze peptidyl prolyl-tRNA2Pro in vitro. At low Pth-His6 concentrations (1 nM), peptidyl prolyl-tRNA2Pro was hydrolyzed more slowly than the control peptidyl glycyl-tRNA3Gly isolated from cells expressing the secM′ mini-gene (Fig. 1c) 20; 26. However, peptidyl prolyl-tRNA2Pro was rapidly and completely and hydrolyzed at higher concentrations (50 nM) of Pth-His6 (Fig. 1c). Because the intracellular concentration of Pth is ~ 1 μM in E. coli 27, we suspect that there is sufficient activity to efficiently hydrolyze any peptidyl prolyl-tRNA drop-off products in vivo. This conclusion was supported by experiments in which we overproduced wild-type Pth from an arabinose-inducible promoter, and observed no decrease in peptidyl prolyl-tRNA2Pro levels (Fig. 1b). Finally, we fractionated cell lysates using sucrose density ultracentrifugation, and found that peptidyl prolyl-tRNA2Pro partitioned with ribosomes in the pellet fraction, whereas most of the free tRNA2Pro remained in the supernatant fraction (Fig. 1d). We note that peptidyl prolyl-tRNA2Pro levels decayed significantly during the process cell lysis and ultracentrifugation (compare Figs. 1b & 1d). Taken together, these results strongly suggest that peptidyl prolyl-tRNA2Pro is bound the P site of paused ribosomes.
Peptidyl prolyl-tRNA2Pro accumulates in tmRNA+ cells
The C-terminal Pro-Pro nascent peptide induces SsrA-peptide tagging in E. coli 8. During the peptide tagging process, the nascent chain is transferred from tRNAPro to tmRNA, and the paused ribosome is recycled into individual 30S and 50S subunits. Therefore, tmRNA•SmpB-mediated ribosome rescue is predicted to diminish, or perhaps even prevent, the accumulation of peptidyl prolyl-tRNA2Pro. However, the same levels of peptidyl prolyl-tRNA2Pro were observed in tmRNA+ and ΔtmRNA cells (Fig. 1b). It is possible that FLAG-mYbeL-PP overexpression overwhelmed the capacity of tmRNA•SmpB, allowing paused ribosomes to accumulate. To test this proposal, we overproduced tmRNA•SmpB from a multi-copy plasmid and examined peptidyl prolyl-tRNA2Pro levels. Though tmRNA•SmpB was overexpressed ~30-fold compared to wild-type cells, there was no significant decrease in peptidyl prolyl-tRNA2Pro by Northern analysis (data not shown).
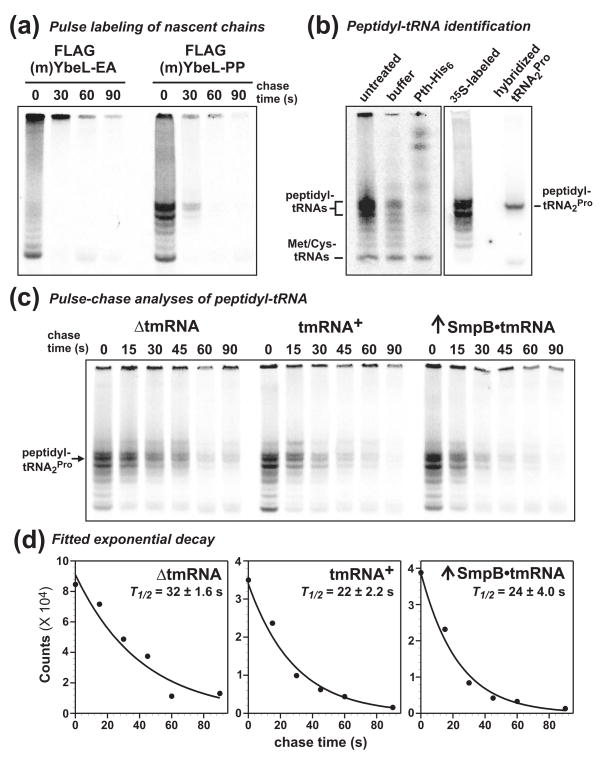
To determine whether static peptidyl-tRNA levels reflect the kinetics of translational pausing, we examined peptidyl prolyl-tRNA2Pro turnover using pulse-chase analysis. Nascent chains were pulse-labeled with [35S]-labeled methionine/cysteine, chased with excess unlabeled amino acids, and peptidyl-tRNA analyzed by gel electrophoresis and autoradiography. A ladder-like pattern of radiolabeled species was observed in samples taken from cells expressing FLAG-(m)YbeL-PP, whereas fewer products were observed in cells expressing FLAG-(m)YbeL-EA (Fig. 2a). In contrast, no high-molecular weight species were radiolabeled in uninduced cells (data not shown). To confirm that radiolabeled products were indeed peptidyl-tRNAs, we treated a sample with purified Pth-His6 in vitro. Pth-His6 converted most of the radiolabeled products into species of lower gel mobility (Fig. 2b). However, the smallest radiolabeled product was unaffected by Pth-His6 treatment (Fig. 2b). These data indicate that the higher molecular weight species are peptidyl-tRNAs, whereas the smallest product is most likely radiolabeled methionyl-tRNAMet and cysteinyl-tRNACys, which are not Pth substrates 28; 29. The multiple peptidyl-tRNA species observed in cells expressing FLAG-(m)YbeL-PP is suggestive of ribosome queuing behind the termination-arrested ribosome. To identify which peptidyl-tRNA species corresponds to peptidyl prolyl-tRNA2Pro, we directly compared the migration position of blotted [35S]-labeled peptidyl-tRNA with that of peptidyl prolyl-tRNA2Pro identified by Northern blot hybridization (Fig. 2b). The lower band of the prominent radiolabeled doublet co-migrated with peptidyl prolyl-tRNA2Pro (Fig. 2b); and therefore the decay of this radiolabeled species was quantified to determine the kinetics of paused ribosome recycling.
Figure 2. Pulse-chase analysis of peptidyl-tRNA turnover.
(a) Pulse labeling of nascent chains. Cells expressing FLAG-(m)YbeL-PP and FLAG-(m)YbeL-EA were pulse labeled with [35S]-methionine/cysteine, then chased with unlabeled amino acids for the indicated times. Peptidyl-tRNA was precipitated from cell lysates, resolved on acid-urea polyacrylamide gels, and visualized by phosphorimaging. High molecular mass radiolabeled species were only detected in cells expressing FLAG-(m)YbeL-PP. (b) Peptidyl-tRNA identification. An [35S]-labeled sample was treated with purified Pth-His6 in vitro, confirming that higher molecular weight radiolabeled species were peptidyl-tRNAs. [35S]-labeled and unlabeled samples from cells expressing FLAG-(m)YbeL-PP were resolved by acid-urea gel, and blotted to nylon membrane. The unlabeled sample lane was cut from the membrane, hybridized to [32P]-labeled tRNA2Pro probe, and then re-aligned to the [35S]-labeled sample blot for phosphorimaging. (c) Pulse-chase analysis of peptidyl-tRNA turnover. tmRNA+ and ΔtmRNA cells expressing FLAG-(m)YbeL-PP were pulse labeled, and peptidyl-tRNA turnover examined as a function of time. The rates of peptidyl-tRNA turnover were similar in ΔtmRNA and tmRNA+ cells, as well as cells overexpressing tmRNA•SmpB. (d) Kinetics of peptidyl prolyl-tRNA2Pro turnover. Radiolabeled peptidyl prolyl-tRNA2Pro was quantified by phosphorimager, and double exponential decay equations were fitted to calculate composite half-life (T1/2) values. Averaged half-life values (± standard error) were determined from at least three independent pulse-chase experiments.
Pulse-chase experiments showed that peptidyl prolyl-tRNA2Pro turnover was similar in tmRNA+ and ΔtmRNA cells (Fig. 2c). Although peptidyl-tRNA turnover was adequately described by single exponential decay, we chose to fit double exponentials to the data because there are at least two peptidyl prolyl-tRNA2Pro decay processes in tmRNA+ cells: RF-mediated termination and tmRNA•SmpB-mediated ribosome rescue. For consistent analyses, and because the fits were generally better, double exponentials were also fitted to data from ΔtmRNA cells. The composite half-life (T1/2) of peptidyl prolyl-tRNA2Pro decay was 22 ± 2.2 s in tmRNA+ cells, and 32 ± 1.6 s in ΔtmRNA cells (Table 1 and Fig. 2d). Overproduction of tmRNA•SmpB did not increase the rate of peptidyl prolyl-tRNA2Pro turnover (Table 1, Figs. 2c, and 2d), indicating that peptidyl-tRNA turnover is not limited by insufficient tmRNA•SmpB capacity. Moreover, overexpression of Pth had no effect on peptidyl prolyl-tRNA2Pro turnover in ΔtmRNA cells (Table 1).
Table 1.
Peptidyl-tRNA decay half-livesa
|
Pro-Pro-stop mRNA (T1/2 in sec) |
nonstop mRNA (t1/2 in sec) |
||
|---|---|---|---|
| Expressed translation factor | ΔtmRNA | tmRNA+ | ΔtmRNA |
| none | 32 ± 1.6 | 22 ± 2.2 | 61 ± 7.3 |
| SmpB•tmRNA | NDb | 24 ± 4.0 | NDb |
| RF-1 | NFc | NFc | 71 ± 10 |
| RF-3 | 33 ± 5.2 | 15 ± 0.3 | 60 ± 0.4 |
| RRF | 28 ± 0.9 | 25 ± 3.2 | 65 ± 11 |
| RF3-RRF | 26 ± 0.4 | 20 ± 1.8 | 84 ±13 |
| Pth | 30 ± 4.8 | 25 ± 2.9 | 54 ± 4.6 |
T1/2 values were determined from fitted double-exponential, and t1/2 values from single-exponential decay equations as described in Methods. Reported values are the mean ± the standard error of measurement (SEM).
Not determined.
Not fitted. Peptidyl-tRNA decay was too rapid for exponential fitting.
Overexpression of RF-1 accelerates peptidyl prolyl-tRNA2Pro turnover during translation termination
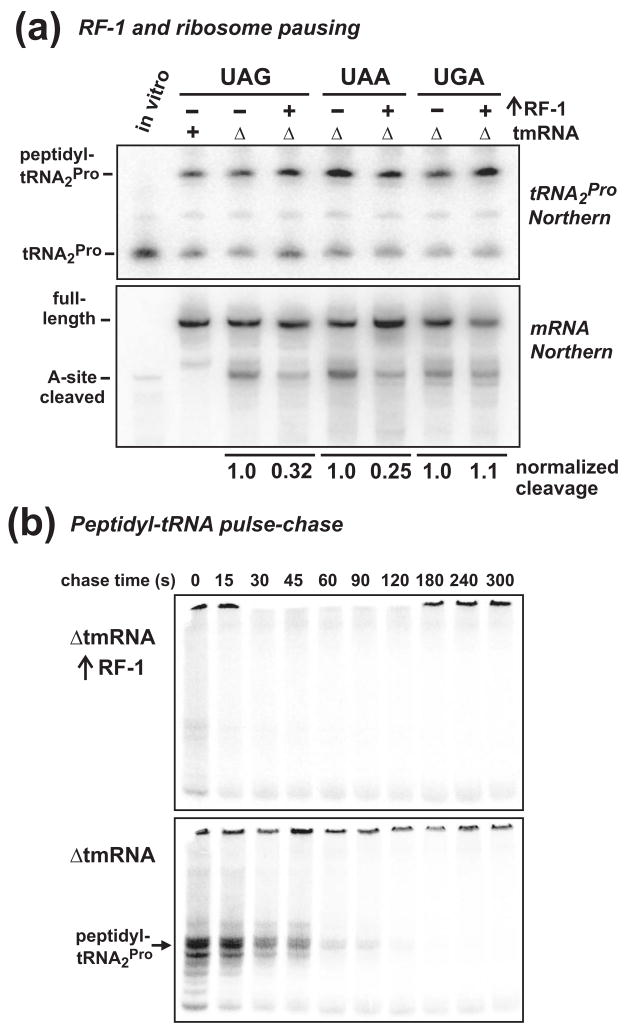
Previous work has shown that RF-1 overexpression suppresses both A-site mRNA cleavage and SsrA-peptide tagging in the YbeL-PP system, presumably by increasing translation termination efficiency 8; 9. Because RF-1 decodes UAA and UAG, but not UGA stop codons, we predicted that peptidyl prolyl-tRNA2Pro levels would be reduced in a stop codon-specific manner upon RF-1 overexpression. However, RF-1 had no effect on peptidyl prolyl-tRNA2Pro accumulation during translation termination at all three stop codons (Fig. 3a). Despite this unexpected result, RF-1 overexpression clearly suppressed A-site cleavage at UAG and UAA stop codons, but not at the UGA codon (Fig. 3a), consistent with codon-specific relief of pausing during translation termination. To resolve this apparent inconsistency, we analyzed peptidyl-tRNA turnover using pulse-chase experiments, which showed that RF-1 overexpression dramatically increased the rate of peptidyl-tRNA turnover in both ΔtmRNA and tmRNA+ cells (Fig. 3b, and data not shown). In fact, pulse-labeled peptidyl-tRNA was difficult to detect when RF-1 was overproduced (Fig. 3b). The disparity between static and kinetic views of peptidyl-tRNA may be a consequence of ribosome queuing. Soon after peptidyl-tRNA hydrolysis and/or paused ribosome recycling, a queued ribosome would rapidly advance to the stop codon and pause, thereby maintaining peptidyl prolyl-tRNA2Pro at high static levels. Because of the discrepancy between peptidyl-tRNA analyses in this instance, we implemented pulse-chase assays for the remainder of our studies.
Figure 3. RF-1 overexpression and nascent peptide-induced ribosome pausing.
(a) Northern blot analysis of tRNA2Pro and mRNA during translational pausing. FLAG-(m)YbeL-PP was synthesized from constructs containing UAG, UAA, and UGA stop codons, with and without co-expression of RF-1. Peptidyl prolyl-tRNA2Pro levels were similar for all constructs, and were not affected by RF-1 overproduction. Northern blot of mRNA showed that RF-1 overexpression significantly reduced A-site cleavage at UAG (32% of control) and UAA (25% of control) stop codons, but not at the UGA (105% of control) codon. A-site cleavage products do not accumulate in tmRNA+ cells. (b) RF-1 overexpression and peptidyl-tRNA turnover in tmRNA cells. FLAG-(m)YbeL-PP nascent chains were pulse labeled with [35S]-methionine/cysteine, then chased with unlabeled amino acid for the indicated times. RF-1 overexpression dramatically increased the rate of peptidyl prolyl-tRNA2Pro turnover in both ΔtmRNA and tmRNA+ cells (data not shown).
The Pro-Pro nascent peptide inhibits RF-1 activity
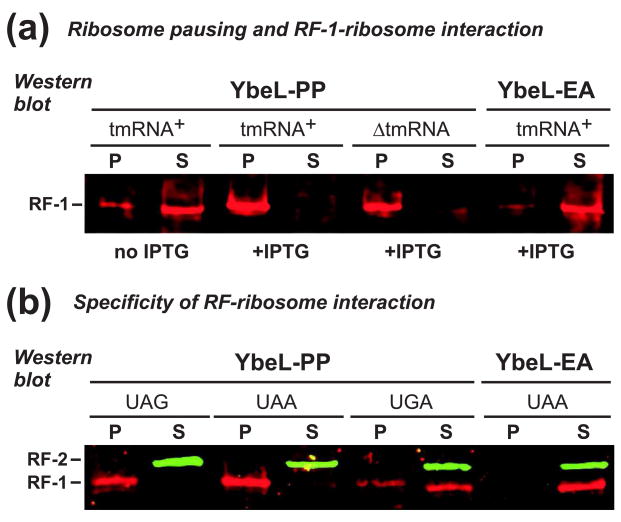
In principle, the Pro-Pro nascent peptide could inhibit RF-mediated peptidyl-tRNA hydrolysis, or alternatively, decrease RF binding to the ribosomal A site. Based on two precedents, we hypothesized that this nascent peptide inhibits subsequent peptidyl-tRNA hydrolysis. The E. coli TnaC and the human cytomegalovirus UL4 uORF2 nascent peptides both contain C-terminal Pro residues that are critical for ribosome pausing during translation termination 30; 31. Both TnaC- and UL4-arrested ribosomes have RF bound stably in the A site, but fail to hydrolyze peptidyl-tRNA 32; 33. To determine whether RFs bind paused ribosomes in our system, we fractionated cell lysates using sucrose density ultracentrifugation, and analyzed the supernatant and pellet fractions by Western blot using antibodies specific for E. coli RF-1. We used full-length YbeL proteins in these experiments because the longer nascent chain helped stabilize paused ribosome complexes during ultracentrifugation (data not shown). In the absence of YbeL-PP expression, most RF-1 was found in the supernatant fraction (Fig. 4a). Induction of YbeL-PP expression in both tmRNA+ and tmRNA cells caused RF-1 to partition in the pellet fraction (Fig. 4a). In contrast, nearly all RF-1 was found in the supernatant fraction from cells expressing YbeL-EA (Fig. 4a). These results suggest that RF-1 is associated with termination-paused ribosomes. To determine whether RF-1 is bound in the ribosomal A site, we examined RF partitioning in cells expressing YbeL-PP from messages containing UAG, UAA, and UGA stop codons. RF-1 was found in the pellet fraction only when YbeL-PP was synthesized from messages containing UAG or UAA stop codons (Fig. 4b), indicating that RF-1 is likely bound to the A site in codon-specific manner. In contrast, RF-2 was found primarily in the supernatant fraction irrespective of stop codon (Fig. 4b).
Figure 4. RF-1 interacts with the ribosome during translational pausing.
(a) Anti-RF-1 Western blot. Cell lysates were fractionated by sucrose density ultracentrifugation, and the pellet (P) and supernatant (S) fractions analyzed by Western blot. RF-1 was found primarily in the supernatant fraction of uninduced cells (no IPTG), and cells expressing YbeL-EA. In contrast, RF-1 partitioned to the pellet fraction in tmRNA+ and ΔtmRNA cells expressing YbeL-PP. (b) Anti-RF-1/RF-2 Western blot. Lysates from tmRNA+ cells expressing YbeL-PP from messages with UAG, UAA, and UGA stop codons were fractionated by ultracentrifugation, and analyzed by Western blot. RF-1 partitioned in the pellet fraction upon expression of the UAG and UAA constructs, but was found in the supernatant with the UGA message. RF-2 was found in the supernatant fraction for all constructs.
Effect of RF-3 and RRF on ribosome pausing during translation termination
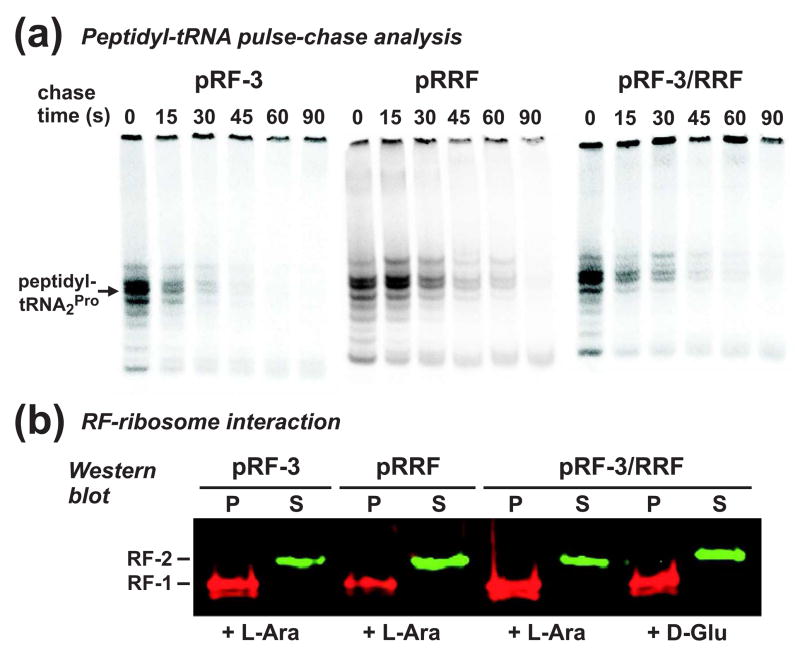
We have previously shown that RF-3 and RRF modulate SsrA-peptide tagging during inefficient translation termination 8. Presumably, these factors facilitate efficient translation termination and canonical ribosome recycling; although several reports have shown that RF-3 and RRF collaborate to remove intact peptidyl-tRNAs from the ribosome 21; 25; 34; 35. We examined the effects of RF-3 and RRF on translational pausing during termination using pulse-chase analysis of peptidyl-tRNA turnover. RF-3 overexpression increased the rate of peptidyl prolyl-tRNA2Pro turnover in tmRNA+ cells, but had no significant effect on ΔtmRNA cells (Table 1 and Fig. 5a). This result perhaps suggests that RF-3 increases termination efficiency during translation of the SsrA peptide tag, which would allow tmRNA to recycle more rapidly. Overexpression of RRF did not dramatically change peptidyl prolyl-tRNA2Pro turnover in either tmRNA+ or ΔtmRNA cells, whereas the coexpression of RF-3 and RRF slightly increased turnover in ΔtmRNA cells (Table 1 and Fig. 5a). We also used density ultracentrifugation to examine RF-ribosome interaction in cells overexpressing RF-3 and/or RRF, and found no changes compared to control cells in which RF-3/RRF expression was not induced (Fig. 5b).
Figure 5. Effect of RF-3 and/or RRF overexpression on nascent peptide-induced ribosome pausing.
(a) Peptidyl-tRNA turnover in tmRNA+ cells overexpressing RF-3 and/or RRF. FLAG-(m)YbeL-PP nascent chains were pulse labeled with [35S]-methionine/cysteine, then chased with unlabeled amino acids for the indicated time. The T1/2 of peptidyl prolyl-tRNA2Pro turnover was 15 ± 0.3 s during RF-3 overexpression, 25 ± 3.2 s during RRF overexpression, and 20 ± 1.8 s during RF-3/RRF co-overexpression. (b) RF-3 and RRF overexpression and RF-ribosome interaction. Lysates from tmRNA+ cells co-expressing YbeL-PP (UAA) along with RF-3 and/or RRF were fractionated by density ultracentrifugation, and analyzed by Western blot. Expression of RF-3 and/or RRF (+ L-Ara) had no effect on the RF-1-ribosome interaction, compared to control cells without RF-3/RRF induction (+ D-Glu).
Peptidyl-tRNA turnover during translational pausing on nonstop mRNA
We next examined the kinetics of peptidyl-tRNA turnover from ribosomes paused on nonstop messages, which lack in-frame stop codons. We removed the stop codon from flag-(m)ybeL-PP and fused the open reading frame to the rrnB T1 transcription terminator (Fig. 6a). The rrnB T1 terminator effectively terminates T7 RNA polymerase transcription 36, and should produce an mRNA with no in-frame stop codons (Fig. 6a). Proteins synthesized from nonstop messages are efficiently tagged by the tmRNA•SmpB system 12; 37, and therefore we confirmed SsrA-tagging of FLAG-(m)YbeL-rrnB in cells expressing tmRNA(His6). tmRNA(His6) encodes the SsrA(His6) peptide tag, which is resistant to proteolysis and allows purification and identification of tagged proteins 38; 39. We used mass spectrometry to identify SsrA(His6)-peptide tagging sites after residues Gly74, Leu75, and Ser76 (Fig. 6b), consistent with translation of a nonstop message truncated at or near these codons. In ΔtmRNA cells, we identified peptidyl-tRNAs by Northern blot using probes specific for tRNA1Ser, tRNA2Leu, and tRNA3Gly, consistent with the expression of a bona fide nonstop message (Figs. 6a, 6c, and data not shown). Although each peptidyl-tRNA species was not abundant, its accumulation was dependent upon nonstop message expression and was not detected in tmRNA+ cells (Fig. 6c, and data not shown). Additionally, a negative control blot for tRNA1BAla showed no evidence of peptidyl-tRNA (Fig. 6c). Pulse-chase analysis showed that nonstop-paused ribosomes turned over much more rapidly in tmRNA+ cells compared to tmRNA cells (Fig. 6d). Because the number of ribosome-recycling pathways is unknown for nonstop mRNA, we fitted single exponential decay equations to these data. Peptidyl-tRNA turned over with t1/2 = 61 ± 7.3 s in ΔtmRNA cells, whereas peptidyl-tRNA could not be detected in tmRNA+ cells (Table 1 and Fig. 6d). These results are consistent with in vitro data showing that tmRNA•SmpB rescues ribosomes from truncated messages much more efficiently than from full-length mRNA 22.
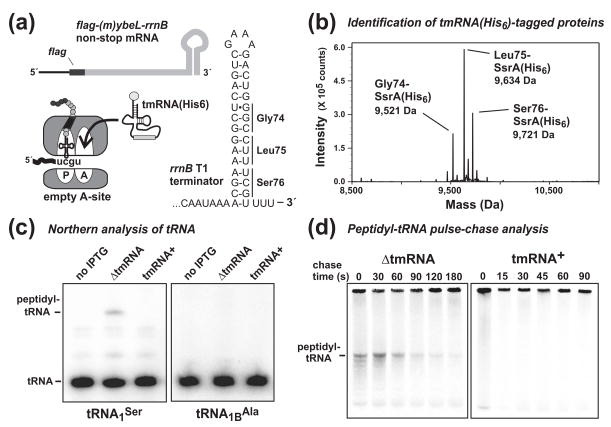
Figure 6. Characterization of the flag-(m)ybeL-rrnB nonstop message.
(a) Schematic of the flag-(m)ybeL-rrnB nonstop mRNA. The nucleotide sequence of the E. coli rrnB T1 intrinsic transcription terminator is shown, along with the positions of codons encoding residues Gly74, Leu75, and Ser76. Ribosomes translate to the 3′ end on nonstop messages, allowing efficient tmRNA•SmpB recruitment to the empty A site. (b) Identification of SsrA(His6)-tagging sites by mass spectrometry. FLAG-(m)YbeL-rrnB was expressed in tmRNA(His6) cells, and SsrA(His6)-tagged proteins purified by Ni2+-NTA affinity chromatography. Mass spectrometry identified three species corresponding to tagging after residues Gly74 (calculated mass, 9,522.7 Da), Leu75 (calculated mass, 9,635.8 Da), and Ser76 (calculated mass, 9,722.9 Da). (c) Northern analysis of peptidyl-tRNA. Peptidyl seryl-tRNA1Ser was dependent upon FLAG-(m)YbeL-rrnB expression, and not observed in tmRNA+ cells. A negative control blot for tRNA1BAla detected no peptidyl-tRNA. (d) Pulse-chase analysis of peptidyl-tRNA turnover during translational pausing on nonstop mRNA. Nascent chains were radiolabeled with [35S]-methionine and cysteine, then chased with unlabeled amino acids. Radiolabeled peptidyl-tRNA decayed with t1/2 = 61 ± 7.3 s in ΔtmRNA cells, but was not detected in tmRNA+ cells.
Translation termination factors do not influence ribosome pausing on nonstop mRNA
In contrast to translational pausing at stop codons, ribosomes arrested on nonstop mRNA are unable to turnover via canonical RF-mediated translation termination. Indeed, overexpression of RF-1 had no effect on peptidyl-tRNA turnover from nonstop-paused ribosomes (Table 1). RRF has recently been proposed to promote peptidyl-tRNA drop-off from nonstop-paused ribosomes arrested in both ΔtmRNA and tmRNA+ cells 40. However, we found that the rate of peptidyl-tRNA turnover from nonstop-paused ribosomes was unaffected in ΔtmRNA cells overexpressing either RF-3 or RRF; and the turnover rate actually decreased when RF-3 and RRF were co-expressed (Table 1). These results demonstrate that RRF and RF-3 do not accelerate ribosome recycling from nonstop mRNA. To further examine the role of RRF and RF-3 in paused ribosome recycling, we asked whether these factors influence tmRNA•SmpB activity on nonstop-arrested ribosomes. We reasoned that if RRF and RF-3 possess robust ribosome rescue activity, then they should effectively compete with tmRNA•SmpB for the ribosomal A site, and inhibit SsrA-peptide tagging. To test this hypothesis, we used a well-characterized nonstop mRNA encoding λN-trpAt, which is the N-terminal domain of λ phage cI repressor fused to the trp operon attenuator-transcription terminator (Fig. 7a) 13; 37. Nearly all λN-trpAt chains are SsrA-tagged in tmRNA+ cells, and these products are stable in cells lacking the ClpP protease, which is responsible for the bulk of SsrA-tagged protein degradation in E. coli 13; 37. In contrast, untagged λN-trpAt accumulates to high levels in ΔtmRNA cells (Fig. 7a). SsrA-tagged and untagged λN-trpAt proteins are readily resolved from one another by SDS-PAGE, allowing SsrA-tagging efficiency to be assessed by quantifying the ratio of tagged to untagged protein (Fig. 7a). We first used pulse-chase analysis to confirm that peptidyl-tRNA accumulated in ΔtmRNA cells expressing λN-trpAt, but not in tmRNA+ cells (Fig. 7b). We then co-expressed λN-trpAt with RF-1, RF-3, RRF, and RF-3/RRF in tmRNA+ cells deleted for the ClpP protease. Examination of whole cell lysates by SDS-PAGE and Coomassie staining showed that SsrA-peptide tagging was not inhibited by any of the overexpressed translation factors (Fig. 7c). Analysis of purified λN-trpAt by SDS-PAGE confirmed that SsrA-tagging efficiency was unaltered under each condition (Fig. 7c). We note that the overproduced translation factors were readily identified on Coomassie stained gels of cell lysates (Fig. 7c). In fact, RRF was expressed at approximately the same level as λN-trpAt (Fig. 7c). These results indicate that RRF and RF-3 play no significant role in ribosome rescue from nonstop mRNA.
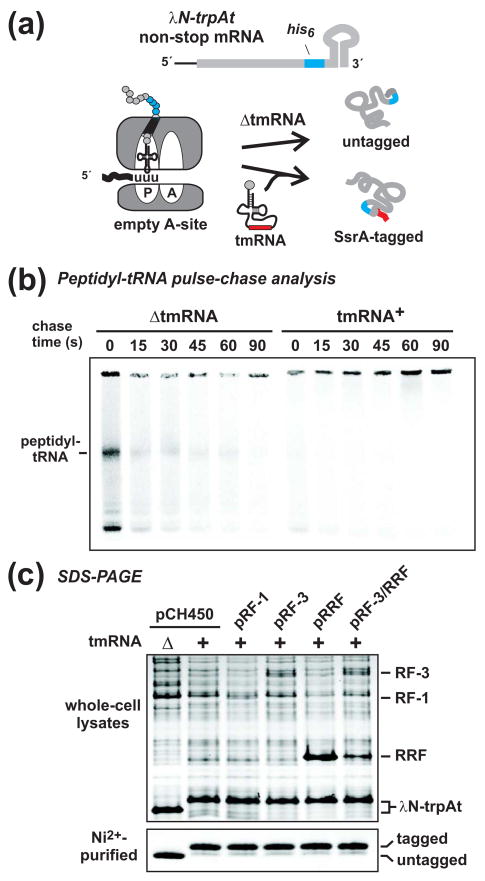
Figure 7. Translation termination and recycling factors do not induce peptidyl-tRNA “drop-off” during ribosome pausing on nonstop mRNA.
(a) Schematic of the λN-trpAt nonstop message and the fates of λN-trpAt nascent chains during ribosome arrest. In tmRNA+ cells, nearly all λN-trpAt chains are SsrA-tagged. In ΔtmRNA cells, untagged chains are released from the paused ribosome by an unknown mechanism. If overproduced translation factors facilitate peptidyl-tRNA “drop-off”, then untagged λN-trpAt chains should accumulate in tmRNA+ cells. (b) Peptidyl-tRNA turnover during ribosome pausing on the λN-trpAt nonstop mRNA. Nascent chains were pulse-labeled with [35S]-methionine/cysteine in cells expressing λN-trpAt, and chased with unlabeled amino acids for the indicated time. Peptidyl-tRNA was only detected in ΔtmRNA cells, consistent with ribosome pausing on a nonstop message. (c) SsrA-tagging of λN-trpAt in cells overexpressing translation termination and recycling factors. Cell lysates and Ni2+-NTA purified λN-trpAt were examined by Coomassie staining of SDS-polyacrylamide gels. Untagged λN-trpAt accumulated ΔtmRNA cells, whereas SsrA-tagged λN-trpAt accumulated in tmRNA+ ΔClpP cells. Overexpression of translation termination and recycling factors had no effect on SsrA-tagging compared to control cells carrying vector plasmid (pCH450). The gel migration positions of RF-3, RF-1, RRF, and λN-trpAt are indicated.
Effects of nonstop and nascent peptide-induced ribosome pausing on cell growth
Finally, we examined the effects of ribosome pausing on E. coli growth and viability. To avoid the general growth inhibition inherent to T7 RNA polymerase-based expression systems, we used IPTG-inducible Ptrc promoter constructs based on plasmid pPW500 13. Two new constructs were made, encoding λN-PP and λN-LA proteins, which are analogous to YbeL-PP and YbeL-EA described above. We confirmed that the C-terminal Pro-Pro nascent peptide of λN-PP induced A-site cleavage of the stop codon and SsrA-peptide tagging, whereas no translational pausing or SsrA-tagging was apparent during λN-LA expression (data not shown). Induction of both λN-PP and λN-LA in ΔtmRNA cells led to a loss of viability, although the effect was far more severe for λN-PP (Figs. 8a & 8b). In contrast, tmRNA+ cells were unaffected by λN-LA expression, whereas λN-PP expression led to abnormal colony morphology but only a minor loss of tmRNA+ cell viability (Figs. 8a & 8b). We sought to rescue viability in ΔtmRNA cells by overproducing RF-1, RF-3, RRF, or a combination of both RF-3 and RRF. None of these translation factors had any effect on the viability of either ΔtmRNA or tmRNA+ cells expressing λN-LA and λN-PP (Figs. 8a & 8b). This result was unexpected given that RF-1 overexpression significantly increased peptidyl-tRNA turnover from termination-paused ribosomes (Fig. 3b). In fact, co-expression of λN-PP and RF-1 actually decreased tmRNA+ cell viability, compared to cells expressing either of the proteins individually (Fig. 8b). This synthetic phenotype was not observed in tmRNA+ cells expressing both λN-LA and RF-1 (Fig. 8a).
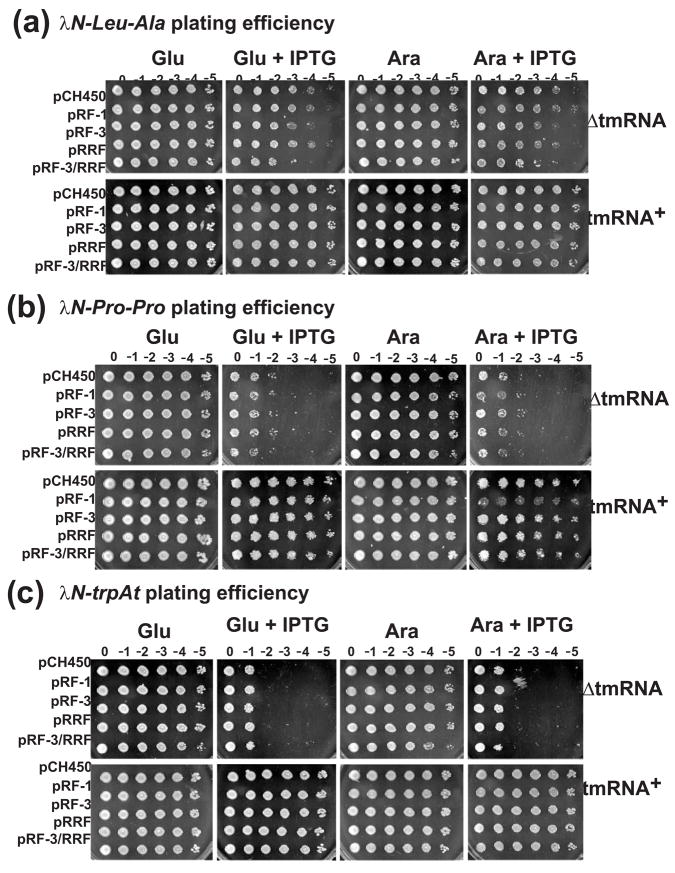
Figure 8. Translational pausing and cell viability.
Cultures were serially diluted (10-fold), and plated on LB agar plates containing: glucose to suppress PBAD expression; glucose + IPTG to induce λN expression; arabinose to induce translation factor expression; or L-arabinose + IPTG to induce expression of λN variants and PBAD-dependent translation factors. (a) Cell viability during λN-LA overexpression. Expression of λN-LA had no effect on tmRNA+ cells, but caused ~ 100-fold decrease ΔtmRNA cell viability. Expression of translation termination/recycling factors had no significant effect on cell viability under all conditions. (b) Cell viability during λN-PP overexpression. Expression of λN-PP resulted in ~ 10,000-fold decrease in ΔtmRNA cell viability, and led to altered colony morphology in tmRNA+ cells. Overexpression of RF-3 and/or RRF had no effect on cell viability. However, co-expression of RF-1 with λN-PP led to a significant loss of tmRNA+ viability compared to cells expressing the proteins individually. (c) Cell viability during λN-trpAt expression from nonstop mRNA. Expression of λN-trpAt resulted in ~ 10,000-fold decrease in ΔtmRNA cell viability, but had no effect on tmRNA+ cells. Expression of translation termination/recycling factors had no significant effect on cell viability under all conditions.
We also examined the viability of cells expressing nonstop mRNA using the λN-trpAt expression construct (Fig. 7a). Expression of the λN-trpAt nonstop mRNA decreased ΔtmRNA cell viability by approximately four orders of magnitude, but had no effect on tmRNA+ cells (Fig. 8c). As was found with λN-LA and λN-PP, the co-expression of RF-1, RF-3, and RRF was unable to restore viability to ΔtmRNA cells expressing λN-trpAt (Fig. 8c). Finally, the overproduction of Pth, tRNA2Pro, and IF-3 had no effect on the viability of ΔtmRNA or tmRNA+ cells expressing any of the λN protein variants (data not shown).
Discussion
These results represent the first comparative analysis of ribosome turnover kinetics during translational pausing on full-length and nonstop mRNAs. We used the previously characterized Pro-Pro nascent peptide to pause ribosomes during translation termination. Translational pausing at this nascent peptide motif resulted in the accumulation of peptidyl prolyl-tRNA2Pro, which was exploited as specific marker of termination-paused ribosomes. Pulse-chase analysis of peptidyl prolyl-tRNA2Pro turnover revealed the time scale of translational pausing, and uncovered evidence of ribosome queuing. Somewhat unexpectedly, ribosome turnover during inefficient translation termination was similar in ΔtmRNA and tmRNA+ cells. We propose that at least two processes account for these findings. Firstly, tmRNA•SmpB is not recruited to ribosomes paused on full-length mRNA, and therefore the message must be cleaved prior to ribosome rescue 22. We have recently shown that this cleavage is largely mediated by 3′×5′ exoribonucleases that degrade mRNA to the border of the paused ribosome (Garza-Sánchez et al., in press). Therefore, tmRNA•SmpB-mediated peptidyl prolyl-tRNA2Pro turnover is probably rate-limited by exonucleolytic processing of mRNA. This model is consistent with the very rapid peptidyl-tRNA turnover from nonstop mRNA-paused ribosomes in tmRNA+ cells. Because nonstop messages are “pre-cleaved”, ribosome rescue occurs immediately. Secondly, though its activity is reduced by the nascent peptide motif, RF-1 probably mediates the majority of ribosome turnover during inefficient termination in both tmRNA+ and ΔtmRNA cells. Approximately 30% of YbeL-PP nascent chains are SsrA-tagged in tmRNA+ cells 8, and therefore the remaining 70% presumably undergo canonical RF-1 mediated translation termination. This reasoning implies that chains destined for SsrA-tagging in tmRNA+ cells are turned over by some other process in ΔtmRNA cells. This tmRNA-independent process may simply be canonical RF-mediated termination, or alternatively may be the activity of novel ribosome recycling system. We are unable to distinguish between these two possibilities because the protein products are presumably identical for each process. However, there is good evidence that tmRNA-independent ribosome recycling occurs during translation of nonstop messages in ΔtmRNA cells. Proteins are readily overproduced from nonstop mRNA in ΔtmRNA cells (see Fig. 7c), even though the translating ribosomes cannot undergo canonical termination. Moreover, peptidyl-tRNA turns over relatively rapidly from nonstop mRNA-arrested ribosomes. Taken together, these data suggest the activity of a tmRNA-independent ribosome recycling system. The existence of such an alternative ribosome rescue pathway was initially proposed when tmRNA activity was discovered, because tmRNA is not essential in E. coli 12; 41. This is not true of all bacteria as tmRNA is essential for the viability of Neisseria gonorrhoeae, Mycoplasma genitalium, and other species 11. Presumably, these bacteria lack a back-up system to recycle stalled ribosomes in the absence of tmRNA.
What is the tmRNA-independent ribosome recycling mechanism? A number of tmRNA-independent recycling pathways have been described over the past 15 years. Each pathway involves the activity of canonical translation factors, including RF-3, RRF, IF-1, IF-2, and IF-3 21; 25; 34; 35. In general, these translation factors remove small peptidyl-tRNAs (usually containing fewer than ten amino acid residues) from the ribosome in a phenomenon known as peptidyl-tRNA “drop-off”. It is unclear whether factor-mediated drop-off also occurs with larger peptidyl-tRNAs, such as the nascent polypeptides examined here. Moreover, direct peptidyl-tRNA drop-off is probably not feasible when the nascent chain contains a folded domain on the cytoplasmic side of the ribosomal exit tunnel. However, a recent report by Varshney and colleagues suggests that RRF mediates the drop-off of large peptidyl-tRNAs (>200 residues) from nonstop-arrested ribosomes in tmRNA+ cells 40. Our data are inconsistent with the conclusions of Singh et al. 40. Firstly, gratuitous overproduction of RRF did not inhibit SsrA-peptide tagging of λN protein synthesized from nonstop mRNA, indicating that RRF does not compete with tmRNA•SmpB for binding to nonstop-paused ribosomes. Secondly, overexpression of RF-3 and/or RRF did not accelerate peptidyl-tRNA turnover during ribosome pausing on nonstop mRNA. If ribosome recycling is an essential function as has been suggested 42, then the ubiquitous RRF is an unlikely candidate for a recycling system that is apparently absent in several species 11. Based on this evidence, we propose that another uncharacterized tmRNA-independent recycling pathway(s) exists in E. coli and other bacteria. This pathway could be an intrinsic activity of the ribosome itself. Two recent reports provide evidence that E. coli ribosomes can recycle from truncated messages in the absence of exogenous factors 43; 44.
Singh et al. proposed RRF-mediated ribosome rescue, in part, based on evidence that RRF overexpression suppressed the toxicity of a nonstop message expressed in tmRNA+ cells 40. We are unsure why their nonstop message was lethal to tmRNA+ cells, because we observed no loss of cell viability upon expression of the well-characterized λN-trpAt nonstop mRNA in tmRNA+ cells. Perhaps the message described in Singh et al. is toxic for reasons unrelated to ribosome pausing. Because the ribosome arrest site was not experimentally confirmed in that paper, we cannot fully explain why our results differ. Regardless of mechanism, we note that the protective effect observed during RRF overexpression in Singh et al. was not dramatic 40. Because plasmid-borne RRF is typically expressed at very high levels (see Fig. 7c) 8, perhaps RRF synthesis monopolized ribosome activity, leading to less translation of the toxic “nonstop” mRNA. In our viability assays, we observed no protective effect from RRF under any of the examined conditions. Instead, we find that tmRNA is the most important protective factor during ribosome pausing on both nonstop and full-length messages. Additionally, ΔtmRNA cells lost significant viability upon expressing a control message that causes no detectable ribosome pausing. This finding suggests that all heterologous protein overexpression is accompanied by subtle translational problems that tmRNA•SmpB typically resolves. Surprisingly, RF-1 overexpression failed to restore viability to cells expressing λN-PP. In fact, tmRNA+ cells actually lost viability when co-expressing RF-1 and λN-PP. This effect was not seen when either λN-trpAt or λN-LA were overexpressed, suggesting that the synthetic toxicity is specific to the nascent peptide-induced ribosome pause. This is perplexing because RF-1 was the only translation factor to dramatically increase peptidyl prolyl-tRNA2Pro turnover. We are unsure of the mechanism, but perhaps RF-1 prevents entry of the tmRNA-independent recycling factor into the ribosomal A site. For this model to be tenable, toxicity would have to result from a small subpopulation of ribosomes that require the proposed tmRNA-independent system for turnover. Clearly, cell viability losses are not necessarily related to the severity of translational pausing. These results illustrate that caution must be exercised when interpreting cell growth data in terms of molecular mechanism.
Our findings also show that the Pro-Pro nascent peptide does not inhibit RF-1 binding to the ribosomal A site. Indeed, essentially all of the cellular RF-1 can be isolated on ribosomes from cells overexpressing YbeL-PP. This is due to high-level YbeL-PP expression from the T7 RNA polymerase system, and suggests that the sequestration of RF-1 on paused ribosomes produces a second population of termination-paused ribosomes that have empty A sites. Overexpressed RF-1 presumably suppresses this secondary ribosome pausing, leading to more rapid peptidyl prolyl-tRNA2Pro turnover. RF-3 overexpression has a similar, yet less pronounced, effect because it promotes RF-dissociation from post-termination ribosomes, and thereby increases the free RF-1 concentration. Even in the absence of RF overproduction, RF-1 must be exchanging between ribosomal A sites in vivo, because YbeL-PP protein is expressed at very high levels despite the pause during termination 8; 9. Ribosome complexes isolated by ultracentrifugation contained much less peptidyl prolyl-tRNA2Pro than expected given the high level of ribosome-associated RF-1. This result suggests that the isolated ribosomes were, in fact, mostly post-termination complexes containing RF-1 in the A site. Given that peptidyl prolyl-tRNA2Pro turned over with T1/2 ~ 20 – 30 s in vivo, we suspect that RF-1 continued hydrolysis of peptidyl prolyl-tRNA2Pro during the cell lysis and ultracentrifugation procedures. RF-1 probably remained bound to the ribosome due to a combination of low temperature, and physical separation from soluble RF-3 during ultracentrifugation. RF-3 accelerates RF-1 and RF-2 dissociation from the post-termination A site. Additionally, RF-1 has significantly higher affinity for the ribosomal A site than RF-2 45, which may explain why RF-1 readily co-purified with ribosomes whereas RF-2 did not. Nevertheless, RF-1 was not ribosome-associated during YbeL-EA overexpression, strongly suggesting that RF-1 binds to the A site during ribosome pausing, but is unable to efficiently hydrolyze the peptidyl-tRNA bond.
What is the basis of nascent peptide-induced ribosome pausing during translation termination? C-terminal Pro residues are important for regulated ribosome pausing in E. coli and human cells 31; 46. Given the divergence between bacteria and mammals, we suspect that the inherently slow rate of peptidyl prolyl-tRNAPro hydrolysis has been exploited to regulate protein synthesis in these two systems. The C-terminal Pro-Pro sequence inhibits translation termination in several genetic contexts 8, suggesting that the intrinsic chemical properties of proline are responsible for translational pausing, rather than specific interactions between the nascent chain and ribosome. Given that Pro residues have the unique ability to adopt and stabilize cis peptide bonds in proteins, one possibility is that the C-terminal Pro residue facilitates trans to cis isomerization of the final peptide bond, thereby inhibiting translation termination. Peptidyl-prolyl isomerization may also explain why the penultimate residue of the nascent chain also influences ribosome pausing 6; 8. Presumably, peptide bonds are formed by the ribosome in the trans conformation, which is the predominate stereoisomer in mature proteins. Isomerization to the cis conformation could conceivably alter the geometry of the peptidyl-tRNA ester bond and prevent nucleophilic attack by water during translation termination, or by aminoacyl-tRNAs during translation elongation. Indeed, recent data from the Ito and Rodnina groups have shown that peptidyl prolyl-tRNA reacts inefficiently with puromycin, which is a mimic of aminoacyl-tRNA 47; 48. However, we have been unable to detect ribosome pausing at internal Pro-Pro sequences, indicating that this nascent peptide sequence is not problematic during elongation. Perhaps the rapid delivery of ternary complexes does not allow enough time for peptidyl-prolyl isomerization during elongation. In contrast, translation termination is significantly slower than elongation 49, which may allow for trans to cis isomerization. Because translation termination is 10–100-fold more rapid than peptidyl-prolyl isomerization in solution, trans-cis isomerization would have to be accelerated on the ribosome for this model to be tenable. This is certainly plausible given that peptidyl-prolyl isomerization can be dramatically increased by desolvation, or by hydrogen bonding to the imide nitrogen of Pro residues 50; 51. Importantly, the peptidyl-prolyl isomerization is reversible, and would eventually allow RF-mediated termination to occur, consistent with our findings in this study. Although the translational termination pause characterized here has no known function, we propose that nascent peptidyl-prolyl isomerization can be used as a molecular switch to regulate translation termination.
Materials and Methods
Bacterial strains and plasmids
All bacterial strains were derivatives of Escherichia coli strain X90 and are listed in Table 2. The prfB(FLAG) allele encoding the FLAG epitope at the C-terminus of release factor-2 (RF-2) was introduced into the prfB locus by Red-mediated homologous recombination as described 8; 52. The resulting strain was confirmed by whole-cell PCR and anti-FLAG Western blot analyses. For routine isolation of RNA and protein, 10 mL cultures were grown at 37 °C with aeration in Luria broth (LB) supplemented with the appropriate antibiotics (150 μg/mL ampicillin, and 25 μg/mL tetracycline) to maintain plasmids. The expression of YbeL and λN proteins was induced with 1.5 mM isopropyl β-D-thiogalactopyranoside (IPTG), and the expression of RF-1, RF-3, RRF, Pth, and IF-3 was induced with 0.2% to 0.4% L-arabinose.
Table 2.
Bacterial strains and plasmids.
| Strain or plasmid | Descriptiona | Reference |
|---|---|---|
| Strains | ||
| X90 | F prime; lacIq lac′ pro′/ara Δ[lac-pro] nalA argE[am] rifr thi-1 | |
| CH12 | X90 (DE3) | (8) |
| CH22 | X90 ssrA::cat, Cmr | (39) |
| CH94 | X90 clpP::cat, Cmr | (13) |
| CH113 | X90 (DE3) ssrA::cat, Cmr | (8) |
| CH2016 | CH12 Δrna ΔslyD::kan, Kanr | (53) |
| CH4119 | CH12 prfB(FLAG) | This study |
| CH4120 | CH113 prfB(FLAG), Cmr | This study |
| Plasmids | ||
| pYbeL-PP | pET11d::ybeL(E159P), T7 expression of YbeL-PP, Ampr | (8) |
| pYbeL-EA | pET11d::ybeL(P160A), T7 expression of YbeL-EA, Ampr | (8) |
| pYbeL-PP(uag) | YbeL-PP expressed from mRNA with UAG stop codon, Ampr | This study |
| pYbeL-PP(uga) | YbeL-PP expressed from mRNA with UGA stop codon, Ampr | This study |
| pFLAG-(m)YbeL-PP | Expresses C-terminal 49 residues of YbeL-PP fused to FLAG, Ampr | This study |
| pFLAG-(m)YbeL-PP(uag) | Expresses FLAG-(m)YbeL-PP from mRNA with UAG stop codon, Ampr | This study |
| pFLAG-(m)YbeL-PP(uga) | Expresses FLAG-(m)YbeL-PP from mRNA with UGA stop codon, Ampr | This study |
| pFLAG-(m)YbeL-rrnB | Expresses FLAG-(m)YbeL-rrnB from a nonstop mRNA, Ampr | This study |
| pFLAG-(m)YbeL-EA | Expresses C-terminal 49 residues of YbeL-EA fused to FLAG, Ampr | This study |
| pET21b::pth | pET plasmid expressing His6-tagged E. coli Pth, Ampr | This study |
| pSecM′ | pFG21b::secM′, Ampr | (20) |
| pPW500 | pTrc99a derivative expressing the N-terminal domain of phage λ cI repressor (λN) from nonstop mRNA. Ampr | (13) |
| pλN-PP | pTrc99a derivative expressing λN with C-terminal Pro-Pro. Ampr | This study |
| pλN-LA | pTrc99a derivative expressing λN with C-terminal Leu-Ala. Ampr | This study |
| pCH405Δ | pACYC184 derivative containing multi-cloning site, Tetr | (20) |
| pCH450 | pACYC184 derivative encoding araC and araBAD promoter, Tetr | (9) |
| pPth | pCH410::pth, arabinose-inducible Pth expression, Tetr | This study & (9) |
| pRF-1 | pACYC184 derivative expressing RF-1 under araBAD promoter, Tetr | (9) |
| pRF-3 | pCH450::prfC, arabinose-inducible RF-3 expression, Tetr | This study |
| pRRF | pCH450::frr, arabinose-inducible RF-3 expression, Tetr | This study |
| pRF-3/RRF | pCH450::prfC-frr, arabinose-inducible RF-3 and RRF expression, Tetr | This study |
| pIF-3 | pCH450::infC, arabinose-inducible IF-3 expression, Tetr | This study |
| pSmpB•tmRNA | pCH405Δ::smpB-ssrA, constitutive expression of SmpB•tmRNA, Tetr | This study |
| ptRNA2Pro | pCH405Δ::proL constitutive expression of tRNA2Pro, Tetr | (20) |
Abbreviations used: Ampr, ampicillin resistant; Cmr, chloramphenicol resistant; Kanr, kanamycin resistant; Tetr, tetracycline resistant.
Strains for viability plating assay were grown in LB medium (supplemented with 150 μg/mL ampicillin and 25 μg/mL tetracycline) to late log phase at 37 °C with aeration. Cell densities were adjusted to OD600 = 1.0, and ten-fold serial dilutions were plated onto LB agar plates containing: i) 0.1% D-glucose; ii) 0.1% D-glucose + 0.05 mM IPTG; iii) 0.1% L-arabinose; or iv) 0.1% L-arabinose + 0.05 mM IPTG. In addition, all LB agar plates contained 150 μg/mL ampicillin and 5 μg/mL tetracycline. Plates were incubated for 15 – 17 h at 37 °C.
All plasmids used in this study are listed in Table 2. Plasmids pYbeL(PP), pYbeL(EA), pRF-1, ptRNA2Pro, and pPW500 have been described previously 8; 9; 13; 20. The (m)ybeL-PP and (m)ybeL-EA mini-genes were generated by PCR amplification of plasmids pYbeL(PP) and pYbeL(EA) using oligonucleotides, ybeL(49)-NdeI, (5′ - GGA CCT CAA TCA TAT GGG GGT TTA TCA CAG) and pET-EcoRI, (5′ - CGT CTT CAA GAA TTC TCA TGT TTG ACA GC), digested with NdeI and EcoRI (restriction endonuclease site underlined), and ligated to plasmid pFG11 53. The resulting plasmids express the C-terminal 49 residues of YbeL(PP) and YbeL(EA) fused to an N-terminal FLAG epitope. Full-length and mini-gene versions of ybeL-PP containing TAG and TGA stop codons were made by PCR using primer pET-PstI, (5′ - CAA GGA ATG GTG CAT GCC TGC AGA TGG CGC CC); with ybeL(PP)uag-BamHI, (5′ - GCC GGA TCC TAC TAG GGC GGA AAC GGG), and ybeL(PP)uga-BamHI, (5′ - GCC GGA TCC TAT CAG GGC GGA AAC GGG), respectively. The λN-PP construct was generated by PCR of pPW500 using primers lacI-HpaI (5′ - TAT CCC GCC GTT AAC TAG TAT CAA ACA GGA TTT TCG C), and λN-PP-SacI, (5′ - AAT GAG CTC AAT TAG GGC GGA TGA TGG TGA TGA TGG TGC). The λN-LA PCR fragment was made using oligonucleotide, λN-LA-SacI, (5′ - ACC GAG CTC AAT TAT GCC AGA TGA TGG TGA TGA TGG), and lacI-HpaI. The resulting PCR fragments were digested with HpaI and SacI and ligated to plasmid pTrc99A (GE Healthcare).
The E. coli pth gene was PCR amplified with primers, pth-NdeI, (5′ - GGA CAA AAA CAT ATG ACG ATT AAA TTG ATT GTC G), and pth-KpnI, (5′ - GAC GGT ACC TTA TTG CGC TTT AAA GGC GTG C), digested with NdeI and KpnI and ligated to plasmid pCH410, which contains araC and the araBAD promoter 9. The Pth-His6 overexpression construct was generated by PCR of pth with primers, pth-XhoI, (5′ - AAC CTC GAG TTG CGC TTT AAA GGC GTG CAA TCG G), and pth-NdeI, followed by ligation to plasmid pET21b (Novagen-EMD). The prfC gene was subcloned from plasmid pCH405::prfC 8 into pCH450 using SacI and KpnI restriction sites, to generate pRF-3. The E. coli frr gene, encoding RRF, was PCR amplified using oligonucleotides, frr-KpnI, (5′ - TCT GGT ACC ACT GAG ACA AGT TTT CAA GG), and frr-PstI, (5′ - GAT CTG CAG GGA TCT ACT GAG CGG CG), followed by ligation to plasmids pCH450 and pRF-3 to generate plasmids pRRF and pRF-3/RRF, respectively. The E. coli infC gene, encoding IF-3, was PCR amplified using oligonucleotides, infC-NdeI, (5′ - GAG GAA TAA CAT ATG AAA GGC GGA AAA CGA GTT CAA ACG); and infC-XhoI, (5′ - CTT CTC GAG ATT ACT GTT TCT TCT TAG G), and ligated to pCH450 to generate plasmid pIF-3. The E. coli smpB and ssrA genes, encoding SmpB and tmRNA, were PCR amplified using oligonucleotides, smpB-SacI, (5′ - CGC GAG CTC TTT AAA CAC GCG ACC AAA GGC G), and ssrA-XhoI, (5′ - CAA CTC GAG GGC ACA AAA AAG CCC GCA GGG C), and ligated to pCH405Δ to generate plasmid pSmpB-tmRNA. All plasmid constructs were confirmed by DNA sequencing.
Analysis of RNA and protein
Total RNA was isolated and Northern blot analyses were conducted as previously described 20; 53. The following oligonucleotides were 5′ end-labeled with [γ-32P]-ATP, and used as probes for Northern blot hybridizations: pET-rbs probe, (5′ - GTA TAT CTC CTT CTT AAA GTT AAA C) for mRNA; proL probe (5′ - CAC CCC ATG ACG GTG CG) for tRNA2Pro; glyV probe, (5′ - CTT GGC AAG GTC GTG CT) for tRNA3Gly; alaT probe, (5′ - TCC TGC GTG CAA AGC AG) for tRNA1BAla; serT probe, (5′ - AAC CCT TTC GGG TCG CCG GTT TTC) for tRNA1Ser; and leuU probe, (5′ - CCC GTA AGC CCT ATT GGG CA) for tRNA2Leu.
Total cell protein was isolated by freeze-thaw lysis in buffered 8M urea as previously described 38. Protein samples were quantified by Bradford protein reagent and run on Tris-tricine-SDS 10% polyacrylamide gels. Western blotting was performed as described 53, and blots were imaged using a LI-COR® infrared imager and quantified using Odyssey software. Polyclonal antisera were raised against C-terminal His6-tagged RF-1 from E. coli. Anti-FLAG monoclonal antibodies (Sigma-Aldrich) were used to detect FLAG-tagged RF-2 in double labeling Western blot experiments. All RF-2 experiments were verified using wild-type RF-2 and anti-RF-2 polyclonal antisera (a generous gift from Dr. Richard Buckingham).
Pulse-chase analysis of peptidyl-tRNA turnover
E. coli strains were grown at 37 °C with aeration in MOPS defined media 54 containing 0.4% glucose and supplemented with 100 μg/mL L-arginine and 20 μg/mL of all other amino acids except L-methionine and L-cysteine. Once cultures attained a cell density of OD600 ~ 0.7, mRNA synthesis was induced with 1.5 mM IPTG for 20 – 30 min, followed by pulse-labeling with 20 μCi/mL of [35S]-radiolabeled L-methionine and L-cysteine (MP Biomedicals –1175 Ci/mmol) for 1 – 2 min. Radiolabel was chased with 0.2 mg/mL of unlabeled L-methionine and L-cysteine, and 1.0 mL samples were removed at the indicated time intervals and quenched with ice-cold trichloroacetic acid (1% final concentration). Cells were collected by centrifugation, washed once with 50 mM Tris-acetate (pH 7.0) – 1 mM EDTA, and lysed in 100 μL of 1% SDS – 50 mM Tris-acetate (pH 7.0) 1 mM EDTA for 20 min at ambient temperature. Cell lysates (45 μL) were mixed with 500 μL of 2% cetyltriethylammonium bromide (CTABr) and 500 μL of 0.5 M sodium acetate (pH 5.0), and placed on ice for 20 min. Samples were then incubated at 30 °C for 10 min and subsequently centrifuged at 13,000 rpm for 15 min in a microcentrifuge. The resulting pellets were washed with 1 mL of 100% ice-cold acetone, and dissolved in 8 M urea – 10 mM sodium acetate (pH 5.2) – 1 mM EDTA – 0.01% bromophenol blue for analysis on acid-urea polyacrylamide gels 20. Gels were dried onto filter paper, and the data visualized and quantified by phosphorimager using the Quantity One software package (BioRad). Exponential decay equations were fitted to the pulse-chase data using DeltaGraph (RedRock Software). Composite half-life values (T1/2) from double-exponential decay fits were determined for termination-paused ribosomes, and simple half-lives (t1/2) were determined from single-exponential fits to nonstop-paused turnover data.
Cell fractionation and ribosome isolation
Cells were grown in 100 mL of LB were grown to an OD600 of 0.7 – 1.0 and induced with 1.5 mM IPTG for 30 min. Cells over-producing RF-3 and/or RRF were grown in LB supplemented with 0.4% L-arabinose, and induced with IPTG as indicated above. Cultures were harvested over ice, centrifuged at 4 °C to collect cells, washed with S30 buffer [60 mM ammonium acetate – 10 mM Tris-acetate (pH 7.0) – 10 mM magnesium acetate], and frozen at −80 °C. Frozen cells were resuspended in 10 mL of S30 buffer and broken by French press passage at 20,000 psi. Lysates were clarified by centrifugation at 30,000 μg at 4 °C for 15 min, loaded onto 40% sucrose in S30 buffer, and centrifuged in either a SW50.1 or MLS50 rotor for 1 – 4 hr at 40,000 rpm. RNA and protein were isolated from high-speed supernatant and pellet fractions.
Purification and in vitro activity of Pth-His6
His6-tagged peptidyl-tRNA hydrolase (Pth-His6) purification was performed according to Bonin et al. with minor modifications 55. Recombinant Pth-His6 was overexpressed using a T7 RNA polymerase expression system in cells deleted for RNase I and SlyD 53. Cells were resuspended in Pth buffer [50 mM sodium phosphate (pH 8.0) – 300 mM sodium chloride – 5 mM β-mercaptoethanol – 10% glycerol], and broken by one passage through French press at 20,000 psi. Lysates were clarified by centrifugation at 30,000 μg for 15 min at 4 °C. Clarified lysates were incubated with nickel-nitrilotriacetic acid (Ni2+-NTA) agarose resin for 4 h at 4 °C. Ni2+-NTA resin was collected and batch washed twice with Pth buffer containing 20 mM imidazole. Washed resin was loaded into column and washed with Pth buffer containing 20 mM imidazole until the eluate contained no detectable protein. Pth-His6 was eluted in Pth buffer containing 250 mM imidazole. Pooled fractions were exhaustively dialyzed against 2 μ Pth buffer, diluted with an equal volume of glycerol, and stored at −20 °C. Pth-His6 was judged to be >95% pure by SDS-PAGE, and quantified by absorbance at 280 nm using a molar extinction coefficient of 13,980 M−1 cm−1.
Total cellular RNA (500 μg/mL final concentration) was treated with purified Pth-His6 in Pth reaction buffer [20 mM Tris-acetate (pH 7.0) – 10 mM magnesium chloride – 0.2% BSA – 0.1 mM EDTA – 10 mM β-mercaptoethanol] at 37 °C. Reactions were quenched with an equal volume of gel loading buffer [8M urea – 10 mM sodium acetate – 1 mM EDTA – 0.01% bromophenol blue 0.01% xylene cyanol] and placed on ice. Samples were incubated at 95 °C for 3 min, then loaded onto acid-urea 6% polyacrylamide gels for blotting and Northern hybridization 20. [35S] pulse-labeled samples were treated similarly, but the acid-urea gel was dried onto filter paper and visualized by phosphorimaging.
Acknowledgments
We thank Elie Diner, Fernando Garza-Sánchez, and Laura Holberger for helpful discussions; Les Wilson, Stu Feinstein, and Chuck Samuel for the use of equipment; and Richard Buckingham for providing anti-RF-2 antisera. We extend additional thanks to Fernando Garza-Sánchez for identification of SsrA(His6)-tagged proteins by mass spectrometry, and to Laura Holberger for providing the N-PP and N-LA plasmid constructs. This work was supported by the National Institutes of Health through grant GM078634.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laurberg M, Asahara H, Korostelev A, Zhu J, Trakhanov S, Noller HF. Structural basis for translation termination on the 70S ribosome. Nature. 2008;454:852–857. doi: 10.1038/nature07115. [DOI] [PubMed] [Google Scholar]
- 2.Weixlbaumer A, Jin H, Neubauer C, Voorhees RM, Petry S, Kelley AC, Ramakrishnan V. Insights into translational termination from the structure of RF2 bound to the ribosome. Science. 2008;322:953–956. doi: 10.1126/science.1164840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavlov MY, Freistroffer DV, MacDougall J, Buckingham RH, Ehrenberg M. Fast recycling of Escherichia coli ribosomes requires both ribosome recycling factor (RRF) and release factor RF3. EMBO J. 1997;16:4134–4141. doi: 10.1093/emboj/16.13.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freistroffer DV, Pavlov MY, MacDougall J, Buckingham RH, Ehrenberg M. Release factor RF3 in E. coli accelerates the dissociation of release factors RF1 and RF2 from the ribosome in a GTP-dependent manner. EMBO J. 1997;16:4126–4133. doi: 10.1093/emboj/16.13.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zavialov AV, Hauryliuk VV, Ehrenberg M. Splitting of the posttermination ribosome into subunits by the concerted action of RRF and EF-G. Mol Cell. 2005;18:675–686. doi: 10.1016/j.molcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Bjornsson A, Mottagui-Tabar S, Isaksson LA. Structure of the C-terminal end of the nascent peptide influences translation termination. EMBO J. 1996;15:1696–1704. [PMC free article] [PubMed] [Google Scholar]
- 7.Mottagui-Tabar S, Bjornsson A, Isaksson LA. The second to last amino acid in the nascent peptide as a codon context determinant. EMBO J. 1994;13:249–257. doi: 10.1002/j.1460-2075.1994.tb06255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes CS, Bose B, Sauer RT. Proline residues at the C terminus of nascent chains induce SsrA tagging during translation termination. J Biol Chem. 2002;277:33825–33832. doi: 10.1074/jbc.M205405200. [DOI] [PubMed] [Google Scholar]
- 9.Hayes CS, Sauer RT. Cleavage of the A site mRNA codon during ribosome pausing provides a mechanism for translational quality control. Mol Cell. 2003;12:903–911. doi: 10.1016/s1097-2765(03)00385-x. [DOI] [PubMed] [Google Scholar]
- 10.Moore SD, Sauer RT. The tmRNA system for translational surveillance and ribosome rescue. Annu Rev Biochem. 2007;76:101–124. doi: 10.1146/annurev.biochem.75.103004.142733. [DOI] [PubMed] [Google Scholar]
- 11.Keiler KC. Biology of trans-translation. Annu Rev Microbiol. 2008;62:133–151. doi: 10.1146/annurev.micro.62.081307.162948. [DOI] [PubMed] [Google Scholar]
- 12.Keiler KC, Waller PR, Sauer RT. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science. 1996;271:990–993. doi: 10.1126/science.271.5251.990. [DOI] [PubMed] [Google Scholar]
- 13.Gottesman S, Roche E, Zhou Y, Sauer RT. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 1998;12:1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman C, Thevenet D, Bouloc P, Walker GC, D’Ari R. Degradation of carboxy-terminal-tagged cytoplasmic proteins by the Escherichia coli protease HflB (FtsH) Genes Dev. 1998;12:1348–1355. doi: 10.1101/gad.12.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choy JS, Aung LL, Karzai AW. Lon protease degrades transfer-messenger RNA-tagged proteins. J Bacteriol. 2007;189:6564–6571. doi: 10.1128/JB.00860-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundermeier TR, Dulebohn DP, Cho HJ, Karzai AW. A previously uncharacterized role for small protein B (SmpB) in transfer messenger RNA-mediated trans-translation. Proc Natl Acad Sci U S A. 2005;102:2316–2321. doi: 10.1073/pnas.0409694102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundermeier TR, Karzai AW. Functional SmpB-ribosome interactions require tmRNA. J Biol Chem. 2007;282:34779–34786. doi: 10.1074/jbc.M707256200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunohara T, Abo T, Inada T, Aiba H. The C-terminal amino acid sequence of nascent peptide is a major determinant of SsrA tagging at all three stop codons. RNA. 2002;8:1416–1427. doi: 10.1017/s1355838202020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sunohara T, Jojima K, Yamamoto Y, Inada T, Aiba H. Nascent-peptide-mediated ribosome stalling at a stop codon induces mRNA cleavage resulting in nonstop mRNA that is recognized by tmRNA. RNA. 2004;10:378–386. doi: 10.1261/rna.5169404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garza-Sánchez F, Janssen BD, Hayes CS. Prolyl-tRNAPro in the A-site of SecM-arrested ribosomes inhibits the recruitment of transfer-messenger RNA. J Biol Chem. 2006;281:34258–34268. doi: 10.1074/jbc.M608052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong M, Cruz-Vera LR, Yanofsky C. Ribosome recycling factor and release factor 3 action promotes TnaC-peptidyl-tRNA dropoff and relieves ribosome stalling during tryptophan induction of tna operon expression in Escherichia coli. J Bacteriol. 2007;189:3147–3155. doi: 10.1128/JB.01868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanova N, Pavlov MY, Felden B, Ehrenberg M. Ribosome rescue by tmRNA requires truncated mRNAs. J Mol Biol. 2004;338:33–41. doi: 10.1016/j.jmb.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 23.Dong H, Nilsson L, Kurland CG. Co-variation of tRNA abundance and codon usage in Escherichia coli at different growth rates. J Mol Biol. 1996;260:649–663. doi: 10.1006/jmbi.1996.0428. [DOI] [PubMed] [Google Scholar]
- 24.Vogel Z, Vogel T, Zamir A, Elson D. The protection by 70 S ribosomes of N-acyl-aminoacyl-tRNA against cleavage by peptidyl-tRNA hydrolase and its use to assay ribosomal association. Eur J Biochem. 1971;21:582–592. doi: 10.1111/j.1432-1033.1971.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 25.Heurgue-Hamard V, Karimi R, Mora L, MacDougall J, Leboeuf C, Grentzmann G, Ehrenberg M, Buckingham RH. Ribosome release factor RF4 and termination factor RF3 are involved in dissociation of peptidyl-tRNA from the ribosome. EMBO J. 1998;17:808–816. doi: 10.1093/emboj/17.3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muto H, Nakatogawa H, Ito K. Genetically encoded but nonpolypeptide prolyl-tRNA functions in the A site for SecM-mediated ribosomal stall. Mol Cell. 2006;22:545–552. doi: 10.1016/j.molcel.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Cruz-Vera LR, Toledo I, Hernandez-Sanchez J, Guarneros G. Molecular basis for the temperature sensitivity of Escherichia coli pth(Ts) J Bacteriol. 2000;182:1523–1528. doi: 10.1128/jb.182.6.1523-1528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutka S, Meinnel T, Lazennec C, Mechulam Y, Blanquet S. Role of the 1–72 base pair in tRNAs for the activity of Escherichia coli peptidyl-tRNA hydrolase. Nucleic Acids Res. 1993;21:4025–4030. doi: 10.1093/nar/21.17.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiloach J, Lapidot Y, de Groot N. The specificity of peptidyl-tRNA hydrolase from E. coli. FEBS Lett. 1975;57:130–133. doi: 10.1016/0014-5793(75)80700-9. [DOI] [PubMed] [Google Scholar]
- 30.Cao J, Geballe AP. Coding sequence-dependent ribosomal arrest at termination of translation. Mol Cell Biol. 1996;16:603–608. doi: 10.1128/mcb.16.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruz-Vera LR, Yanofsky C. Conserved residues Asp16 and Pro24 of TnaC-tRNAPro participate in tryptophan induction of Tna operon expression. J Bacteriol. 2008;190:4791–4797. doi: 10.1128/JB.00290-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janzen DM, Frolova L, Geballe AP. Inhibition of translation termination mediated by an interaction of eukaryotic release factor 1 with a nascent peptidyl-tRNA. Mol Cell Biol. 2002;22:8562–8570. doi: 10.1128/MCB.22.24.8562-8570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruz-Vera LR, Rajagopal S, Squires C, Yanofsky C. Features of ribosome-peptidyl-tRNA interactions essential for tryptophan induction of tna operon expression. Mol Cell. 2005;19:333–343. doi: 10.1016/j.molcel.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Dincbas V, Heurgue-Hamard V, Buckingham RH, Karimi R, Ehrenberg M. Shutdown in protein synthesis due to the expression of mini-genes in bacteria. J Mol Biol. 1999;291:745–759. doi: 10.1006/jmbi.1999.3028. [DOI] [PubMed] [Google Scholar]
- 35.Heurgue-Hamard V, Dincbas V, Buckingham RH, Ehrenberg M. Origins of minigene-dependent growth inhibition in bacterial cells. EMBO J. 2000;19:2701–2709. doi: 10.1093/emboj/19.11.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christiansen J. The 9S RNA precursor of Escherichia coli 5S RNA has three structural domains: implications for processing. Nucleic Acids Res. 1988;16:7457–7476. doi: 10.1093/nar/16.15.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roche ED, Sauer RT. SsrA-mediated peptide tagging caused by rare codons and tRNA scarcity. EMBO J. 1999;18:4579–4589. doi: 10.1093/emboj/18.16.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayes CS, Bose B, Sauer RT. Stop codons preceded by rare arginine codons are efficient determinants of SsrA tagging in Escherichia coli. Proc Natl Acad Sci U S A. 2002;99:3440–3445. doi: 10.1073/pnas.052707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roche ED, Sauer RT. Identification of endogenous SsrA-tagged proteins reveals tagging at positions corresponding to stop codons. J Biol Chem. 2001;276:28509–28515. doi: 10.1074/jbc.M103864200. [DOI] [PubMed] [Google Scholar]
- 40.Singh NS, Ahmad R, Sangeetha R, Varshney U. Recycling of ribosomal complexes stalled at the step of elongation in Escherichia coli. J Mol Biol. 2008;380:451–464. doi: 10.1016/j.jmb.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 41.Komine Y, Kitabatake M, Yokogawa T, Nishikawa K, Inokuchi H. A tRNA-like structure is present in 10Sa RNA, a small stable RNA from Escherichia coli. Proc Natl Acad Sci U S A. 1994;91:9223–9227. doi: 10.1073/pnas.91.20.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C, Wolfgang M, Withey J, Koomey M, Friedman D. Charged tmRNA but not tmRNA-mediated proteolysis is essential for Neisseria gonorrhoeae viability. EMBO J. 2000;19:1098–1107. doi: 10.1093/emboj/19.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuroha K, Horiguchi N, Aiba H, Inada T. Analysis of nonstop mRNA translation in the absence of tmRNA inEscherichia coli. Genes Cells. 2009 doi: 10.1111/j.1365-2443.2009.01304.x. [DOI] [PubMed] [Google Scholar]
- 44.Szaflarski W, Vesper O, Teraoka Y, Plitta B, Wilson DN, Nierhaus KH. New features of the ribosome and ribosomal inhibitors: non-enzymatic recycling, misreading and back-translocation. J Mol Biol. 2008;380:193–205. doi: 10.1016/j.jmb.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 45.Pavlov MY, Freistroffer DV, Dincbas V, MacDougall J, Buckingham RH, Ehrenberg M. A direct estimation of the context effect on the efficiency of termination. J Mol Biol. 1998;284:579–590. doi: 10.1006/jmbi.1998.2220. [DOI] [PubMed] [Google Scholar]
- 46.Cao J, Geballe AP. Inhibition of nascent-peptide release at translation termination. Mol Cell Biol. 1996;16:7109–7114. doi: 10.1128/mcb.16.12.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muto H, Ito K. Peptidyl-prolyl-tRNA at the ribosomal P-site reacts poorly with puromycin. Biochem Biophys Res Commun. 2008;366:1043–1047. doi: 10.1016/j.bbrc.2007.12.072. [DOI] [PubMed] [Google Scholar]
- 48.Wohlgemuth I, Brenner S, Beringer M, Rodnina MV. Modulation of the rate of peptidyl transfer on the ribosome by the nature of substrates. J Biol Chem. 2008;283:32229–32235. doi: 10.1074/jbc.M805316200. [DOI] [PubMed] [Google Scholar]
- 49.Freistroffer DV, Kwiatkowski M, Buckingham RH, Ehrenberg M. The accuracy of codon recognition by polypeptide release factors. Proc Natl Acad Sci U S A. 2000;97:2046–2051. doi: 10.1073/pnas.030541097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagradova N. Enzymes catalyzing protein folding and their cellular functions. Curr Protein Pept Sci. 2007;8:273–282. doi: 10.2174/138920307780831866. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y, Ke H. Mechanistic implication of crystal structures of the cyclophilin-dipeptide complexes. Biochemistry. 1996;35:7362–7368. doi: 10.1021/bi960278x. [DOI] [PubMed] [Google Scholar]
- 52.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garza-Sánchez F, Gin JG, Hayes CS. Amino acid starvation and colicin D treatment induce A-site mRNA cleavage in Escherichia coli. J Mol Biol. 2008;378:505–519. doi: 10.1016/j.jmb.2008.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neidhardt FC, Bloch PL, Smith DF. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonin PD, Choi GH, Trepod CM, Mott JE, Lyle SB, Cialdella JI, Sarver RW, Marshall VP, Erickson LA. Expression, purification, and characterization of peptidyl-tRNA hydrolase from Staphylococcus aureus. Protein Expr Purif. 2002;24:123–130. doi: 10.1006/prep.2001.1540. [DOI] [PubMed] [Google Scholar]