Abstract
Biologic scaffold materials composed of extracellular matrix (ECM) are routinely used for a variety of clinical applications ranging from the treatment of chronic skin ulcers to hernia repair and orthopaedic soft tissue reconstruction. The tissues and species from which the ECM is harvested vary widely as do the methods used to remove the cellular component of the source tissues. The efficacy of decellularization procedures can be quantified by examination of the DNA that remains in the ECM. The objective of the present study was to determine the DNA content and fragment length in both laboratory produced and commercially available ECM scaffold materials. Results showed that the majority of DNA is removed from ECM devices but that small amounts remained in most tested materials.
Introduction
Biologic scaffold materials composed of mammalian extracellular matrix (ECM) are commonly used for the surgical repair and reconstruction of musculotendinous, dermal, cardiovascular, gastrointestinal, and lower urinary tract tissues, among others (1-14). These ECM scaffolds are harvested from a variety of tissues including porcine dermis, small intestine, and urinary bladder, bovine dermis, equine pericardium, and human dermis. The methods used to remove the cellular component of each tissue vary widely. Decellularization is considered important because of the potential adverse immune response elicited by cell membrane epitopes, allogeneic or xenogeneic DNA, and damage associated molecular pattern (DAMP) molecules (15-17). Despite the broad clinical success of ECM scaffolds (1-14), porcine DNA remnants have been implicated as the cause of “inflammatory reactions” following the implantation of porcine derived scaffolds for orthopaedic applications (18). Several commercially available ECM scaffold materials, in particular Restore™ (porcine small intestinal submucosa (SIS)), GraftJacket™ (human dermis), and TissueMend™ (bovine dermis), have been shown to contain trace amounts of DNA (18, 19). Currently, the U.S. Federal Drug Administration does not regulate the limits for DNA in biologic scaffold materials.
The objective of the present study was to identify and quantify the DNA content and fragment length in several commercially available ECM scaffold materials and to compare the results with the DNA content in ECM scaffold materials produced in a laboratory setting. The presence of DNA was assessed qualitatively based upon histologic analysis with H&E and DAPI for nuclear DNA specific staining. In addition, quantitative measurements of total DNA content and fragment length were determined by the Picogreen Assay and gel electrophoresis, respectively.
Materials & Methods
Commercially available ECM scaffold materials
The commercially available ECM scaffold materials evaluated in the present study included Oasis™ (Cook Biotech, Inc.), a device consisting of a single layer of porcine SIS-ECM that has not been subjected to chemical crosslinking, Restore™ (DePuy Orthopedics Inc.), a 10-layer multilaminate device consisting of porcine SIS-ECM that has not been subjected to chemical crosslinking, the ACell Vet device (Acell, Inc.) consisting of non-crosslinked porcine urinary bladder matrix (UBM), Alloderm™ (LifeCell Corporation) consisting of human dermis that has not been subjected to chemical crosslinking, GraftJacket™ (Wright Medical Technology Inc.) consisting of a non-crosslinked decellularized human dermis, and the Zimmer™ collagen repair patch, consisting of crosslinked porcine dermis. (Table 1)
Table 1.
Source tissue and processing methods for seven commercially available and three laboratory produced extracellular matrix scaffolds
| Test Article | Species/Tissue of Origin | Crosslinking | Sterilization Method |
|---|---|---|---|
| Oasis™ (Cook Biotech, Inc.) | Porcine small intestinal submucosa | n/a | Ethylene oxide |
| Restore™ (DePuy Orthopaedics) | Porcine small intestinal submucosa | n/a | Electron beam irradiation |
| Acell Vet (Acell, Inc.) | Porcine urinary bladder basement membrane and mucosa | n/a | Electron beam irradiation |
| Alloderm™ (Lifecell, Corp.) | Human dermis | n/a | None, regulated as a tissue transplant by Food and Drug Administration |
| GraftJacket™ (Wright Medical Techology) | Human dermis | n/a | None, regulated as a tissue transplant by Food and Drug Administration |
| Zimmer Collagen Repair Patch™ (Zimmer, Inc.) | Porcine dermis | Isocyantate | Gamma irradiation |
| SIS-ECM | Porcine small intestinal submucosa | n/a | Ethylene oxide |
| UBM-ECM | Porcine urinary bladder basement membrane and mucosa | n/a | Ethylene oxide |
| LS-ECM | Porcine liver stroma | n/a | Ethylene oxide |
Preparation of ECM scaffold materials in the laboratory
Porcine small intestine, urinary bladder, and liver were harvested from market weight pigs (~110-130kg) immediately after euthanasia. Preparation of SIS-ECM (20, 21), urinary bladder matrix (UBM-ECM) (22, 23), and liver stroma (LS-ECM) (23, 24) has been previously described, and these methods are reviewed briefly below.
Preparation of SIS-ECM
After rinsing the small intestine, the tunica muscularis externa and the majority of the tunica mucosa were removed. The remaining tunica submucosa, muscularis mucosa, and basilar portion of the tunica mucosa represented SIS and the constituent cells. The SIS-ECM was then disinfected and decellularized in 0.1% (v/v) peracetic acid (σ), 4% (v/v) ethanol, and 96% (v/v) deionized water for 2 h. The SIS-ECM material was then washed twice for 15 min with PBS (pH = 7.4) and twice for 15 min with deionized water.
Preparation of UBM-ECM
The urinary bladder was trimmed to remove external connective tissues, including adipose tissue, and all residual urine was removed by repeated washes with tap water. The urothelial layer was removed by soaking of the bladder in 1 N saline. The tunica serosa, tunica muscularis externa, tunica submucosa, and most of the muscularis mucosa were mechanically delaminated from the bladder tissue. The remaining basement membrane of the tunica epithelialis mucosa and the subjacent tunica propria, collectively termed urinary bladder matrix (UBM), were then decellularized and disinfected by immersion in 0.1% (v/v) peracetic acid (σ), 4% (v/v) ethanol, and 96% (v/v) deionized water for 2 h. The UBM-ECM material was then washed twice for 15 min with PBS (pH = 7.4) and twice for 15 min with deionized water.
Preparation of liver stroma
The lobes of the liver were separated and frozen at -80°C for at least 24 hours. The frozen lobes were then cut into 3 mm sections using a rotating blade. The liver slices were decellularized by placement in deionized (DI) water and continuous vigorous shaking for 30 minutes at room temperature. This process was repeated with fresh DI water three times. The slices were then manually massaged after this step and all subsequent steps to hasten the lysis of hepatocytes and removal of cell remnants. The slices were then immersed in 0.02% trypsin/0.05% EDTA (Invitrogen, Carlsbad, CA) at 37°C for 1 hour, followed by rinsing with DI water. Next, the liver slices were exposed to a solution of 3% Triton X-100 (Spectrum Chemicals, New Brunswick, NJ) on a shaker for 1 hour at room temperature, again followed by rinsing. Finally, the slices were placed in 4% sodium deoxycholic acid (Spectrum) on a shaker for 1 hour at room temperature, followed by rinsing. The remaining tissue consisted almost exclusively of ECM and was referred to as liver stroma (LS-ECM). The LS-ECM was then disinfected and further decellularized in 0.1% (v/v) peracetic acid (σ), 4% (v/v) ethanol, and 96% (v/v) deionized water for 2 h followed by rinsing twice for 15 min with PBS (pH = 7.4) and twice for 15 min with DI water.
Histology
The laboratory prepared samples of SIS-ECM, UBM-ECM, and LS-ECM were harvested at each stage of the decellularization process and were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned and stained by DAPI and H&E. Commercially available ECM devices were also subjected to similar fixation and staining. Images were taken using a Nikon eclipse E600 microscope and a Spot RTslider camera, and saved with the MetaView software program. All images were taken at 200X magnification.
DNA quantification and fragment length analysis
To assess total DNA content within each of the test articles, the ECM scaffold materials were cut into thin strips and digested with Proteinase K at 37°C for up to 144 hours or until no visible scaffold material remained. Digested scaffolds were then centrifuged at 2980 g for 30 minutes to precipitate any remaining proteins. Supernatants were purified with Phenol-Chloroform-Isoamyl alcohol (25:24:1) followed by centrifugation at 9000 × G for 30 minutes. Aqueous layers were removed and ethanol precipitated at -20°C for at least 8 hours to isolate any DNA present. Samples were dehydrated in a vacuum manifold and rehydrated in 1X TE buffer. DNA content was quantified using the Picogreen DNA assay (Invitrogen) following manufacturer’s instructions. To determine DNA fragment size, samples were separated by electrophoresis on a 3% LMP agarose gel with Ethidium Bromide at 60V for 1 hour stained and visualized with ultraviolet transillumination.
Results
Most of the commercially available ECM scaffold materials contained measurable amounts of DNA as determined either by histologic staining, gel electrophoresis for DNA, or through the PicoGreen assay. The only biologic materials that were not found to contain DNA with any of the methods used were Alloderm™ and the Zimmer Collagen Patch™.
The Restore™ scaffold material showed the most nuclear staining with H&E and DAPI. The Restore™ device was found to contain 1.13 ± 0.03 ng DNA/mg dry weight by PicoGreen (Figure 1). Gel electrophoresis of the DNA showed that the majority of the DNA material was present in fragments primarily in the size range of 100 to 200 bp (Figure 2). Oasis™, which consists of the same material source with similar processing showed less DNA (0.42 ± 0.01 ng DNA/mg dry weight; p<0.05) (Figure 1) and showed no positive staining for cellular material by histologic examination. The DNA fragment size for Oasis™ was primarily between 100 and 200 bp (Figure 2). The Acell Vet material consists of porcine urinary bladder matrix, but is also decellularized using PAA. The amount of DNA for Acell Vet was less than the Oasis™ material (0.08 ± 0.003 ng DNA/mg dry weight) (Figure 1). Gel electrophoresis for DNA from the Acell Vet product showed a similar pattern of fragment sizes (Figure 2).
Figure 1.
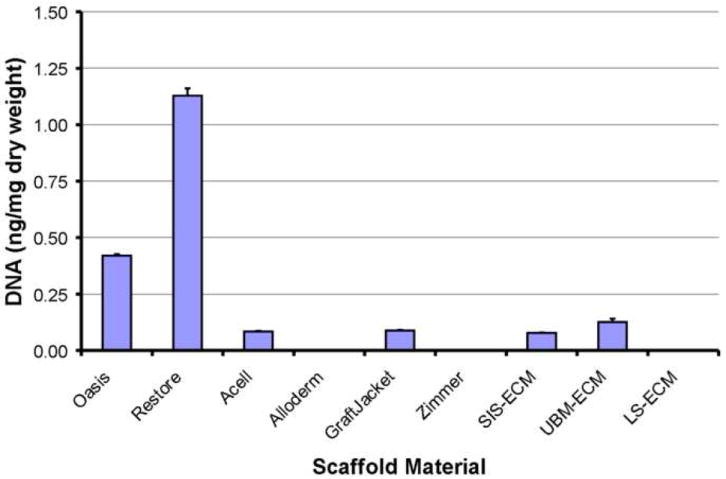
DNA content for commercially available and laboratory produced ECM scaffold materials as determined with the PicoGreen Assay.
Figure 2.
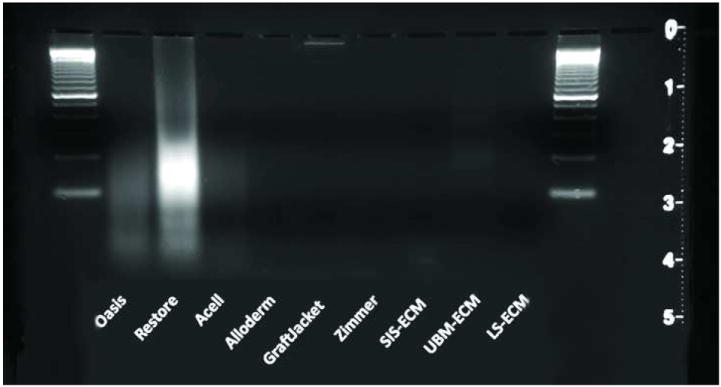
Gel electrophoresis of DNA isolated from commercially available and laboratory produced ECM scaffold materials.
Alloderm™ and GraftJacket™ are both derived from human dermis and are similarly processed. The DNA content in Alloderm™ was undetectable, and in GraftJacket™, the DNA was 0.09 ± 0.003 ng DNA/g dry weight (Figure 1). By gel electrophoresis, the DNA in GraftJacket appeared to remain intact through processing with a fragment size larger than the largest standard in the ladder used in the study (2072 bp) (Figure 2).
The DNA content of native SIS, UBM, and LS were found to be 10.1 ± 0.3 ng DNA/mg dry weight, 3.0 ± 0.1 ng DNA/mg dry weight, and 5.3 ± 0.5 ng DNA/mg dry weight, respectively.
After processing, the laboratory produced SIS-ECM and UBM-ECM showed limited positive staining for nuclear material histologic staining with H&E and DAPI. The positive staining tended to be localized in the stratum compactum of SIS-ECM and in muscularis mucosa tissue in the UBM-ECM, both representing regions of dense connective tissue within the materials. The PicoGreen assay showed 0.08 ± 0.002 ng DNA/g dry weight of SIS-ECM and 0.13 ± 0.02 ng DNA/g dry weight of UBM (Figure 1). Gel electrophoresis showed limited residual DNA with fragment lengths similar to Restore™ and ACell Vet (Figure 2). The laboratory produced LS-ECM showed no positive staining for nuclear material by histology, gel electrophoresis, or PicoGreen Assay (Figures 1 and 2).
Discussion
This study shows that most of the commercially available naturally occurring ECM devices tested contain at least trace amounts of DNA. Even on a laboratory scale where decellularization should theoretically be more complete, small amounts of DNA were detectable. The extent of DNA removal did vary considerably, with Restore™ showing prominent histologic staining for nuclear material and 0.1% DNA compared to 0.01% DNA for most other devices. Both Alloderm™ and Zimmer™ collagen patch have no detectable DNA by the methods used in this study.
Despite the presence of DNA in many of the commercially available ECM devices, the clinical efficacy of these devices for their intended application has been largely positive (1-14). It therefore appears unlikely that the remaining DNA fragments within these biologic scaffold materials contribute to any adverse host response or are a cause for concern. Considering the manner in which cells are embedded within tissue ECM, especially relatively dense tissues like the dermis, it seems unlikely that complete removal of all cellular contents is possible even with the most rigorous processing methods. The current study evaluated only DNA, but it is plausible and likely that other intracellular cytoplasmic proteins and membrane components are retained in the scaffold materials through the processing steps.
It is clear that cell products are capable of eliciting a host inflammatory response and/or stimulating an immune reaction. However, it may be possible that a threshold amount of material is required to adversely affect the remodeling response. It is also possible that processing/decellularization methods alter any remaining cell products to the extent that they no longer stimulate adverse events in host tissue (17).
The presence of small amounts of cellular antigens may be insufficient to elicit the type of proinflammatory or immune response that could adversely affect biologic scaffold remodeling. For example, small amounts of the Galα1,3 epitope, a known cause of hyperacute rejection in xenogeneic organ transplants (25, 26), has been suggested to contribute to adverse tissue reactions following implantation of selected porcine origin ECM scaffolds (27-29). However, the amount of Galα1,3 epitope in an ECM scaffold was insufficient to cause in vitro activation of complement in human plasma (30). Therefore, the presence of 1 ng of DNA/mg dry weight, the DNA content in the Restore™ device, may or may not be significant in terms of eliciting a clinically significant host response.
In most of the biologic scaffold materials that were investigated in the current study, the DNA that was present consisted of fragments less than 300 bp in length. This length of DNA is likely too short to be of concern. The only device that appeared to contain full DNA strands was GraftJacket™, which is derived from human dermis. In addition to the small amount of DNA and abbreviated length of the DNA fragments, the noncrosslinked forms of ECM scaffolds are degraded rapidly after placement in vivo (31-33). Any residual DNA is logically subject to the same degradation fate via enzymatic breakdown as the rest of the ECM components. Toll-like receptors may play an important role in this regard as they bind soluble DNA so that they can be broken down into nucleotides for future use by the cells (34, 35).
It is possible that a portion of the DNA that is present in biologic scaffold materials represent deleterious nucleic acid from viruses or prions, although the amount of any such DNA would be exceedingly small. Peracetic acid, one of the chemicals used for removal of cellular material from biologic scaffold materials, has been shown to essentially eliminate the viral load (36). Furthermore, based on previous studies, it seems unlikely that the DNA fragments would be capable of transmitting the disease. Several studies have investigated the transmission of porcine endogenous retrovirus (PERV) from apoptotic porcine cells to human cells both in vitro and in vivo (37-39). Even with direct co-culture, porcine DNA was found in only 0.22% of human cells immediately following exposure and no DNA was detectable by 4 weeks (37). This result strongly suggests that any viral DNA present in the ECM could not transmit the disease to the host.
In summary, the present study shows that most commercially available biologic scaffold materials contain small amounts of remnant DNA. The study also showed that remaining DNA is typically present as small fragments, which indicates that it is highly unlikely that these fragments play a causative role in any adverse tissue remodeling response. More thorough methods of tissue decellularization would be desirable and any quality assurance steps that assure removal of cell remnants would be welcome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 2.Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22:534–538. doi: 10.1016/j.arthro.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Brigido SA. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16-week pilot study. Int Wound J. 2006;3:181–187. doi: 10.1111/j.1742-481X.2006.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler CE, Prieto VG. Reduction of adhesions with composite AlloDerm/polypropylene mesh implants for abdominal wall reconstruction. Plast Reconstr Surg. 2004;114:464–473. doi: 10.1097/01.prs.0000132670.81794.7e. [DOI] [PubMed] [Google Scholar]
- 5.Catena F, Ansaloni L, Leone A, De Cataldis A, Gagliardi S, Gazzotti F, Peruzzi S, Agrusti S, D’Alessandro L, Taffurelli M. Lichtenstein repair of inguinal hernia with Surgisis inguinal hernia matrix soft-tissue graft in immunodepressed patients. Hernia. 2005;9:29–31. doi: 10.1007/s10029-004-0273-y. [DOI] [PubMed] [Google Scholar]
- 6.Coons DA, Alan Barber F. Tendon graft substitutes-rotator cuff patches. Sports Med Arthrosc. 2006;14:185–190. doi: 10.1097/00132585-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Harper C. Permacol: clinical experience with a new biomaterial. Hosp Med. 2001;62:90–95. doi: 10.12968/hosp.2001.62.2.2379. [DOI] [PubMed] [Google Scholar]
- 8.Lee MS. GraftJacket augmentation of chronic Achilles tendon ruptures. Orthopedics. 2004;27:s151–153. doi: 10.3928/0147-7447-20040102-15. [DOI] [PubMed] [Google Scholar]
- 9.Liyanage SH, Purohit GS, Frye JN, Giordano P. Anterior abdominal wall reconstruction with a Permacol implant. Br J Plast Surg. 2005 doi: 10.1016/j.bjps.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Metcalf MH, Savoie FH, Kellum B. Surgical technique for xenograft (SIS) augmentation of rotator-cuff repairs. Oper Tech Orthop. 2002;12:204–208. [Google Scholar]
- 11.Parker DM, Armstrong PJ, Frizzi JD, North JH., Jr Porcine dermal collagen (Permacol) for abdominal wall reconstruction. Curr Surg. 2006;63:255–258. doi: 10.1016/j.cursur.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Sclafani AP, Romo T, 3rd, Jacono AA, McCormick S, Cocker R, Parker A. Evaluation of acellular dermal graft in sheet (AlloDerm) and injectable (micronized AlloDerm) forms for soft tissue augmentation. Clinical observations and histological analysis. Arch Facial Plast Surg. 2000;2:130–136. doi: 10.1001/archfaci.2.2.130. [DOI] [PubMed] [Google Scholar]
- 13.Smart N, Immanuel A, Mercer-Jones M. Laparoscopic repair of a Littre’s hernia with porcine dermal collagen implant (Permacol) Hernia. 2007 doi: 10.1007/s10029-007-0197-4. [DOI] [PubMed] [Google Scholar]
- 14.Ueno T, Pickett LC, de la Fuente SG, Lawson DC, Pappas TN. Clinical application of porcine small intestinal submucosa in the management of infected or potentially contaminated abdominal defects. J Gastrointest Surg. 2004;8:109–112. doi: 10.1016/j.gassur.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Lotze MT. Damage-associated molecular pattern molecules. Clin Immunol. 2007;124:1–4. doi: 10.1016/j.clim.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61–67. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 19.Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. 2006;88:2665–2672. doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert TW, Sacks MS, Grashow JS, Woo SL-Y, Badylak SF, Chancellor MB. Fiber kinematics of small intestinal submucosa under biaxial and uniaxial stretch. J Biomech Eng. 2006;128:890–898. doi: 10.1115/1.2354200. [DOI] [PubMed] [Google Scholar]
- 21.Zantop T, Gilbert TW, Yoder MC, Badylak SF. Extracellular matrix scaffolds are repopulated by bone marrow-derived cells in a mouse model of Achilles tendon reconstruction. J Orthop Res. 2006;24:1299–1309. doi: 10.1002/jor.20071. [DOI] [PubMed] [Google Scholar]
- 22.Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, Freytes DO, Thapa A, Gilbert TW, Nieponice A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87–97. doi: 10.1016/j.jss.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Brown B, Lindberg K, Reing J, Stolz DB, Badylak SF. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12:519–526. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 24.Lin P, Chan WC, Badylak SF, Bhatia SN. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10:1046–1053. doi: 10.1089/ten.2004.10.1046. [DOI] [PubMed] [Google Scholar]
- 25.Galili U. The alpha-gal epitope (Gal alpha 1-3Gal beta 1-4GlcNAc-R) in xenotransplantation. Biochimie. 2001;83:557–563. doi: 10.1016/s0300-9084(01)01294-9. [DOI] [PubMed] [Google Scholar]
- 26.Platt JL, Fischel RJ, Matas AJ, Reif SA, Bolman RM, Bach FH. Immunopathology of hyperacute xenograft rejection in a swine-to-primate model. Transplantation. 1991;52:214–220. doi: 10.1097/00007890-199108000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Konakci KZ, Bohle B, Blumer R, Hoetzenecker W, Roth G, Moser B, Boltz-Nitulescu G, Gorlitzer M, Klepetko W, Wolner E, Ankersmit HJ. Alpha-Gal on bioprostheses: xenograft immune response in cardiac surgery. Eur J Clin Invest. 2005;35:17–23. doi: 10.1111/j.1365-2362.2005.01441.x. [DOI] [PubMed] [Google Scholar]
- 28.Stone KR, Ayala G, Goldstein J, Hurst R, Walgenbach A, Galili U. Porcine cartilage transplants in the cynomolgus monkey. III. Transplantation of alpha-galactosidase-treated porcine cartilage. Transplantation. 1998;65:1577–1583. doi: 10.1097/00007890-199806270-00007. [DOI] [PubMed] [Google Scholar]
- 29.Stone KR, Walgenbach AW, Turek TJ, Somers DL, Wicomb W, Galili U. Anterior cruciate ligament reconstruction with a porcine xenograft: a serologic, histologic, and biomechanical study in primates. Arthroscopy. 2007;23:411–419. doi: 10.1016/j.arthro.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 30.McPherson TB, Liang H, Record RD, Badylak SF. Galalpha(1,3)Gal epitope in porcine small intestinal submucosa. Tissue Eng. 2000;6:233–239. doi: 10.1089/10763270050044416. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89:621–630. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 32.Record RD, Hillegonds D, Simmons C, Tullius R, Rickey FA, Elmore D, Badylak SF. In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials. 2001;22:2653–2659. doi: 10.1016/s0142-9612(01)00007-2. [DOI] [PubMed] [Google Scholar]
- 33.Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;88:2673–2686. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- 34.Bennett RM, Gabor GT, Merritt MM. DNA binding to human leukocytes. Evidence for a receptor-mediated association, internalization, and degradation of DNA. J Clin Invest. 1985;76:2182–2190. doi: 10.1172/JCI112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCoy SL, Kurtz SE, Hausman FA, Trune DR, Bennett RM, Hefeneider SH. Activation of RAW264.7 macrophages by bacterial DNA and lipopolysaccharide increases cell surface DNA binding and internalization. J Biol Chem. 2004;279:17217–17223. doi: 10.1074/jbc.M303837200. [DOI] [PubMed] [Google Scholar]
- 36.Hodde JP, Hiles M. Virus safety of a porcine-derived medical device: evaluation of a viral inactivation method. Biotechnol Bioeng. 2002;79:211–216. doi: 10.1002/bit.10281. [DOI] [PubMed] [Google Scholar]
- 37.Bisset LR, Boni J, Lutz H, Schupbach J. Lack of evidence for PERV expression after apoptosis-mediated horizontal gene transfer between porcine and human cells. Xenotransplantation. 2007;14:13–24. doi: 10.1111/j.1399-3089.2006.00351.x. [DOI] [PubMed] [Google Scholar]
- 38.Di Nicuolo G, van de Kerkhove MP, Hoekstra R, Beld MG, Amoroso P, Battisti S, Starace M, di Florio E, Scuderi V, Scala S, Bracco A, Mancini A, Chamuleau RA, Calise F. No evidence of in vitro and in vivo porcine endogenous retrovirus infection after plasmapheresis through the AMC-bioartificial liver. Xenotransplantation. 2005;12:286–292. doi: 10.1111/j.1399-3089.2005.00226.x. [DOI] [PubMed] [Google Scholar]
- 39.Hermida-Prieto M, Domenech N, Moscoso I, Diaz T, Ishii J, Salomon DR, Manez R. Lack of cross-species transmission of porcine endogenous retrovirus (PERV) to transplant recipients and abattoir workers in contact with pigs. Transplantation. 2007;84:548–550. doi: 10.1097/01.tp.0000275203.91841.23. [DOI] [PubMed] [Google Scholar]


