Abstract
The presentation of antigenic peptides by class I molecules of the major histocompatibility complex begins in the endoplasmic reticulum where the coordinated action of molecular chaperones, folding enzymes and class I-specific factors ensure that class I molecules are loaded with high affinity peptide ligands that will survive prolonged display at the cell surface. Once assembled, class I molecules are released from the quality control machinery of the ER for export to the plasma membrane where they undergo dynamic endocytic cycling and turnover. We review recent progress in our understanding of class I assembly, anterograde transport and endocytosis and highlight some of the events targeted by viruses as a means to evade detection by cytotoxic T cells and natural killer cells.
Keywords: endoplasmic reticulum, molecular chaperones, peptide loading complex, antigen presentation, endocytosis, membrane traffic, ubiquitylation
Major histocompatibility complex (MHC) class I molecules bind peptide fragments of intracellular proteins and display them at the cell surface where they function to report the presence of viruses or tumors to CD8+ T cells. They also function in NK cell recognition by providing inhibitory signals that are overcome when class I molecules are down-regulated as a consequence of viral infection or tumor transformation. Central to the function and stability of class I molecules is their remarkable ability to bind a diverse array of peptides and to do so with the high affinity required to survive trafficking through the secretory pathway and prolonged display at the cell surface. This is accomplished within the endoplasmic reticulum (ER) through a complex machinery that involves components of general glycoprotein quality control including the lectin-chaperones calnexin (Cnx), calreticulin (Crt) and their associated thiol oxidoreductase, ERp57, and MHC class I-specific components including the chaperone tapasin and the ABC peptide transporter, TAP (1). Loading of high affinity peptides triggers release of class I molecules from this quality control system and subsequent export of class I from the ER along the conventional secretory pathway (2). In the first part of this review, we discuss the functions of the various ER quality control components as well as the current state of knowledge concerning class I export from the ER and trafficking within the early secretory pathway. In the second part, we examine the endocytic trafficking route followed by class I and how viral and cellular ubiquitin ligases regulate class I turnover.
Overview of MHC Class I Assembly
MHC class I molecules are heterotrimers consisting of a glycosylated transmembrane heavy chain (HC), the soluble subunit β2-microglobulin (β2m) and an 8-10 residue peptide ligand. As shown in Fig. 1A, the newly translocated HC first associates with the transmembrane chaperone Cnx and associated ERp57. Cnx and its soluble paralog Crt recognize glycoprotein folding intermediates through a lectin site specific for monoglucosylated Glc1Man5-9GlcNAc2 oligosaccharides (3, 4) as well as through a polypeptide binding site selective for non-native conformers (5). Reconstitution of mouse class I assembly in the presence or absence of Cnx in Drosophila cells or treatment of mouse cells with castanospermine to block formation of monoglucosylated oligosaccharides has suggested that Cnx functions to promote HC folding and inhibit aggregation as well as retain unassembled HC in the ER (6). Milder phenotypes were observed in human cells with a modest slowing of HC disulfide formation in the absence of Cnx association (7). Interestingly, Cnx-deficient human cells exhibit no class I biosynthetic phenotype, possibly due to compensation by Crt or other ER chaperones such as the Hsp70 BiP which are upregulated in these cells (8). ERp57 has clearly been shown to be involved in the formation of HC disulfides since siRNA knockdown results in an ~10-fold slowing of HC oxidative folding. Surprisingly, ERp57 did not require interaction with Cnx or Crt to effect its functions on HC folding although this has been shown to be a requirement for ERp57 activity towards several other substrates (9).
Figure 1. Intracellular Assembly and Quality Control of MHC Class I Molecules.
A) Newly synthesized class I HC initially binds to the lectin-chaperone calnexin and associated thiol oxidoreductase, ERp57, an interaction that retains free HC in the ER and promotes formation of the two HC disulfides. Upon binding of β2-microglobulin, most HC-β2m heterodimers are unstable and require entry into the peptide loading complex for stability and acquisition of high affinity peptide ligands. Peptides that bind to class I molecules are generated in the cytosol through the action of the proteasome. Upon TAP-mediated import into the ER lumen, peptides are further trimmed by ER aminopeptidases until the appropriate length of 8-10 residues is achieved. Following loading of high affinity peptides, class I is released from the peptide loading complex and packaged into COPII vesicles for export from the ER. Since the cytosolic tails of most class I HC lack anterograde transport signals, their export may involve cargo receptors such as Bap31 which interacts with both HC and tapasin and cycles between the ER and ERGIC compartments. B) Interactions that stabilize the peptide loading complex. Most of the indicated interactions have been well mapped (see (71) for review). Tapasin makes multipoint contacts within the PLC; through transmembrane interactions with TAP, through two points of contact with the luminal segment of the HC, and extensive contacts with the two redox-active domains of ERp57, including a stable mixed disulfide bond (S-S). Calreticulin also makes multiple contacts which include interaction with ERp57 through the tip of its extended arm domain, binding to monoglucosylated oligosaccharides (G) of the HC through the lectin site of its globular domain, and polypeptide-based interactions with the HC through an undefined site in the globular domain. Bap31 interacts with both the HC and tapasin, at least in part through transmembrane interactions, but is not required for PLC assembly. Estimates of the stoichiometry of TAP:tapasin:HC within the PLC range from 1:2:1 to 1:4:4.
The second stage of class I assembly occurs at the time of HC association with β2m (Fig. 1A). At this point, newly assembled HC-β2m heterodimers enter the peptide loading complex (PLC) in a process typically accompanied by the loss of Cnx-ERp57 and its replacement with Crt-ERp57. The PLC consists of HC-β2m heterodimer, Crt, ERp57, the type I transmembrane glycoprotein tapasin (Tpn) and the polytopic peptide transporter associated with antigen processing (TAP). As the name implies, the PLC serves to transport proteasome-generated peptides across the ER membrane and facilitate the loading of appropriate peptides onto class I molecules. The functions and stability of the PLC are regulated by a complex series of interactions between the various components as illustrated in Fig. 1B (1). The centerpiece of the PLC is tapasin which interacts both with TAP and the class I HC, bridging the two (10). Tapasin also forms a stable mixed disulfide bond with ERp57 between Cys 95 of tapasin and Cys 57 of ERp57 (11). ERp57 simultaneously associates with Crt which in turn uses its lectin and polypeptide binding sites to interact with the class I HC (12).
Functions of the Peptide Loading Complex
The functions of the various PLC components have largely been revealed through the study of component-negative cells. The ABC transporter TAP transports proteasome-generated peptides from the cytosol into the ER lumen with a preference for peptides of ~8-16 residues in length (13). In TAP-/- mice or cells, class I molecules are not loaded with high affinity peptides (although low affinity peptides can be detected (14)) and consequently most CD8+ T cell responses are profoundly impaired (15, 16). These class I molecules that lack peptide or contain low affinity peptides exhibit prolonged association with remaining components of the PLC and are largely retained in the ER or cycle between the ER and cis-Golgi (17-19). Only small amounts are exported to the cell surface where they are unstable at 37°C and dissociate rapidly (20). Thus binding of high affinity peptide is crucial for the release of class I molecules from the PLC, for efficient export beyond the ER-cis-Golgi recycling compartments and for class I stability at physiological temperature.
Tapasin acts as a bridge between peptide-receptive HC-β2m heterodimers and TAP, and its loss impacts both molecules (21). In the absence of tapasin, the TAP transporter is less stable, resulting in lower expression and decreased peptide transport into the ER lumen (22). Tapasin deficiency also results in class I molecules not being recruited into the PLC in proximity to TAP, and indeed, class I molecules in Tpn–/– cells exhibit reduced loading of high affinity peptides, reduced stability and reduced cell surface expression, although not to the same extent as observed with TAP deficiency (23). Strikingly, Cresswell and colleagues showed that a soluble version of tapasin that does not interact with TAP but retains the ability to bind to class I molecules still supports normal peptide loading and surface class I expression, suggesting that tapasin promotes loading of high affinity peptides by a mechanism independent of recruiting class I molecules in proximity to TAP (24). Studies by Elliott and co-workers have demonstrated that tapasin acts to optimize the repertoire of peptides bound by class I molecules over time, in a process known as peptide editing, with abundant low affinity peptides that bind initially being exchanged in favor of those that bind with a long half-life (25, 26). Two in vitro systems have provided mechanistic insights into this behavior, both employing novel methods to overcome the weak intrinsic affinity observed between soluble forms of tapasin and peptide-free class I molecules. Wearsch and Cresswell discovered that when a purified mixed disulfide conjugate of tapasin and ERp57 was added to digitonin lysates of tapasin-deficient cells, it supported full PLC assembly and the loading of exogenously added peptides onto class I molecules (27). They found that Tpn-ERp57 stabilized peptide-receptive class I molecules and promoted the loading of exogenous peptides whereas Tpn alone was ineffective. Furthermore, Tpn-ERp57 promoted more efficient loading of high affinity peptides in the presence of a large excess of lower affinity competitors and enhanced the dissociation of pre-loaded low affinity peptides (27). In an alternative approach, Chen and Bouvier created Jun and Fos fusions with soluble Tpn and HLA-B*0801 HC, respectively, to stabilize their interaction (28). It was observed that the presence of Tpn caused peptides to associate with and dissociate from the HC binding groove more rapidly, probably through an effect of widening the groove. Since peptides lacking a COOH terminus exhibited the same decay kinetics in the presence or absence of Tpn, it was suggested that Tpn acts to disrupt H-bond interactions between HC and peptide primarily at the C-terminal end of the groove. The authors further suggest that the net result of Tpn action is to permit binding of a larger pool of candidate peptides, ultimately favoring those that bind with sufficient energy to effect a conformational change that disengages Tpn (28). As yet, the conformational changes accompanying the binding of high affinity peptides that lead to dissociation of the PLC remain elusive.
Knockout experiments have also provided insights into the role of ERp57 within the PLC. In mice, ERp57 gene deletion is lethal, but in cultured B cells or fibroblasts lacking ERp57 the PLC was shown to be unstable, peptide loading was inefficient and surface class I expression was reduced (29). Mutagenesis experiments to inactivate the two redox active sites of ERp57 have demonstrated that the enzymatic activity of ERp57 is not required for its functions within the PLC (9, 30), indicating that it plays a structural rather than catalytic role within the PLC. These findings have been confirmed by the recent crystal structure of the ERp57-Tpn mixed disulfide conjugate which showed that both ERp57 active sites are buried in the interaction interface with Tpn (31). It has been suggested that the structural function of ERp57 within the PLC is to provide additional stabilization by interacting with Crt that is simultaneously bound to the class I HC (31) (Fig. 1B). However, ERp57 that has been mutated to prevent Crt binding functioned just as well as wild type ERp57 (9), leading to the alternative possibility that ERp57 binding to Tpn stabilizes the class I interaction sites(s) recently delineated on Tpn (31). Despite these findings, Crt also contributes significantly to PLC function. Crt knockout is lethal in mice and, in Crt–/– fibroblasts, peptide loading is again inefficient, class I molecules lacking high affinity ligands are prematurely exported from the ER and class I expression at the cell surface is reduced (32). Interestingly, all of these phenotypes can be complemented by lectin-deficient mutants of Crt, demonstrating the importance of polypeptide-based modes of Crt -substrate interaction in addition to lectin-oligosaccharide binding (12).
Export of Class I Molecules from the ER
It is well established that upon acquiring high affinity peptide, class I molecules are released from the PLC (2). This can be monitored in living cells by feeding exogenous peptide (which can reach the ER) and using FRAP to detect an increase in GFP-tagged class I diffusion rate as it is released from the very large PLC (33). However, there is some controversy as to whether peptide-receptive class I molecules remain solely in the ER before acquiring peptide ligand or whether they are exported to the ERGIC or cis-Golgi and recycle back to the ER for peptide loading. Several studies have documented PLC components in the ERGIC/cis-Golgi at steady state including peptide-receptive class I molecules, TAP, tapasin, and Crt (34-36). The latter two proteins possess C-terminal -KKXX and -KDEL retrieval sequences, respectively, that could effect recycling of associated peptide-receptive class I molecules. Indeed, mutation of these retrieval sequences in tapasin (36) and Crt3 results in reduced peptide loading efficiency. Furthermore, both peptide-occupied and peptide-receptive class I molecules have been shown to be packaged into COPII vesicles with similar efficiencies in wild type lymphocytes (34). These findings suggest that the quality control machinery that retains peptide-receptive class I molecules intracellularly extends beyond the ER. In contrast, Edidin and co-workers found that GFP-tagged tapasin could not be detected in ERGIC/cis-Golgi compartments and was actually excluded from ER exit sites (37). Furthermore, they monitored the dynamics of GFP-fused class I molecules in the presence and absence of exogenous peptides and found that upon release from the PLC, peptide-loaded class I clusters and accumulates at ER exit sites (38). These findings suggest that peptide binding is not rate-limiting for ER export and that there are subsequent events that limit export rate such as binding to cargo receptors. With the notable exception of the non-classical HLA-F molecule which possesses ER export signals in its cytoplasmic tail (39), the cytoplasmic tails of classical class I HCs lack anterograde trafficking signals such as dihydrophobic or diacidic motifs for incorporation into COPII vesicles. This suggests that cargo receptors may participate in packaging classical class I molecules for export. A candidate receptor is Bap31, a transmembrane protein that spans the membrane three times and includes a cytoplasmic tail with a -KKXX retrieval motif (Fig. 1). Bap31 has been implicated both in the anterograde transport of some proteins from the ER as well as the retention/retrieval of others. It binds both human and mouse class I molecules and its overexpression increases both the ER to Golgi trafficking rate of human class I molecules as well as their expression levels at the cell surface (40). Bap31 deficiency or knockdown results in a modest slowing of mouse class I ER to Golgi transport with no effect on surface expression, suggesting that Bap31 facilitates but is not essential for anterograde class I trafficking (41). Presumably, additional redundant cargo receptors exist for class I molecules.
Viral Subversion of Class I Biogenesis
Given the complexity of the machinery that facilitates the assembly of class I molecules and monitors their integrity within the ER, it is not surprising that viruses have evolved a multitude of strategies to subvert these processes as a means to downregulate surface class I and evade detection by CD8+ T cells. There have been many excellent reviews on this topic in recent years so only a brief overview will be included here (42, 43). The human cytomegalovirus (HCMV) US2 and US11 proteins act at the earliest stages of class I biogenesis. US2 and US11 bind to class I HCs soon after their translocation into the ER, diverting them into the ER-associated degradation (ERAD) pathway which retrotranslocates HCs to the cytosol where they are ubiquitylated and rapidly degraded by the proteasome. Similarly, the mK3 protein of mouse herpesvirus 68 is an E3 ligase that binds to both TAP and class I HC, ubiquitylating the HC and diverting it to ERAD. Farther along the assembly pathway, tapasin is a target of the HCMV US3 protein which inhibits tapasin-dependent peptide loading and peptide optimization. Also within the PLC, the herpes simplex virus protein ICP47 binds to the cytoplasmic face of TAP and prevents peptide binding, effectively cutting off the supply of proteasome-generated peptides to the ER lumen. The HCMV protein US6 accomplishes the same thing by interacting with the ER luminal face of TAP and transducing a conformational change that prevents ATP binding and hydrolysis through the cytosolic nucleotide binding domains of TAP. Finally, the adenovirus E3-19K and cowpox CPXV203 proteins bind to mature class I molecules, retaining them within the ER thereby reducing their expression at the cell surface. Although down-regulation of class I molecules offers viruses protection from CD8+ T cells, it renders them susceptible to NK cells since class I molecules act as ligands for inhibitory NK receptors. To avoid NK cell recognition, some viruses encode class I-like surrogate ligands for inhibitory NK receptors such as HCMV UL18 protein and the MCMV m144 and m157 proteins (reviewed in (44)).
MHC Class I at the Plasma Membrane
Upon arrival at the cell surface, fully-assembled peptide-loaded class I functions to present the array of endogenously-generated peptides to the immune system sentinels, the NK and CD8+ T cells. However, class I is not a static plasma membrane resident as it is continually being removed from the cell surface by endocytosis. The fate of internalized class I, whether it is recycled back to the plasma membrane or routed to degradation, and the exposure of class I to different environments in the endosome might regulate class I turnover and present an opportunity for class I modification and in some instances (dendritic cells) cross-presentation of exogenous antigens. In cross-presentation, exogenous antigens are taken up by endocytic mechanisms such as phagocytosis and are either transferred to the cytosol for degradation by the proteasome followed by peptide loading onto class I within the ER or, alternatively, are degraded by endosomal proteases with peptide products binding to recycling class I within endocytic compartments. Cross-presentation is an important process whereby dendritic cells acquire exogenous antigens from other afflicted cells and then migrate to lymphoid tissues to active immature T cells (45). Class I may also as a consequence of this endocytic trafficking take on an open conformation lacking peptide and/or β2m resulting in trans-interactions with other receptors and plasma membrane proteins.
Constitutive Endocytosis and Recycling of Class I
The heavy chain of class I has a short cytoplasmic domain that lacks amino acid sorting sequences necessary for clathrin- and adaptor protein-dependent endocytosis. Thus, for many years it was assumed that class I was not brought into cells by endocytosis but remained at the cell surface. However, class I has been shown in antibody internalization assays to enter cells by a clathrin- and dynamin-independent endocytic pathway (46, 47). This pathway exists in all cell types examined but has been most studied in HeLa cells where it is associated with and influenced by the activities of the Arf6 GTP-binding protein (see Fig. 2). After endocytosis, vesicles carrying class I fuse with the Rab5-associated “classical” early endosomes containing clathrin-derived cargo such as the transferrin receptor (46). After this, some class I molecules go on to late endosomes and lysosomes where they are degraded. Other class I molecules move on with transferrin receptor to a juxtanuclear endocytic recycling compartment and then emerge in microtubular-associated membrane tubules that carry class I back to the PM. In HeLa cells, with each round of endocytosis of class I, approximately half goes on to late endosomes for degradation and half is recycled (46). CD1a, a class I-related molecule that presents amphipathic lipid antigens to T cells, also travels along this endosomal pathway (48). In addition to MHC class I and CD1a, many other cargo proteins have been shown to traffic along this clathrin-independent endocytic and recycling pathway including integrins, CD59, CD44, CD98, ICAM1, the glucose transporter 1 (49) and, in transfected cells, the IL2 receptor alpha (Tac) (46, 47). Interestingly, similar endocytic uptake and recycling has been demonstrated for peptide-loaded MHC Class II molecules in B cells and dendritic cells (50).
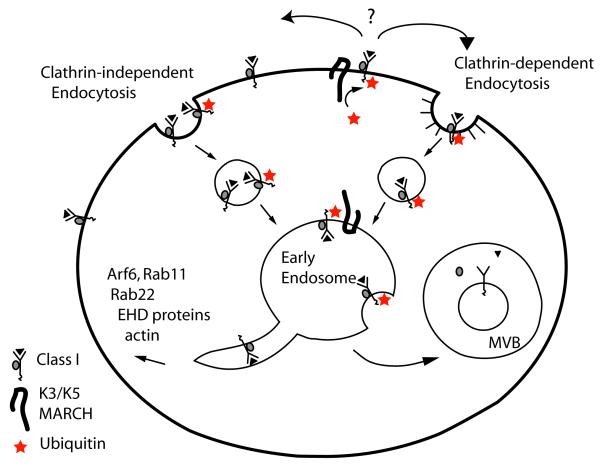
Figure 2. Endosomal Trafficking of MHC Class I.
Class I undergoes constitutive internalization by clathrin-independent endocytosis. After internalization, vesicles containing class I fuse with an early endosomal compartment which is associated with Rab5 and is mildly acidic. Class I can either be routed to the late endosomal/MVB pathway and degraded or recycled back to the cell surface in recycling endosomes. In many cells, this recycling is dependent upon activation of several GTPases (Arf6, Rab11, Rab22), EHD proteins and actin. The viral proteins K3 and K5 and the endogenous MARCH proteins are E3 RING-CH ubiquitin ligases that ubiquitylate class I (red stars). This may cause ubiquitylated class I to now enter via clathrin-dependent endocytosis in some cases. These E3 ligases may also be present on endosomal or lysosomal membranes. Proteins tagged with monoubiquitylation (shown here) or lysine-63-linked ubiquitylation are subsequently targeted to intralumenal vesicles in the forming MVBs and degraded.
There has been increased interest in how these clathrin-independent modes of endocytosis are regulated and the fates of cargo proteins internalized by these mechanisms (51, 52). Endocytosis of class I and other clathrin-independent cargo proteins is dependent upon free PM cholesterol and is stimulated in cells expressing a constitutively active form of Arf6, Q67L, which activates a phosphoinositide kinase that generates phosphatidylinositol 4,5 bisphosphate (PIP2) (46, 53). Once internalized, however, the vesicle-associated GTP-bound Arf6 must be inactivated and PIP2 metabolized so that the vesicles can fuse with the Rab5 early endosomes that have phosphoinositide 3-phosphate (PI3P) and the tethering protein EEA1 associated with them (53). Within this early or sorting endosome, cargo proteins, whether entering from clathrin-dependent or -independent pathways, are sorted either towards degradative compartments or towards recycling pathways (Fig. 2). The mild acidification of this early endosome may facilitate this sorting (54). Additionally, ubiquitylation of the cytoplasmic domains on class I and other plasma membrane proteins plays a role in routing proteins into the late endosome pathway (see below).
Whether there are positive signals for recycling or whether lack of ubiquitylation allows recycling is not known but entry into the recycling endosomes and back out to the PM is mediated by multiple GTPases and other factors (Fig. 2). In Hela cells, Rab11 and Rab22 are required for recycling of class I (55) and CD1a (50). Activation of Arf6 is also required for class I recycling and part of this requirement is for Arf6 to activate phospholipase D to generate phosphatidic acid (56). Eps 15 homology domain proteins (EHDs) are associated with the recycling endosomes and facilitate PM recycling of class I (57, 58). In addition, at least in HeLa cells, there are many signaling proteins associated with the recycling endosome that may affect recycling including Rac, Ras, Src and Erk (51, 59). Recycling of these membrane proteins, taken up by clathrin-independent endocytosis, is critical for PM maintenance and processes involving the cortical actin cytoskeleton.
Downregulation of Class I by Ubiquitylation
How cells normally regulate the turnover of PM proteins including class I is not known. However some surface receptors, such as the EGF and growth hormone receptor, are tagged with a single ubiquitin moiety after ligand activation which causes these receptors to be removed from the PM and targeted to degradation. The ESCRT complex of proteins recognize mono-ubiquitin-tagged membrane proteins and sort them into the invaginating membranes forming the multi-vesicular body (MVB) (for a review of ESCRT function and MVB formation see (60)). Proteins are selected for ubiquitylation by enzymes called E3 ubiquitin ligases that couple with E2 conjugating enzymes. Class I molecules are also subject to ubiquitylation of the HC that leads to downregulation. This modification of class I was first shown to be induced by the viral immune modulators, K3 and K5, expressed by the Kaposi sarcoma associated herpes virus (KSHV), but related, endogenous cellular proteins containing this activity have now been identified (61).
The K3 and K5 proteins encode integral membrane E3 ubiquitin ligases containing RING (for “really interesting new genes”) domains and zinc-finger (CH) motifs. The KSHV K3 protein catalyzes ubiquitylation of class I and causes its downregulation by increased degradation (62). By contrast, K5 ubiquitylates and downregulates many other surface proteins in addition to class I. These include CD1, and the cell adhesion molecules ICAM-1, PECAM, and the co-stimulatory molecule B7.2. The class I substrates for K3 and K5 ubiquitylation are those that are in post-ER compartments. Thus it appears that this modification does not occur in the secretory pathway but occurs at the PM or in endosomal compartments (61). The downregulation of class I requires lysines in the cytoplasmic tail of the HC, which can be either mono-ubiquitylated or lysine 63-linked ubiquitylation (63). Expression of ESCRT protein mutants block K3 and K5-mediated degradation of class I (61) indicating that degradation is through the MVB/lysosomal and not through the proteasomal pathway. The downregulation of class I induced by K3 and K5 allows virally-infected cells to escape recognition by CD8+ T cells (42). Additionally K5 causes downregulation of AICI, MICA and MICB, proteins which are ligands for activating receptors on NK cells, thus avoiding NK cell activation (64).
The search for human and mouse homologs of these RING-CH viral proteins identified the membrane-associated RING-CH (MARCH) family of proteins (65). There are 11 of these genes in humans that encode proteins with RING domains followed by two or more trans-membrane segments. MARCH IV and IX are related in sequence and when overexpressed, each one causes ubiquitylation and downregulation of class I, CD4 and ICAM-1 (65, 66). MARCH I and VIII also are related and their overexpression causes ubiquitylation of MHC class II (61). Some of the MARCH proteins are widely expressed (MARCH II and IX) whereas others are more restricted (IV to brain and placenta) (65). There is great interest in identifying substrates for all the MARCH proteins and to understand how their expression is regulated. Furthermore, more details are needed to understand where and how MARCH IV and IX ubiquitylate class I (endosomes or PM?). Is mono or polyubiquitin required for endocytosis and/or for trafficking into the MVB pathway? Is ubiquitylated class I internalized by clathrin-independent or -dependent pathways? In some instances ubiquitylated class I may not be targeted for degradation as there are de-ubiquinating enzymes in cells that can remove ubiquitin moieties, allowing class I to recycle back to the PM.
There may also be other proteins and factors that influence turnover of class I at the PM that need to be identified. For example, the amyloid precursor-like protein 2, a type I membrane protein implicated in neurite outgrowth and epithelial cell migration, interacts with class I and increases its endocytosis and turnover (67). Another viral immune modulator that downregulates class I is HIV nef. Most evidence indicates that nef alters the trafficking of biosynthetic class I at the TGN using the clathrin adaptor protein 1, diverting it to the late endosome instead of the plasma membrane (68). Nef, like K3 and K5, also down regulates other molecules including CD4 and CD28 (42).
Alternative activities for Class I
In addition to its role in antigen presentation, class I molecules have been shown to interact with self, forming homo-oligomers, and with other receptors at the PM. Indeed, prior to the discovery that class I proteins were involved in antigen presentation much of the studies on class I centered on their interactions with growth factor receptors (see comment in (69) ). The existence of open conformers of class I which lack β2m and/or peptide at the cell surface has been documented by several investigators. It is these open conformers that can interact laterally with the insulin and EGF receptors, the IL2 receptor a subunit (Tac), and ICAM-1 (70). Although internalization of class I and entry into acidic compartments might enhance formation of open conformers and thus facilitate formation of these hybrid complexes, studies have shown that these cis-interactions can occur at the PM. These open conformers of class I also interact in trans with the LIR-1 and Ly49C receptors on NK cells that recognize peptide-free class I. Since MHC class I is an abundant cell surface protein expressed on all nucleated cells, these unconventional class I interactions might be important for modulating the level of peptide-loaded class I at the cell surface available for antigen presentation. In addition these unconventional forms of class I may have important functions in physiology and development.
Acknowledgments
We thank Sebastian Springer for sharing unpublished work and apologize to colleagues whose work we were unable to cite due to length restrictions. DBW acknowledges support from the Canadian Cancer Society and the Canadian Institutes of Health Research. JGD is supported by the Intramural Research Program of the National Heart, Lung and Blood Institute, NIH.
Footnotes
Howe, C, Gartska, MA, Al-Balushi, M, Ghanem, E., Scheeweiss, C, Antoniou, AN, Kontouli, N, Williams, A, Elliott, T, Springer, S. Manuscript submitted.
References
- 1.Peaper DR, Cresswell P. Regulation of MHC class I assembly and peptide binding. Annu Rev Cell Dev Biol. 2008;24:343–368. doi: 10.1146/annurev.cellbio.24.110707.175347. [DOI] [PubMed] [Google Scholar]
- 2.Suh WK, Cohen-Doyle MF, Fruh K, Wang K, Peterson PA, Williams DB. Interaction of MHC class I molecules with the transporter associated with antigen processing. Science. 1994;264(5163):1322–1326. doi: 10.1126/science.8191286. [DOI] [PubMed] [Google Scholar]
- 3.Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci U S A. 1994;91(3):913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vassilakos A, Michalak M, Lehrman MA, Williams DB. Oligosaccharide binding characteristics of the molecular chaperones calnexin and calreticulin. Biochemistry. 1998;37(10):3480–3490. doi: 10.1021/bi972465g. [DOI] [PubMed] [Google Scholar]
- 5.Williams DB. Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. J Cell Sci. 2006;119(Pt 4):615–623. doi: 10.1242/jcs.02856. [DOI] [PubMed] [Google Scholar]
- 6.Vassilakos A, Cohen-Doyle MF, Peterson PA, Jackson MR, Williams DB. The molecular chaperone calnexin facilitates folding and assembly of class I histocompatibility molecules. Embo J. 1996;15(7):1495–1506. [PMC free article] [PubMed] [Google Scholar]
- 7.Tector M, Salter RD. Calnexin influences folding of human class I histocompatibility proteins but not their assembly with beta 2-microglobulin. J Biol Chem. 1995;270(33):19638–19642. doi: 10.1074/jbc.270.33.19638. [DOI] [PubMed] [Google Scholar]
- 8.Sadasivan BK, Cariappa A, Waneck GL, Cresswell P. Assembly, peptide loading, and transport of MHC class I molecules in a calnexin-negative cell line. Cold Spring Harb Symp Quant Biol. 1995;60:267–275. doi: 10.1101/sqb.1995.060.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Kozlov G, Pocanschi CL, Brockmeier U, Ireland BS, Maattanen P, Howe C, Elliott T, Gehring K, Williams DB. ERp57 does not require interactions with calnexin and calreticulin to promote assembly of class I histocompatibility molecules, and it enhances peptide loading independently of its redox activity. J Biol Chem. 2009;284(15):10160–10173. doi: 10.1074/jbc.M808356200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5(2):103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 11.Peaper DR, Wearsch PA, Cresswell P. Tapasin and ERp57 form a stable disulfide-linked dimer within the MHC class I peptide-loading complex. EMBO J. 2005;24(20):3613–3623. doi: 10.1038/sj.emboj.7600814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ireland BS, Brockmeier U, Howe CM, Elliott T, Williams DB. Lectin-deficient calreticulin retains full functionality as a chaperone for class I histocompatibility molecules. Mol Biol Cell. 2008;19(6):2413–2423. doi: 10.1091/mbc.E07-10-1055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Scholz C, Tampe R. The peptide loading complex - antigen translocation and MHC class I loading. Biol Chem. 2009 doi: 10.1515/BC.2009.069. [DOI] [PubMed] [Google Scholar]
- 14.De Silva AD, Boesteanu A, Song R, Nagy N, Harhaj E, Harding CV, Joyce S. Thermolabile H-2Kb molecules expressed by transporter associated with antigen processing-deficient RMA-S cells are occupied by low-affinity peptides. J Immunol. 1999;163(8):4413–4420. [PubMed] [Google Scholar]
- 15.Spies T, DeMars R. Restored expression of major histocompatibility class I molecules by gene transfer of a putative peptide transporter. Nature. 1991;351(6324):323–324. doi: 10.1038/351323a0. [DOI] [PubMed] [Google Scholar]
- 16.Van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell. 1992;71(7):1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 17.Baas EJ, van Santen HM, Kleijmeer MJ, Geuze HJ, Peters PJ, Ploegh HL. Peptide-induced stabilization and intracellular localization of empty HLA class I complexes. J Exp Med. 1992;176(1):147–156. doi: 10.1084/jem.176.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu VW, Yuan LC, Nuchtern JG, Lippincott-Schwartz J, Hammerling GJ, Klausner RD. A recycling pathway between the endoplasmic reticulum and the Golgi apparatus for retention of unassembled MHC class I molecules. Nature. 1991;352(6334):441–444. doi: 10.1038/352441a0. [DOI] [PubMed] [Google Scholar]
- 19.Townsend A, Ohlen C, Bastin J, Ljunggren HG, Foster L, Karre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- 20.Ljunggren HG, Stam NJ, Ohlen C, Neefjes JJ, Hoglund P, Heemels MT, Bastin J, Schumacher TN, Townsend A, Karre K, et al. Empty MHC class I molecules come out in the cold. Nature. 1990;346(6283):476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- 21.Momburg F, Tan P. Tapasin-the keystone of the loading complex optimizing peptide binding by MHC class I molecules in the endoplasmic reticulum. Mol Immunol. 2002;39(3-4):217–233. doi: 10.1016/s0161-5890(02)00103-7. [DOI] [PubMed] [Google Scholar]
- 22.Garbi N, Tiwari N, Momburg F, Hammerling GJ. A major role for tapasin as a stabilizer of the TAP peptide transporter and consequences for MHC class I expression. Eur J Immunol. 2003;33(1):264–273. doi: 10.1002/immu.200390029. [DOI] [PubMed] [Google Scholar]
- 23.Ortmann B, Copeman J, Lehner PJ, Sadasivan B, Herberg JA, Grandea AG, Riddell SR, Tampe R, Spies T, Trowsdale J, Cresswell P. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science. 1997;277(5330):1306–1309. doi: 10.1126/science.277.5330.1306. [DOI] [PubMed] [Google Scholar]
- 24.Lehner PJ, Surman MJ, Cresswell P. Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line .220. Immunity. 1998;8(2):221–231. doi: 10.1016/s1074-7613(00)80474-4. [DOI] [PubMed] [Google Scholar]
- 25.Howarth M, Williams A, Tolstrup AB, Elliott T. Tapasin enhances MHC class I peptide presentation according to peptide half-life. Proc Natl Acad Sci U S A. 2004;101(32):11737–11742. doi: 10.1073/pnas.0306294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams AP, Peh CA, Purcell AW, McCluskey J, Elliott T. Optimization of the MHC class I peptide cargo is dependent on tapasin. Immunity. 2002;16(4):509–520. doi: 10.1016/s1074-7613(02)00304-7. [DOI] [PubMed] [Google Scholar]
- 27.Wearsch PA, Cresswell P. Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer. Nat Immunol. 2007;8(8):873–881. doi: 10.1038/ni1485. [DOI] [PubMed] [Google Scholar]
- 28.Chen M, Bouvier M. Analysis of interactions in a tapasin/class I complex provides a mechanism for peptide selection. Embo J. 2007;26(6):1681–1690. doi: 10.1038/sj.emboj.7601624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garbi N, Tanaka S, Momburg F, Hammerling GJ. Impaired assembly of the major histocompatibility complex class I peptide-loading complex in mice deficient in the oxidoreductase ERp57. Nat Immunol. 2006;7(1):93–102. doi: 10.1038/ni1288. [DOI] [PubMed] [Google Scholar]
- 30.Peaper DR, Cresswell P. The redox activity of ERp57 is not essential for its functions in MHC class I peptide loading. Proc Natl Acad Sci U S A. 2008;105(30):10477–10482. doi: 10.1073/pnas.0805044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong G, Wearsch PA, Peaper DR, Cresswell P, Reinisch KM. Insights into MHC class I peptide loading from the structure of the tapasin-ERp57 thiol oxidoreductase heterodimer. Immunity. 2009;30(1):21–32. doi: 10.1016/j.immuni.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao B, Adhikari R, Howarth M, Nakamura K, Gold MC, Hill AB, Knee R, Michalak M, Elliott T. Assembly and antigen-presenting function of MHC class I molecules in cells lacking the ER chaperone calreticulin. Immunity. 2002;16(1):99–109. doi: 10.1016/s1074-7613(01)00260-6. [DOI] [PubMed] [Google Scholar]
- 33.Marguet D, Spiliotis ET, Pentcheva T, Lebowitz M, Schneck J, Edidin M. Lateral diffusion of GFP-tagged H2Ld molecules and of GFP-TAP1 reports on the assembly and retention of these molecules in the endoplasmic reticulum. Immunity. 1999;11(2):231–240. doi: 10.1016/s1074-7613(00)80098-9. [DOI] [PubMed] [Google Scholar]
- 34.Garstka M, Borchert B, Al-Balushi M, Praveen PV, Kuhl N, Majoul I, Duden R, Springer S. Peptide-receptive major histocompatibility complex class I molecules cycle between endoplasmic reticulum and cis-Golgi in wild-type lymphocytes. J Biol Chem. 2007;282(42):30680–30690. doi: 10.1074/jbc.M701721200. [DOI] [PubMed] [Google Scholar]
- 35.Kleijmeer MJ, Kelly A, Geuze HJ, Slot JW, Townsend A, Trowsdale J. Location of MHC-encoded transporters in the endoplasmic reticulum and cis-Golgi. Nature. 1992;357(6376):342–344. doi: 10.1038/357342a0. [DOI] [PubMed] [Google Scholar]
- 36.Paulsson KM, Jevon M, Wang JW, Li S, Wang P. The double lysine motif of tapasin is a retrieval signal for retention of unstable MHC class I molecules in the endoplasmic reticulum. J Immunol. 2006;176(12):7482–7488. doi: 10.4049/jimmunol.176.12.7482. [DOI] [PubMed] [Google Scholar]
- 37.Pentcheva T, Spiliotis ET, Edidin M. Cutting edge: Tapasin is retained in the endoplasmic reticulum by dynamic clustering and exclusion from endoplasmic reticulum exit sites. J Immunol. 2002;168(4):1538–1541. doi: 10.4049/jimmunol.168.4.1538. [DOI] [PubMed] [Google Scholar]
- 38.Spiliotis ET, Manley H, Osorio M, Zuniga MC, Edidin M. Selective export of MHC class I molecules from the ER after their dissociation from TAP. Immunity. 2000;13(6):841–851. doi: 10.1016/s1074-7613(00)00081-9. [DOI] [PubMed] [Google Scholar]
- 39.Boyle LH, Gillingham AK, Munro S, Trowsdale J. Selective export of HLA-F by its cytoplasmic tail. J Immunol. 2006;176(11):6464–6472. doi: 10.4049/jimmunol.176.11.6464. [DOI] [PubMed] [Google Scholar]
- 40.Ladasky JJ, Boyle S, Seth M, Li H, Pentcheva T, Abe F, Steinberg SJ, Edidin M. Bap31 enhances the endoplasmic reticulum export and quality control of human class I MHC molecules. J Immunol. 2006;177(9):6172–6181. doi: 10.4049/jimmunol.177.9.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paquet ME, Cohen-Doyle M, Shore GC, Williams DB. Bap29/31 influences the intracellular traffic of MHC class I molecules. J Immunol. 2004;172(12):7548–7555. doi: 10.4049/jimmunol.172.12.7548. [DOI] [PubMed] [Google Scholar]
- 42.Hansen TH, Bouvier M. MHC class I antigen presentation: learning from viral evasion strategies. Nat Rev Immunol. 2009;9(7):503–513. doi: 10.1038/nri2575. [DOI] [PubMed] [Google Scholar]
- 43.Lilley BN, Ploegh HL. Viral modulation of antigen presentation: manipulation of cellular targets in the ER and beyond. Immunol Rev. 2005;207:126–144. doi: 10.1111/j.0105-2896.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 44.Jonjic S, Babic M, Polic B, Krmpotic A. Immune evasion of natural killer cells by viruses. Curr Opin Immunol. 2008;20(1):30–38. doi: 10.1016/j.coi.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 46.Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell. 2004;15(8):3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139(1):49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barral DC, Cavallari M, McCormick PJ, Garg S, Magee AI, Bonifacino JS, De Libero G, Brenner MB. CD1a and MHC Class I Follow a Similar Endocytic Recycling Pathway. Traffic. 2008;9(9):1446–1457. doi: 10.1111/j.1600-0854.2008.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eyster CA, Higginson JD, Huebner R, Porat-Shliom N, Weigert R, Wu WW, Shen RF, Donaldson JG. Discovery of New Cargo Proteins that Enter Cells through Clathrin-Independent Endocytosis. Traffic. 2009;10(5):590–599. doi: 10.1111/j.1600-0854.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walseng E, Bakke O, Roche PA. MHC class II-peptide complexes internalize using a clathrin- and dynamin-independent endocytosis pathway. J Biol Chem. 2008;283:14717–14727. doi: 10.1074/jbc.M801070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donaldson JG, Porat-Shliom N, Cohen LA. Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling. Cell Signal. 2009;21(1):1–6. doi: 10.1016/j.cellsig.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8(8):603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naslavsky N, Weigert R, Donaldson JG. Convergence of Non-clathrin- and Clathrin-derived Endosomes Involves Arf6 Inactivation and Changes in Phosphoinositides. Mol Biol Cell. 2003;14(2):417–431. doi: 10.1091/mbc.02-04-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5(2):121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 55.Weigert R, Yeung AC, Li J, Donaldson JG. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell. 2004;15(8):3758–3770. doi: 10.1091/mbc.E04-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jovanovic OA, Brown FD, Donaldson JG. An effector domain mutant of Arf6 implicates phospholipase D in endosomal membrane recycling. Mol Biol Cell. 2006;17(1):327–335. doi: 10.1091/mbc.E05-06-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caplan S, Naslavsky N, Hartnell LM, Lodge R, Polishchuk RS, Donaldson JG, Bonifacino JS. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. Embo J. 2002;21(11):2557–2567. doi: 10.1093/emboj/21.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grant BD, Caplan S. Mechanisms of EHD/RME-1 protein function in endocytic transport. Traffic. 2008;9(12):2043–2052. doi: 10.1111/j.1600-0854.2008.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robertson SE, Setty SR, Sitaram A, Marks MS, Lewis RE, Chou MM. Erk Signaling Regulates Clathrin-independent Endosomal Trafficking. Mol Biol Cell. 2005 doi: 10.1091/mbc.E05-07-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nathan JA, Lehner PJ. The trafficking and regulation of membrane receptors by the RING-CH ubiquitin E3 ligases. Exp Cell Res. 2009;315(9):1593–1600. doi: 10.1016/j.yexcr.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 62.Coscoy L, Sanchez DJ, Ganem D. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J Cell Biol. 2001;155(7):1265–1273. doi: 10.1083/jcb.200111010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duncan LM, Piper S, Dodd RB, Saville MK, Sanderson CM, Luzio JP, Lehner PJ. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. Embo J. 2006;25(8):1635–1645. doi: 10.1038/sj.emboj.7601056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas M, Boname JM, Field S, Nejentsev S, Salio M, Cerundolo V, Wills M, Lehner PJ. Down-regulation of NKG2D and NKp80 ligands by Kaposi’s sarcoma-associated herpesvirus K5 protects against NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2008;105(5):1656–1661. doi: 10.1073/pnas.0707883105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartee E, Mansouri M, Nerenberg BT Hovey, Gouveia K, Fruh K. Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J Virol. 2004;78(3):1109–1120. doi: 10.1128/JVI.78.3.1109-1120.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoer S, Smith L, Lehner PJ. MARCH-IX mediates ubiquitination and downregulation of ICAM-1. FEBS Lett. 2007;581(1):45–51. doi: 10.1016/j.febslet.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 67.Tuli A, Sharma M, McIlhaney MM, Talmadge JE, Naslavsky N, Caplan S, Solheim JC. Amyloid precursor-like protein 2 increases the endocytosis, instability, and turnover of the H2-K(d) MHC class I molecule. J Immunol. 2008;181(3):1978–1987. doi: 10.4049/jimmunol.181.3.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roeth JF, Williams M, Kasper MR, Filzen TM, Collins KL. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J Cell Biol. 2004;167(5):903–913. doi: 10.1083/jcb.200407031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bowness P, Caplan S, Edidin M. MHC molecules lead many lives. Workshop on MHC Class I Molecules at the interface between Biology & Medicine. EMBO Rep. 2009;10(1):30–34. doi: 10.1038/embor.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arosa FA, Santos SG, Powis SJ. Open conformers: the hidden face of MHC-I molecules. Trends Immunol. 2007;28(3):115–123. doi: 10.1016/j.it.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Wearsch PA, Cresswell P. The quality control of MHC class I peptide loading. Curr Opin Cell Biol. 2008;20(6):624–631. doi: 10.1016/j.ceb.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]