Abstract
Nicotinic acetylcholine receptors (AChRs) are pentameric proteins that form agonist-gated cation channels through the plasma membrane. AChR agonists and antagonists are potential candidates for the treatment of neurodegenerative diseases. Cembranoids are naturally occurring diterpenoids that contain a 14-carbon ring. These diterpenoids interact with AChRs in complex ways: as irreversible inhibitors at the agonist sites, as noncompetitive inhibitors, or as positive modulators, but no cembranoid was ever shown to have agonistic activity on AChRs. The cembranoid eupalmerin acetate displays positive modulation of agonist-induced currents in the muscle-type AChR and in the related gamma-aminobutyric acid (GABA) type A receptor. Moreover, cembranoids display important biological effects, many of them mediated by nicotinic receptors. Cembranoids from tobacco are neuroprotective through a nicotinic anti-apoptotic mechanism preventing excitotoxic neuronal death which in part could result from anti-inflammatory properties of cembranoids. Moreover, tobacco cembranoids also have anti-inflammatory properties which could enhance their neuroprotective properties. Cembranoids from tobacco affect nicotine-related behavior: they increase the transient initial ataxia caused by first nicotine injection into naive rats and inhibit the expression of locomotor sensitization to repeated injections of nicotine. In addition, cembranoids are known to act as anti-tumor compounds. In conclusion, cembranoids provide a promising source of lead drugs for many clinical areas, including neuroprotection, smoking-cessation, and anti-cancer therapies.
Keywords: cembranoid, neuroprotection, apoptosis, soft coral and tobacco
Introduction
The octocorals are a subclass of anthozoans that feature polyps with eight-fold symmetry. The octocorallia currently include 3 orders, 45 families and close to 4000 estimated species. The order Alcyonacea is the most abundant and contains about two-thirds of the octocoral families. Alcyonacea include the so-called “soft” corals and two suborders of sea fans or “gorgonians”. The octocorals are ideal organisms to search for bioactive metabolites that could be used by them for chemical defense. Octocorals are immobile, apparently defenseless organisms without the rigid carbonate skeletons that protect their cousins, the scleractinian or “hard” corals. Therefore, without chemical defenses the octocorals would seem to be easy nutrient sources for marine predators. In addition, some octocorals are brightly colored. In the words of the biologist Edward O. Wilson: “…if a small and otherwise unknown organism is strikingly beautiful, it is probably poisonous; and if it is not only beautiful, but also easy to catch, it is probably deadly” (Wilson 2003). The role of octocoral secondary metabolites in defensive mechanisms has been documented (Sammarco and Coll 1992). Among these metabolites are many examples of terpenoids. This review will limit itself to a subgroup of diterpenoids known as cembranoids.
Cembranoids are diterpenoids that contain a 14-carbon or “cembrane” ring that has varying degrees of oxygenation (Fig. 1). More than 300 naturally occurring cembranoids have been described (Wahlberg and Eklund 1992). Although cembranoids have been isolated from plants, including tobacco, insects and even vertebrates, marine invertebrates have been, by far, the richest source of cembranoids. Most of these compounds have been isolated from Caribbean or Pacific gorgonians in which cembranoids comprise up to 25% of their identified secondary metabolites (Rodríguez 2001). The occurrence of cembranoids in octocorals was discovered nearly 50 years ago by the Ciereszko lab (Ciereszko et al 1960), where they successfully isolated and characterized eunicin (Fig. 1) from the Caribbean gorgonian Eunicea mammosa.
Figure 1.
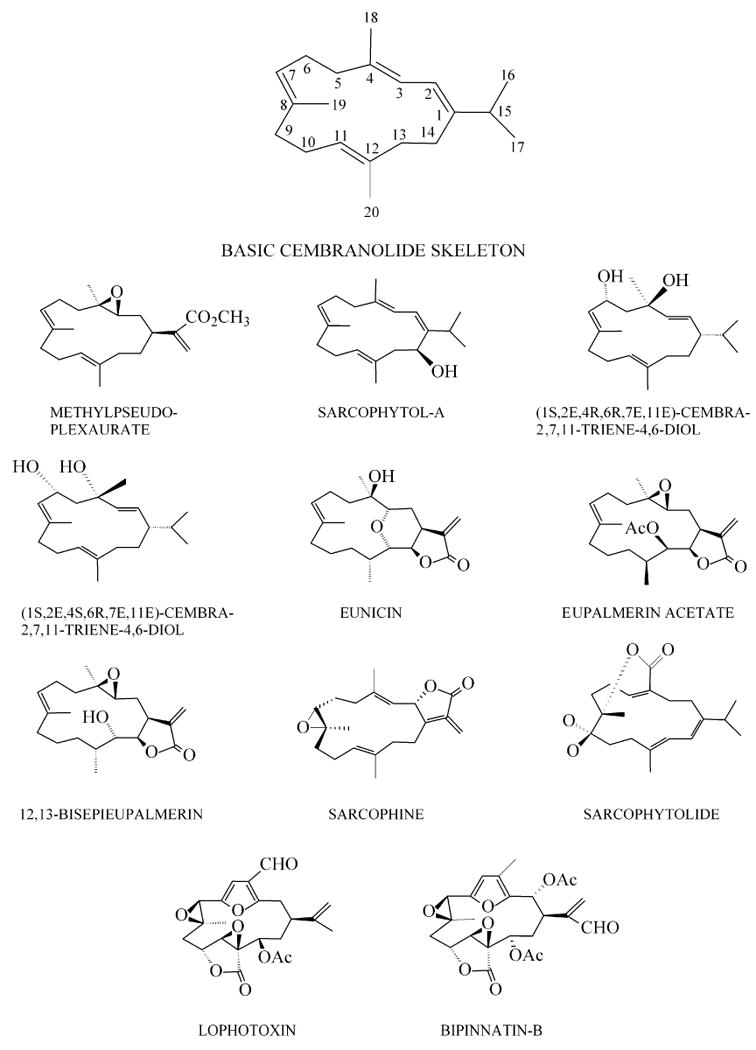
Cembranoid structures
Nicotinic acetylcholine receptors (AChRs) are pentameric transmembrane proteins that form agonist-gated cation channels through the plasma membrane (Karlin 2002). They are members of the ligand-gated ion channel superfamily that also includes gamma-aminobutyric acid (GABA) type A, glycine and serotonin (5HT) type 3 receptors. Each AChR subunit features a large extracellular N-terminal segment followed by 4 transmembrane segments, M1 - M4, with M2 lining the transmembrane ion channel. The large extracellular segments form the agonist sites at their subunit interfaces where agonists and competitive antagonists bind. Noncompetitive AChR antagonists bind outside the agonist cavity thereby and prevent conformational changes of the protein necessary for channel opening.
There are many AChR subtypes and the subunit stoichiometry is not known in all cases. The AChR found in muscle and electric organ is a heteropentamer that has a subunit stoichiometry of α2βγδ or α2βεδ. In contrast, the α7 AChR, which is found both inside and outside the nervous system, is a homopentameric molecule. Each muscle-type AChR has two non-identical agonist-binding sites that are located in the receptor's extracellular domain at the αγ or αε and αδ subunit interfaces (Pedersen and Cohen 1990). Differences in the homologous non-α subunit segments are what give the αγ, αε and αδ sites different affinities for certain agonists and competitive antagonists such as tubocurarine (Pedersen and Cohen 1990) and α-conotoxins MI and GI (Hann et al 1994). Biochemical studies, done mostly on electric organ AChR, first established that the α subunit contributes three segments (A, B and C) to each site while the γ or ε and δ subunits contribute four segments each (D, E, F, and G) (Sine 2002). Based on the crystal structure of a soluble mollusk acetylcholine binding protein reported in 2001 (Brejc et al 2001), it was proposed that the structure of the extracellular domain of every AChR subunit was formed by an N-terminal α-helix followed by 10 antiparallel β strands that fold into a “β sandwich”. This structure was recently confirmed crystallographically for a muscle-type AChR subunit (Dellisanti et al 2007). It is now clear that α subunit segments A, B and C are largely unstructured segments that lie between β strands 4 and 5, 7 and 8, and 9 and 10, respectively. Segment G residues on γ or ε and δ subunits are also present in an unstructured segment between β strands 8 and 9, while segments D, E and F residues are actually part of beta strands 1, 2 and 5/6, respectively (Sine 2002). For further information on AChR structure and function, the reader is referred to two excellent reviews (Kalamida et al 2007; Karlin 2002). This review will discuss the effects of octocoral and tobacco cembranoids on AChRs and related ligand-gated ion channels.
Mechanisms of cembranoid actions on nicotinic receptors
Actions on muscle-type nicotinic receptors
The first evidence that cembranoids affect nicotinic receptors was presented in 1981 with the discovery and characterization of lophotoxin (LTX, Fig. 1), a cembranoid isolated from Pacific gorgonians of the genus Lophogorgia (Culver and Jacobs 1981; Fenical et al 1981). This cembranoid produced slow irreversible block at the neuromuscular junction in rat diaphragm preparations. In addition to its very slow onset, the neuromuscular inhibition by LTX was recognized early as being unusual because LTX lacked a cationic moiety present in all AChR agonists and competitive antagonists known at that time (Culver and Jacobs 1981). Indeed, the lack of a cationic moiety led to early doubts that LTX was a competitive inhibitor, despite the resemblance of its inhibition to that of the better-characterized inhibition of muscle AChR by the competitive antagonists α-neurotoxins (Atchison et al 1984; Langdon and Jacobs 1983). These doubts proved to be unjustified when it was subsequently shown that LTX is a competitive inhibitor that binds irreversibly and preferentially to one of the two agonists sites on embryonic mouse muscle AChR. This site is the one displaying lower affinity for tubocurarine, that is now known to be at the αδ interface (Culver et al 1984).
Activity similar to that of LTX was also identified in five of its structural analogs that were isolated from the Caribbean gorgonian Pseudopterogorgia bipinnata (Culver et al 1985). One of these cembranoids, bipinnatin B (BPB, Fig. 1), was comparable to LTX in its binding affinity to embryonic mouse muscle AChR and was more potent than LTX in binding to Torpedo californica electric organ AChR. Both [3H]-LTX and [3H]-BPB covalently labeled the T. californica AChR α subunit (Abramson et al 1988). [3H]-BPB was later shown to covalently react with alpha Y190 which is now known to contribute an important electrophilic aromatic group to the agonist site from segment C of the α subunit (Abramson et al 1989).
A structure-activity study on 25 LTX analogs, 12 of which displayed substantial activity on T. californica AChR, identified a pharmacophore in which the electron-deficient epoxide carbons at C7 and C8 mimic the cationic nitrogen group that is usually found in AChR agonists and competitive antagonists while the lactone oxygens mimic the ester group of acetylcholine (Abramson et al 1991). It was later shown that the nematode Caenorhabditis elegans AChR expressed in Xenopus oocytes is resistant to BPB due to the substitution of proline for tyrosine at the homologous position on the C. elegans α-like subunit, unc38 (Tornoe et al 1996).
The reason for the slow onset of inhibition in the AChR agonist sites became clearer when three bipinnatins (A, B and C) were shown to be relatively inactive protoxins that required pre-incubation in aqueous buffer for several hours to become active (Groebe et al 1994). The main active solvolysis products of BPA and BPC (and presumably of BPB) featured the replacement of the acetate group present on C2 of all three analogues with a hydroxyl group (Hyde et al 1995a). This reaction was subsequently shown to occur through an SN1 type reaction involving the rate-limiting generation of a carbocation at C2 followed by the addition of hydroxide from solvent (Hyde et al 1995b). However, LTX did not display this same protoxin phenomenon; its activity was about the same with or without preincubation in aqueous buffer (Groebe and Abramson 1995). LTX does not carry an acetate or hydroxyl group at C2 position but instead has a saturated carbon at that position. LTX is intrinsically a very slow-binding irreversible inhibitor that selectively labels the mouse embryonic muscle AChR αδ interface site due to both a higher reversible affinity and a faster rate of irreversible inhibition at the αδ site (Groebe and Abramson 1995).
Inspired by the findings on LTX and the bipinnatins, this laboratory studied 3 cembranoids that were isolated from Caribbean gorgonians of the Eunicea genus, eunicin, eupalmerin acetate (EUAC) and 12,13-bis-epi-eupalmerin (BEEP)(Fig. 1). These cembranoids inhibited agonist-induced currents through the embryonic mouse muscle AChR expressed in Xenopus oocytes with IC50s in the low μmolar range (Eterovic et al 1993a). EUAC was also shown to partially block agonist-induced currents through T. californica electric organ AChR expressed in oocytes (Eterovic et al 1993b). However, unlike the inhibition by LTX and the bipinnatins, the AChR inhibition by these three cembranoids proved to be non-competitive in nature. None of the cembranoids inhibited binding of the competitive antagonist α-bungarotoxin to electric organ AChR, ruling out their binding to agonist sites on the AChR. On the other hand, all 3 cembranoids completely and competitively inhibited high-affinity binding of [3H]-phencyclidine (PCP) to the AChR from T. californica electric organ (Eterovic et al 1993a). The most potent in this activity was eunicin [Fig. 1], the first cembranoid identified in octocorals (Ciereszko et al 1960), with an IC50 near 1μM. This suggested a channel site for cembranoid binding because PCP had been shown to bind with high-affinity inside the ion channel of the desensitized muscle-type AChR (Oswald et al 1983). In retrospect, it is not surprising that the inhibitory mechanism of eunicin, EUAC and BEEP is different from that of LTX and the bipinnatins. Comparison of the structures of the Eunicea cembranoids with LTX and its analogues reveals that, although all 3 Eunicea cembranoids possess a lactone ring, they lack the 7,8 epoxide of the LTX pharmacophore that reacts covalently with αY190 in the muscle-type agonist sites (Abramson et al 1991). The Eunicea cembranoids also lack the furan ring responsible for giving compact rigidity to the LTX molecule.
Further structure-activity studies on a series of 20 cembranoids isolated from various octocorals showed that all 20 cembranoids, including lophotoxin, were able to completely block PCP binding to the T. californica AChR (Hann et al 1998). The IC50 values for this inhibition ranged from 0.9 μM for methylpseudoplexaurate [Fig. 1] to 372 μM for lophotoxin. Furthermore, eunicin and methylpseudoplexaurate competed with each other for the PCP site, which confirmed that the observed inhibition of PCP binding involved a direct interaction between cembranoids and the AChR and was not due to a non-specific membrane effect. There was a reasonably good correlation between potency and cembranoid hydrophobicity, as measured by high-performance thin-layer chromatography mobility, which is consistent with cembranoid binding to a hydrophobic site on the AChR. All 7 of the cembranoids in this series that were tested electrophysiologically inhibited agonist-induced currents through the T. californica AChR expressed in oocytes with low micromolar potency.
Complete inhibition of the binding to T. californica AChR of [3H]-tenocyclidine (TCP), an analogue of PCP, was obtained with two cembranoids of tobacco origin: (1S,2E,4R,6R,7E,11E)-cembra-2,7,11-triene-4,6-diol (4R) and its stereoisomer, 4S (Fig. 1) (Ferchmin et al 2001). These two cembranoids differ only in the stereochemistry of their hydroxyl group at C4 position and yet 4R displayed nearly an order of magnitude higher inhibitory potency than 4S, confirming that inhibitory cembranoid binding to the AChR is not due to nonspecific hydrophobic interactions. These two tobacco cembranoids along with two octocoral cembranoids, EUAC and BEEP, completely and noncompetitively inhibited agonist-induced 86Rb efflux through the embryonic human muscle AChR (Ferchmin et al 2001) with potencies similar to those observed for inhibition of agonist-induced current on embryonic mouse muscle AChR (Eterovic et al 1993a) and of PCP or TCP binding to the T. californica AChR (Ferchmin et al 2001; Hann et al 1998).
Radioligand-receptor assays confirmed the presence of a cembranoid-binding site on the T. californica AChR that sterically overlaps the TCP/PCP site and is probably located in the ion channel (Pagan et al 2001). This inhibitory site on T. californica AChR also appears to overlap the site for cationic noncompetitive inhibitors such as procaine and quinacrine.
Electrophysiological studies on oocyte-expressed T. californica and embryonic mouse muscle AChRs revealed that EUAC and PCP interact with different residues on the M2 segments in the ion channels in these two muscle-type receptors to produce their inhibitory effects (Eterovic et al 1999).
In addition to their inhibitory effect, eunicin, EUAC and BEEP increased the rate of desensitization of the embryonic mouse muscle AChR (Eterovic et al 1993a). EUAC affected the desensitization process differently in the muscle and electocyte AChRs and produced an unusual secondary response in the muscle AChR (Eterovic et al 1993b). These were the first indications that more than one cembranoid binding site may exist on the AChR. Evidence was also seen for the presence on the T. californica AChR of one or more additional cembranoid sites which allosterically modulate the affinity of cembranoid for its inhibitory channel site as well as the affinity of long-chain alkanol general anesthetics (Pagan et al 2001).
The mechanism of EUAC actions on the embryonic mouse muscle AChR in BC3H-1 cells was studied by using whole-cell and single-channel patch-clamp current measurements (Ulrich et al 2008). EUAC did not act as an agonist on this receptor as revealed by whole-cell recording experiments. Co-application of 30 μM EUAC with 50 μM, 100 μM, or 500 μM carbamoylcholine (CCh) reversibly inhibited the current amplitude, whereas, with 20 μM CCh, current was increased above control values in the presence of the cembranoid. EUAC concentration curves (0.01-40 μM) obtained with 100 μM and 500 μM CCh displayed slope coefficients, nH, significantly smaller than one, suggesting that EUAC bound to several sites with widely differing affinities on the receptor molecule. The apparent rate of receptor desensitization in the presence of EUAC and CCh was either slower than or equal to that obtained with CCh alone. The major finding from single-channel studies was that EUAC did not affect single-channel conductance or the ability of CCh to interact with the receptor. Instead, EUAC acted by increasing the channel closing rate constant. The results are not consistent with a simple competitive model for EUAC inhibition, with the sequential open-channel block model, or with inhibition by increased desensitization. The data are best accounted for by a model in which EUAC acts by closed-channel block at low concentrations, by positive modulation at intermediate concentrations, and by negative allosteric modulation of the open channel at high concentrations.
Actions on neuronal-type nicotinic acetylcholine receptors
The first evidence that LTX irreversibly blocks neuronal AChRs was acquired in frog sympathetic ganglia and in guinea pig ileum sections with parasympathetic innervation (Langdon and Jacobs 1985). This inhibition by LTX displayed the same time course and potency as the neuromuscular block discovered earlier. It was later shown that LTX is a high-affinity competitive antagonist of ganglionic AChRs (Sorenson et al 1987). Later studies on rat neuronal AChR subunit combinations expressed in Xenopus oocytes showed that BPB completely blocked α4β2 transmission at the same concentration that it blocked embryonic mouse muscle AChR (Luetje et al 1990). At the same concentration, BPB only partially blocked α2β2 and α3β2 transmission. The mechanism of this inhibition was not determined and there have been no further studies reported on the neuronal AChR blocking action of the LTX family of cembranoids.
Cembranoids isolated both from octocorals (EUAC and BEEP, Fig. 1) and from tobacco (4R and 4S, Fig. 1) were tested on human α4β2 neuronal AChRs and on human α3β4 ganglionic AChRs expressed in different cell lines (Ferchmin et al 2001). All four cembranoids completely and noncompetitively inhibited agonist-induced 86Rb flux through these receptors, displaying slightly higher potency on the α3β4 AChR (IC50 values ranging between 2 - 9 μM) than on the α4β2 AChR (IC50 values ranging between 10 - 33 μM). 4R was more potent than 4S with both receptors. However, later studies on rat α4β2 neuronal AChRs expressed in Xenopus oocytes showed no difference in the potencies of 4R and 4S to inhibit agonist-induced currents; both isoforms inhibited nicotine-induced response more effectively than acetylcholine-induced reponse and the inhibition was significantly increased by preincubation with cembranoid for 1 minute (Eaton et al 2004).
In an exciting related development, it was recently reported that EUAC at micromolar concentrations potentiated the rat α1β2γ2L GABAA receptor expressed in HEK 293 cells (Li et al 2008). Site-directed mutagenesis and pharmacological approaches led to the conclusion that EUAC potentiates GABA-induced ion flow by binding to an allosteric site. The mode of action was similar to that of neurosteroids. The cembranoid may either interact with the neurosteroid binding site on the GABAA receptor or act on common down-stream signal transduction targets. As mentioned above, the GABAA receptor is a member of the ligand-gated ion channel superfamily to which nicotinic AChRs also belong. Therefore, cembranoids may also represent a novel class of activity modulators for this AChR-related neuronal receptor.
Neuroprotective effects of cembranoids
Agonists and antagonist selective for AChR subtypes have been used in experimental and clinical research. Some of those compounds are potential candidates for the treatment of neurodegenerative disease such as Alzheimer's disease, Parkinson's disease and others. A growing list of in vivo and in vitro research suggest that AChRs modulators are gaining importance as clinically relevant neuroprotective drugs (Mudo et al 2007).
The most detailed study of neuroprotection by cembranoids was done not with marine but with tobacco cembranoids (Ferchmin et al 2005). This work was performed with acute hippocampal slices using bath applied N-methyl-D-aspartate (NMDA) as the excitotoxic stimulus. NMDA as a specific agonist of the NMDA subtype of glutamate receptors, mimics the effects of excitotoxicity in stroke and in certain neurodegenerative diseases. The decreased capability to produce synaptically elicited population spikes was used as the endpoint of excitotoxicity rather than the late event of neuronal death. A large body of literature supports the validity of this paradigm and its relevance to in vivo events (Kerr et al 1999; Shinno et al 1997). The cembranoid used was 4R, one of the mayor cembranoids found in tobacco leaves. Application of 4R before or after application of NMDA prevented the loss of the physiological competence of pyramidal neurons in the CA1 area of hippocampal slices.
4R protected the function of the hippocampal slice in a dose dependent manner with an EC50 of 0.24 μM. In order to elucidate the neuroprotective mechanism, cell signaling pathways were studied by Western blot analysis of phosphorylated protein kinases in the presence and in the absence of inhibitors of the relevant cell signaling steps. These experiments revealed that 4R neuroprotection was mediated by the activation of the PI3-kinase/Akt antiapoptotic cascade. Consequently, the proapoptotic enzyme glycogen synthase kinase 3-β (GSK3-β) was inactivated, leading to the reversal of apoptosis induced by NMDA application. The Raf/MEK/ERK cascade was not involved in the 4R-mediated neuroprotection.
A model of the synaptic mechanism intervening in 4R neuroprotection was proposed. According to this model, the synaptic mechanism is triggered by 4R inhibition of the α7 AChR located on GABAergic interneurons (Alkondon and Albuquerque 2001; Frazier et al 1998). The inhibition of α7 AChRs decreases the GABAergic tone causing increased ACh release into the synaptic cleft (Giorgetti et al 2000), which then activates the α4β2 AChRs located post-synaptically. Several facts support this model. The selective α7 inhibitor methyllycaconitine (Ivy Carroll et al 2007; Sharples and Wonnacott 2001) mimics, at least in part, the neuroprotective effect of 4R (Ferchmin et al 2003). Other in vivo and in vitro studies confirm that α7 inhibition can be neuroprotective (de Fiebre and de Fiebre 2005; Laudenbach et al 2002; Martin et al 2004). The involvement of the α4β2 nAChR was inferred on the basis of the complete inhibition of 4R-mediated neuroprotection by dihydro-β-erythroidine, a selective α4β2 inhibitor (Raggenbass and Bertrand 2002; Sharples and Wonnacott 2001).
The marine cembranoid sarcophytolide (Fig. 1), isolated from the soft coral Sarcophyton glaucum, was reported to be neuroprotective against glutamate-induced excitotoxicity leading to apoptosis of neuronal cortical primary cultures (Badria et al 1998). Sarcophytolide was neuroprotective when applied prior to 1 mM glutamate but did not rescue the viability of neurons when applied after glutamate. Application of sarcophytolide for 30 min suppressed most of the Ca2+ influx mediated by 0.1 mM glutamate. In addition, sarcophytolide increased the expression of the antiapoptotic protein Bcl-2. The authors proposed that sarcophytolide was neuroprotective by stabilizing Ca2+ homeostasis and activating the anti-apoptotic protein Bcl-2.
It is difficult to compare the two cembranoids with neuroprotective action, 4R and sarcophytolide, since different experimental paradigms were used to study the behavior of each compound. There are, however, apparent similarities between both compounds in the effective dose and in the mechanism of neuroprotection. The most effective neuroprotective concentrations of 4R and sarcophytolide were 40 and 32 μM, respectively, and both cembranoids apparently have antiapoptotic activities. We have demonstrated that 4R-induced neuroprotection is based on a nicotinic mechanism. For neuroprotection by sarcophytolide a similar mechanism is suggested in view of its structural similarity with other cembranoids interacting with AChRs.
Neuroprotective action by cembranoids could be mediated by their anti-inflammatory, anti-oxidant, cytoprotective, and anti-apoptotic activities (Candelario-Jalil et al 2005; Castellanos et al 2002; del Zoppo et al 2000; Dirnagl et al 1999; Iadecola and Alexander 2001; Lees et al 2006; Nakayama et al 1998; Ren et al 2003). Several cembranoids are endowed with these properties. The tobacco cembranoids 4S and 4R were reported to inhibit prostaglandin synthesis with lower IC50 than acetylsalicylic acid (Olsson et al 1993). El Sayed and colleagues studied the anti-tumor activity of sarcophytol A, sarcophine, and the derivatives produced in their laboratory (El Sayed et al 1998b). Sarcophytol A suppressed oxidant formation, decreased infiltration of phagocytes, and alleviated TPA-induced inflammation. Activation of macrophages and microglia are very relevant to neuroprotection (del Zoppo et al 2000; Iadecola and Alexander 2001; Vila et al 2003), and cembranoids participate in decreasing this toxic process. Sarcophytol protected against DNA damage in MRC5 cells induced by oxygen radicals that were generated by stimulated phagocytes (Weitberg and Corvese 1999). Sarcophine and derivatives produced by bioconversion decreased the release of superoxide from lypopolysaccharide-activated microglia (Sawant et al 2006). It remains to be determined whether the AChR-mediated neuroprotection by 4R is the exception or a general property of most cembranoids. In conclusion, marine and terrestrial cembranoids could be a source of lead compounds for neuroprotection in neurodegenerative diseases.
Cembranoids and nicotine-related behavior
Injection of cembranoids of marine or terrestrial origin did not overtly alter behavior of rats in open field nor did intramuscular injection of tobacco cembranoids in the caudal thigh muscle impair movement or the capacity to perform complex and strength requiring tasks (unpublished results). The behavioral effects of cembranoids in the context of behavioral sensitization to nicotine were studied for the first time in our laboratory (Ferchmin et al 2001). Sensitization to a drug means that repeated exposures to the same dose produce greater responses; in rats, sensitization to nicotine manifests as increased locomotor activity. Cembranoids robustly inhibited the expression of nicotine sensitization in rats. To induce sensitization, nicotine was injected subcutaneously for seven days. Immediately after each injection, the rats were transferred to a maze where exploration was measured. Sensitization could be maintained by four weekly nicotine injections for several months. We used a specific type of maze called Greek cross with bright and dark compartments (DeNelsky and Denenberg 1967; Ferchmin and Eterovic 1990; Ferchmin et al 1993) but most areas that allows for exploration are suitable for this purpose. Sensitization was measured by injecting saline 30 minutes before challenging the rats with either 0.2 or 0.4 mg/kg nicotine. Control exploratory activity was measured in sensitized rats injected with saline instead of nicotine. The central nicotinic antagonist 1 mg/kg mecamylamine injected 30 min before nicotine inhibited the expression of sensitization to the level of sensitized rats injected with saline instead of nicotine. Two octocoral cembranoids, eunicine and EUAC, and the tobacco cembranoid 4R at a dose of 6 mg/ kg inhibited the expression of sensitization to the level of saline controls and mecamylamine injected sensitized rats (Ferchmin et al 2001).
Nicotine injected to nicotine-naive rats induces ataxia and although enhanced locomotion by nicotine begins with the first administration, the increased exploratory activity is seen only after the 5 to 20 min when the nicotine ataxia decreases (Clarke and Kumar 1983a; b). The intensity and length of this initial ataxia depends on the dose of nicotine. After the second or third daily nicotine injection, the ataxia disappears altogether. The initial ataxia is mediated by the nucleus Accumbens and is a different phenomenon from sensitization (Benwell and Balfour 1992). This central effect of nicotine appears to be more complex than initially expected since it depends on the circadian rhythm and the receptors involved remain to be identified (Kita et al 1988). Interestingly, 4R injected with nicotine to naive rats dramatically increased the initial nicotine mediated ataxia (unpublished results). In another study, 4R cembranoid inhibited nicotine effects in planarian flatworms (Pagán et al., in preparation).
The fact that cembranoids are present in cigarette smoke (Saito et al 1985) and modulate the effect of nicotine in vivo suggests a plausible manner to manipulate tobacco use.
Other biological activities of cembranoids
The anti-growth effects of several marine and semisynthetic cembranoids for different types of cancer cell lines have been studied since 1989 (Fujiki et al 1989). Sarcophine and sarcophytol, cembranoids isolated from Sarcophyton glaucum, are among the best characterized. Sarcophine isolated in large amounts from the Red Sea soft coral has been studied in detail for its potential as chemotherapeutics. As shown in its chemical structure (Fig.1), sarcophine contains the 7-8 epoxide similar to lophotoxin which suggests that it has the potential to act directly at the agonist site of AChRs. The high yield of the cembranoid obtained from the coral together with its promising activity makes it particularly interesting for modification and generation of a structurally diverse library of sarcophine derivatives. Hydroxylated and sulfur-containing derivatives of sarcophine were synthesized and some of these derivatives demonstrated improved biological activity (El Sayed et al 1998a), (Sawant et al 2006).
In summary, though the mechanism of antimitotic and anti-inflammatory actions of cembranoids is not clear, it is intriguing to speculate that both events rely on intracellular calcium-dependent signaling pathways that may be triggered by the activation of AChR, especially the α7 subtype that is highly permeable to this ion. Whether the mechanism of action for the particular cembranoids is as a direct receptor antagonist similar to lophotoxin, as an allosteric modulator such as the tobacco cembranoids, and which intracellular signaling pathways are effected as a result, promises to be an exciting area of research in the near future.
Perspectives
Cembranoids are a promising source of pharmacologically active lead compounds. The main relevant pharmacological activities of cembranoids are the well studied anticancer activity and the more recently discovered nicotinic activity that appears to mediate neuroprotection.
The anticancer activity was paradoxically attributed to apparently antagonistic activities like cytotoxicity, cytoprotection, anti-inflammatory and antioxidant properties. Neuroprotection was also attributed to antioxidant and antiapoptotic activity. The inflammatory and immune responses which are part of the response to cancer and neurodegenerative disease generate toxic reactive oxygen species are also under nicotinic control (Razani-Boroujerdi et al 2007). We proposed that the antiapoptotic activity was triggered by a nicotinic signal transduction cascade. It is possible that both, the anticancer activity and neuroprotection are actually mediated by nicotinic receptor modulation. Nicotinic receptors are involved in a complex manner in these processes.
On the basis of the above considerations we propose that the most successful research on clinical applications of cembranoids will relate to the study of signal transduction mechanisms triggered by nicotinic receptors. In addition, finding cembranoids with selective affinity for nicotinic receptors subtypes could provide powerful tools for research and medicinal uses. These studies will be challenging because of the multiplicity of subtypes of nAChRs and the variety of downstream effectors present in different tissues.
Acknowledgments
The authors are thankful to L.T. Sulikowski for invaluable IT support. Authors work reviewed here was supported by the NINDS/NCRR/SNRP NS39408 (to VAE, PAF and RMH); NIH/NIGMS/MBRS 2 S06 GM050695 (to RMH and PAF); NIH/NCRR/INBRE P20RR16470 (to ACS and VAE); NIH/RCMI G12 RR03035 (to the UCC); Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) No. 06/61285-9 (to H.U.); Fellowship support by Conselho Nacional de Desenvolvimento Cientifico e Tecnológico, Brazil (CNPq; to H.U.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
P.A. Ferchmin, Department of Biochemistry, Universidad Central del Caribe, Bayamon, PR
Oné R. Pagán, Department of Biology, West Chester University, West Chester, PA
Henning Ulrich, Departamento de Bioquímica, Instituto de Química, Universidade de São Paulo, São Paulo, Brazil.
Ada C. Szeto, Department of Biochemistry, Universidad Central del Caribe, Bayamon, PR
Richard M. Hann, Department of Biochemistry, Universidad Central del Caribe, Bayamon, PR
Vesna A. Eterović, Department of Biochemistry, Universidad Central del Caribe, Bayamon, PR
References
- Abramson SN, Culver P, Kline T, Li Y, Guest P, Gutman L, Taylor P. Lophotoxin and related coral toxins covalently label the alpha-subunit of the nicotinic acetylcholine receptor. J Biol Chem. 1988;263(34):18568–18573. [PubMed] [Google Scholar]
- Abramson SN, Li Y, Culver P, Taylor P. An analog of lophotoxin reacts covalently with Tyr190 in the alpha-subunit of the nicotinic acetylcholine receptor. J Biol Chem. 1989;264(21):12666–12672. [PubMed] [Google Scholar]
- Abramson SN, Trischman JA, Tapiolas DM, Harold EE, Fenical W, Taylor P. Structure/activity and molecular modeling studies of the lophotoxin family of irreversible nicotinic receptor antagonists. J Med Chem. 1991;34(6):1798–1804. doi: 10.1021/jm00110a007. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic Acetylcholine Receptor alpha 7 and alpha 4beta 2 Subtypes Differentially Control GABAergic Input to CA1 Neurons in Rat Hippocampus. J Neurophysiol. 2001;86(6):3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Atchison WD, Narahashi T, Vogel SM. Endplate blocking actions of lophotoxin. Br J Pharmacol. 1984;82(3):667–672. doi: 10.1111/j.1476-5381.1984.tb10805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badria FA, Guirguis AN, Perovic S, Steffen R, Muller WE, Schroder HC. Sarcophytolide: a new neuroprotective compound from the soft coral Sarcophyton glaucum. Toxicology. 1998;131(23):133–143. doi: 10.1016/s0300-483x(98)00124-3. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. British journal of pharmacology. 1992;105(4):849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411(6835):269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Mhadu N, Gonzalez-Falcon A, Garcia-Cabrera M, Munoz E, Leon O, Fiebich B. Effects of the cyclooxygenase-2 inhibitor nimesulide on cerebral infarction and neurological deficits induced by permanent middle cerebral artery occlusion in the rat. Journal of Neuroinflammation. 2005;2(1):3. doi: 10.1186/1742-2094-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos M, Castillo J, Garcia MM, Leira R, Serena J, Chamorro A, Davalos A. Inflammation-Mediated Damage in Progressing Lacunar Infarctions: A Potential Therapeutic Target. 2002;33(4):982–987. doi: 10.1161/hs0402.105339. [DOI] [PubMed] [Google Scholar]
- Ciereszko LS, Sifford DH, Weinheimer AJ. Chemistry of coelenterates. I. Occurrence of terpenoid compounds in gorgonians. Ann N Y Acad Sci. 1960;90:917–919. doi: 10.1111/j.1749-6632.1960.tb26437.x. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Kumar R. Characterization of the locomotor stimulant action of nicotine in tolerant rats. British journal of pharmacology. 1983a;80(3):587–594. doi: 10.1111/j.1476-5381.1983.tb10733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. British journal of pharmacology. 1983b;78(2):329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver P, Burch M, Potenza C, Wasserman L, Fenical W, Taylor P. Structure-activity relationships for the irreversible blockade of nicotinic receptor agonist sites by lophotoxin and congeneric diterpene lactones. Mol Pharmacol. 1985;28(5):436–444. [PubMed] [Google Scholar]
- Culver P, Fenical W, Taylor P. Lophotoxin irreversibly inactivates the nicotinic acetylcholine receptor by preferential association at one of the two primary agonist sites. J Biol Chem. 1984;259(6):3763–3770. [PubMed] [Google Scholar]
- Culver P, Jacobs RS. Lophotoxin: a neuromuscular acting toxin from the sea whip (Lophogorgia rigida) Toxicon. 1981;19(6):825–830. doi: 10.1016/0041-0101(81)90078-7. [DOI] [PubMed] [Google Scholar]
- de Fiebre NC, de Fiebre CM. alpha7 Nicotinic acetylcholine receptor knockout selectively enhances ethanol-, but not beta-amyloid-induced neurotoxicity. Neurosci Lett. 2005;373(1):42–47. doi: 10.1016/j.neulet.2004.09.054. [DOI] [PubMed] [Google Scholar]
- del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellisanti CD, Yao Y, Stroud JC, Wang ZZ, Chen L. Crystal structure of the extracellular domain of nAChR alpha1 bound to alpha-bungarotoxin at 1.94 A resolution. Nat Neurosci. 2007;10(8):953–962. doi: 10.1038/nn1942. [DOI] [PubMed] [Google Scholar]
- DeNelsky GY, Denenberg VH. Infantile stimulation and adult exploratory behavior: effects of handling upon tactual variation seeking. Journal of comparative and physiological psychology. 1967;63(2):309–312. doi: 10.1037/h0024365. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Ospina CA, Rodriguez AD, Eterovic VA. Differential inhibition of nicotine- and acetylcholine-evoked currents through alpha4beta2 neuronal nicotinic receptors by tobacco cembranoids in Xenopus oocytes. Neurosci Lett. 2004;366(1):97–102. doi: 10.1016/j.neulet.2004.05.019. [DOI] [PubMed] [Google Scholar]
- El Sayed KA, Hamann MT, Waddling CA, Jensen C, Lee SK, Dunstan CA, Pezzuto JM. Structurally Novel Bioconversion Products of the Marine Natural Product Sarcophine Effectively Inhibit JB6. Cell Transformation. 1998a;63(21):7449–7455. doi: 10.1021/jo9813134. [DOI] [PubMed] [Google Scholar]
- El Sayed KA, Hamann MT, Waddling CA, Jensen C, Lee SK, Dunstan CA, Pezzuto JM. Structurally Novel Bioconversion Products of the Marine Natural Product Sarcophine Effectively Inhibit JB6 Cell Transformation. J Org Chem. 1998b;63(21):7449–7455. doi: 10.1021/jo9813134. [DOI] [PubMed] [Google Scholar]
- Eterovic VA, Hann RM, Ferchmin PA, Rodriguez AD, Li L, Lee YH, McNamee MG. Diterpenoids from Caribbean gorgonians act as noncompetitive inhibitors of the nicotinic acetylcholine receptor. Cell Mol Neurobiol. 1993a;13(2):99–110. doi: 10.1007/BF00735367. [DOI] [PubMed] [Google Scholar]
- Eterovic VA, Li L, Ferchmin PA, Lee YH, Hann RM, Rodriguez AD, McNamee MG. The ion channel of muscle and electric organ acetylcholine receptors: differing affinities for noncompetitive inhibitors. Cell Mol Neurobiol. 1993b;13(2):111–121. doi: 10.1007/BF00735368. [DOI] [PubMed] [Google Scholar]
- Eterovic VA, Lu R, Eakin AE, Rodriguez AD, Ferchmin PA. Determinants of phencyclidine potency on the nicotinic acetylcholine receptors from muscle and electric organ. Cell Mol Neurobiol. 1999;19(6):745–757. doi: 10.1023/a:1006905106834. [DOI] [PubMed] [Google Scholar]
- Fenical W, Okuda RK, Bandurraga MM, Culver P, Jacobs RS. Lophotoxin: a novel neuromuscular toxin from Pacific sea whips of the genus Lophogorgia. Science. 1981;212(4502):1512–1514. doi: 10.1126/science.6112796. [DOI] [PubMed] [Google Scholar]
- Ferchmin PA, Eterovic VA. Putrescine decreases exploration of a black and white maze. Pharmacology, biochemistry, and behavior. 1990;37(3):445–449. doi: 10.1016/0091-3057(90)90010-f. [DOI] [PubMed] [Google Scholar]
- Ferchmin PA, Hao J, Perez D, Penzo M, Maldonado HM, Gonzalez MT, Rodriguez AD, de Vellis J. Tobacco cembranoids protect the function of acute hippocampal slices against NMDA by a mechanism mediated by alpha4beta2 nicotinic receptors. J Neurosci Res. 2005;82(5):631–641. doi: 10.1002/jnr.20666. [DOI] [PubMed] [Google Scholar]
- Ferchmin PA, Lukas RJ, Hann RM, Fryer JD, Eaton JB, Pagan OR, Rodriguez AD, Nicolau Y, Rosado M, Cortes S, Eterovic VA. Tobacco cembranoids block behavioral sensitization to nicotine and inhibit neuronal acetylcholine receptor function. Journal of neuroscience research. 2001;64(1):18–25. doi: 10.1002/jnr.1049. [DOI] [PubMed] [Google Scholar]
- Ferchmin PA, Perez D, Eterovic VA, de Vellis J. Nicotinic receptors differentially regulate N-methyl-D-aspartate damage in acute hippocampal slices. J Pharmacol Exp Ther. 2003;305(3):1071–1078. doi: 10.1124/jpet.102.048173. [DOI] [PubMed] [Google Scholar]
- Ferchmin PA, Rivera E, Eterovic VA. alpha-Difluoromethylornithine does not antagonize the behavioral effects of putrescine. Pharmacology, biochemistry, and behavior. 1993;45(4):967–971. doi: 10.1016/0091-3057(93)90149-n. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine Activates an alpha -Bungarotoxin-Sensitive Nicotinic Current in Rat Hippocampal Interneurons, But Not Pyramidal Cells. J Neurosci. 1998;18(4):1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H, Suganuma M, Suguri H, Yoshizawa S, Takagi K, Kobayashi M. Sarcophytols A and B inhibit tumor promotion by teleocidin in two-stage carcinogenesis in mouse skin. J Cancer Res Clin Oncol. 1989;115(1):25–28. doi: 10.1007/BF00391595. [DOI] [PubMed] [Google Scholar]
- Giorgetti M, Bacciottini L, Giovannini MG, Colivicchi MA, Goldfarb J, Blandina P. Local GABAergic modulation of acetylcholine release from the cortex of freely moving rats. Eur J Neurosci. 2000;12(6):1941–1948. doi: 10.1046/j.1460-9568.2000.00079.x. [DOI] [PubMed] [Google Scholar]
- Groebe DR, Abramson SN. Lophotoxin is a slow binding irreversible inhibitor of nicotinic acetylcholine receptors. J Biol Chem. 1995;270(1):281–286. doi: 10.1074/jbc.270.1.281. [DOI] [PubMed] [Google Scholar]
- Groebe DR, Dumm JM, Abramson SN. Irreversible inhibition of nicotinic acetylcholine receptors by the bipinnatins. Toxin activation and kinetics of receptor inhibition. J Biol Chem. 1994;269(12):8885–8891. [PubMed] [Google Scholar]
- Hann RM, Pagan OR, Eterovic VA. The alpha-conotoxins GI and MI distinguish between the nicotinic acetylcholine receptor agonist sites while SI does not. Biochemistry (Mosc) 1994;33(47):14058–14063. doi: 10.1021/bi00251a014. [DOI] [PubMed] [Google Scholar]
- Hann RM, Pagan OR, Gregory L, Jacome T, Rodriguez AD, Ferchmin PA, Lu R, Eterovic VA. Characterization of cembranoid interaction with the nicotinic acetylcholine receptor. J Pharmacol Exp Ther. 1998;287(1):253–260. [PubMed] [Google Scholar]
- Hyde EG, Boyer A, Tang P, Xu Y, Abramson SN. Irreversible inhibitors of nicotinic acetylcholine receptors: isolation and structural characterization of the biologically active solvolysis products of bipinnatin-A and bipinnatin-C. J Med Chem. 1995a;38(12):2231–2238. doi: 10.1021/jm00012a023. [DOI] [PubMed] [Google Scholar]
- Hyde EG, Thornhill SM, Boyer AJ, Abramson SN. Evidence for a carbocation intermediate during conversion of bipinnatin-A and -C into irreversible inhibitors of nicotinic acetylcholine receptors. J Med Chem. 1995b;38(23):4704–4709. doi: 10.1021/jm00023a010. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Alexander M. Cerebral ischemia and inflammation. Curr Opin Neurol. 2001;14:89–94. doi: 10.1097/00019052-200102000-00014. [DOI] [PubMed] [Google Scholar]
- Ivy Carroll F, Ma W, Navarro HA, Abraham P, Wolckenhauer SA, Damaj MI, Martin BR. Synthesis, nicotinic acetylcholine receptor binding, antinociceptive and seizure properties of methyllycaconitine analogs. Bioorg Med Chem. 2007;15(2):678–685. doi: 10.1016/j.bmc.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamida D, Poulas K, Avramopoulou V, Fostieri E, Lagoumintzis G, Lazaridis K, Sideri A, Zouridakis M, Tzartos SJ. Muscle and neuronal nicotinic acetylcholine receptors. Structure, function and pathogenicity. Febs J. 2007;274(15):3799–3845. doi: 10.1111/j.1742-4658.2007.05935.x. [DOI] [PubMed] [Google Scholar]
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3(2):102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- Kerr DS, Briggs DM, Saba HI. A neurophysiological method of rapid detection and analysis of marine algal toxins. Toxicon. 1999;37(12):1803–1825. doi: 10.1016/s0041-0101(99)00124-5. [DOI] [PubMed] [Google Scholar]
- Kita T, Nakashima T, Shirase M, Asahina M, Kurogochi Y. Effects of nicotine on ambulatory activity in mice. Japanese journal of pharmacology. 1988;46(2):141–146. doi: 10.1254/jjp.46.141. [DOI] [PubMed] [Google Scholar]
- Langdon RB, Jacobs RS. Quantal analysis indicates an alpha-toxin-like block by lophotoxin, a non-ionic marine natural product. Life Sci. 1983;32(11):1223–1228. doi: 10.1016/0024-3205(83)90191-1. [DOI] [PubMed] [Google Scholar]
- Langdon RB, Jacobs RS. Irreversible autonomic actions by lophotoxin suggest utility as a probe for both C6 and C10 nicotinic receptors. Brain Res. 1985;359(12):233–238. doi: 10.1016/0006-8993(85)91433-7. [DOI] [PubMed] [Google Scholar]
- Laudenbach V, Medja F, Zoli M, Rossi FM, Evrard P, Changeux JP, Gressens P. Selective activation of central subtypes of the nicotinic acetylcholine receptor has opposite effects on neonatal excitotoxic brain injuries. FASEB J. 2002;16(3):423–425. doi: 10.1096/fj.01-0532fje. [DOI] [PubMed] [Google Scholar]
- Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, Grotta J, Lyden P, Shuaib A, Hardemark HG, Wasiewski WW. NXY-059 for acute ischemic stroke. The New England journal of medicine. 2006;354(6):588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- Li P, Reichert DE, Rodriguez AD, Manion BD, Evers AS, Eterovic VA, Steinbach JH, Akk G. Mechanisms of potentiation of the mammalian GABA(A) receptor by the marine cembranoid eupalmerin acetate. Br J Pharmacol. 2008;153(3):598–608. doi: 10.1038/sj.bjp.0707597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetje CW, Wada K, Rogers S, Abramson SN, Tsuji K, Heinemann S, Patrick J. Neurotoxins distinguish between different neuronal nicotinic acetylcholine receptor subunit combinations. J Neurochem. 1990;55(2):632–640. doi: 10.1111/j.1471-4159.1990.tb04180.x. [DOI] [PubMed] [Google Scholar]
- Martin SE, de Fiebre NEC, de Fiebre CM. The [alpha]7 nicotinic acetylcholine receptor-selective antagonist, methyllycaconitine, partially protects against [beta]-amyloid1-42 toxicity in primary neuron-enriched cultures. Brain Research. 2004;1022(12):254–256. doi: 10.1016/j.brainres.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Mudo G, Belluardo N, Fuxe K. Nicotinic receptor agonists as neuroprotective/neurotrophic drugs. Progress in molecular mechanisms. J Neural Transm. 2007;114(1):135–147. doi: 10.1007/s00702-006-0561-z. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Uchimura K, Zhu RL, Nagayama T, Rose ME, Stetler RA, Isakson PC, Chen J, Graham SH. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci USA. 1998;95:10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson E, Holth A, Kumlin E, Bohlin L, Wahlberg I. Structure-related inhibiting activity of some tobacco cembranoids on the prostaglandin synthesis in vitro. Planta medica. 1993;59(4):293–295. doi: 10.1055/s-2006-959684. [DOI] [PubMed] [Google Scholar]
- Oswald RE, Heidmann T, Changeux JP. Multiple affinity states for noncompetitive blockers revealed by [3H]phencyclidine binding to acetylcholine receptor rich membrane fragments from Torpedo marmorata. Biochemistry (Mosc) 1983;22(13):3128–3136. doi: 10.1021/bi00282a015. [DOI] [PubMed] [Google Scholar]
- Pagan OR, Eterovic VA, Garcia M, Vergne D, Basilio CM, Rodriguez AD, Hann RM. Cembranoid and long-chain alkanol sites on the nicotinic acetylcholine receptor and their allosteric interaction. Biochemistry (Mosc) 2001;40(37):11121–11130. doi: 10.1021/bi0112255. [DOI] [PubMed] [Google Scholar]
- Pedersen SE, Cohen JB. d-Tubocurarine binding sites are located at alpha-gamma and alphadelta subunit interfaces of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1990;87(7):2785–2789. doi: 10.1073/pnas.87.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggenbass M, Bertrand D. Nicotinic receptors in circuit excitability and epilepsy. J Neurobiol. 2002;53(4):580–589. doi: 10.1002/neu.10152. [DOI] [PubMed] [Google Scholar]
- Razani-Boroujerdi S, Boyd RT, Davila-Garcia MI, Nandi JS, Mishra NC, Singh SP, Pena-Philippides JC, Langley R, Sopori ML. T cells express alpha7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. J Immunol. 2007;179(5):2889–2898. doi: 10.4049/jimmunol.179.5.2889. [DOI] [PubMed] [Google Scholar]
- Ren M, Senatorov VV, Chen RW, Chuang DM. Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. PNAS. 2003;100(10):6210–6215. doi: 10.1073/pnas.0937423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez AD. The natural products chemistry of West Indian gorgonian octocorals. Tetrahedron. 2001;51(16):4571–4618. doi: 10.1016/0040-4020(95)00216-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Takizawa H, Konishi S, Yoshida D, Mizusaki S. Identification of cembratriene-4,6-diol as antitumor-promoting agent from cigarette smoke condensate. Carcinogenesis. 1985;6(8):1189–1194. doi: 10.1093/carcin/6.8.1189. [DOI] [PubMed] [Google Scholar]
- Sammarco PW, Coll JC. Chemical adaptations in the Octocorallia: evolutionary considerations. Marine Ecology Progress Series. 1992;88:93–104. [Google Scholar]
- Sawant S, Youssef D, Mayer A, Sylvester P, Wali V, Arant M, El Sayed K. Anticancer and anti-inflammatory sulfur-containing semisynthetic derivatives of sarcophine. Chem Pharm Bull (Tokyo) 2006;54(8):1119–1123. doi: 10.1248/cpb.54.1119. [DOI] [PubMed] [Google Scholar]
- Sharples CGV, Wonnacott S. Neuronal Nicotinic Receptors. Tocris Reviews. 2001;19(October 2001):1–12. [Google Scholar]
- Shinno K, Zhang L, Eubanks JH, Carlen PL, Wallace MC. Transient ischemia induces an early decrease of synaptic transmission in CA1 neurons of rat hippocampus: electrophysiologic study in brain slices. J Cereb Blood Flow Metab. 1997;17(9):955–966. doi: 10.1097/00004647-199709000-00005. [DOI] [PubMed] [Google Scholar]
- Sine SM. The nicotinic receptor ligand binding domain. J Neurobiol. 2002;53(4):431–446. doi: 10.1002/neu.10139. [DOI] [PubMed] [Google Scholar]
- Sorenson EM, Culver P, Chiappinelli VA. Lophotoxin: selective blockade of nicotinic transmission in autonomic ganglia by a coral neurotoxin. Neuroscience. 1987;20(3):875–884. doi: 10.1016/0306-4522(87)90248-x. [DOI] [PubMed] [Google Scholar]
- Tornoe C, Holden-Dye L, Garland C, Abramson SN, Fleming JT, Sattelle DB. Lophotoxin-insensitive nematode nicotinic acetylcholine receptors. J Exp Biol. 1996;199(Pt 10):2161–2168. doi: 10.1242/jeb.199.10.2161. [DOI] [PubMed] [Google Scholar]
- Ulrich H, Akk G, Nery AA, Trujillo CA, Rodriguez AD, Eterovic VA. Mode of cembranoid action on embryonic muscle acetylcholine receptor. J Neurosci Res. 2008;86(1):93–107. doi: 10.1002/jnr.21468. [DOI] [PubMed] [Google Scholar]
- Vila N, Castillo J, Davalos A, Esteve A, Planas AM, Chamorro A. Levels of Anti-Inflammatory Cytokines and Neurological Worsening in Acute Ischemic Stroke. 2003;34(3):671–675. doi: 10.1161/01.STR.0000057976.53301.69. [DOI] [PubMed] [Google Scholar]
- Wahlberg I, Eklund AM. Cembranoids, pseudopteranoids, and cubitanoids of natural occurrence. In: Herz W, K G, Moore RE, Steglich W, Tamm C, editors. Prog Chem Org Natural Prod. New York: Springer Verlag; 1992. pp. 141–276. [Google Scholar]
- Weitberg AB, Corvese D. The effect of epigallocatechin galleate and sarcophytol A on DNA strand breakage induced by tobacco-specific nitrosamines and stimulated human phagocytes. J Exp Clin Cancer Res. 1999;18(3):433–437. [PubMed] [Google Scholar]
- Wilson EO. The Future of Life: Vintage 2003 [Google Scholar]


