Abstract
Neuropeptide S (NPS) and its cognate receptor were reported to mediate anxiolytic-like and arousal effects. NPS receptors are predominantly expressed in the brain, especially in limbic structures, including amygdala, olfactory nucleus, subiculum and retrosplenial cortex. In contrast, the NPS precursor is expressed in only a few brainstem nuclei where it is co-expressed with various excitatory transmitters, including glutamate. The current study investigates interactions of the NPS system with glutamatergic neurotransmission. It has been suggested that dysfunctions in glutamatergic neurotransmission via N-methyl-D-aspartate (NMDA) receptors might be involved in the pathophysiology of schizophrenia since NMDA receptor antagonists, such as MK-801, have been shown to induce psychotic-like behavior in humans and animal models. Also, MK-801 is known to produce histological changes such as cytoplasmic vacuoles in retrosplenial cortex neurons where NPS receptors are highly expressed. In this study we show that NPS is able to alleviate neuropathological, neurochemical and behavioral changes produced by NMDA receptor antagonists. NPS treatment attenuated MK-801-induced vacuolization in the rat retrosplenial cortex in a dose dependent manner that can be blocked by an NPS receptor-selective antagonist. NPS also suppressed MK-801-induced increases of extracellular acetylcholine levels in the retrosplenial cortex. In the prepulse inhibition (PPI) assay, animals pretreated with NPS recovered significantly from MK-801-induced disruption of PPI. Our study suggests that NPS may have protective effects against the neurotoxic and behavioral changes produced by NMDA receptor antagonists and that NPS receptor agonists may elicit antipsychotic effects.
Keywords: glutamate, NMDA, microdialysis, neuropeptide, schizophrenia, prepulse inhibition, retrosplenial cortex
1. Introduction
Neuropeptide S (NPS) is a recently identified peptide transmitter that activates a G protein–coupled receptor, termed NPSR. Activation of NPSR induces calcium influx and increased levels of intracellular cyclic AMP, indicating an excitatory profile of action (Reinscheid et al., 2005a). NPS precursor mRNA is expressed in a few discrete nuclei in the brainstem, most prominently in a previously undefined cluster of cells located adjacent to the noradrenergic locus coeruleus (LC) (Xu et al., 2004). The NPS-expressing cells in the LC area also contain vesicular glutamate transporter mRNA, indicating that these NPS-positive neurons are glutamatergic (Xu et al., 2007). In addition, NPS precursor expression is detected in the lateral parabrachial nucleus (LPB) and the principle sensory 5 nucleus (Pr5) of the trigeminal tract. In the LPB, NPS is found co-expressed with corticotropine-releasing hormone while the NPS-positive neurons in Pr5 appear to be glutamatergic. Thus, NPS is co-localized with excitatory neurotransmitters. In contrast, NPS receptor mRNA is widely expressed in corticolimbic areas, such as the amygdala, subiculum, thalamus, hypothalamus, and retrosplenial cortex. Central administration of NPS induces arousal and produces an anxiolytic-like profile in a variety of behavioral models (Xu et al., 2004; Leonard et al., 2008; Rizzi et al., 2008). Considering the distribution of NPS and NPS receptors in corticolimbic structures, it is likely that the NPS system may play a role in the pathophysiology of neuropsychiatric disorders as well as alertness and mood (Reinscheid et al., 2005b).
N-methyl-D-aspartate (NMDA) receptor antagonists, such as phencyclidine (PCP) and ketamine, induce schizophrenia-like symptoms in humans (Javitt and Zukin, 1991). Several lines of evidence suggest that a hypofunctional state of NMDA receptor neurotransmission could be involved in the pathophysiology of schizophrenia (Javitt and Zukin, 1991; Olney and Farber, 1995; Hashimoto et al., 2004) and recent advances in the genetics of schizophrenia have identified a number of candidate genes that are involved in glutamatergic neurotransmission or developmental control of glutamatergic neurons (Straub et al., 2002; Chumakov et al., 2002; Stefansson et al., 2002). Along the NMDA receptor hypofunctional hypothesis, Olney et al. (1989) first reported neuropathological changes that produce cytoplasmic vacuoles in neurons of the rat retrosplenial cortex caused by systemic administration of NMDA receptor antagonists. The retrosplenial cortex is a component of limbic circuits that has been shown to play a pivotal role in discriminative avoidance learning and spatial memory processes (Lukoyanov and Lukoyanova, 2006; Keene and Bucci, 2008). It has been hypothesized that cognitive dysfunctions produced by NMDA receptor antagonists may, at least in part, result from neuropathological changes in this brain structure (Sutherland and Hoesing, 1993; Maddock, 1999). The neurotoxic effects of NMDA receptor antagonists are mediated by complex polysynaptic pathways involving neuronal disinhibition and are associated with excessive release of acetylcholine and glutamate in the retrosplenial cortex (Kim et al., 1999). Interestingly, neuropathological changes induced by the NMDA-receptor antagonist MK-801 (synonymous: dizocilpine) can be restored by the atypical antipsychotic clozapine, but not haloperidol (Farber et al., 1996; Hashimoto et al., 2000). Therefore, these neuropathological changes have been used as one of the predictive models of potential atypical antipsychotic properties (Hashimoto et al., 2004).
Prepulse inhibition (PPI) of the acoustic startle reflex is considered the most reliable operational paradigm for the measurement of sensorimotor gating mechanisms. PPI is the reduction of a startle reflex that occurs when the startling stimulus is preceded by a weak, non-startling prestimulus (Braff et al., 2001). Deficits in PPI are observed in patients with schizophrenia (Braff et al., 2001) and are thought to reflect dysfunctional sensorimotor gating mechanisms in this disorder. Animals treated with NMDA receptor antagonists are known to present PPI deficits, which can be restored by antipsychotics like clozapine. Thus the potency to attenuate MK-801-induced PPI deficits has been used as a reliable screening assay for antipsychotics (Geyer et al., 2001; Geyer and Ellenbroek, 2003).
Considering the distribution of NPS and NPS receptors in the corticolimbic system including the retrosplenial cortex, it is of great interest to study the effects of NPS on animal models of schizophrenia. In addition, as the majority of NPS-expressing neurons appear to be glutamatergic, interaction of the two transmitters at the cellular, circuit or systems level can be assumed. In the present study, we examined the effects of NPS on neuropathological changes in rat retrosplenial cortex and PPI deficits in mice after administration of MK-801. We also studied the effects of NPS on the release of acetylcholine (ACh) in rat retrosplenial cortex after administration of MK-801 since excessive MK-801-induced release of ACh has been correlated with neuropathological changes in this area (Kim et al., 1999; Okamura et al., 2003; 2004).
2. Materials and Methods
2.1 Animals
Female Sprague-Dawley rats (11 − 12 weeks) were used for neuropathology and microdialysis studies, and male ddY mice (9 − 10 weeks) were used for PPI experiments. Rats and mice for neuropathological studies, microdialysis and PPI were obtained from Japan SLC (Hamamatsu, Shizuoka, Japan). One group of rats used for microdialysis experiments was obtained from Charles River Laboratories (Wilmington, MA). Animals were housed separately under a 12-h light/12-h dark cycle (lights on: 7 AM) with free access to food and water. Animal rooms were maintained at 21 ± 2 °C and 60 ± 5 % relative humidity. All experiments were carried out in accordance with the Guide for Animal Experimentation, Chiba University Graduate School of Medicine and the Institutional Animal Care and Use Committee of the University of California, Irvine.
2.2 Drugs
MK-801 [dizocilpine maleate; (5R,10S)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo [a,d]cyclohepten-5,10-imine hydrogen maleate] and clozapine [8-Chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e][1,4]-diazepine] were purchased from Sigma-RBI (St. Louis, MO, USA) and dissolved in 0.9% saline as vehicle. A stock solution of MK-801 in 0.8% acetic acid was used to prepare working solutions. MK-801 doses were calculated as free base. NPS was synthesized at the Peptide Proteomic Centre, Brain Research Centre, University of British Columbia (Vancouver, BC, Canada). NPS was dissolved in phosphate-buffered saline (PBS, pH 7.4) containing 0.1% bovine serum albumin, and injected intracerebroventricularly (i.c.v.; total volume: 2 μl for mice, 5 μl for rats). The NPSR antagonist SHA 68 was synthesized as described (Okamura et al., 2008) and dissolved in PBS containing 10% Cremophor EL (Sigma). SHA 68 was administered by intraperitoneal (i.p.) injection (100 μl per animal).
2.3 Neuropathology
The neuropathological experiments were performed as described previously (Okamura et al., 2004). Briefly, rats were deeply anesthetized with pentobarbital (50 mg/kg; i.p.; Sigma) and implanted with a stainless steel guide cannula for i.c.v. administration (AMI microinjection cannula system; inner diameter: 0.15 mm; Eicom, Kyoto, Japan) five days prior to the experiment. Guide cannulae were aimed at the lateral ventricle (A = −0.9 mm, L = 1.4 mm, D = 3.3 mm; all coordinates relative to bregma). Guides were fastened to the skull with dental cement and stainless steel microscrews and dummy cannulae (Eicom) were inserted. Animals received postoperative buprenorphine (0.05 mg/kg s.c., twice daily) for one day. Ventricular localization was controlled by measuring the dipsogenic effects of 1 pmole angiotensin II dissolved in PBS containing 0.1% bovine serum albumin two days before the experiment. On the test day, vehicle (PBS containing 0.1% bovine serum albumin; 5 μl), NPS (0.01, 0.1, 1, or 10 nmole; 5 μl), or clozapine (150 nmole/5 μl) was injected at a rate of 2 μl/min using a microsyringe (1701RN; Hamilton, Reno, NV) operated by an infusion pump (KDS100, KD Scientific, Holliston, MA), followed by manual i.p. injection with MK-801 (0.5 mg/kg). A separate group of rats was injected with either vehicle or the NPSR antagonist SHA 68 (50 mg/kg, i.p.) 15 min before NPS (10 nmole, i.c.v.) or vehicle administration, followed by MK-801 treatment as described above. The dose of SHA 68 (50 mg/kg) and the pretreatment interval (15 min) was chosen based on our previous studies in which this dose and protocol was shown effective to block NPS-induced hyperlocomotion (Okamura et al., 2008).
Four hours after administration of MK-801 or vehicle, the animals were perfused with saline, followed by 4% paraformaldehyde. Brains were removed, processed by graded ethanol dehydration and embedded in paraffin. 4 μm-thick coronal slices were sectioned and stained with hematoxylin and eosin. Sections were analyzed at the level of bregma −5.80 mm. The number of vacuolized neurons in layers III and IV of the retrosplenial cortex of each side of the brain were counted and averaged for each subject from two slides per subject. Counting was performed blind with respect to the treatment group.
2.4 In vivo microdialysis
In vivo microdialysis and quantification of ACh was performed as described previously (Okamura et al., 2004). Rats were implanted with two guide cannulae for i.c.v. injection and dialysis (i.c.v. injection: A = −0.9 mm, L = 1.4 mm, D = 3.3 mm; dialysis: A = −5.8 mm, L = 0.5 mm, D = 1.5 mm; all coordinates relative to bregma). Rats were allowed to recover from surgery for 48 h and in vivo microdialysis was carried out in awake animals. A concentric I-type microdialysis probe was used (0.22 mm diameter; 1.5 mm exposed membrane; Eicom Co., Kyoto, Japan). The perfusion rate was 2 μl/min, using Ringer's solution containing the cholinesterase inhibitor eserine sulfate (0.2 mM physostigmine, Sigma). Dialysate was collected every 20 min and the first three samples were used to establish baseline ACh levels. NPS (1 nmole) or vehicle (PBS, 0.1% bovine serum albumin) were injected i.c.v through a guide cannula placed in the lateral ventricle (2 μl/min by infusion pump) as described above and 5−10 min later rats were injected with MK-801 or vehicle (i.p). ACh was measured by electrochemical detection using high-performance liquid chromatography (HPLC) equipped with a post-column enzyme reactor (Eicom Co., Kyoto, Japan) as described before (Okamura et al., 2004).
2.5 Prepulse Inhibition
Mice were briefly anesthetized with isoflurane and administered either vehicle (PBS; 2 μl) or NPS (0.01, 0.1, or 1 nmole; 2 μl) by acute i.c.v. injection as described previously (Xu et al., 2004). Another group of mice received 5 mg/kg clozapine (i.p.) dissolved in 0.9% saline. Systemic injection of vehicle or MK-801 (0.1 mg/kg, i.p.) followed 15 min after the first drug injection. Each subject was placed in the test chamber 15 min after MK-801 injection. The apparatus used for detection of startle reflexes consisted of a clear non-restrictive Plexiglas cylinder resting on a platform inside of a ventilated and illuminated chamber (SR-Lab, San Diego Instruments, San Diego, CA, USA). Background noise (a 65-dB broadband noise) was presented alone for 15 min. The test session included twelve blocks of trials, a total of 70 trials. Blocks one and twelve consisted of five pulses alone (a 40-ms 120-dB broadband burst). Blocks two to eleven each contained six trials of each type described below that were presented in a pseudo-randomized sequence.
Six trial types were presented: a 40-ms broadband 120-dB burst (pulse-alone); four prepulse + pulse trials in which 20-ms-long 69-dB, 73-dB, 77-dB, or 81-dB (4, 8, 12, and 16 dB above background) stimuli preceded the 120-dB pulse by 100 ms (onset to onset); and a no-stimulus trial. Each trial was presented with a false-random intertrial interval (average of 15 s). Startle magnitude was calculated and expressed as the average response to the pulse-alone trials presented during each block of the session. PPI data were calculated and expressed as both percentage and difference scores. Only percentage scores are presented as both measurements showed comparable results. Percent PPI was derived from the calculation: 100 − ((prepulse + pulse) / pulse alone) × 100.
2.6 Statistical analysis
For vacuolization studies, the differences between treatment groups were examined by analysis of variance (ANOVA), followed by Dunnett's t-test for multiple comparisons as post hoc analysis. For in vivo microdialysis, ACh output was expressed as a percentage of basal concentration. For each rat, a basal ACh concentration was determined by obtaining the mean value for the first three samples collected prior to the drug injection. The extracellular ACh concentration of each sample was then calculated as percentage of baseline ACh levels. Differences between the different treatment groups were evaluated by repeated measure two-way ANOVA (time point × treatment group) seeking a significant interaction of two variables. ACh levels at individual time points between 0 − 200 min post drug administration were compared between the three treatment groups by one-way ANOVA followed by Bonferroni's post-hoc test, where appropriate. For data analysis of prepulse inhibition tests, an initial two-way ANOVA (prepulse intensity × treatment group) was conducted, followed by simple main effect one-way ANOVA and/or Dunnett's post hoc test, as appropriate. The criteria for significance were p < 0.05 or p < 0.01. All data are presented as means ± standard error of mean (S.E.M.).
3. Results
3.1 Effect of NPS on MK-801-induced neurotoxicity in the retrosplenial cortex
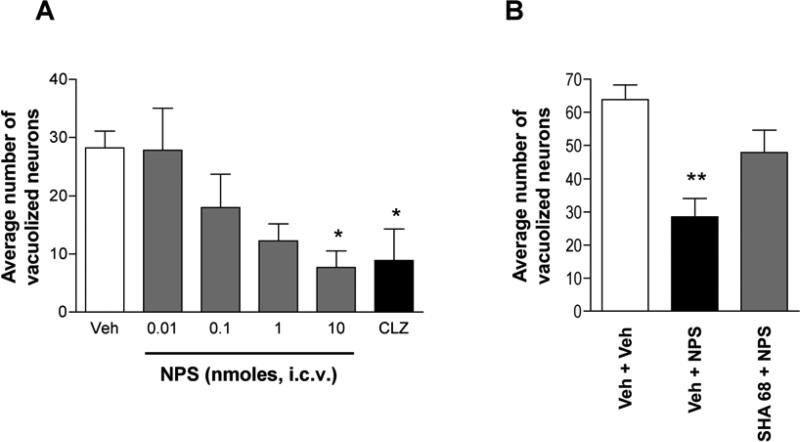
Administration of MK-801 (0.5 mg/kg, i.p.) caused neuronal vacuolization in layers III-IV of the retrosplenial cortex of the rat brain 4 h after injection (Fig. 1A). NPS decreased the number of MK-801-induced vacuolized neurons (Fig. 1B). One-way ANOVA showed significant differences between five treatment groups (F[4, 34] = 2.795, p < 0.05), and the highest dose of NPS (10 nmole, i.c.v.) significantly decreased the number of vacuolized neurons (p < 0.05; Fig. 2A). Administration of the atypical antipsychotic clozapine (150 nmole, i.c.v.) showed similar protective effects as 10 nmole NPS, producing a significant reduction in vacuolized neurons (Fig. 1C) as compared to the PBS + MK-801 group (t[13] = 2.728, p < 0.05) (Fig. 2A). In a separate experiment, the specificity of the NPS-mediated effect was tested by co-administration of the NPS receptor antagonist SHA 68. As shown in Fig. 2B, MK-801 administration increased the number of neuronal vacuoles in the retrosplenial cortex in rats that otherwise received only vehicle injections. The number of vacuolized neurons was significantly reduced in rats treated with 10 nmole NPS while co-administration of the NPS receptor antagonist SHA 68 attenuated the protective effect of NPS (Fig. 2B). One-way ANOVA revealed significant differences across the three treatment groups (F[2, 27] = 5.25, p = 0.012) and post hoc comparisons demonstrated a significant difference between vehicle- and NPS-treated animals (p < 0.01) while SHA 68 + NPS-treated rats were not different from controls (Veh + Veh) or NPS + Veh-treated animals. These data indicate that NPS might have neuroprotective effects against NMDA receptor antagonist-induced neuronal toxicity in the retrosplenial cortex and that these effects are dependent on NPS receptor activation.
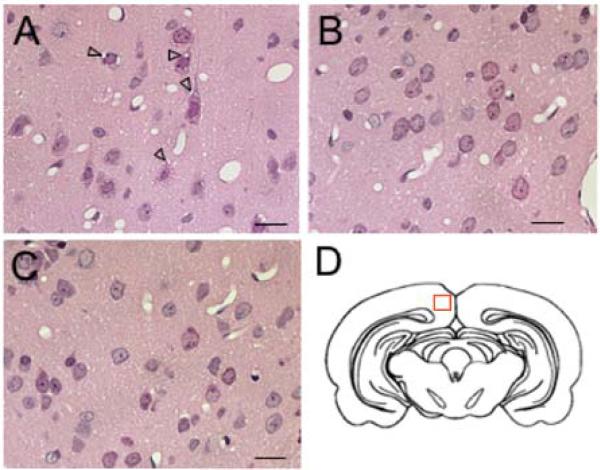
Figure 1.
Neuropathological changes in the rat retrosplenial cortex of rats. Systemic MK-801 administration induces vacuoles in pyramidal layer III-IV neurons of the retrosplenial cortex indicated by open arrowheads (A). These cytotoxic changes can be attenuated by pretreatment with NPS (B) or clozapine (C). (D) Schematic drawing of a coronal section of the rat brain at bregma −5.80. The red box indicates the location of areas that are shown in (A), (B) and (C), which are high power magnifications of layer III-IV of the retrosplenial cortex. Scale bar = 10 μm.
Figure 2.
Effects of NPS on MK-801-induced neurotoxicity in the rat retrosplenial cortex. All rats were injected with MK-801 (0.5 mg/kg, i.p.). (A) NPS decreased the number of MK-801-induced vacuolized neurons in a dose-dependent manner. Clozapine (CLZ) was used as a positive control. Data are presented as mean ± S.E.M. of 5 to 10 animals per group. * p < 0.05 in post-hoc tests compared with vehicle group (Veh). (B) Blockade of NPS receptors by the selective antagonist SHA 68 (50 mg/kg, i.p.) reverses the protective effect of 10 nmole NPS (i.c.v.) on MK-801-induced neurotoxicity. Animal numbers: Veh + Veh, n = 6; Veh + NPS, n = 8; SHA 68 + NPS, n = 14. ** p < 0.01 vs. Veh + Veh group. Data are means ± S.E.M.
Throughout the experiments, behavioral responses were monitored after administration of 0.5 mg/kg of MK-801 to the rats. Five minutes after drug administration rats exhibited head weaving and biting in a stationary posture, accompanied by increased locomotion. Rats then exhibited occasional movements in a flat-body posture until the end of the neuropathological experiment (4 h after injection). Co-administration of NPS did not alter abnormal behaviors induced by MK-801, although we did not evaluate specific behaviors quantitatively.
3.2 NPS blocks MK-801-induced release of ACh in the retrosplenial cortex
In vivo microdialysis indicated that administration of MK-801 (0.5 mg/kg, i.p.) significantly increased extracellular levels of ACh in rat retrosplenial cortex, as previously reported (Kim et al., 1999). Pretreatment with NPS (1 nmole, i.c.v.) significantly suppressed the MK-801-induced increase of ACh release with significant two-way interaction (F = 3.18, p < 0.0001) and main effect of treatment (F[2, 140] = 15.18, p = 0.0003) and time (F[10, 140] = 5.39, p < 0.0001). Administration of 1 nmole NPS alone (i.c.v.) had no effect on ACh release (Fig. 3).
Figure 3.
Effects of NPS on extracellular ACh levels in the retrosplenial cortex after administration of MK-801 or vehicle. NPS (1 nmole, i.c.v.) significantly attenuated MK-801-induced extracellular ACh increase while having no effect on its own. Levels of ACh are expressed as percentage of basal ACh concentration (mean ± S.E.M. of 5 to 7 animals per group). Average molar baseline concentrations of ACh were: 44.7 ± 25.2 nM (Veh + MK-801), 11.0 ± 2.3 nM (NPS + MK-801), and 6.82 ± 3.7 nM (NPS + Veh), calculated from a standard curve of increasing ACh concentrations. * p < 0.05 and **p < 0.01 compared to PBS + MK-801 group after one-way ANOVA at individual time points followed by Bonferroni's post hoc test.
3.3 Effects of NPS on MK-801-induced PPI disruption
Prepulse inhibition of auditory startle reflexes in mice was significantly disrupted by administration of MK-801 (0.1 mg/kg, i.p.). Two-way ANOVA (prepulse intensity × treatment) showed significant differences between the PBS + vehicle group and PBS + MK-801 group (F[1, 22] = 26.130, p < 0.01) (Fig. 4A). Pretreatment with NPS significantly reversed the MK-801-induced PPI deficit. Two-way ANOVA revealed significant main effect of pretreatment without two-way interaction between four groups which are: PBS + MK-801, NPS (0.01 nmole) + MK-801, NPS (0.1 nmole) + MK-801, and NPS (1 nmole) + MK-801 (F[3, 53] = 2.790, p < 0.05). Dunnett's post hoc test showed a significant difference between PBS + MK-801 treated animals and the NPS (1 nmole) + MK-801 treatment group (p < 0.01). Clozapine pretreatment reversed the MK-801-induced PPI deficit to a similar extent as 1 nmole NPS. Two-way ANOVA showed no significant difference between NPS (1 nmole) + MK-801 group and clozapine + MK-801 treatment groups (F[1, 23] =1.933, p > 0.05)
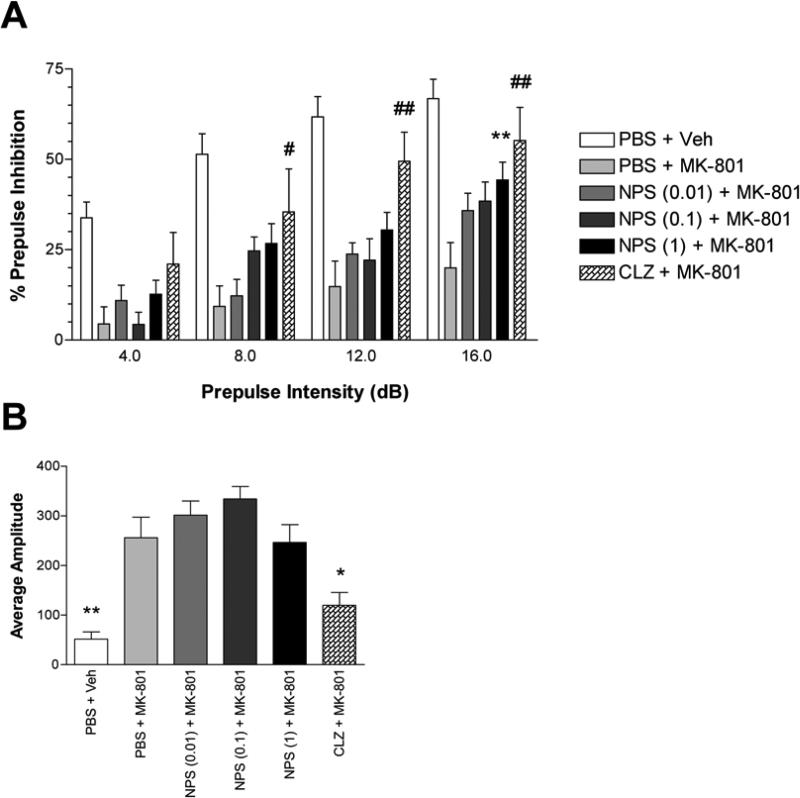
Figure 4.
(A) Effects of NPS on MK-801-induced PPI deficits in mice. NPS (1 nmole) reversed MK-801-induced PPI deficits in ddY mice. Data are shown as average amplitude of PPI and are presented as mean ± S.E.M. Dunnett's post hoc test showed a significant difference between the PBS + MK-801 group and the NPS (1) + MK-801 group (**p < 0.01). Groups of mice treated with PBS + vehicle (n = 9) or clozapine + MK-801 (n = 10) served as negative or positive controls, respectively. #p < 0.05 and ##p < 0.01 in Student's t-test compared to PBS + MK801 group. Doses of NPS (in nanomoles; i.c.v.) are shown in parenthesis. CLZ, clozapine. (B) Acoustic startle response (ASR) without prepulse. MK-801 significantly increased ASR (**p < 0.01, PBS + vehicle group versus PBS + MK-801 group; Student's t-test). NPS preadministration did not change MK-801 effects on ASR. Two-way ANOVA showed no significant difference between four treatment groups (F[3, 53] = 1.451, p > 0.05). Preadministration of clozapine reduced ASR, as clozapine + MK801 treatment produced significantly lower ASR compared to PBS + MK-801 treatment group (*p < 0.05; Student's t-test). Data were collected from the same animals used in PPI experiments.
Looking at each prepulse level individually, one-way ANOVA revealed significant differences between four treatment groups at 8 dB prepulse level (F[3, 53] = 3.081, p < 0.05) and at 16 dB prepulse level (F[3,53] = 3.562, p < 0.05). Dunnett's post hoc test indicated that there were significant differences between the PBS + MK-801 group and NPS (0.1 nmole) + MK-801 treated animals at 16 dB prepulse level (p < 0.01). Clozapine treatment produced significant improvement of PPI in MK-801 treated mice at 8, 12, and 16 dB prepulse levels (Student's t-test, compared to PBS + MK-801 group). There was no significant effect of NPS treatment on acoustic startle response (ASR) without prepulse (Fig 4B). Administration of MK-801 alone (PBS + MK-801) significantly increased ASR when compared to vehicle-treated (PBS + Veh) animals (t[22]=3.712, p < 0.01). On the other hand, clozapine pretreatment significantly reduced startle amplitude in MK-801 treated mice. T-test comparison between PBS + MK801 treatment group and clozapine + MK801 treatment group revealed a significant difference (t[23]=2.798, p < 0.05).
4. Discussion
In the present study we show that central administration of NPS can significantly attenuate MK-801-induced neuropathological and neurochemical changes in rat retrosplenial cortex. NPS also significantly improved MK-801-induced PPI deficits in mice. It has been reported that clozapine can attenuate neuropathological changes (Farber et al., 1996; Hashimoto et al., 2000) as well as PPI deficits (Bakshi et al., 1994; Geyer and Ellenbroek, 2003) produced by administration of MK-801. Our present data demonstrate that in these paradigms NPS shows a pharmacological profile similar to that of clozapine. Since these paradigms have been validated as models for antipsychotic drug effects under conditions of hypo-glutamatergic neurotransmission, our current data indicate that NPS may have potential atypical antipsychotic activity that might be useful for treatment of schizophrenia.
Although the mechanisms and neuroanatomical substrates underlying the ability of NPS to alleviate MK-801-induced PPI deficits are currently unknown, it is possible that the enhanced arousal produced by NPS could lead to improved behavioral attention that might be required during the acoustic startle reflex. Indeed, studies in human subjects have demonstrated that directed attention can enhance PPI (Heekeren et al., 2004). An alternative hypothesis to explain the observed effects of NPS on MK-801-induced neuropathological, neurochemical and behavioral changes can be deduced from the abundant co-localization of NPS and glutamate in NPS-positive neurons of the LC area, implying co-release of both transmitters under certain conditions. The present study indicates that under conditions of attenuated neurotransmission at NMDA-type glutamate receptors, NPS is able to overcome the specific deficits or effects produced by the NMDA receptor antagonist MK-801. This observation suggests that under normal conditions NPS might act as a co-transmitter at glutamatergic synapses with potentially synergistic or potentiating functions. Such a functional interaction of NPS receptors and glutamate receptors in postsynaptic neurons might lead to enhanced glutamatergic neurotransmission. Similar interactions have been described for dopamine D1 receptors and NMDA-type glutamate receptors (Lee et al., 2002) or between prolactin-releasing peptide receptors and GluR2 or GluR3 AMPA receptors (Lin et al., 2001). Obviously, further neuroanatomical and physiological investigations are needed to support this hypothesis.
The major limitation in psychosis research is the lack of a comprehensive animal model that resembles all aspects of the human disorder. Therefore, scientists have developed separate models for specific dysfunctions of the syndrome that can be validated for pharmacological intervention. Sensory-motor gating deficits are commonly found in schizophrenic patients but are also observed in Huntington's disease, Tourette's syndrome or obsessive-compulsive disorder patients. In the animal model, disruption of sensory-motor gating is induced pharmacologically (either using glutamate antagonists, dopamine agonists or serotonin agonists) or by developmental manipulations (e.g. isolation rearing). The different models of disrupted PPI display different sensitivity to pharmacological treatment and a number of inconsistencies regarding the effect of the prototypical antipsychotic clozapine have been reported from animal studies (reviewed in Geyer et al., 2001). However, it is generally accepted that the ability of a drug to reverse PPI disruption has predictive validity as a potential antipsychotic effect. We used male ddY mice for PPI experiments because this species and strain has shown robust acoustic startle with reliable prepulse inhibition (Furuya et al., 1999; Tohmi et al., 2005; Sakaue et al., 2003, Zhang et al., 2007). Furthermore, these mice display significant disruption of PPI after MK-801 administration (Sakaue et al., 2003; Zhang et al., 2007) and other competitive NMDA receptor antagonist (Sakaue et al., 2003). On the other hand, it has been shown earlier that female Sprague-Dawley rats are significantly more sensitive to the neurotoxic effects of NMDA antagonists than male rats (Fix et al., 1995; Auer 1996). Also, most studies showing NMDA antagonist-induced ACh release in the retrosplenial cortex have used female Sprague-Dawley rats (Kim et al., 1999; Farber et al., 2002) and we, therefore, chose the same gender and strain of rats for our neuropathology and microdialysis studies. The neurobiological basis for the documented differences across species, strain or gender in the different experimental models is currently unknown but certainly rewards further research.
The neuropathological changes produced by NMDA receptor antagonists are thought to be mediated by a complex polysynaptic mechanism involving the interference of GABAergic disinhibition resulting in excessive release of ACh (Olney and Farber, 1995; Farber et al., 2002; 2003). It has been reported that systemic administration of MK-801 (0.5 mg/kg) significantly increases the extracellular levels of ACh in the rat retrosplenial cortex, and that the α2-adrenergic agonist clonidine (Kim et al., 1999), the metabotropic glutamate receptor mGluR type II agonist LY 379268 (Okamura et al., 2003), and adenosine A1 receptor agonists (Okamura et al., 2004) significantly suppress both MK-801-induced neuronal vacuolization and MK-801-induced ACh release in rat retrosplenial cortex, suggesting that excessive release of ACh by systemic administration of MK-801 may partly contribute to the neuropathological damages in this region. In this study, we found that central injection of NPS significantly attenuates the increase of ACh levels and neurotoxicity in rat retrosplenial cortex after administration of MK-801. This effect might be mediated by NPS receptors that are particularly expressed in neurons of the retrosplenial cortex and anterior thalamic nuclei (Xu et al., 2004; 2007). Interestingly, it has been reported that bilateral injection of MK-801 (5, 10, 15, 20 μg/μL per side) into the anterior thalamus induced the expression of heat shock protein HSP-70, a reversible marker of neuronal injury, in pyramidal neurons in deep layer III of the rat retrosplenial cortex (Tomitaka et al., 2000), suggesting that the circuit from anterior thalamus to retrosplenial cortex is implicated in the neuropathological changes observed after administration of MK-801. It is thus likely that inhibitory projections to the retrosplenial cortex from anterior thalamic nuclei that express NPS receptors may play a role in the neuroprotective effects of NPS. Alternatively, it is possible that NPS may modulate glutamatergic neurotransmission directly at the postsynaptic level as discussed above. In the current study we have also investigated the specificity of NPS-induced neuroprotective effects in the retrosplenial cortex by co-administration of the NPSR antagonist SHA 68. However, SHA 68 did not fully antagonize the protective effects of NPS in this assay. Such an incomplete reversal of NPS-induced effects by SHA 68 has been observed previously for NPS-induced hyperlocomotion (Okamura et al., 2008) and may be caused by limited availability of SHA 68 at NPSR due to its lipophilic character.
A number of studies demonstrated that NMDA receptor antagonists disrupt PPI in rodents, mimicking the clinically observed PPI deficits in schizophrenia patients and supporting the role of glutamatergic systems in the neural circuitry of sensorimotor gating (Geyer et al., 2001). Most importantly, clozapine improves MK-801-induced PPI deficits in rodents (Geyer and Ellenbroek, 2003) and PPI deficits in schizophrenic patients (Oranje et al., 2002), suggesting that NMDA receptor antagonist-induced PPI deficits may serve as a model for sensorimotor gating deficits in schizophrenia. Therefore, the potency to restore MK-801-induced PPI deficits has been used as one of the most reliable screening assays for atypical antipsychotics (Geyer and Ellenbroek, 2003). Our results show that NPS significantly improved MK-801-induced PPI deficits in mice similar to clozapine, suggesting that activation of NPS receptors might have antipsychotic-like effects. It should be noted that psychostimulants such as cocaine or amphetamine enhance PPI disruption, although less robust than dopamine D2 receptor agonists or NMDA receptor antagonists (Geyer et al., 2001). Therefore, the present data support the hypothesis that NPS might not be a typical psychostimulant although some of its effects, such as stimulation of locomotor responses and suppression of sleep, resemble the pharmacological profile of the classical stimulants.
In conclusion, the present results suggest that activation of NPS receptors elicits protective effects against neurotoxicity and PPI deficits produced by the NMDA receptor antagonist MK-801. The ability of NPS to alleviate MK-801-induced neuropathological, neurochemical and behavioral symptoms across three experimental models in two different species suggests that the NPS receptor might be an interesting target for development of novel antipsychotic drugs.
Acknowledgements
We are grateful to Ms. Yuko Fujita for technical assistance, Dr. James Belluzzi for help with statistical analysis and Dr. Yan-Ling Xu for helpful suggestions and discussions. This study was supported in part by grants from the National Institute of Mental Health (MH-71313 to R.K.R.), a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression, NARSAD (R.K.R.), a postdoctoral fellowship grant from the Mitsubishi Pharma Research Foundation (N.O.), and a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan (K.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auer RN. Effect of age and sex on N-methyl-D-aspartate antagonist-induced neuronal necrosis in rats. Stroke. 1996;27:743–746. doi: 10.1161/01.str.27.4.743. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Swerdlow NR, Geyer MA. Clozapine antagonizes phencyclidine-induced deficits in sensorimotor gating of the startle response. J. Pharmacol. Exp. Ther. 1994;271:787–794. [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, et al. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13675–13680. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber NB, Foster J, Duhan NL, Olney JW. Olanzapine and fluperlapine mimic clozapine in preventing MK-801 neurotoxicity. Schizophr. Res. 1996;21:33–37. doi: 10.1016/0920-9964(96)00024-2. [DOI] [PubMed] [Google Scholar]
- Farber NB, Jiang X, Dikranian K, Nemmers B. Muscimol prevents NMDA antagonist neurotoxicity by activating GABAA receptors in several brain regions. Brain Res. 2003;993:90–100. doi: 10.1016/j.brainres.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Farber NB, Kim SH, Dikranian K, Jiang XP, Heinkel C. Receptor mechanisms and circuitry underlying NMDA antagonist neurotoxicity. Mol. Psychiatry. 2002;7:32–43. doi: 10.1038/sj.mp.4000912. [DOI] [PubMed] [Google Scholar]
- Fix AS, Wozniak DF, Truex LL, McEwen M, Miller JP, Olney JW. Quantitative analysis of factors influencing neuronal necrosis induced by MK-801 in the rat posterior cingulate/retrosplenial cortex. Brain Res. 1995;696:194–204. doi: 10.1016/0006-8993(95)00842-e. [DOI] [PubMed] [Google Scholar]
- Furuya Y, Kagaya T, Ogura H, Nishizawa Y. Competitive NMDA receptor antagonists disrupt prepulse inhibition without reduction of startle amplitude in a dopamine receptor-independent manner in mice. Eur. J. Pharmacol. 1999;364:133–140. doi: 10.1016/s0014-2999(98)00839-5. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fujimura M, Yamagami K. Dizocilpine-induced neuropathological changes in rat retrosplenial cortex are reversed by subsequent clozapine treatment. Life Sci. 2000;66:1071–1078. doi: 10.1016/s0024-3205(00)00410-0. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Okamura N, Shimizu E, Iyo M. Glutamate hypothesis of schizophrenia and approach for possible therapeutic drugs. Curr. Med. Chem. - CNS Agents. 2004;4:147–154. [Google Scholar]
- Heekeren K, Meincke U, Geyer MA, Gouzoulis-Mayfrank E. Attentional modulation of prepulse inhibition: a new startle paradigm. Neuropsychobiology. 2004;49:88–93. doi: 10.1159/000076416. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Involvement of the retrosplenial cortex in processing multiple conditioned stimuli. Behav. Neurosci. 2008;122:651–658. doi: 10.1037/0735-7044.122.3.651. [DOI] [PubMed] [Google Scholar]
- Kim SH, Price MT, Olney JW, Farber NB. Excessive cerebrocortical release of acetylcholine induced by NMDA antagonists is reduced by GABAergic and alpha2-adrenergic agonists. Mol. Psychiatry. 1999;4:344–352. doi: 10.1038/sj.mp.4000529. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, et al. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Leonard SK, Dwyer JM, Sukoff Rizzo SJ, Platt B, Logue SF, Neal SJ, Malberg JE, Beyer CE, Schechter LE, Rosenzweig-Lipson S, Ring RH. Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology (Berl) 2008;197:601–611. doi: 10.1007/s00213-008-1080-4. [DOI] [PubMed] [Google Scholar]
- Lin SH, Arai AC, Wang Z, Nothacker HP, Civelli O. The carboxyl terminus of the prolactin-releasing peptide receptor interacts with PDZ domain proteins involved in alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor clustering. Mol. Pharmacol. 2001;60:916–923. doi: 10.1124/mol.60.5.916. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Lukoyanova EA. Retrosplenial cortex lesions impair acquisition of active avoidance while sparing fear-based emotional memory. Behav. Brain Res. 2006;173:229–236. doi: 10.1016/j.bbr.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Okamura N, Hashimoto K, Shimizu E, Koike K, Ohgake S, Koizumi H, Kumakiri C, Komatsu N, Iyo M. Protective effect of LY379268, a selective group II metabotropic glutamate receptor agonist, on dizocilpine-induced neuropathological changes in rat retrosplenial cortex. Brain Res. 2003;992:114–119. doi: 10.1016/j.brainres.2003.08.045. [DOI] [PubMed] [Google Scholar]
- Okamura N, Hashimoto K, Shimizu E, Kumakiri C, Komatsu N, Iyo M. Adenosine A1 receptor agonists block the neuropathological changes in rat retrosplenial cortex after administration of the NMDA receptor antagonist dizocilpine. Neuropsychopharmacology. 2004;29:544–550. doi: 10.1038/sj.npp.1300351. [DOI] [PubMed] [Google Scholar]
- Okamura N, Habay SA, Zeng J, Chamberlin AR, Reinscheid RK. Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor. J. Pharmacol. Exp. Ther. 2008;325:893–901. doi: 10.1124/jpet.107.135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen, Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- Oranje B, Van Oel CJ, Gispen-De Wied CC, Verbaten MN, Kahn RS. Effects of typical and atypical antipsychotics on the prepulse inhibition of the startle reflex in patients with schizophrenia. J. Clin. Psychopharmacol. 2002;22:359–365. doi: 10.1097/00004714-200208000-00005. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Xu YL, Okamura N, Zeng J, Chung S, Pai R, Wang Z, Civelli O. Pharmacological characterization of human and murine neuropeptide S receptor variants. J. Pharmacol. Exp. Ther. 2005a;315:1338–1345. doi: 10.1124/jpet.105.093427. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Xu YL, Civelli O. Neuropeptide S: a new player in the modulation of arousal and anxiety. Mol. Interv. 2005b;5:42–46. doi: 10.1124/mi5.1.8. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Vergura R, Marzola G, Ruzza C, Guerrini R, Salvadori S, Regoli D, Calo G. Neuropeptide S is a stimulatory anxiolytic agent: a behavioural study in mice. Br. J. Pharmacol. 2008;154:471–479. doi: 10.1038/bjp.2008.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue M, Ago Y, Baba A, Matsuda T. The 5-HT1A receptor agonist MKC-242 reverses isolation rearing-induced deficits of prepulse inhibition in mice. Psychopharmacology (Berl) 2003;170:73–79. doi: 10.1007/s00213-003-1515-x. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. Am. J. Hum. Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am. J. Hum. Genet. 2002;71:337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, Hoesing JM. Posterior cingulate cortex and spatial memory: a microlimnology analysis. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus. Birkhauser; Boston: 1993. pp. 461–477. [Google Scholar]
- Tohmi M, Tsuda N, Mizuno M, Takei N, Frankland PW, Nawa H. Distinct influences of neonatal epidermal growth factor challenge on adult neurobehavioral traits in four mouse strains. Behav. Genet. 2005;35:615–629. doi: 10.1007/s10519-005-5357-7. [DOI] [PubMed] [Google Scholar]
- Tomitaka S, Tomitaka M, Tolliver BK, Sharp FR. Bilateral blockade of NMDA receptors in anterior thalamus by dizocilpine (MK-801) injures pyramidal neurons in rat retrosplenial cortex. Eur. J. Neurosci. 2000;12:1420–1430. doi: 10.1046/j.1460-9568.2000.00018.x. [DOI] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of Neuropeptide S receptor mRNA and neurochemical characteristics of Neuropeptide S expressing neurons in the rat brain. J. Comp. Neurol. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shirayama Y, Iyo M, Hashimoto K. Minocycline attenuates hyperlocomotion and prepulse inhibition deficits in mice after administration of the NMDA receptor antagonist dizocilpine. Neuropsychopharmacology. 2007;32:2004–2010. doi: 10.1038/sj.npp.1301313. [DOI] [PubMed] [Google Scholar]