Abstract
The brain has enormous anabolic needs during early postnatal development. This study presents multiple lines of evidence showing that endogenous brain insulin-like growth factor 1 (Igf1) serves an essential, insulin-like role in promoting neuronal glucose utilization and growth during this period. Brain 2-deoxy-d- [1-14C]glucose uptake parallels Igf1 expression in wild-type mice and is profoundly reduced in Igf1−/− mice, particularly in those structures where Igf1 is normally most highly expressed. 2-Deoxy-d- [1-14C]glucose is significantly reduced in synaptosomes prepared from Igf1−/− brains, and the deficit is corrected by inclusion of Igf1 in the incubation medium. The serine/threonine kinase Akt/PKB is a major target of insulin-signaling in the regulation of glucose transport via the facilitative glucose transporter (GLUT4) and glycogen synthesis in peripheral tissues. Phosphorylation of Akt and GLUT4 expression are reduced in Igf1−/− neurons. Phosphorylation of glycogen synthase kinase 3β and glycogen accumulation also are reduced in Igf1−/− neurons. These data support the hypothesis that endogenous brain Igf1 serves an anabolic, insulin-like role in developing brain metabolism.
Keywords: mental retardation, glycogen synthase kinase, glucose transporter, GLUT4, Akt
The brain requires enormous supplies of fuel to support neuroglial growth and process formation during early postnatal development. Murine and human brains consume over half the energy available to the organism as a whole during this critical period (1), when undernutrition may result in permanent intellectual deficit (2). How the developing brain competes so successfully with peripheral tissues for resources is unknown. Insulin preferentially enhances fuel and substrate utilization by peripheral tissues, but does not seem to be involved in the regulation of brain metabolism (3). Very little insulin is synthesized within the brain (4) and little circulating insulin crosses the blood–brain barrier (5). However, insulin-like growth factor 1 (Igf1), an insulin homologue, is abundant in the developing brain (6), where it is concentrated in large projection neurons (7–9). Igf1 and insulin receptors are also homologous, with nearly identical signal-transducing domains engaging many of the same intracellular pathways (10). The Igf1 receptor (Igf1R) is widely expressed in the brain (11–14) where it is most abundant in Igf1-expressing neurons (15), suggesting a local autocrine/paracrine mode of action for neuronal Igf1. Although Igf1's precise role in brain development is unknown, its importance seems clear because IGF1 gene deletion in humans results in mental retardation (16). Based on Igf1/Igf1R expression patterns in the developing brain and on the functional homology with the insulin/insulin receptor dyad, we hypothesized that neuronal Igf1 serves an anabolic, literally “insulin-like” role in brain development (17). To test this hypothesis, we investigated glucose utilization in Igf1-targeted gene deletion mice.
Experimental Procedures
The mice used in this study were from an Igf1 deletion line derived and genotyped as previously described (18). This line has been bred into an outbred CD1 line for more than 10 generations to obtain the mice used in this study. Sprague–Dawley rats used for cerebral Igf1 injection and middle cerebral artery occlusion experiments were obtained from Taconic Farms (Germantown, NY). Animals were studied under protocols approved by the National Institute of Child Health and Human Development Animal Use and Care Committee.
2-Deoxy-d-Glucose Uptake (2DGU).
Wild-type (WT) and Igf1−/− mice of postnatal day 10 (P10) were injected at 10:00 a.m. with 2-deoxy-d-[1-14C]glucose [1 μCi/g i.p. (1 Ci = 37 GBq); Amersham Pharmacia] and were killed 45 min later by decapitation without anesthesia. Blood was obtained at the time of death for radioactivity and glucose determinations. Brains rapidly were dissected and snap frozen. Coronal sections (10-μm thick) were cut at −20°C, thaw-mounted on to poly-l-lysine-coated slides, and dried on a 60°C plate for 15 min. Anatomically matched sections were exposed to a single piece of Hyperfilm-βmax (Amersham Pharmacia) for 5 days. Slides from WT and Igf1−/− mice were apposed to film in random order. Brain images were digitized by using a solid-state video camera. The signal intensity in different brain structures of Igf1−/− and WT mice was compared after subtraction of background film signal with the National Institutes of Health IMAGE 1.57 program.
To evaluate the effects of Igf1 injection on cerebral glucose uptake, adult (250 g) male Sprague–Dawley rats were anesthetized with Chloropent (0.3 ml/kg 4.25% chloral hydrate/0.9% pentobarbital, i.p.) and their heads were placed in a stereotactic apparatus. With the skull exposed, bilateral holes were drilled 1-mm lateral to the bregma. A 25-gauge needle was lowered through each hole to a depth of 3 mm from the surface of the parietal cortex. Afterward, 5 μl of PBS alone or PBS containing 10 ng of recombinant IGF1, nerve growth factor, or fibroblast growth factor 2 (Upstate Biotechnology, Lake Placid, NY) was injected into the cortex over 10 min, while the needle slowly was retracted. Immediately after needle withdrawal, 2DG was injected i.p. at a dose of 1 μCi/g. Animals were killed 45 min later, before awakening from anesthesia. Brains were flash frozen and sectioned at 16-μm thickness for film autoradiography. Surgical procedures for middle cerebral artery occlusion studies were performed as described (19). The rats were allowed to recover for 6 days after the surgery, at which time they were injected with 2DG (1 μCi/g), then killed 45 min later; brains were sectioned and subjected to autoradiography as described above.
Synaptosomes were prepared from whole brain (20) and used for in vitro 2DGU (21) as previously described. Synaptosomes were washed once with glucose-free Hepes-buffered physiologic saline solution, then incubated at 37°C for 5 min in the same solution containing 0.5 mmol of glucose. The synaptosomes were diluted 1:5 with glucose-free suspension buffer containing deoxy-d-glucose,2-[1,2-3H(N)] (NEN) at a concentration of 1 μCi/ml. Uptake was assayed in the presence or absence of Igf1 (1 μg/ml). Cytochalasin B (80 μM; Sigma) was included in parallel reactions to determine non-carrier-mediated uptake. To terminate the experiments, samples were centrifuged at 16,000 × g for 25 sec, the supernatant was discarded, the pellet was rinsed twice with 0.5 ml of ice-cold buffer, and then radioactivity was measured by scintillation counter. Protein content was determined with a Bio-Rad kit and data were expressed as fmol/mg of protein per min.
Hexokinase Activity Assay.
Hexokinase activity assay was performed by adding 0.2 mg of freshly prepared synaptosomes to a solution containing 0.1 M Mops⋅Tris (pH7.5), 0.1% Triton X-100, 8 mM MgCl2, 0.4 mM NADP+, 5 mM d-glucose, 5 units of glucose-6-phosphate dehydrogenase, and 1 mM ATP. The reaction was initiated with the addition of ATP. The linear rate of NADP+ reduction was measured after the initial lag of 1 min by continuously monitoring the increase in absorbance at 340 nm in a spectrophotometer for 4 min. Data were presented as nmol of product formed/min per mg of protein added.
Histochemistry and Immunoblotting.
The protocol for in situ hybridization and the generation of 35S-labeled sense and antisense RNA probes for IGF1 and GLUT4 were described previously (8, 22). GLUT4 mRNA was quantified in Purkinje cells of WT and Igf1-null mice by a blinded observer counting grains overlying 50 cells per section in two to three sections for each mouse, with five to six mice per group. After subtraction of background signal, group means were obtained and compared by unpaired t test. Immunohistochemistry was performed by the avidin–biotin–immunoperoxidase method as described previously (23). Immunoblotting was performed also as described previously (23). Antibodies were obtained from the following sources: GSK3β-pan (Transduction Laboratories, Lexington, KY; catalog no. G22320); GSK3β-ser9, GLUT1, and GLUT3 (Chemicon; catalog nos. AB16981, AB1340, and AB1344, respectively); GLUT4 (Biogenesis, Bournemouth, U.K.; catalog no. RAIRGT); Akt-pan (New England Biolabs; catalog no. 9272); and Akt-ser473 and Akt-thr308 (New England Biolabs; catalog nos. 9271S and 9275, respectively).
Histological staining of glycogen was performed by the periodic acid–Schiff (PAS) reaction. Paraffin-embedded sections first were deparaffinized in xylene, rehydrated, and oxidized in 0.1% periodic acid for 5 min. After washing in water, slides were stained in 0.5% Schiff's solution (Sigma) for 15 min and counterstained with hematoxylin. For negative controls, parallel brain sections were treated with 0.5% α-amylase (Sigma) at 37°C for 90 min before PAS staining. For quantitation, anatomically matched sagittal sections from WT and Igf1−/− brains were examined. PAS “positivity” was determined by the presence of fuchsia-colored staining. PAS-stained positive and negative Purkinje and mitral cells were counted manually at ×200 magnification. Data were presented as percentage of total cells that are PAS-positive. Two sections were evaluated for each animal (WT = 7; Igf1−/− = 6). Data are expressed as means ± SEM. Differences in groups' means were evaluated by ANOVA followed by Scheffé t test.
Results
Endogenous Igf1 Expression and Glucose Utilization.
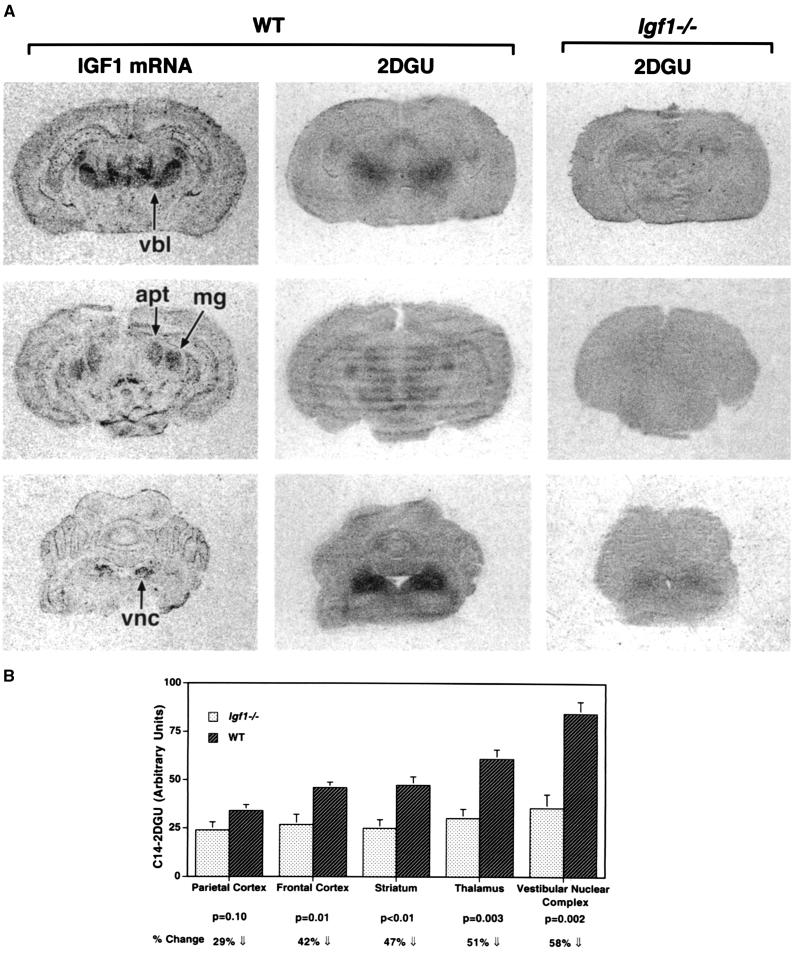
Brain Igf1 expression is highest during early postnatal development (8), corresponding to a time of peak energy requirements for the developing brain (24). If endogenous Igf1 promotes brain glucose utilization, then glucose utilization should be impaired in Igf1-null brains, and the deficit should be most evident at the time point when Igf1 expression is normally highest. Therefore, we compared brain 2DGU in Igf1−/− and WT littermate mice at P10. Regional 2DGU patterns parallel Igf1 expression in the developing WT mouse brain (Fig. 1), and 2DGU is significantly reduced in the Igf1−/− brain, most profoundly in those structures where Igf1 is highly expressed in the WT. For example, 2DGU is reduced by >50% in thalamic and brainstem sensory nuclei (Fig. 1B). 2DGU also was reduced by ≈50% in the cerebellar cortex (P = 0.005; data not shown). There were no significant differences in blood 14C-2DG or glucose levels between the groups by the end of the 45-min experiment. Because of the small size of the mice, it was not possible to assay for blood radioactivity levels during the course of the experiment, so the clearance rate of 14C-2DG in the two groups is not known. However, it seems unlikely that the reduced 2DGU in Igf1−/− brains could be caused by increased metabolic clearance of 2DG in the null mice. Arguing against any major clearance effects, 2DGU in the tibialis anterior muscle from the same animals was equal in WT and in Igf1−/− mice, and cardiac 2DGU was significantly increased in the Igf1−/− mice (our unpublished data).
Figure 1.
Glucose utilization is dramatically reduced in the Igf1−/− brain. (A) Anatomically matched coronal sections from forebrain (Top), midbrain (Middle), and hindbrain (Bottom) are shown. 2DGU is globally reduced in the Igf1−/− brains, most notably in the brain regions where Igf1 is most abundant in WT (left column). apt, anterior pretectal nuclei; mg, medial geniculate nuclei; vbl, ventrobasolateral complex; vnc, vestibular nuclear complex. (B) 14C-2DGU was quantified from film autoradiographs. The thalamus was measured at the level of the ventrobasolateral nuclear complex. Data are presented as mean ± SEM (arbitrary density units). n = 4–5 per group.
Because very little Igf1 crosses the blood–brain barrier (5), systemic Igf1 treatment is not an effective way to determine whether Igf1 can reverse the diminished brain glucose utilization in Igf1−/−mice. Therefore, glucose uptake was investigated in isolated nerve terminals, or synaptosomes, prepared from Igf1−/− and WT brains. Synaptosomes from Igf1−/− brains demonstrate significantly decreased 2DGU in vitro, which is corrected by addition of Igf1 to the incubation medium (Table 1). These data also argue against a major role for differences in 2DG clearance rates in the reduced 2DGU seen in the Igf1−/− brains in vivo.
Table 1.
Glucose uptake in WT and in Igf1−/− synaptosomes
| Treatments | WT | Igf1−/− | Δ | P |
|---|---|---|---|---|
| Control medium | 544.3 ± 24.8 | 376.0 ± 25.7 | 31% ⇓ | 0.006 |
| Medium + Igf1 | 502.7 ± 53.4 | 494.3 ± 38.9 | ⇔ | 0.9 |
Whole brain synaptosome preparations were incubated with 3H-labeled 2DG (1 μCi/ml) in the absence (control medium) or presence of Igf1. After centrifugation and washing, the radioactivity absorbed by the synaptosomal pellet was measured by scintillation counting. In control incubation, 2DGU was significantly reduced in Igf1−/− synaptosomes. When Igf1 was added to the incubation, 2DGU in the Igf1−/− was equal to WT. Results are expressed as means ± SEM in fmol/mg of protein per min. WT, n = 3; Igf1−/−, n = 4.
Igf1 deletion results in significantly decreased postnatal brain growth in both homozygous and heterozygous genotypes. Igf1−/− brain weight is not significantly different from WT at birth but is decreased by ≈40% by P40 (473 ± 5 mg for WT vs. 287 ± 3 mg for Igf1−/−, with n = 16 vs. n = 7, respectively; P < 0.0001) after which the size differential remains constant. Igf1−/+ brains are ≈10% smaller than WT brains at P40 (427 ± 7; n = 11; P < 0.0001 vs. WT). Despite the reduction in size, Igf1−/− brain cell numbers are remarkably normal, except for a selective reduction in size of the dentate gyrus (25) and olfactory bulb (23). The large part of the size reduction seems attributable to a reduction in neuropil, that is, nerve processes (23, 25).
Molecular Mechanisms of Igf1 Action in Brain Metabolism.
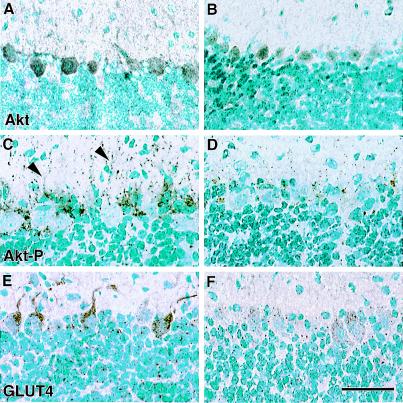
The homology between insulin and Igf1 receptors suggested that known insulin-signaling pathways could be involved in Igf1's actions regulating brain glucose metabolism. In peripheral tissues, insulin receptor activation triggers a kinase cascade leading through phosphatidylinositol 3-kinase to phosphorylation of the serine/threonine kinase Akt (also known as protein kinase B or RAC-PK) (26). Activation of this kinase regulates the synthesis and translocation of a specific glucose transporter, GLUT4, which enhances glucose entry into cells (27). We focused these investigations on the cerebellar cortex, because Purkinje neurons express very high levels of Igf1 during early postnatal development (7, 8) and because its relatively simple laminar structure allows ready visualization of the Purkinje cells and their dendrites. Akt expression is, like Igf1, selectively concentrated in Purkinje cells in the cerebellar cortex (Fig. 2 A–C). Akt immunoreactivity revealed by a phosphorylation-insensitive antibody is homogeneously distributed in neuronal perikarya and processes and is very similar in WT and Igf1−/− brains (Fig. 2 A and B). The active, phosphorylated form of this kinase, in contrast, is concentrated in granular deposits in WT neuronal processes, but is barely detectable in Igf1−/− neurons (Fig. 2 C and D). Antibodies specific for phosphoserine473 and phosphothreonine308 Akt identified the same pattern of subcellular localization in the WT and profound reduction of phospho-Akt in Igf1−/− brains. In the WT brain, GLUT4 immunoreactivity is concentrated in neuronal processes of Igf1-expressing neurons in a pattern similar to phospho-Akt. In Igf1−/− brains, however, GLUT4 immunoreactivity is reduced and is largely confined to perikarya (Fig. 2 E and F). Supporting the decreased GLUT4 immunoreactivity, GLUT4 mRNA as revealed by in situ hybridization also was decreased in Igf1−/− Purkinje cells (16.6 ± 1.5 grains for WT vs. 9.9 ± 1.0 for Igf1−/− brains; P < 0.01; n = 6–7). Expression of GLUT1 and GLUT3 also was investigated in these studies by using immunoblotting of whole brain homogenates and membrane fractions. GLUT1 and GLUT3 protein levels were not reduced in the Igf1-null brains (data not shown).
Figure 2.
Akt phosphorylation and GLUT4 localization in WT and Igf1−/− cerebellar cortex. Total Akt immunoreactivity is homogeneously distributed in Purkinje perikarya and processes in WT (A) and Igf1−/− (B) brains. Phospho-specific Akt immunoreactivity (Thr308), in contrast, is granular-appearing and is detected predominantly in Purkinje dendritic processes in the WT (C, arrowhead) and is scarcely detected in the Igf1−/− brain (D). GLUT 4 immunoreactivity is concentrated in Purkinje dendrites and the cytoplasm just around the dendrite's origin (E). GLUT4 is present in Purkinje perikarya in the Igf1−/− cerebellum, but is not preferentially localized in dendrites (F). (Bar = 100 μm.)
Hexokinase I is responsible for glucose-6 phosphorylation in brain and is thus essential for neuronal glucose utilization. Hexokinase I protein levels were equal in Igf1-null and WT brains by immunoblot analysis (data not shown), but hexokinase activity was reduced by 22% (33.6 ± 1.4 vs. 43.2 ± 2.1 nmol/mg of protein per min; n = 4 for each group; P < 0.0001) in synaptosomal fractions from Igf1-null brains. Thus, decreased glucose utilization in the Igf1-null brain may be explained by reduced hexokinase activity and/or by reduced GLUT4 expression and translocation.
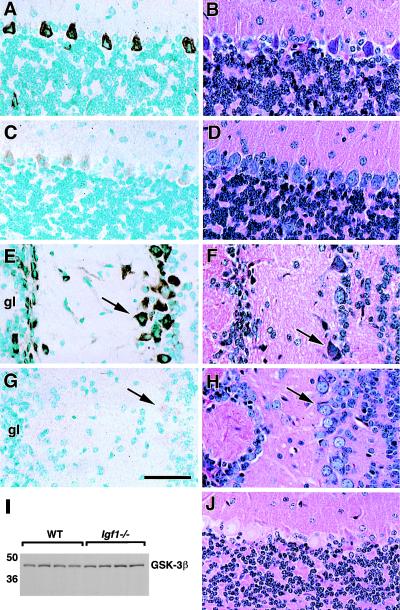
Insulin-induced stimulation of glycogen synthesis in liver and muscle involves the phosphorylation of glycogen synthase kinase 3β (GSK3β) on serine9 (28, 29). This phosphorylation prevents GSK3β-induced inhibition of glycogen synthase, thus promoting glycogen synthesis. Immunoreactivity specific for ser9-phospho-GSK3β is concentrated in the perikarya of large, Igf1-expressing projection neurons in WT brain and is associated with prominent glycogen accumulation in the same neurons (Fig. 3). In Igf1−/− brains, however, ser9-phospho-GSK3β is barely detectable, and glycogen stores are profoundly reduced (Fig. 3 C, D, G, and H). The percentage of glycogen-positive Purkinje cells, for example, is 72 ± 2.6% in WT vs. 14.5 ± 2.1% in Igf1−/− brains (P < 0.0001). A phosphorylation-insensitive antibody, however, shows that GSK3β protein levels are equal in WT and Igf1−/− brains (Fig. 3I).
Figure 3.
(A–H) GSK3β-ser9 phosphorylation and glycogen synthesis in cerebellar cortex (A–D) and olfactory bulb (E–H). Representative micrographs show immunostaining specific for ser9-phosphorylated GSK3β on the left-hand side of the figure, and glycogen-PAS staining is shown on the right side. WT sections are shown in A, B, E, and F, and Igf1−/− sections are shown in C, D, G, and H. Intense ser9-phospho-GSK3β staining of projection neurons (arrows) is seen in the WT but is barely detectable in the Igf1−/− brain. (I) GSK3β total protein levels assessed by immunoblot are the same in WT and Igf1−/−. (J) This section was pretreated with α-amylase to remove tissue glycogen before PAS staining. gl, glomerular layer. (Bar = 100 μm.)
Igf1 and Glucose Utilization in the Mature Brain.
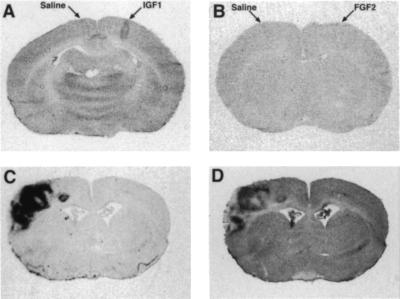
Murine brain Igf1 expression is greatly reduced by P40 (6–9) at which time there are no statistically significant differences in 2DGU between Igf1−/− and WT brains (data not shown). To test Igf1's ability to regulate mature brain glucose utilization, Igf1 was microinjected into the cerebral cortex of anesthetized normal adult animals while saline was injected into the contralateral cortex (Fig. 4 A and B). Igf1 produced a marked increase in local 2DGU that was not seen with saline or with neuroactive peptides such as nerve growth factor or fibroblast growth factor 2. As previously noted, Igf1 expression is minimal in the normal adult murine brain, but it is highly induced in glial cells reacting to injury, such as focal infarction caused by middle cerebral artery occlusion (19). Here we show that this local Igf1 expression closely parallels increased 2DGU in the injured brain (Fig. 4 C and D). In this setting, the injury zone is densely populated with Igf1-expressing glial cells engaged in scar formation (19). Thus, Igf1 expression by neurons or glial cells correlates with extraordinary brain metabolic activity.
Figure 4.
(A and B) Igf1 increases cerebrocortical glucose uptake. Igf1 (A) or fibroblast growth factor 2 (FGF2) (B) was injected into the cerebral cortex of anesthetized rats with vehicle injected simultaneously into the contralateral side. After the cerebral injections, 2DG was administered i.p. and the animals were killed 45 min later. Representative film autoradiographs from sections at the injection site show that only Igf1 produced increases in 2DGU, which is readily appreciated along the needle track. These findings were replicated in three animals. (C and D) Parallel patterns for Igf1 expression (C) and 14C-2DGU (D) in the adult rat brain 6 days after middle cerebral artery occlusion. Other growth factors such as Igf2 and platelet-derived growth factor did not show a similar correlation with 2DGU (data not shown).
Discussion
This study provides multiple lines of evidence suggesting that Igf1 serves in an insulin-like manner to augment brain glucose utilization during brain development. We have shown that regional 2DGU parallels Igf1 expression in the developing brain, and that glucose utilization is significantly reduced in the Igf1−/− brain, particularly in structures where Igf1 expression is normally most abundant. The defect in glucose utilization in Igf1−/− brains is demonstrable at the nerve terminal level in vitro, suggesting that the defect is primarily neuronal and is independent of synaptic activity. Because Igf1 expression is minimal in the normal mature brain, it has little role in glucose utilization under normal conditions in the adult. Exogenous Igf1 by microinjection, however, increases brain glucose utilization, and endogenous Igf1 induction in response to injury is correlated with increases in local glucose utilization in the mature brain.
The mechanism(s) whereby Igf1 augments brain glucose utilization seem to be similar to those used by insulin in the periphery. The present data demonstrate the selective colocalization of phospho-Akt and GLUT4 in WT neuronal processes, whereas Akt phosphorylation and GLUT4 are diminished and GLUT4 is largely confined to the perikarya in Igf1−/− neurons. These data suggest, as a matter of speculation, that Igf1-induced Akt phosphorylation may regulate GLUT4 synthesis and translocation during neuronal development. In contrast, we found no evidence for reduced GLUT1 or GLUT3 levels related to Igf1 deletion. However, hexokinase activity is significantly reduced in Igf1-null brain tissue, suggesting a role for Igf1 in regulation of brain hexokinase activity. This possibility is supported by the observation that insulin, acutely injected into the cerebral cortex, is able to increase hexokinase activity (30). Insulin and Igf1 are reported to stimulate GSK3β phosphorylation in cultured human neurons (31). The present study supports this role for Igf1 in vivo, because ser9-phospho-GSK3β is abundant in Igf1-expressing neurons in WT brains, but is barely detected in these neurons in Igf1−/− brains. The presence of phospho-GSK3β in WT neurons is correlated with abundant glycogen stores, consistent with the view that Igf1 acts in an autocrine, insulin-like manner to promote glucose uptake and storage as glycogen in developing projection neurons. Supporting this view, neuronal glycogen synthesis is largely a perinatal phenomenon that is spatiotemporally correlated with Igf1 expression (8, 32).
Because endogenous brain Igf1 expression peaks during postnatal development and is barely detected in the normal adult murine brain (6–8), Igf1 probably does not play a role in the moment-to-moment, activity-dependent glucose metabolism of the adult brain. In normal mature brains, increased glucose utilization primarily is associated with increased synaptic activity (33). During brain development and injury responses, however, glucose utilization for biosynthetic and reparative processes may exceed that for synaptic activity. The defect in glucose utilization in the Igf1−/− brain in vivo is paralleled by decreased glucose uptake by synaptosomes in vitro, supporting a metabolic defect at the nerve terminal level, independent of synaptic activity, that is absent in isolated nerve terminals. Moreover, the specific changes in glucose transport- and metabolizing enzymes in Igf1-expressing neurons resemble the effects of insulin on muscle glucose metabolism, supporting a primary metabolic role for Igf1 in the brain. By analogy with the activity-dependent glycogen depletion seen in muscle, if reduced neuronal activity were the primary defect in the Igf1−/− brain, there should be glycogen accumulation rather than depletion in Igf1−/− neurons. Finally, the coordinate increase in Igf1 and 2DGU expression after focal ischemia occurs in glial cells involved in scar formation in the absence of synaptic activity (19).
In vitro studies have suggested many potential roles for Igf1 in brain development and function (34–36). The specific effects exerted by Igf1 in vivo, however, are determined by where and when the peptide and its receptor are actually expressed. Brain Igf1 and Igf1 receptor expression are most abundant in maturing sensory and cerebellar projection neurons during neuronal process growth and synaptogenesis (7–9, 15). The Igf1−/− brain demonstrates a relative preservation of cell number but a generalized reduction in neuropil (23, 25), suggesting that decreased process growth and synapse formation account for much of the growth deficit. Likewise, transgenic Igf1 brain expression after normal endogenous Igf1 has subsided is associated with a generalized increase in neuropil (37) and 2DGU (38), suggesting that Igf1 may promote process growth and synapse formation in mature as well as in developing brains.
Igf1 expression is most abundant in growing projection neurons (e.g., Purkinje cells), destined to be the largest and most anatomically complex cells in the brain. It thus seems possible that Igf1's local insulin-like, anabolic effects suggested in the present work may promote this extraordinary growth. Interestingly, this is essentially the same effect whereby Igf1 promotes longitudinal bone growth by amplifying growth plate chondrocyte hypertrophy (39). These Igf1-expressing neurons grow not just large perikarya and long axons but also exuberant dendritic arbors typifying the sensory and cerebellar projection systems where Igf1 expression excels (8). Absence of Igf1 during murine brain development results in smaller cells with fewer processes and synapses (R. F. Mervis, J. McKean, S. Zats, C.M.C., S. Wray, A. Gum, and C.A.B., unpublished data) and in humans is associated with mental retardation (16). These observations suggest, as a matter of speculation, that therapeutic augmentation of brain Igf1 expression may enhance the potential for cognitive development.
Acknowledgments
We thank Lyn Powell-Braxton and Genentech for providing the Igf1−/− line, Ricardo Dreyfuss for expert photomicrography, Kent Morest and Miles Herkenham for seminal discussions, and John Wilson for help with hexokinase determinations.
Abbreviations
- Igf1
insulin-like growth factor 1
- 2DG
2-deoxy-d-glucose
- 2DGU
2DG uptake
- WT
wild type
- PAS
periodic acid–Schiff
- Pn
postnatal day n
- GSK3β
glycogen synthase kinase 3β
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.170008497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.170008497
References
- 1.Gibbons A. Science. 1998;280:1345–1347. doi: 10.1126/science.280.5368.1345. [DOI] [PubMed] [Google Scholar]
- 2.Chase H P, Martin H P. N Engl J Med. 1970;282:933–939. doi: 10.1056/NEJM197004232821701. [DOI] [PubMed] [Google Scholar]
- 3.Baskin D G, Figlewicz D P, Woods S C, Porte D, Jr, Dorsa D M. Annu Rev Physiol. 1987;49:335–347. doi: 10.1146/annurev.ph.49.030187.002003. [DOI] [PubMed] [Google Scholar]
- 4.Coker G T, 3rd, Studelska D, Harmon S, Burke W, O'Malley K L. Brain Res Mol Brain Res. 1990;8:93–98. doi: 10.1016/0169-328x(90)90052-f. [DOI] [PubMed] [Google Scholar]
- 5.Reinhardt R R, Bondy C A. Endocrinology. 1994;35:1753–1761. doi: 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- 6.Rotwein P, Burgess S K, Milbrandt J D, Krause J E. Proc Natl Acad Sci USA. 1988;85:265–269. doi: 10.1073/pnas.85.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson I K, Edwall D, Norstedt G, Rozell B, Skottner A, Hansson H A. Acta Physiol Scand. 1988;132:167–173. doi: 10.1111/j.1748-1716.1988.tb08314.x. [DOI] [PubMed] [Google Scholar]
- 8.Bondy C A. J Neurosci. 1991;11:3442–3455. doi: 10.1523/JNEUROSCI.11-11-03442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartlett W P, Li X S, Williams M, Benkovic S. Dev Biol. 1991;147:239–250. doi: 10.1016/s0012-1606(05)80021-1. [DOI] [PubMed] [Google Scholar]
- 10.De Meyts P, Wallach B, Christoffersen C T, Urso B, Gronskov K, Latus L J, Yakushiji F, Ilondo M M, Shymko R M. Horm Res. 1994;42:152–169. doi: 10.1159/000184188. [DOI] [PubMed] [Google Scholar]
- 11.Bohannon N J, Corp E S, Wilcox B J, Figlewicz D P, Dorsa D M, Baskin D G. Brain Res. 1988;444:205–213. doi: 10.1016/0006-8993(88)90931-6. [DOI] [PubMed] [Google Scholar]
- 12.Lesniak M A, Hill J M, Kiess W, Rojeski M, Pert C B, Roth J. Endocrinology. 1988;123:2089–2099. doi: 10.1210/endo-123-4-2089. [DOI] [PubMed] [Google Scholar]
- 13.Bondy C A, Werner H, Roberts C T, Jr, LeRoith D. Mol Endocrinol. 1990;4:1386–1398. doi: 10.1210/mend-4-9-1386. [DOI] [PubMed] [Google Scholar]
- 14.Marks J L, Porte D, Jr, Baskin D G. Mol Endocrinol. 1991;5:1158–1168. doi: 10.1210/mend-5-8-1158. [DOI] [PubMed] [Google Scholar]
- 15.Bondy C A, Werner H, Roberts C T, LeRoith D. Neuroscience. 1992;46:909–923. doi: 10.1016/0306-4522(92)90193-6. [DOI] [PubMed] [Google Scholar]
- 16.Woods K A, Camacho-Hubner C, Savage M O, Clark A J. N Engl J Med. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- 17.Bondy C A, Lee W H. Ann N Y Acad Sci. 1993;692:33–43. doi: 10.1111/j.1749-6632.1993.tb26203.x. [DOI] [PubMed] [Google Scholar]
- 18.Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart T A. Genes Dev. 1993;7:2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- 19.Lee W H, Clemens J A, Bondy C A. Mol Cell Neurosci. 1992;3:36–43. doi: 10.1016/1044-7431(92)90006-n. [DOI] [PubMed] [Google Scholar]
- 20.Dodd P R, Hardy J A, Oakley A E, Edwardson J A, Perry E K, Delaunoy J P. Brain Res. 1981;226:107–118. doi: 10.1016/0006-8993(81)91086-6. [DOI] [PubMed] [Google Scholar]
- 21.Erecinska M, Nelson D, Chance B. Proc Natl Acad Sci USA. 1991;88:7600–7604. doi: 10.1073/pnas.88.17.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin E, Zhou J, Bondy C A. J Clin Invest. 1993;91:1810–1815. doi: 10.1172/JCI116392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng C M, Joncas G, Reinhardt R R, Farrer R, Quarles R, Janssen J, McDonald M P, Crawley J N, Powell-Braxton L, Bondy C A. J Neurosci. 1998;18:5673–5681. doi: 10.1523/JNEUROSCI.18-15-05673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nehlig A. Diabetes Metab. 1997;23:18–29. [PubMed] [Google Scholar]
- 25.Beck K D, Powell-Braxton L, Widmer H R, Valverde J, Hefti F. Neuron. 1995;14:717–730. doi: 10.1016/0896-6273(95)90216-3. [DOI] [PubMed] [Google Scholar]
- 26.Summers S A, Birnbaum M J. Biochem Soc Trans. 1997;25:981–988. doi: 10.1042/bst0250981. [DOI] [PubMed] [Google Scholar]
- 27.Kohn A D, Summers S A, Birnbaum M J, Roth R A. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 28.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Nature (London) 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 29.Shaw M, Cohen P, Alessi D R. FEBS Lett. 1997;416:307–311. doi: 10.1016/s0014-5793(97)01235-0. [DOI] [PubMed] [Google Scholar]
- 30.Hoyer S, Prem L, Sorbi S, Amaducci L. NeuroReport. 1993;4:991–993. doi: 10.1097/00001756-199307000-00039. [DOI] [PubMed] [Google Scholar]
- 31.Hong M, Lee V M. J Biol Chem. 1997;272:19547–19553. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- 32.Borke R C, Nau M E. Brain Res. 1984;318:277–284. doi: 10.1016/0165-3806(84)90032-4. [DOI] [PubMed] [Google Scholar]
- 33.Sokoloff L. J Cereb Blood Flow Metab. 1981;1:7–36. doi: 10.1038/jcbfm.1981.4. [DOI] [PubMed] [Google Scholar]
- 34.Caroni P, Grandes P. J Cell Biol. 1990;110:1307–1317. doi: 10.1083/jcb.110.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aizenman Y, de Vellis J. Brain Res. 1987;406:32–42. doi: 10.1016/0006-8993(87)90766-9. [DOI] [PubMed] [Google Scholar]
- 36.Feldman E L, Sullivan K A, Kim B, Russell J W. Neurobiol Dis. 1997;4:201–214. doi: 10.1006/nbdi.1997.0156. [DOI] [PubMed] [Google Scholar]
- 37.Yao D L, Liu X, Hudson L D, Webster H D. Proc Natl Acad Sci USA. 1995;92:6190–6194. doi: 10.1073/pnas.92.13.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutierrez-Ospina G, Saum L, Calikoglu A S, Diaz-Cintra S, Barrios F A, D'Ercole A J. NeuroReport. 1997;8:2907–2911. doi: 10.1097/00001756-199709080-00021. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Zhou J, Bondy C A. FASEB J. 1999;13:1985–1990. doi: 10.1096/fasebj.13.14.1985. [DOI] [PubMed] [Google Scholar]