Abstract
Objective
Assess the effect of pregnancy and first vaginal delivery on urethral striated sphincter neuromuscular function.
Study Design
Quantitative electromyographic (EMG) interference pattern analysis of the urethral sphincter of 23 nulligravidas and 31 third trimester primigravidas allowed comparison of mean motor unit parameters before term vaginal delivery and post partum.
Results
Mean electromyographic interference pattern parameters in the primigravidas were significantly lower than nulligravidas even antepartum, with decreased turns, lower amplitude, and less activity. The only significant change at 6 months post partum was further decline in number of turns resulting in a further decrease in turns:amplitude. All other electromyographic abnormalities persisted at six months post partum and remained abnormal compared to the nulligravidas.
Conclusion
Urethral sphincter neuromuscular function changed significantly during pregnancy and these changes persisted post partum. Lack of recovery 6 months post partum suggests a physiologic impact of pregnancy itself on future risk of urinary incontinence.
Keywords: Urethra, Pregnancy, Pelvic floor, Electromyography, Urinary incontinence
INTRODUCTION
Electrophysiological studies using a variety of methods have suggested that a major component of urinary incontinence in women is neurologic injury to the pelvic floor resulting from obstetric delivery.[1,2] Specific risk factors for urinary incontinence and the actual mechanism(s) of injury are unclear. Rat models of parturition have demonstrated local tissue hypoxia in the urethral sphincter in response to vaginal distension mimicking descent of the fetal head [3] with further experiments identifying pudendal nerve crush injury as having a distinct, additional deleterious effect.[4] However, a woman who does not deliver vaginally may not be protected from urinary symptoms. The Childbirth and Pelvic Symptoms study (CAPS), a contemporary human cohort study of primiparas, yielded the interesting finding that 23% of women having cesarean section without labor still reported urinary incontinence symptoms at 6 months post partum.[5] This finding raises the question of a distinct effect of pregnancy itself on urinary function.
We undertook this study to assess urethral sphincter neuromuscular function in a group of primigravidas having vaginal delivery. Our hypothesis was driven by prior observations of neurogenic injury in other muscles of the pelvic floor after various modes of obstetrical delivery. [6,7] We sought to investigate the effect of pregnancy and vaginal delivery on the neuromuscular function of the urethral striated sphincter in a group of primigravidas. We hypothesized that we would observe a) significant neuromuscular changes in the urethral sphincter 6 weeks after first vaginal delivery and b) these changes would be consistent with a neurogenic injury.
MATERIALS AND METHODS
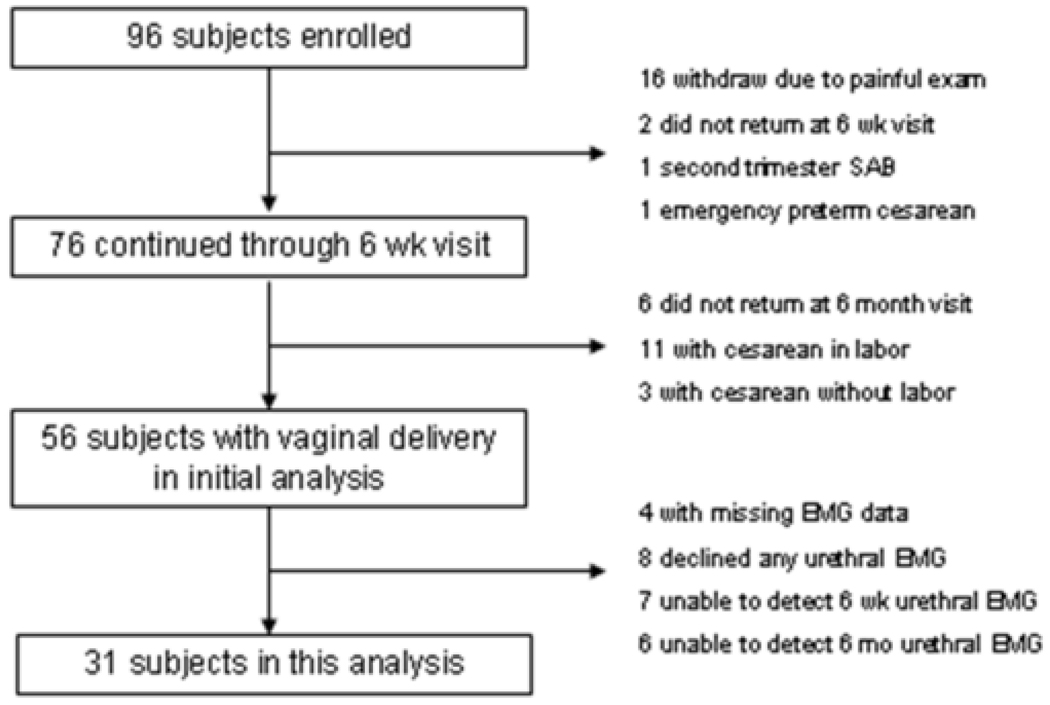
Primigravid women who presented to the Duke obstetrical clinics from 2001–2004 were approached to participate in a larger study of neuromuscular function in the pelvic floor after receipt of full Duke Medical Center Institutional Review Board approval. We recruited 96 singleton primigravidas less than 34 weeks gestational age who denied a history of pelvic surgery, pre-pregnancy pelvic floor symptoms, diabetes or neuromuscular disorder, and tested neuromuscular function using EMG and MRI before and after vaginal delivery. Attrition in the original primigravida group of 96 was fairly high and is summarized in Figure 1. Seventy subjects completed the final visit at 6 months post partum. Fourteen of those underwent cesarean delivery and were excluded. Of the remaining 52 with data sufficient for analysis, 8 declined to have urethral EMG performed. Thirteen more subjects permitted initial urethral assessment at the antepartum visit but no recordable motor units were detected at the urethral site at either the 6 week or 6 month post partum visit, leaving 31 primigravid subjects for this analysis. Twenty three nulligravid volunteers with no pelvic symptoms or prior pelvic surgery were used as controls.
Figure 1.
Progress of primigravid subjects through the study.
EMG Acquisition and Analysis
At 28–34 weeks gestation, primigravid subjects underwent baseline concentric needle EMG assessment of the striated urethral sphincter by a single examiner (ACW). [6,7] EMG signals were recorded from one suprameatal needle site using a 0.45 mm needle with 0.07 mm2 recording area, with the muscle at rest and with moderate and maximal activity. Recordings were made on a Synergy-2 channel electromyograph (Oxford Instruments Medical Systems, Hawthorne, NY). Position of the needle was confirmed by auditory signal and motor unit action potential morphology. This same EMG assessment was performed again at 6 weeks and 6 months postpartum. Nulligravidas underwent a single, identical EMG examination. A pelvic organ prolapse quantitation (POPQ) [8] and a clinical assessment of voluntary pelvic floor contraction was also performed in all subjects. [9] Each subject completed the long form of the Pelvic Floor Distress Inventory and Impact Questionnaire (PFDI/PFIQ) at each visit. [10]
We used interference pattern analysis to sample and analyze the EMG data as we have previously reported. [6] This type of analysis is sensitive to changes in motor unit changes that occur after neuromuscular injury. Typically, acute neuropathic injury causes loss of motor units, and summation action potentials generated by the remaining motor units have fewer electrical potential reversals (“turns/second”) and lower amplitude. As reinnervation and recovery occur over time, action potentials become larger and more complex, with abnormally high amplitude. Thus, a muscle that has been reinnervated after neuropathic injury will typically generate an interference pattern with stable or decreased number of turns/second coupled with very high amplitude. This makes the ratio of turns:amplitude (abbreviated here as T/A) low. In contrast, myopathic injury typically causes early recruitment of many low amplitude motor units, generating an interference pattern with a high number turns/second and low amplitude, and therefore a high T/A ratio.
Recordings from nulligravid subjects’ urethral EMG were pooled to calculate mean and standard deviation for representative motor unit parameters: amplitude in µVolts, number of turns/second, and the T/A ratio. We performed a similar analysis of the 31 primigravid subjects at each visit (antepartum, 6 weeks post partum, and 6 months post partum) and tested for differences at each time point from the antepartum group using the Wilcoxon rank sum test.
We further calculated the mean and the 95% confidence interval for T/A for the 23 nulligravidas as a group, and T/A for each individual primiparous subject at each postpartum visit. Each site was assessed to determine whether it was judged “normal” or “abnormal” at either postpartum visit. A site was considered to be “low abnormal” if the entire 95% confidence interval of T/A was below the lower limit of the range defined by the nulligravidas, and a “high abnormal” T/A if the 95% confidence interval value exceeded the upper limit of normal as defined by the nulligravid group.
Obstetrical Measures
Each primigravid subject’s labor and delivery were managed by her obstetrician, following a protocol conforming to accepted practices of active management of labor. [11] The decision whether or not to use regional anesthetic was left to the subject and her physician, though once placed, epidurals were managed using a standard protocol of ropivicaine/fentanyl-loading dose and a subject controlled analgesic pump. Routine episiotomy was not performed. Because of our desire to standardize the effect of the delivery on the urethral sphincter to the degree possible, only subjects having spontaneous or operative vaginal delivery were included in this analysis.
Statistical Analysis
We used the Wilcoxon rank sum test to assess differences between groups and the Wilcoxon signed rank test to assess within-group changes.
RESULTS
The two groups were similar in age with a mean 28 yrs for nulligravidas vs. 29.5 yrs for primigravidas. Nine (39%) of the nulligravidas were Caucasian vs. 20 (65%) of the primigravidas. Mean BMI at enrollment differed appropriately due to pregnancy (25.6 vs 28.9 kg/m2). Mean neonatal weight was 3330 ± 534 g and the mean duration of second stage of labor 75 ± 48 min, with 25 subjects having spontaneous vaginal delivery and 6 having operative delivery (3 via vacuum and 3 via forceps).
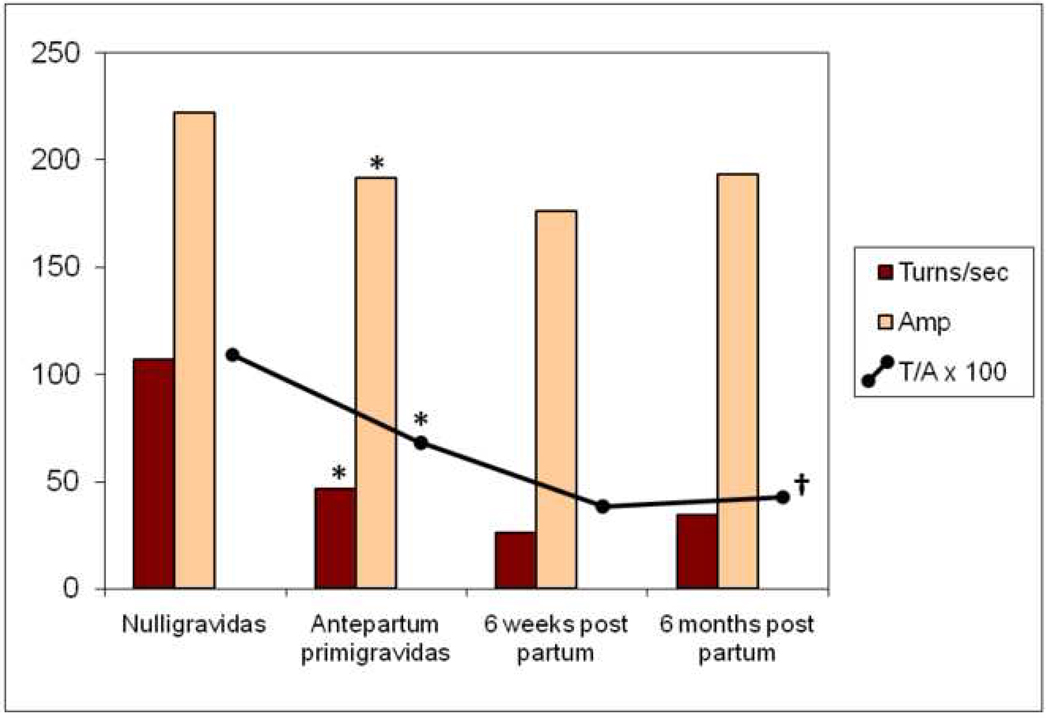
We found significant differences in urethral striated sphincter neuromuscular function between the nulligravidas and the primigravida group even before obstetric delivery. These findings are summarized in Table I and depicted in Figure 2. The EMG parameter of number of turns/second, a measure of the number of motor units activated during a defined time period of muscular contraction, was 43% less in primigravidas than in nulligravidas. EMG signal amplitude was also significantly less in the primigravidas even before delivery. Because the absolute decrease in number of turns/second was greater than that of amplitude, the T/A in primigravidas was significantly less than that of nulligravidas.
Table I.
Mean motor unit parameters (a) and median PFDI QOL scores (b) for nulligravid group vs. primigravida/primipara group. (a) The most global differences in both turns/sec and amplitude were noted between nulligravidas and antepartum primigravidas. After vaginal delivery, number of turns/sec declined further, yielding a further decline in the ratio of turns/amplitude that persisted at 6 months post partum.
(b) Median QOL pelvic floor distress inventory scores indicated more distress in antepartum primigravidas than nulligravidas. Post partum, distress scores improved significantly only in the urinary domain.
(Motor unit parameters shown are mean (SD), QOL scores are median (range). Tests of significance by Wilcoxon rank sum and signed rank respectively.)
| Nulligravidas | Primigravida/Primiparas | |||||||
|---|---|---|---|---|---|---|---|---|
| a. Motor unit parameter at maximum effort |
Antepartum | p Nullipara vs Antepartum |
6wks post partum |
p Antepartum vs 6 wks post partum |
6mos post partum |
p Antepartum vs 6 mos post partum |
p 6wks vs 6 mos post partum |
|
| Turns/second | 106.9 (84.8) | 46.6 (43.2) | 0.004 | 26.1 (41.5) | 0.120 | 34.4 (51.1) | 0.187 | 0.534 |
| Amplitude (µV) | 221.6 (60.7) | 191.5 (95.4) | 0.003 | 176.1 (52.3) | 0.497 | 193.1 (62.4) | 0.667 | 0.225 |
| T/A (µV−1) | 1.08 (0.57) | 0.67 (0.44) | 0.005 | 0.39 (0.31) | 0.064 | 0.41 (0.38) | 0.018 | 0.912 |
| b. QOL scores | ||||||||
| UDI | 0 (0–21.2) | 7.1 (0–89.1) | 0.007 | 0 (0–39.8) | 0.006 | 0 (0–44.6) | 0.006 | 0.124 |
| POPDI | 8.3 (0–29.2) | 16.1 (0–65.4) | 0.021 | 16.7 (0–52.4) | 0.193 | 8.3 (0–78.6) | 0.082 | 0.478 |
| CRADI | 8.3 (0–68.1) | 15.4 (0–78.1) | 0.135 | 20.3 (0–139.2) | 0.035 | 17.9 (0–92.9) | 0.267 | 0.338 |
Figure 2.
Mean urethral EMG parameters in nulligravidas and primigravidas antepartum and after delivery. Mean urethral EMG turns, amplitude, and T/A were significantly lower in antepartum subjects compared to controls. (*p ≤ 0.005) These changes persisted post partum, without significant recovery. T/A at 6 mos post partum was significantly lower than at antepartum. (†p=0.018)
(Mean motor unit parameters shown are turns=turns/second, amp=amplitude in µVolts. Tests of significance by Wilcoxon rank sum with * denotes nulligravidas vs. antepartum primigravidas, † denotes antepartum primigravidas vs 6 months post partum.)
Six weeks after vaginal delivery, the urethral EMG T/A declined further because of a further decrease in the number of motor units activated when subjects were asked to contract their pelvic floor muscles. At six months post partum, there was no significant change in the primiparas’ neuromuscular function of the urethra from the status at six weeks.
The normal range of urethral T/A defined as the 95% confidence interval for the nulligravid group was 0.82–0.92 µV−1. Twenty one of the 31 primigravid subjects had mean urethral T/A significantly lower than this normal range at the antepartum visit and that abnormality persisted in 15/21 at 6 weeks and 19/21 at 6 months post partum.
Because of differences in the racial composition of our groups, we performed a two-way ANOVA to assess the significance of race (African-American vs Caucasian) and group (nulligravidas vs primigravidas) as predictors of urethral EMG parameters. The difference between groups was not different for African-Americans and Caucasians. Race was not a significant predictor for number of turns (p=0.28) or T/A (p=0.68), but was a significant predictor of amplitude. African-American nulligravidas had significantly higher mean amplitude of urethral EMG signal than Caucasians (244±73 µV vs. 194±34 µV, p=0.006).
Quality of life scores as measured by the PFDI were significantly higher (indicative of worse distress) in primigravidas than nulligravidas in the urinary (UDI) and prolapse (POPDI) domains, though not the colorectal (CRADI) domain. (see Table I) At six weeks post partum, UDI scores were actually significantly improved over those reported during the antepartum period, with a decline in median score back to zero. This improvement was maintained at six months post partum. At six months post partum POPDI and CRADI scores were not significantly better or worse than those reported by the primigravidas during the antepartum period. The only domain that actually improved in median score was urinary (UDI).
COMMENT
Our data indicate an impressive difference in urethral sphincter neuromuscular function between nulligravid women and primigravid women of similar age, and emphasize the potential impact of pregnancy itself on urethral function. Our findings do not support our hypothesis that a) significant changes in neuromuscular function of the urethral sphincter would be evident at 6 weeks post partum, and b) such changes would be consistent with a neurogenic pattern of injury. In fact, our findings were much more consistent with a lasting effect of the pregnancy itself on the neuromuscular integrity of the urethral sphincter. We showed that when asked to contract their pelvic floor muscles, the primigravid women were able to recruit fewer urethral sphincter motor units (represented by number of turns/second) than nulligravid women, even before delivery. The finding that the mean amplitude of these motor units in the primigravid women was also smaller is consistent with the turns data, since activation of fewer motor units results in a smaller summation electrical potential (measured as amplitude). Furthermore, these changes were not clearly associated with temporary physiologic changes of pregnancy. Following term vaginal delivery, the antepartum changes in muscle performance essentially persisted if not actually slightly worsened, with minimal recovery at 6 months post partum.
These findings were not particularly consistent with quality of life scores as measured by the validated PFDI. The UDI and CRADI scores did improve, however, with UDI declining back to a median score equivalent to that of the nulligravid group. We found this finding particularly interesting in light of our EMG data, which indicated poor urethral function antepartum which did not recover during the post partum period. Possible explanations for this finding could be that post partum urinary symptoms do not become evident until a threshold amount of damage has occurred, such as with subsequent deliveries. Our quality of life questionnaire, while validated for use with women with pelvic floor dysfunction, may not have been sufficiently responsive to measure the relatively mild symptom severity typical of the primiparous woman. A physiologic explanation of this finding could also be that the increased intra-abdominal pressure of third trimester pregnancy and the post partum relief of this effect overshadow the negative effect of one vaginal delivery on urethral function.
We hypothesized that we would show a classic pattern of neurogenic injury in the urethra at 6 weeks post partum followed by partial recovery via reinnervation at 6 months. However, our findings overall do not support a neurogenic injury mechanism either during pregnancy or after delivery, since such an injury typically results in an increase in EMG signal amplitude after reinnervation of injured motor units. This kind of recovery should have been evident at 6 months post partum, and we found no such evidence. This finding is in direct contrast to our previous reports of a neurogenic injury pattern in the levator ani in post partum primiparas, with remarkable increase in EMG signal amplitude. [6,7] Neither are our findings in this report consistent with myopathic injury, in which muscle fibers themselves atrophy, since such an injury tends to increase the number of turns during a standardized effort, as amplitude either decreases or remains stable. We observed instead that the number of contracting motor units, represented by the number of turns, actually decreased.
We considered several scenarios that might explain these results. The most obvious is related to the difference in proportions of African-Americans and Caucasians in our two groups. Our nulligravid group had relatively more African-Americans, who had higher mean EMG signal amplitude than their Caucasian counterparts. This could result from 1) larger more complex motor units comprised of larger muscle fibers and/or more muscle fibers, or 2) earlier recruitment of more motor units during the contraction effort. We favor the former explanation, since the number of turns/second was not significantly different between African-Americans and Caucasians and that parameter should have increased if subjects were recruiting more muscle fibers for the same effort. Howard et al found significantly higher urethral pressure and overall urethral volume in African-American women compared to Caucasians [12], findings that would also support a larger, stronger urethral muscle. We do not attribute all of our findings to racial differences, however, because subject race was significantly associated with only one of our three EMG parameters.
A failure on the part of the electromyographer to properly position the needle close to motor units can decrease both the number of turns and the amplitude. This seems unlikely because the same experienced examiner performed all the EMGs and only recorded signals with a crisp sound and a sharp rise from electrical baseline. Both nulligravid and primigravid groups were recruited during the same time period using the same methods. Another possible scenario involves the electrical masking of the signal of small motor units by larger ones; this could have occurred if the primigravid women were recruiting larger, higher threshold motor units to accomplish a similar volitional effort. The larger motor unit action potentials of big motor units can artifactually decrease the number of turns. In this scenario, however, the EMG signal amplitude should also increase, but we observed an actual decrease in amplitude, even antepartum.
Edema in the extracellular space around muscle fibers can increase the volume of muscle and create a similar effect as poor needle position. This is an attractive explanation for our findings in the primigravid women, as we would expect some tissue edema in such a physiologic state. However, it does not fit with the persistence of our post partum changes after pregnancy-related tissue edema would have cleared. Were edema to have been the major factor underlying our interesting finding in the antepartum subjects, we should have seen a post partum increase in both turns and amplitude at 6 weeks, and we did not. A combination of antepartum tissue edema and post partum neurogenic injury should have resulted in a post partum increase in amplitude relative to nulligravidas. Unfortunately, fully delineating a combination mechanism of changes to urethral neuromuscular function is beyond the scope of this initial study, and possibly the subject of future research.
We chose interference pattern analysis for this study because it enables electromyographic assessment of a muscle without standardization of force or the subject’s volitional exertion effort. The potential benefits of this for assessment of the pelvic floor muscles are readily apparent; though some researchers have used mechanical devices to measure force generated by pelvic floor contraction[13], which are not universally available. We recognize that we cannot be certain that each subject cooperated fully with the request for maximal effort, but our protocol included practicing the maneuver and we have no reason to believe otherwise. Data not presented in this report show that the magnitude of difference between effort levels for our EMG parameters was appropriate, and similar between groups. Interference pattern analysis has the additional benefit of being relatively less labor intensive than individual motor unit potential analysis, and yields data that describes motor unit function over the whole range of contraction effort and which is readily amenable to statistical analysis.
The limitations of this study are apparent. We did not expect such a dramatic difference in sphincter function in the antepartum period, therefore we did not design our study to isolate discrete effects of pregnancy versus those of the obstetrical delivery itself, and have no baseline measures of our primigravida group before the index pregnancy. This is particularly important given our finding that African-American race was associated with higher mean amplitude on urethral EMG, though the effect of race does not explain all of our findings. We were disappointed in the final number of subjects from the original cohort of 96 primigravidas who were available for inclusion in this analysis. We have no reason to believe that the 16 subjects who withdrew at the outset of the study (the largest attrition group) were substantially different than the rest of the cohort, but those subjects were effectively lost to long term follow up. Our decision to limit our primigravida group to women undergoing vaginal delivery contributed to the decrease in sample size, but we intentionally excluded women undergoing cesarean section in order to provide some standardization of the obstetrical experience and to simplify our analysis. Our 13 primiparous subjects in whom motor unit activity could not be identified at one of the two post partum visits had to be excluded from this study because of its longitudinal nature, but it is reasonable to assume that those subjects would also have had abnormal urethral EMG. Our findings need to be validated in a larger cohort that includes a comparison group of women having cesarean delivery without labor and testing early in pregnancy, and which includes more traditional measures of urinary function such as urodynamics for comparison to EMG findings.
This study documents a significant decrease in neuromuscular function of the urethral striated sphincter in primigravid women compared to nulligravid controls. The lack of significant change in urethral EMG acutely at 6 weeks or chronically at 6 months post partum suggests that effects of the pregnancy itself on urethral function may be significant, with possible future implications for urinary continence for the woman regardless of events of labor or delivery.
Acknowledgments
Supported by NIH grant HD38661-05 and the Charles Hammond Research Fund
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the national meeting of the Society of Gynecologic Surgeons, New Orleans, LA, April 2009.
REFERENCES
- 1.Deindl FM, Vodusek DB, Hesse U, Schussler B A kinesiological EMG study. Pelvic floor activity patterns: comparison of nulliparous continent and parous urinary stress incontinent women. Br J Urol. 1994;73:413–417. doi: 10.1111/j.1464-410x.1994.tb07606.x. [DOI] [PubMed] [Google Scholar]
- 2.Snooks SJ, Badenoch DF, Tiptaft RC, Swash M An electrophysiological study. Perineal nerve damage in genuine stress urinary incontinence. Br J Urol. 1985;57:422–426. doi: 10.1111/j.1464-410x.1985.tb06302.x. [DOI] [PubMed] [Google Scholar]
- 3.Damaser MS, Whitbeck C, Chichester P, Levin RM. Effect of vaginal distension on blood flow and hypoxia of urogenital organs of the female rat. J Appl Physiol. 2005;98:1884–1890. doi: 10.1152/japplphysiol.01071.2004. [DOI] [PubMed] [Google Scholar]
- 4.Jiang HH, Pan HQ, Gustilo-Ashby MA, et al. Dual Simulated Childbirth Injuries Result in Slowed Recovery of Pudendal Nerve and Urethral Function. Neurourol Urodyn. 2008 doi: 10.1002/nau.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borello-France D, Burgio KL, Richter HE, et al. Fecal and urinary incontinence in primiparous women. Obstet Gynecol. 2006;108:863–872. doi: 10.1097/01.AOG.0000232504.32589.3b. [DOI] [PubMed] [Google Scholar]
- 6.Weidner AC, Jamison MG, Branham V, South MM, Borawski KM, Romero AA. Neuropathic injury to the levator ani occurs in 1 in 4 primiparous women. Am J Obstet Gynecol. 2006;195:1851–1856. doi: 10.1016/j.ajog.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 7.South MT, Stinnett SS, Sanders DB, Weidner AC. Levator ani denervation and reinnervation six months after childbirth. Am J Obstet Gynecol. 2009;200(5):519e1–519e7. doi: 10.1016/j.ajog.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 8.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 9.Brink CA, Sampselle CM, Wells TJ, Diokno AC, Gillis GL. A digital test for pelvic muscle strength in older women with urinary incontinence. Nurs Res. 1989;38:196–199. [PubMed] [Google Scholar]
- 10.Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185:1388–1395. doi: 10.1067/mob.2001.118659. [DOI] [PubMed] [Google Scholar]
- 11.Hansen SL, Clark SL, Foster JC. Active pushing versus passive fetal descent in the second stage of labor: a randomized controlled trial. Obstet Gynecol. 2002;99:29–34. doi: 10.1016/s0029-7844(01)01642-8. [DOI] [PubMed] [Google Scholar]
- 12.Howard D, DeLancey JOL, Tunn R, Ashton-Miller JA. Racial differences in the structure and function of the stress urinary continence mechanism. Obstet Gynecol. 2000;95(5):713–717. doi: 10.1016/s0029-7844(00)00786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampselle CM, Miller JM, Mims BL, Delancey JO, Ashton-Miller JA, Antonakos CL. Effect of pelvic muscle exercise on transient incontinence during pregnancy and after birth. Obstet Gynecol. 1998;91:406–412. doi: 10.1016/s0029-7844(97)00672-8. [DOI] [PubMed] [Google Scholar]