Abstract
Background
Alterations in the inhibitory circuitry of the dorsolateral prefrontal cortex (DLPFC) in schizophrenia include reduced expression of the messenger RNA (mRNA) for somatostatin (SST), a neuropeptide present in a subpopulation of γ-aminobutyric acid (GABA) neurons. Neuropeptide Y (NPY) is expressed in a subset of SST-containing interneurons and lower levels of NPY mRNA have also been reported in schizophrenia spectrum disorders. However, whether the alterations in these two transcripts identify the same, particularly vulnerable, subset of GABA neurons has not been examined.
Methods
We used in situ hybridization to quantify NPY mRNA levels in DLPFC gray and white matter from 23 pairs of subjects with schizophrenia or schizoaffective disorder and matched normal control subjects; results were compared to those from a previous study of SST mRNA expression in the same subjects.
Results
In contrast to SST mRNA, NPY mRNA levels were not significantly lower in the gray matter of subjects with schizophrenia or schizoaffective disorder. However, NPY, but not SST, mRNA expression was significantly lower in the superficial white matter of subjects with schizoaffective disorder.
Conclusion
These findings suggest that the alterations in SST-containing interneurons in schizophrenia and schizoaffective disorder are selective for the subset that do not express NPY mRNA, and that lower NPY mRNA expression in the superficial white matter may distinguish subjects with schizoaffective disorder from those with schizophrenia.
Keywords: GABA, interneurons, mood disorder, somatostatin, suicide
I. Introduction
Alterations in the inhibitory circuitry of the dorsolateral prefrontal cortex (DLPFC) appear to be a common feature of schizophrenia (Akbarian and Huang 2006; Torrey et al. 2005). For example, lower levels of the mRNA that encodes for the 67 kDa isoform of glutamic acid decarboxylase (GAD67), an enzyme for GABA synthesis, have been consistently found in the DLPFC of individuals with schizophrenia (Akbarian et al. 1995; Guidotti et al. 2000; Hashimoto et al. 2005; Mirnics et al. 2000; Volk et al. 2000; Straub et al. 2007); this decrease is due to a marked reduction in GAD67 mRNA expression in a minority (~25-35%) of GABA neurons, with apparently normal levels of expression in the remaining neurons (Volk et al. 2000; Akbarian et al. 1995). The affected neurons include the GABA neurons that express the calcium-binding protein, parvalbumin (PV) (Hashimoto et al. 2003), or the neuropeptide, somatostatin (SST) (Morris et al. 2008), whereas the ~50% of GABA neurons that express calretinin (CR) appear to be unaffected (Hashimoto et al. 2003; Sakai et al. 2008; Woo et al. 1998).
In the frontal cortex of rodents, ~40% of SST neurons also express NPY and most NPY neurons contain SST (Hendry et al. 1984;Kubota et al. 1994). Both SST (Morris et al. 2008) and NPY (Caberlotto et al. 2000) mRNAs are expressed by neurons in the gray and white matter of the human DLPFC. Some studies have found lower levels of NPY mRNA (Hashimoto et al. 2008; Mellios et al. 2008) or protein (Gabriel et al. 1996) in homogenates containing both gray and white matter from the DLPFC of subjects with schizophrenia or schizoaffective disorder, suggesting that the NPY-containing subclass of SST neurons is preferentially affected in these illnesses (Morris et al. 2008). However, we found that lower SST mRNA expression in schizophrenia was restricted to cortical layers 2-superficial layer 6 (Morris et al. 2008), whereas most NPY mRNA expression in the human cortex is located in deep layer 6 and the superficial white matter (Caberlotto et al. 2000). Consistent with these observations, the co-localization of SST and NPY mRNAs in the rodent cerebral cortex is most prominent in layer 6 and is uncommon in the superficial layers (Wang et al. 2004).
Consequently, in order to determine if the levels of NPY and SST mRNAs are altered in the same or different populations of DLPFC neurons, we used in situ hybridization and autoradiographic analyses to quantify NPY mRNA expression in the gray and white matter compartments of DLPFC area 9 from 23 pairs of subjects with schizophrenia or schizoaffective disorder and matched normal control subjects in which we had previously measured SST mRNA expression using an identical approach (Morris et al. 2008).
2. Materials and Methods
2.1. Human subjects
Brain tissue specimens were obtained from the Allegheny County Medical Examiner’s Office at the time of autopsy with the consent of the next-of-kin. Subjects with schizophrenia (n = 15) or schizoaffective disorder (n = 8) were each matched with one normal control subject for sex, and as closely as possible for age and postmortem interval (PMI) (Table 1). Subject groups did not differ in mean age, PMI, brain pH, RNA integrity number (RIN), or tissue storage time at -80° C (for all t < 1.61; p > 0.11). Additional demographic and clinical details are provided in our previous study of SST mRNA expression in this subject cohort (Morris et al. 2008). An independent committee of experienced research clinicians made consensus DSMIV (Diagnosis and Statistical Manual of Mental Disorders (American Psychiatric Association 1994)) diagnoses based on structured interviews conducted with family members of the deceased and a review of medical records. All procedures were approved by the University of Pittsburgh’s Institutional Review Board for Biomedical Research and Committee for Oversight of Research Involving the Dead.
Table 1.
Characteristics of subjects
| Pair | Control subjects |
Schizophrenia subjects |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Sex/race | Age | PMI a | RIN | Storage time b | Cause of death c | Case | DSM IV diagnosis | Sex/race | Age | PMI a | RIN | Storage time b | Cause of death c | Antipsychotic medication d | |
| 1 | 592 | M/B | 41 | 22.1 | 9.0 | 100 | ASCVD | 533 | Chronic undifferentiated schizophrenia | M/W | 40 | 29.1 | 8.4 | 110 | Accidental asphyxiation | Typical |
| 2 | 567 | F/W | 46 | 15.0 | 8.9 | 104 | Mitral valve prolapse | 537 | Schizoaffective disorder | F/W | 37 | 14.5 | 8.6 | 109 | Suicide by hanging | None |
| 3 | 516 | M/B | 20 | 14.0 | 8.4 | 111 | Homicide by gun shot | 547 | Schizoaffective disorder | M/B | 27 | 16.5 | 7.4 | 107 | Heat stroke | Typical |
| 4 | 630 | M/W | 65 | 21.2 | 9.0 | 94 | ASCVD | 566 | Chronic undifferentiated schizophrenia e | M/W | 63 | 18.3 | 8.0 | 104 | ASCVD | Atypical |
| 5 | 604 | M/W | 39 | 19.3 | 8.6 | 97 | Hypoplastic coronary artery | 581 | Chronic paranoid schizophreniaf,g | M/W | 46 | 28.1 | 7.9 | 102 | Accidental combined drug overdose | Typical |
| 6 | 546 | F/W | 37 | 23.5 | 8.6 | 108 | ASCVD | 587 | Chronic undifferentiated schizophreniae | F/B | 38 | 17.8 | 9.0 | 101 | Myocardial hypertrophy | Both |
| 7 | 551 | M/W | 61 | 16.4 | 8.3 | 107 | Cardiac tamponade | 625 | Chronic disorganized schizophrenia h | M/B | 49 | 23.5 | 7.6 | 95 | ASCVD | Typical |
| 8 | 685 | M/W | 56 | 14.5 | 8.1 | 87 | Hypoplastic coronary artery | 622 | Chronic undifferentiated schizophrenia | M/W | 58 | 18.9 | 7.4 | 95 | Right MCA infarction | None |
| 9 | 681 | M/W | 51 | 11.6 | 8.9 | 87 | Hypertrophic cardiomyopathy | 640 | Chronic paranoid schizophrenia | M/W | 49 | 5.2 | 8.4 | 93 | Pulmonary embolism | Atypical |
| 10 | 806 | M/W | 57 | 24.0 | 7.8 | 66 | Pulmonary thromboembolism | 665 | Chronic paranoid schizophrenia f | M/B | 59 | 28.1 | 9.2 | 90 | Intestinal hemorrhage | Typical |
| 11 | 822 | M/B | 28 | 25.3 | 8.5 | 63 | ASCVD | 787 | Schizoaffective disorder i | M/B | 27 | 19.2 | 8.4 | 70 | Suicide by gun shot | Typical |
| 12 | 727 | M/B | 19 | 7.0 | 9.2 | 80 | Trauma | 829 | Schizoaffective disorderf,j | M/W | 25 | 5.0 | 9.3 | 61 | Suicide by drug overdose | None |
| 13 | 871 | M/W | 28 | 16.5 | 8.5 | 53 | Trauma | 878 | Disorganized schizophrenia f | M/W | 33 | 10.8 | 8.9 | 52 | Myocardial fibrosis | Atypical |
| 14 | 575 | F/B | 55 | 11.3 | 9.6 | 102 | ASCVD | 517 | Chronic disorganized schizophrenia f | F/W | 48 | 3.7 | 9.3 | 111 | Intracerebral hemorrhage | Atypical |
| 15 | 700 | M/W | 42 | 26.1 | 8.7 | 84 | ASCVD | 539 | Schizoaffective disorder k | M/W | 50 | 40.5 | 8.1 | 109 | Suicide by combined drug overdose | Atypical |
| 16 | 988 | M/W | 82 | 22.5 | 8.4 | 31 | Trauma | 621 | Chronic undifferentiated schizophrenia | M/W | 83 | 16.0 | 8.7 | 95 | Accidental asphyxiation | None |
| 17 | 686 | F/W | 52 | 22.6 | 8.5 | 87 | ASCVD | 656 | Schizoaffective disorder f | F/B | 47 | 20.1 | 9.2 | 91 | Suicide by gun shot | Atypical |
| 18 | 634 | M/W | 52 | 16.2 | 8.5 | 93 | ASCVD | 722 | Undifferentiated schizophreniaj,l | M/B | 45 | 9.1 | 9.2 | 81 | Upper GI bleeding | Typical |
| 19 | 852 | M/W | 54 | 8.0 | 9.1 | 56 | Cardiac tamponade | 781 | Schizoaffective disorder k | M/B | 52 | 8.0 | 7.7 | 71 | Peritonitis | Typical |
| 20 | 987 m | F/W | 65 | 21.5 | 9.1 | 31 | ASCVD | 802 | Schizoaffective disorderf,l | F/W | 63 | 29.0 | 9.2 | 67 | Right ventricular dysplasia | Both |
| 21 | 818 | F/W | 67 | 24.0 | 8.4 | 64 | Anaphylactic reaction | 917 | Chronic undifferentiated schizophrenia | F/W | 71 | 23.8 | 7.0 | 44 | ASCVD | Typical |
| 22 | 857 | M/W | 48 | 16.6 | 8.9 | 55 | ASCVD | 930 | Disorganized schizophreniaj,k | M/W | 47 | 15.3 | 8.2 | 41 | ASCVD | Typical |
| 23 | 739 | M/W | 40 | 15.8 | 8.4 | 79 | ASCVD | 933 | Disorganized schizophrenia | M/W | 44 | 8.3 | 8.1 | 40 | Myocarditis | Atypical |
| Mean | 48.0 | 18.0 | 8.7 | 80.0 | 47.9 | 17.8 | 8.4 | 84.3 | ||||||||
| SD | 15.5 | 5.5 | 0.4 | 23.6 | 14.1 | 9.3 | 0.7 | 23.7 | ||||||||
PMI indicates postmortem interval in hours.
Storage time (months) at −80°C.
ASCVD indicates arteriosclerotic cardiovascular disease.
Indicates prescribed antipsychotic medications at time of death.
Alcohol abuse, in remission at time of death.
Alcohol dependence, current at time of death.
Other substance abuse, current at time of death.
Alcohol abuse, current at time of death.
Other substance dependence, current at time of death.
Other substance abuse, in remission at time of death.
Alcohol dependence, in remission at time of death.
Other substance dependence, in remission at time of death.
History of post-traumatic stress disorder, in remission 39 years at time of death.
2.2. Tissue preparation
For each brain specimen, coronal blocks from the right frontal cortex were immediately frozen and stored at -80°C. Serial sections (20μm) containing the superior frontal gyrus were cut on a cryostat, thaw-mounted onto glass slides and stored at -80°C until processed. Adjacent sections were collected into tubes containing Trizol (Invitrogen Corp, Carlsbad, CA) in order to obtain RNA for RIN measures using the Agilent Bioanalyzer 2100 (Agilent Technologies, Inc., Santa Clara, CA) according to the manufacturer’s protocol as previously described (Hashimoto et al. 2008). The location of DLPFC area 9 was identified by cytoarchitectonic criteria in Nissl-stained sections as previously described (Volk et al. 2000). Three sections per subject, at intervals of approximately 300 μm, were matched for anterior-posterior location within subject pairs, and used to assess NPY mRNA expression.
2.3. In situ hybridization
Templates for the synthesis of the antisense and sense riboprobes for human NPY mRNA were first generated by polymerase chain reaction (PCR). The specific primers amplified a 430 base pair fragment of human NPY. These fragments corresponded to bases 34-463 of the human (GenBank NM_000905) NPY gene. Nucleotide sequencing confirmed 100% homology of the amplified fragment to the previously reported sequence. The fragment was then subcloned into a plasmid (pSTBlue-1, Novagen, Madison, WI). The antisense and sense riboprobes were transcribed in the presence of 35S-CTP (Amersham Biosciences, Piscataway, NJ) using T7 and SP6 RNA polymerase, respectively. DNase I was used to digest the DNA template. The riboprobes were purified using RNeasy mini spin columns (Qiagen, Valencia, CA).
For each matched subject pair, one section from each pair was processed side-by-side in three separate runs. Prior to the hybridization reaction, tissue sections were fixed with 4% paraformaldehyde in PBS solution, acetylated with 0.25% acetic anhydrate in 0.1 M triethanolamine/0.9% NaCl for 10 minutes, dehydrated with a graded alcohol series, and then defatted in chloroform for 10 minutes. The sections were then hybridized with 35S-labeled riboprobes (2.0 × 106 cpm/μl) in hybridization buffer at 56°C for 16 hours. The hybridization buffer contained 50% formamide, 0.75 M NaCl, 20 mM 1,4-piperazine diethane sulfonic acid, pH 6.8, 10 mM EDTA, 10% dextran sulfate, 5X Denhardt’s solution (0.2 mg/ml Ficoll, 0.2 mg/ml polyvinylpyrrolidone, 0.2 mg/ml BSA), 50 mM dithiothreitol, 0.2% SDS, and 100 g/ml yeast tRNA. Following the hybridization reaction, sections were washed in a solution of 0.3 M NaCl, 20 mM Tris-HCl, pH 8.0, 1 mM EDTA, pH 8.0, and 50% formamide at 63°C, treated wi th RNase A (20 μg/ml) at 37°C, washed in 0.1 X SSC (1.5 mM NaCl, 150 M sodium citrate) at 67°C, dehydrated through a graded ethanol series, and air dried. Sections from both subjects in a pair were exposed on the same BioMax MR film (Kodak, Rochester, NY) for 4 days, and then coated with NTB2 emulsion (Kodak) diluted 2:1 with water. The emulsion was exposed for 9 days at a constant temperature of 4°C. The sl ides were developed with D-19 (Kodak) and counterstained with Cresyl-violet.
2.4. Quantification of mRNA expression levels
Each section was randomly coded, so that subject number and diagnosis were unknown to the single rater (RS). Autoradiographic films were trans-illuminated and captured on video camera under controlled conditions, digitized, and analyzed with a Microcomputer Imaging Device (MCID; Imaging Research Inc., London, Ontario, Canada) as previously described (Eggan et al. 2008). Digitized images of adjacent Nissl-stained sections were superimposed onto autoradiographic images to draw contours of the full cortical thickness of the locations in area 9 that were cut perpendicular to the pial surface. Optical density measures within each sampled area were calibrated to radioactive Carbon-14 standards (ARC Inc., St. Louis, MO), exposed on the same autoradiographic film, and expressed as nanocuries per gram (nCi/g) of tissue. The mean (SD) total areas of gray matter sampled for each control and schizophrenia subject were 114 (55) mm2 and 115 (48) mm2, respectively. Optical density measures in the superficial white matter were determined in a zone extending 800 μm below, and with a contour that followed, the layer 6/white matter border of the sampled gray matter zones. The mean (SD) total areas of superficial white matter sampled were 10.7 (5.7) mm2 for control subjects and 12.9 (5.5) mm2 for subjects with schizophrenia. Total white matter was sampled by outlining the gray matter/white matter border and including all white matter on the section. The mean total areas (SD) of white matter sampled were 95 (39) mm2 for control subjects and 105 (49) mm2 for schizophrenia subjects.
2.5. Statistical analyses
Two analyses of covariance (ANCOVA) models were used to test differences in NPY mRNA expression between control subjects and subjects with schizophrenia. The data were averaged across the three sections per subject before statistical analyses. The first ANCOVA model used diagnostic group as the main effect, pair as a blocking effect, and storage time, brain pH, and RIN as covariates. Brain pH and RIN were included as covariates because they may influence mRNA quantity and integrity (Harrison et al. 1995; Stan et al. 2006). The pair effect reflects the matching of individual subject pairs for sex, age and PMI. Subject pairing may be considered an attempt to balance the two diagnostic groups with regard to the experimental factors instead of a true statistical paired design. Thus, to validate the first model, a second ANCOVA model was performed with a main effect of diagnostic group and covariates of sex, age, PMI, brain pH, RIN, and storage time. Storage time as a covariate was not significant in either model and was excluded in the reported analyses. Both models produced comparable results for diagnostic group effect; because age showed trend level effects, the results of the second model are reported.
The potential influence of history of substance abuse/dependence, diagnosis of schizoaffective disorder, medications at time of death, or death by suicide on the within-pair percentage of differences in mRNA expression was assessed by two-sample t-test analyses. Correlations were assessed by Pearson’s correlation analyses.
3. Results
3.1. Specificity of NPY riboprobe
Several lines of evidence confirm the specificity of the riboprobe for NPY mRNA used in this study. First, NPY mRNA expression had the distinctive distribution previously reported for NPY-containing interneurons in the human frontal cortex (Caberlotto et al. 2000). Specifically, NPY mRNA levels were lowest in layer 1, moderate in layer 2, low in layers 3-5, and high in layer 6 and the white matter (Figure 1A). Second, the presence of intensely NPY mRNA-positive neurons in the superficial and deep white matter (Figure 1A) is consistent with previous descriptions of NPY mRNA-positive and immunopositive cell bodies in human frontal cortex (Caberlotto et al. 2000). Third, emulsion-coated sections demonstrated very dense silver grain clusters over large, faintly Nissl-stained neuronal nuclei, whereas the smaller and more intensely stained glial nuclei lacked silver grains (Figure 2). Fourth, sense riboprobes for NPY mRNA showed an absence of signal above background (data not shown).
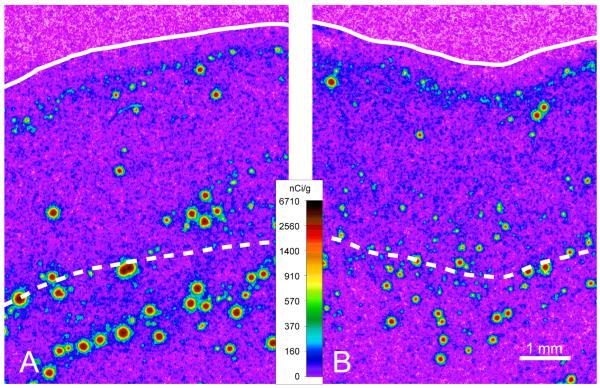
Figure 1.
Representative film autoradiograms from a normal control subject (A) and matched subject with schizoaffective disorder (B). The densities of hybridization signals are presented in a pseudocolor manner according to the calibration bar. The solid and dashed lines indicate the pial surface and the gray/white matter border, respectively. The calibration bar applies to both panels. The expression of NPY mRNA in the superficial white matter appears to be lower in the subject with schizoaffective disorder (B) relative to the matched control subject (A).
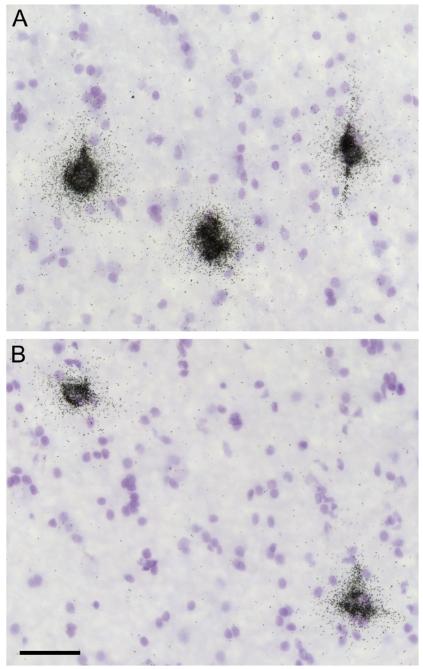
Figure 2.
Representative photomicrographs of silver grains clusters above neuronal nuclei indicating cellular NPY mRNA expression in the superficial white matter of a control subject (A) and the matched subject with schizoaffective disorder (B). Scale bar = 50 μm and applies to both A and B.
3.2. Expression of NPY in area 9 gray and white matter
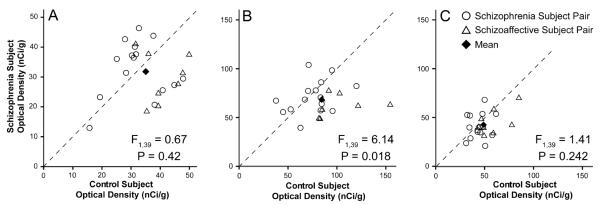
The mean (±SD) optical density (OD) of NPY mRNA expression in the gray matter did not significantly (F(1,39) = 0.67; p = 0.42) differ between the schizophrenia (31.8 ± 9.0 nCi/g) and control (35.0 ± 8.9 nCi/g) groups (Figure 3A). Similarly, the mean OD in the total white matter was not significantly different (F(1,39) = 1.41; p = 0.242) between the schizophrenia (43.1 ± 13.0 nCi/g) and control (49.3 ± 13.3 nCi/g) groups (Figure 3C). In contrast, mean OD in the superficial white matter was significantly reduced (F(1,39) = 6.14; p = 0.018) by 19% in the schizophrenia groups (68.6 ± 15.4 nCi/g) relative to the control groups (84.3 ± 25.3 nCi/g) (Figure 3B).
Figure 3.
NPY mRNA expression levels assessed by autoradiographic film optical density (OD) measures in DLPFC area 9 of matched subject pairs for the gray matter (A), superficial white matter (B), and total white matter (C). Values below the dashed unity line indicate a lower level of NPY mRNA expression in the subjects with schizophrenia or schizoaffective disorder relative to their matched control subject. The key in (C) applies to all graphs. ANCOVAs (see statistical methods) were performed on all 23 pairs and the values are given in the bottom right of each respective graph.
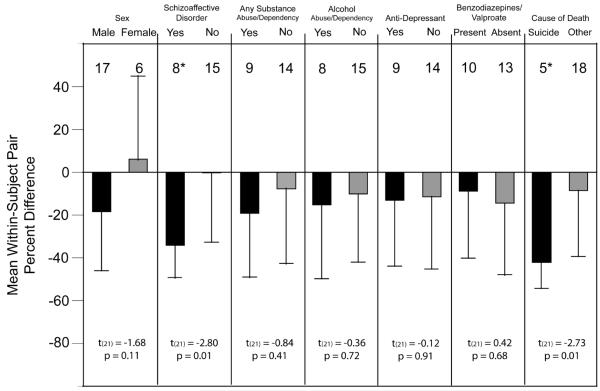
The effect of age on NPY mRNA levels in the superficial white matter showed a trend (F(1,39) = 3.42; p = 0.072) level of significance. Further analyses revealed that OD measures from superficial white matter contours in the schizophrenia group were not significantly correlated with age (r = -0.12; p = 0.58); in contrast, NPY mRNA expression did significantly decline with age in control subjects (r = -0.51; p = 0.013). The within-subject pair percent differences in NPY mRNA expression in the superficial white matter did not differ as a function of sex, history of substance abuse/dependence, or the use of antidepressant medications or benzodiazepines/valproate at time of death (all t(21) < -1.68, all p > 0.11) (Figure 4). However, the mean within-subject pair percent difference in NPY mRNA expression in the superficial white matter was significantly larger for subject pairs with a diagnosis of schizoaffective disorder or death by suicide (t(21) = -2.80, p = 0.01 and t(21) = -2.73, p = 0.01, respectively) (Figure 4).
Figure 4.
The effects of potential confounding factors on the expression differences of NPY mRNA in the superficial white matter. Bars represent the mean (SD) percent differences from control subjects for NPY mRNA within subject pairs, and the numbers above each bar indicate the number of subject pairs. Two-sample t-tests were performed on each potential confound and values are listed below the respective graphs. Neither sex, substance abuse/dependence at time of death, use of antidepressant medications at time of death, or use of benzodiazepines/valproate at time of death affected the expression differences. However, within-subject pair differences in NPY mRNA expression were significantly greater in subjects with a diagnosis of schizoaffective disorder or death by suicide. * Note, five subjects twho died by suicide had a diagnosis of schizoaffective disorder
Consistent with the absence of a group difference in SST mRNA levels in the superficial white matter (Morris et al. 2008), the within-pair percent differences in NPY mRNA expression in the superficial white matter were not significantly correlated (r = 0.006, p = 0.98) with those for SST mRNA in the same subjects.
4. Discussion
The expression of NPY mRNA in the DLPFC of normal control subjects was lowest in layer 1, moderate in layer 2, low in layers 3-5, and high in layer 6 and the white matter; this pattern was distinctively different from the high levels of SST mRNA in layers 2, superficial 3 and 5 in these subjects (Morris et al. 2008). In addition, in contrast to SST mRNA, the expression of NPY mRNA in gray matter and total white matter of DLPFC area 9 did not differ between the schizophrenia and normal control groups. However, NPY (but not SST) mRNA levels were significantly lower in the superficial white matter of the schizophrenia group, a finding that was due to markedly lower levels in subjects with a diagnosis of schizoaffective disorder or death by suicide. Because suicide was not associated with altered NPY mRNA or protein levels in previous studies of the frontal cortex of subjects with psychiatric disorders (Caberlotto and Hurd 1999; Klempan et al. 2009), it seems likely that a diagnosis of schizoaffective disorder is the primary cause of the lower NPY expression. Finally, the NPY mRNA expression differences in the superficial white matter were not correlated with those for SST mRNA in the same subjects. Together, these findings indicate that alterations in NPY and SST mRNA expression in schizophrenia occur 1) in separate populations of neurons, 2) in distinct compartments of the DLPFC, and 3) in different subsets of subjects.
In order to determine if the effect of a schizoaffective disorder diagnosis on superficial white matter NPY mRNA expression was robust, we performed ANCOVAs on the schizophrenia (n = 15 pairs) and schizoaffective disorder (n = 8 pairs) subject pairs separately. Consistent with our interpretation, the mean (±SD) OD of superficial white matter NPY mRNA expression in subjects with schizoaffective disorder (63.8 ± 10.7 nCi/g) was significantly (F1,9 = 8.56; p = 0.017) reduced by 36.7% compared to matched control subjects (100.7 ± 25.6 nCi/g). In contrast, the mean superficial white matter NPY mRNA expression in subjects with “pure” schizophrenia (71.2 ± 17.2 nCi/g) did not significantly (F1,23 = 1.25; p = 0.26) differ from their matched control subjects (75.6 ± 20.9 nCi/g).
The finding of lower NPY mRNA levels does not appear to be attributable to antipsychotic medications since both subjects with schizoaffective disorder (537 and 829) who were off these medications at time of death had lower levels of NPY mRNA expression in the superficial white matter than their matched control subjects. Consistent with this interpretation, chronic administration of either olanzapine, clozapine, or haloperidol to rats was not associated with reduced NPY mRNA expression in the cingulate cortex (Huang et al. 2006).
Although some studies have reported lower levels of NPY protein and mRNA in the frontal cortex of subjects with schizophrenia, these studies evaluated tissue homogenates that included both gray and white matter (Gabriel et al. 1996; Hashimoto et al. 2008; Mellios et al. 2008) and/or included subjects with schizoaffective disorder (Hashimoto et al. 2008). Our results clarify these findings by demonstrating that NPY mRNA expression is not altered in either the gray or white matter compartments in the DLPFC of subjects with “pure” schizophrenia, consistent with another in situ hybridization study of prefrontal gray matter NPY expression (Caberlotto and Hurd 1999), but is reduced in the superficial white matter of subjects with schizoaffective disorder. Interestingly, NPY mRNA expression was previously reported to be lower in the prefrontal cortex of subjects with bipolar disorder, a mood disorder frequently accompanied by psychosis, but not in subjects with schizophrenia or major depressive disorder (Caberlotto and Hurd 1999). These findings, in concert with the results of the present study, suggest that NPY mRNA neurons in the superficial white matter may be preferentially vulnerable in individuals with severe disruptions in both reality testing and mood regulation.
The affected NPY-containing neurons are likely the remnants of the earliest born neurons in the neocortex. During development of the cerebral cortex, the earliest born cells form the preplate which is subsequently split into the marginal zone (adult layer 1) and the subplate (adult deep layer 6 and superficial white matter) by later born neurons which migrate to become the cortical plate (adult layers 2 - superficial 6) (Kostovic and Rakic 1980). Interestingly, NPY protein is present very early in human development in the preplate neurons underlying DLPFC areas 9 and 46; and in adult human prefrontal cortex, NPY protein is most strongly expressed by neurons located in the residual preplate (layer 1, deep layer 6, and the underlying white matter) (Delalle et al. 1997; Uylings and Delalle 1997). Thus, the vulnerable neurons in subjects with schizoaffective disorder, that is those with concurrent psychotic symptoms and a mood disorder, appear to be residual preplate neurons. The relatively low expression of NPY mRNA in layer 1 likely precluded the ability to detect any differences between subjects groups in the residual preplate neurons present in this location. However, other markers of layer 1 interneurons that are co-localized with NPY do suggest that these neurons are affected in individuals with both psychosis and mood alterations. For example, most NPY-containing neurons in layer 1 also express reelin mRNA, at least in the adult mouse brain (Alcantara et al. 1998), and both reelin protein and mRNA levels are lower in subjects with bipolar disorder with a history of psychosis (Guidotti et al. 2000), although the laminar specificity of this finding was not examined.
In contrast, the deficits in SST mRNA expression in the DLPFC of subjects with schizophrenia were restricted to cortical layers that arise from the cortical plate, and were not present in residual preplate neurons (Morris et al. 2008). Together, these findings raise the hypothesis that the phenotypic differences between “pure” schizophrenia and schizoaffective disorder may reflect differences in the types of, or timing of exposure to, environmental factors that are associated with increased risk for a psychotic illness (Lewis and Levitt 2002). That is, although the liability to schizophrenia and schizoaffective disorder (and psychotic bipolar disorder) may arise from shared genetic factors (Potash 2006), the resulting clinical phenotype may reflect the impact of adverse environmental events during development that preferentially affect NPY-containing preplate neurons or SST-containing cortical plate neurons. Testing of this hypothesis might include the determination of whether the nature of the reported altered density [increased in some studies and decreased in others (Akbarian et al. 1996; Eastwood and Harrison 2005; Eastwood and Harrison 2003)], of neurons in the superficial white matter of a subset of subjects with schizophrenia, is associated with a particular clustering of clinical features.
Interestingly, dysfunctional developmental regulation of SST/NPY- and SST-containing interneurons might be another factor leading to the phenotypic differences between “pure” schizophrenia and schizoaffective disorder. Cell type-specific transcription factors seem to play an integral role in interneuron development in terms of both differentiation and migratory properties (for review see Wonders and Anderson 2006). For example, experiments in mice (Cobos et al. 2005) have demonstrated that SST-expressing interneurons maintain the expression of the transcription factor, Lhx6, whereas interneurons that express NPY down-regulate Lhx6 expression during development. Therefore, disturbances of Lhx6 regulation during development might contribute to the emergence of cell type-specific abnormalities and particular clinical phenotypes of schizophrenia.
Acknowledgements
The authors thank the members of the Clinical Services and Diagnostics Core of the Conte Center for the Neuroscience of Mental Disorders (MH084053) for their assistance in diagnostic assessments.
Role of Funding Source
Support for these studies was provided by National Institute of Health grants MH043784 and MH084053 and by a Scottish Rite Dissertation Research Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
David A. Lewis currently receives investigator-initiated research support from the BMS Foundation, Bristol-Myers Squibb, Curridium Ltd and Pfizer and in 2007-2009 served as a consultant in the areas of target identification and validation and new compound development to AstraZeneca, Bristol-Myers Squibb, Hoffman-Roche, Lilly, Merck and Neurogen. Harvey Morris and Rachelle Stopczynski report no conflicts of interest.
References
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr., Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE, Jr., Jones EG. Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry. 1996;53:425–436. doi: 10.1001/archpsyc.1996.01830050061010. [DOI] [PubMed] [Google Scholar]
- Alcantara S, Ruiz M, D’Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . DSM-IV. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition American Psychiatric Association; Washington, D.C.: 1994. p. 4. [Google Scholar]
- Caberlotto L, Fuxe K, Hurd YL. Characterization of NPY mRNA-expressing cells in the human brain: co-localization with Y2 but not Y1 mRNA in the cerebral cortex, hippocampus, amygdala, and striatum. J Chem Neuroanat. 2000;20:327–337. doi: 10.1016/s0891-0618(00)00107-1. [DOI] [PubMed] [Google Scholar]
- Caberlotto L, Hurd YL. Reduced neuropeptide Y mRNA expression in the prefrontal cortex of subjects with bipolar disorder. Neuroreport. 1999;10:1747–1750. doi: 10.1097/00001756-199906030-00022. [DOI] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking DIx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Delalle I, Evers P, Kostovic I, Uylings HB. Laminar distribution of neuropeptide Y-immunoreactive neurons in human prefrontal cortex during development. J Comp Neurol. 1997;379:515–522. doi: 10.1002/(sici)1096-9861(19970324)379:4<515::aid-cne4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: Towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol Psychiatry. 2003;8:769, 821–769, 831. doi: 10.1038/sj.mp.4001399. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Interstitial white matter neuron density in the dorsolateral prefrontal cortex and parahippocampal gyrus in schizophrenia. Schizophr Res. 2005;79:181–188. doi: 10.1016/j.schres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SM, Davidson M, Haroutunian V, Powchik P, Bierer LM, Purohit DP, Perl DP, Davis KL. Neuropeptide deficits in schizophrenia vs. Alzheimer’s disease cerebral cortex. Biol Psychiatry. 1996;39:82–91. doi: 10.1016/0006-3223(95)00066-6. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Heath PR, Eastwood SL, Burnet PWJ, McDonald B, Pearson RCA. The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: Selective mRNA vulnerability and comparison with their encoded proteins. Neurosci Lett. 1995;200:151–154. doi: 10.1016/0304-3940(95)12102-a. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SHC, Jones EG, Emson PC. Morphology, distribution, and synaptic relations of somatostatin- and neuropeptide Y-immunoreactive neurons in rat and monkey neocortex. J Neurosci. 1984;4:2497–2517. doi: 10.1523/JNEUROSCI.04-10-02497.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XF, Deng C, Zavitsanou K. Neuropeptide Y mRNA expression levels following chronic olanzapine, clozapine and haloperidol administration in rats. Neuropeptides. 2006;40:213–219. doi: 10.1016/j.npep.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, Turecki G. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry. 2009;14:175–189. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Cytology and the time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 1980;9:219–242. doi: 10.1007/BF01205159. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994;649:159–173. doi: 10.1016/0006-8993(94)91060-x. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Ann Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.11.019. Epub 2008 Dec 31. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potash JB. Carving chaos: genetics and the classification of mood and psychotic syndromes. Harv Rev Psychiatry. 2006;14:47–63. doi: 10.1080/10673220600655780. [DOI] [PubMed] [Google Scholar]
- Sakai T, Oshima A, Nozaki Y, Ida I, Haga C, Akiyama H, Nakazato Y, Mikuni M. Changes in density of calcium-binding-protein-immunoreactive GABAergic neurons in prefrontal cortex in schizophrenia and bipolar disorder. Neuropathology. 2008;28:143–150. doi: 10.1111/j.1440-1789.2007.00867.x. [DOI] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, Tamminga CA. Human postmortem tissue: What quality markers matter? Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Delalle I. Morphology of neuropeptide Y-immunoreactive neurons and fibers in human prefrontal cortex during prenatal and postnatal development. J Comp Neurol. 1997;379:523–540. doi: 10.1002/(sici)1096-9861(19970324)379:4<523::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Woo T-U, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]