Abstract
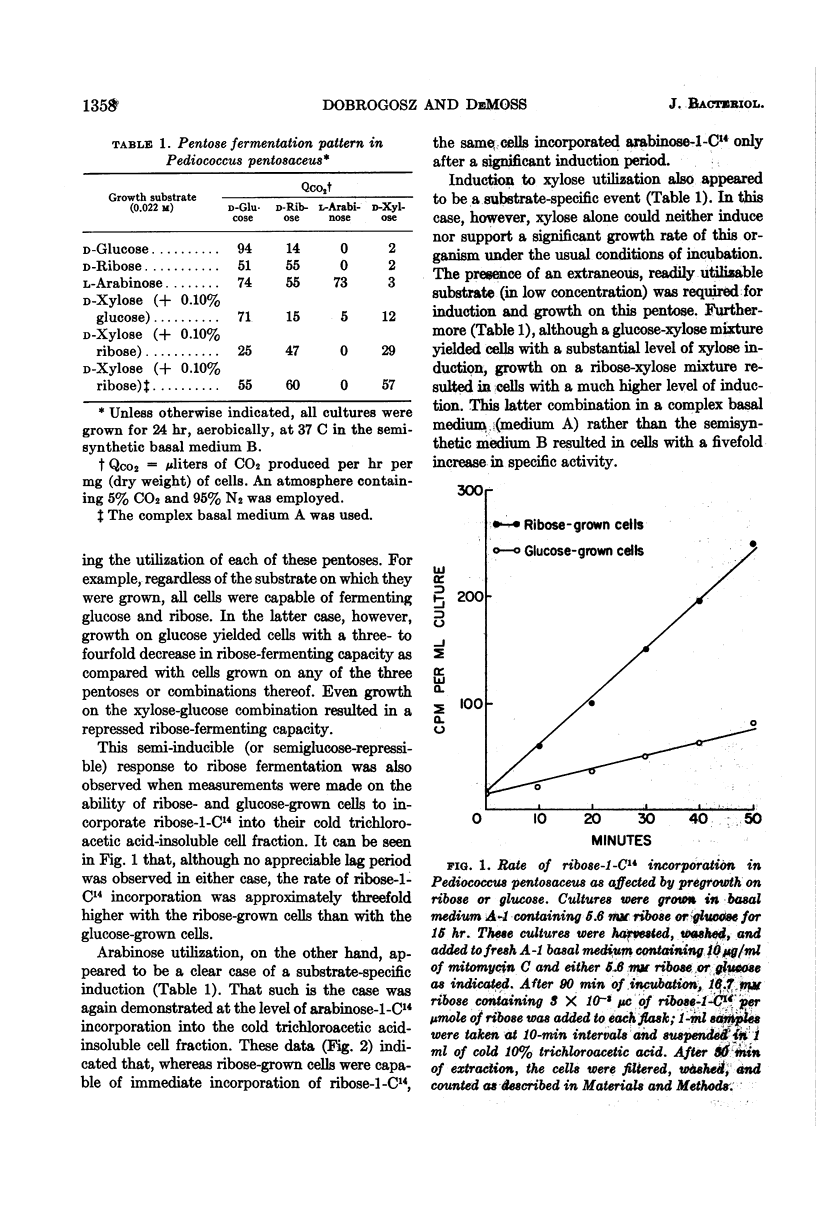
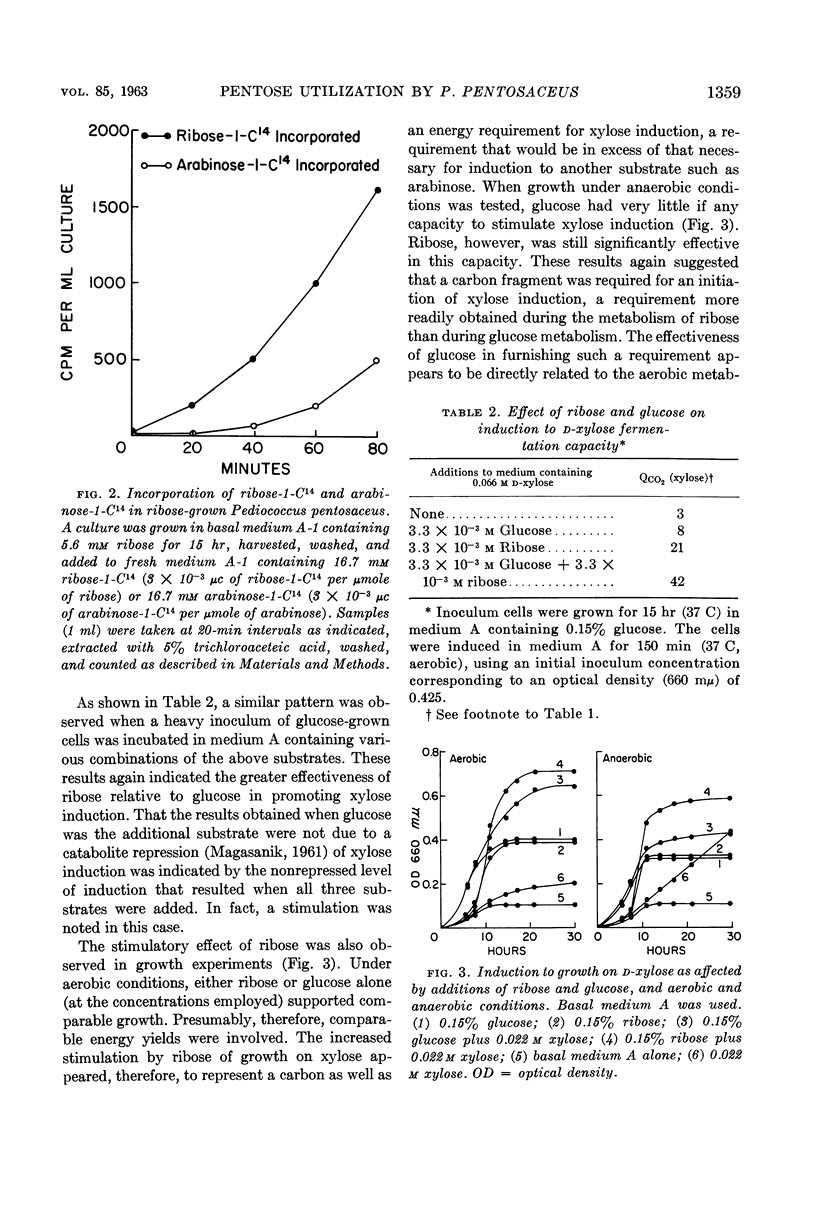
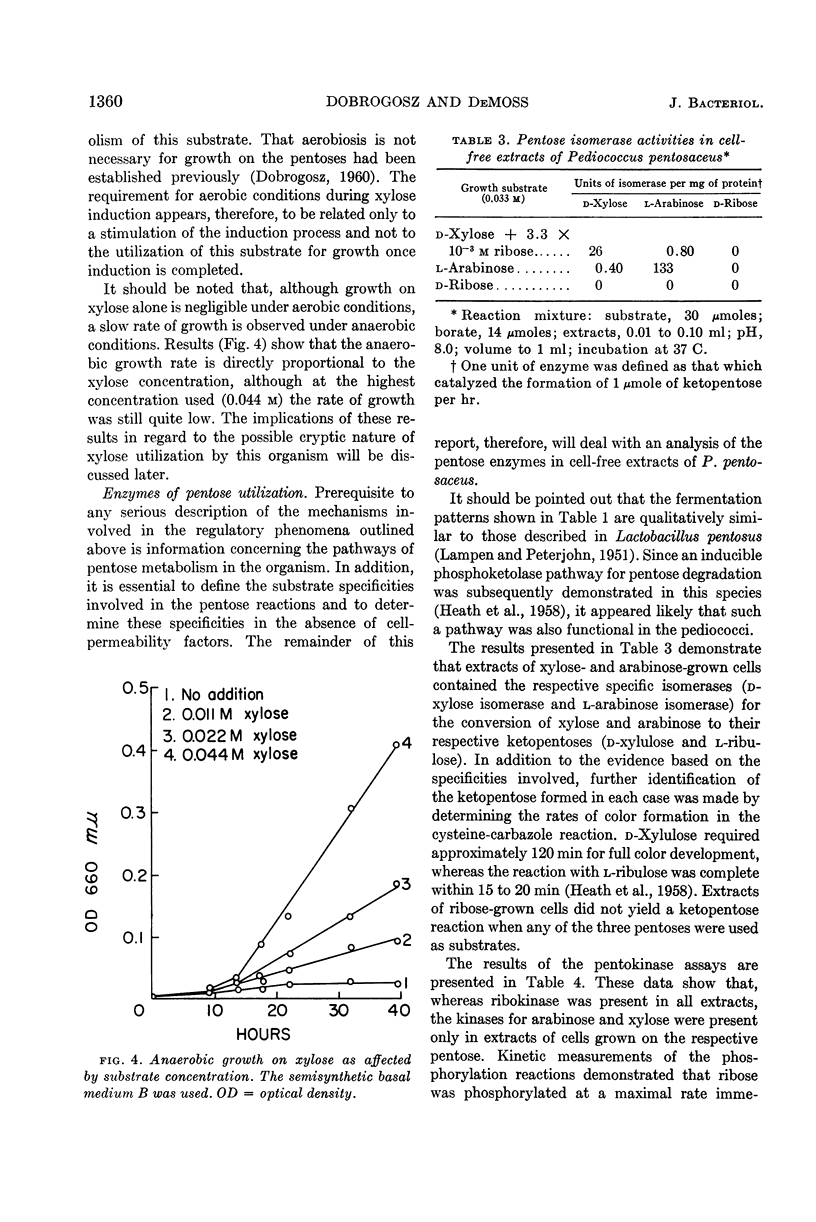
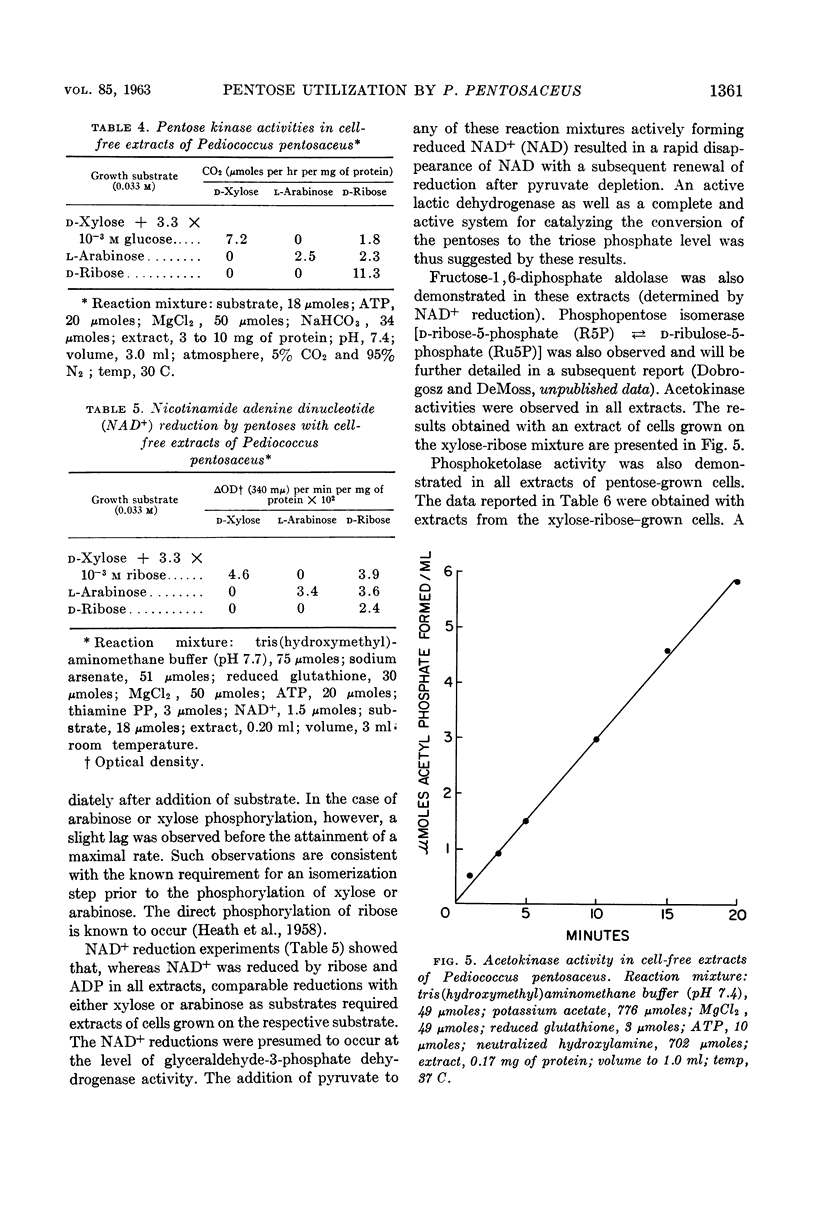
Dobrogosz, Walter J. (University of Illinois, Urbana) and Ralph D. DeMoss. Pentose utilization by Pediococcus pentosaceus. J. Bacteriol. 85:1356–1364. 1963.—Data are presented which indicate that pentoses are metabolized by Pediococcus pentosaceus through the use of an inducible phosphoketolase pathway. The utilization of each of the pentoses (l-arabinose, d-ribose, and d-xylose) appears to involve a different regulatory process. Thus, d-ribose was fermented by cells grown on any pentose or glucose, although growth on a pentose resulted in an increased ribose-fermenting capacity. l-Arabinose fermentation was only observed when cultures were grown in the presence of this pentose. Similarly, d-xylose fermentation was a specific response to the presence of xylose in the growth medium. In this case, however, growth occurred only at a very low rate unless another readily utilizable substrate (in low concentration) was added to the growth medium. Under these conditions, an aerobic atmosphere was more stimulatory than anaerobiosis for induction and growth on xylose. The possible cryptic nature of xylose utilization in this organism is described.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBROGOSZ W. J., STONE R. W. Oxidative metabolism in Pediococcus pentosaceus. I. Role of oxygen and catalase. J Bacteriol. 1962 Oct;84:716–723. doi: 10.1128/jb.84.4.716-723.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBROGOSZ W. J., STONE R. W. Oxidative metabolism in Pediococcus pentosaceus. II. Factors controlling the formation of oxidative activities. J Bacteriol. 1962 Oct;84:724–729. doi: 10.1128/jb.84.4.724-729.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEATH E. C., HURWITZ J., HORECKER B. L., GINSBURG A. Pentose fermentation by Lactobacillus plantarum. I. The cleavage of xylulose 5-phosphate by phosphoketolase. J Biol Chem. 1958 Apr;231(2):1009–1029. [PubMed] [Google Scholar]
- LAMPEN J. O., PETERJOHN H. R. Studies on the specificity of the fermentation of pentoses by Lactobacillus pentosus. J Bacteriol. 1951 Sep;62(3):281–292. doi: 10.1128/jb.62.3.281-292.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. NUCLEIC ACID PRECURSORS AND PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1954 May;40(5):263–270. doi: 10.1073/pnas.40.5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBA S., TERAWAKI A., TAGUCHI T., KAWAMATA J. Selective inhibition of formation of deoxyribonucleic acid in Escherichia coli by mitomycin C. Nature. 1959 Apr 11;183(4667):1056–1057. doi: 10.1038/1831056a0. [DOI] [PubMed] [Google Scholar]
- SIMPSON F. J., WOLIN M. J., WOOD W. A. Degradation of L-arabinose by Aerobacter aerogenes. I. A pathway involving phosphorylated intermediates. J Biol Chem. 1958 Jan;230(1):457–472. [PubMed] [Google Scholar]



