Abstract
Object
To report the clinical features, surgical treatment, and long-term outcomes of adults with moyamoya phenomenon treated at a single institution in the United States.
Methods
Forty-three adult patients with moyamoya disease (mean age of 40+/−11 years; range 18 to 69) were treated with encephaloduroarteriosynangiosis (EDAS). Neurologists examined patients pre- and post-operatively. Follow-up was obtained in-person or by structured telephone interview (median 41 months; range 4 to 126). The following outcomes were collected: transient ischemic attack (TIA), infarction, graft collateralization, change in cerebral perfusion, and functional level according to the modified Rankin scale (mRS). Kaplan-Meier infarction risk was calculated between operated and contralateral hemispheres.
Results
The majority of patients were women (65%), Caucasian (65%), presented with ischemic symptoms (98%), and had bilateral disease (86%). Nineteen patients underwent unilateral and 24 patients bilateral EDAS (n=67). Fifty of 52 (98%) patients with available imaging developed collateral vessels, and 41 of 50 (82%) had increased perfusion on SPECT scan. The incidence of peri-procedural hemisphere infarction (<48 hours) was 3%. In the follow-up period patients experienced 10 TIAs, 6 infarcts, and 1 intracranial hemorrhage. Although the hemisphere selected for surgery was based upon patient symptoms and severity of pathology, the five year infarction free survival rate was 94% in operated hemispheres versus less than 36% in non-operated hemispheres (p=0.007). After controlling for age and sex, operative hemispheres were 89% less likely to experience infarction than contralateral hemispheres (hazard ratio: 0.11; 95% CI 0.02–0.56). Thirty-eight of 43 patients (88%) had preserved or improvement in mRS over baseline status.
Conclusion
In this mixed race population of North American patients, indirect bypass promoted adequate pial collateral development and increased perfusion in the majority of adult patients with moyamoya disease. Patients had low rates of postoperative TIAs, infarction, and hemorrhage, and the majority of patients had preserved or improved functional status.
Keywords: Indirect, bypass, encephaloduroarteriosynangiosis, moyamoya, outcome, stroke
INTRODUCTION
Moyamoya disease is a chronic cerebrovascular disorder defined by progressive occlusion of the intracranial vessels. The stenosis begins with the intracranial carotid arteries and may progress to involve anterior, middle cerebral, and posterior cerebral arteries (PCA). As these arteries gradually stenose, a collateral network of capillaries develops at the base of the brain, giving rise to the characteristic reticulate (“puff of smoke”) on angiography.
In Asian populations, moyamoya disease has a well-defined phenotype. The disease has a bimodal age of presentation, with children developing ischemia secondary to inadequate collaterals and adults presenting with intracranial hemorrhage due to rupture of fragile collateral vessels.25,27 A number of studies have provided evidence that moyamoya disease in the United States may represent a different phenomena.2,4 Patient ethnicities are in proportion to the ethnicities of people in the region of diagnosis and adult patients present with ischemic symptoms rather than intracranial hemorrhage.
Direct superficial temporal artery (STA) to middle cerebral artery (MCA) bypass surgery has been used successfully to augment collateral blood flow in patients with moyamoya disease for over 30 years.10,12,14,18,19 Direct STA-MCA bypass, however, can be difficult in children because of the both the size and progressive occlusion of the MCAs. In contrast, encephaloduroarteriosynangiosis (EDAS) is a method of indirect bypass and cerebral revascularization that has been shown to be beneficial in this patient population.8,10,16,19,3 This operative technique is also considered easier, safer in patients with serious medical comorbidities, and feasible in patients with inadequate recipient or donor artery grafts. There have only been a few small case series case, however, reporting on this operative technique in an adult population (>18 years of age).5,21,4,8,24
To this end, we present our institutional experience using EDAS for the treatment of adult moyamoya patients. The primary goals of our study were to: (1) characterize the demographic characteristics, presentation, and natural history of our patients to determine if they differed from the Asian disease phenotype; (2) determine the rates of post-operative and long-term deficits; (3) evaluate the growth of collaterals and alterations in perfusion after surgery; (4) calculate the follow-up rates of TIA, stroke, infarction, intracranial hemorrhage, and seizures; and (5) determine how surgery and disease progression affect functional independence.
MATERIALS AND METHODS
Patient Population and Management Protocol
From November 1997 to September 2007, 43 patients with symptomatic moyamoya disease were treated with 67 indirect bypass operations at Columbia University Medical Center. All subjects provided consent in this IRB-approved study. All patients were examined by a neurologist immediately prior to and after surgery. Patient evaluation included neurological status, CT, MRI, digital subtraction angiography, and TCD with CO2 reactivity or SPECT with acetazolamide challenge. The subjects were classified according to the Suzuki and Takaku angiographic staging (Table 1).29
Table 1.
Patient characteristics
| Patient Characteristics | Number of Patients (n=43) |
|---|---|
| Age | 40+/−11 (range 18 to 69) |
| Female | 28 (65%) |
| Ethnicity | |
| Caucasian | 28 (65%) |
| African American | 2 (5%) |
| Latino | 4 (9%) |
| Asian | 9 (21%) |
| Antiplatelet treatment | 19 (44%) |
| Anticoagulation treatment | 8 (19%) |
| Oral contraception | 3 (11%)* |
| Presentation/History | |
| Headache | 13 (30%) |
| Weakness/sensory loss | 24 (56%) |
| Dysarthria | 16 (37%) |
| Visual Defect | 5 (12%) |
| Seizure | 6 (14%) |
| Syncope | 4 (9%) |
| TIA | 25 (58%) |
| Stroke | 26 (61%) |
| Intracranial bleed | 5 (12%) |
| Dissection | 1 (2%) |
| Intracranial Aneurysm | 7 (16%) |
| Posterior Circulation Aneurysm | 4 (57%)^ |
| Bilateral Lesions | 37 (86%) |
| Suzuki and Takaku Angiographic Stage† | |
| 1 | 6 (14%) |
| 2 | 3 (7%) |
| 3 | 33 (77%) |
| 4 | 1 (2%) |
| 5 | 0 |
Percent of women
Percent of aneurysms
Stage at initial presentation
Baseline epidemiological moyamoya syndrome risk factors were obtained including history of vasculitis, neurofibromatosis, tuberous sclerosis, retinitis pigmentosa, fibromuscular dysplasia, pseudoxanthoma elasticum, Fanconi’s anemia, antiphospolipid syndrome, Down syndrome, rheumatoid arthritis, other collagen vascular disease, head trauma, meningitis, sickle cell disease, head, neck or skull radiation, or family history of moyamoya disease. Baseline stroke risk factors were obtained, and patients with a diagnosis of atherosclerosis determined through patient history, presence of atherosclerosis in extracranial areas, and angiographic changes were excluded.13 Exclusion criteria were presence of any other disease that might be responsible for the observed vasculopathy.
Patients with active TIAs or strokes were followed until they were without new ischemic events for at least five weeks before receiving operative intervention, but all patients were symptomatic within the 3 months prior to surgery. Intractable TIAs were defined as greater than 5 episodes of reversible focal symptoms. Stroke was defined by a new neurological deficit which persisted for more than 24 hours. Infarction was defined by any new infarction on follow-up radiographic imaging regardless of the presence of new neurological deficit.
After discharge, long-term outcomes were ascertained through either in-person follow-up or a structured telephone interview at a median follow-up of 41 months (range 4 to 126). Neurological outcomes were classified by the modified Rankin scale.26 Any worsening of the patients’ preoperative Rankin scale score after surgery was coded as “new neurological deficit” as previously defined.6 New neurological deficits were classified as “disabling” when Rankin scores were 3, 4, or 5.
Cerebral blood flow was evaluated by SPECT scan after intravenous administration of 21 mCi of Technetium-99m HMPAO, and cerebral blood flow reserve was evaluated with acetazolamide challenge. Patients were followed up with angiography when possible starting 6 months after EDAS and with SPECT scan and acetazolamide challenge or TCD and CO2 challenge 3 months after surgery and at 3 to 12 month intervals.
Surgical Treatment
Sixty-seven EDAS surgeries were performed in 43 patients using either the STA or occipital artery. Briefly, EDAS involves placement of an external carotid artery branch beneath the arachnoid in ischemic territories. Most commonly, the STA is used. In certain circumstances, depending on the territory at risk, the occipital artery may also be used. Preoperatively, Doppler is used to map out the course of the target artery. Intraoperatively, the target artery is dissected completely free, a craniotomy is performed, and the dura opened. The target artery is then sewn to the pia/arachnoid with a 10-0 Prolene suture under microscopic vision after extensive arachnoid dissection. The bone flap is replaced after cutting out entry and exit sites for the envisage artery. In select patients, multiple burr holes with arachnoid and dural incisions were made over the region of interest without vessel transplantation.
Additionally, 15 of the 43 patients received burr holes with arachnoid and pial dissection to increase blood flow to the regions of the anterior cerebral arteries or posterior circulation, not supplied by the EDAS procedure. EDAS and burr holes were carried out on the symptomatic side and in the distribution of perfusion failure on SPECT or TCD.
Intraoperative Doppler ultrasound was used to demonstrate graft patency. Patients received outpatient follow-up, and follow-up imaging was performed with SPECT scan, TCD, angiography, CT, or MRI as clinically indicated. Post-operatively, medical management was optimized to treat vascular risk factors, and patients were treated with antiplatelet therapy (aspirin or clopidogrel). Blood pressure was managed conservatively to avoid relative hypotension and decreased cerebral blood flow in hemodynamically challenged patients.11
Statistical Analysis
Analysis was carried out using unpaired t-test, Chi-square, and Fisher’s exact tests as appropriate. Kaplan-Meier infarction risk was calculated between infarction ipsilateral to a hemisphere not receiving EDAS versus any hemisphere experiencing peri-procedural or follow-up infarction ipsilateral to surgery. The log-rank test was used to assess differences in survival curves and Cox regression was used to assess hazard ratios. P-values of ≤ 0.05 were considered statistically significant.
RESULTS
Forty-three patients underwent indirect bypass using EDAS and/or burr holes for the treatment of symptomatic moyamoya disease. The study cohort included 15 men and 28 women with a mean age of 40 +/− 11 years at the time of surgery (Table 1). Patient ethnicities includes Caucasian (65%), black (5%), Latino, (9%) or Asian (21%). Eight (19%) and 19 patients (44%) had a history of receiving anticoagulation and antiplatelet medications, respectively to treat moyamoya disease. Three of 28 women (11%) had a history of oral contraception use.
The majority of patients presented with ischemic symptoms. All patients had a history of TIA (58%) or stroke (61%) except for 1 patient who presented with intracranial hemorrhage. No patient was treated with indirect bypass emergently (<5 weeks from last event, and in patients receiving more than one operation, operations were separated by a minimum of 3 weeks. Prior to surgery (n=67) 23 patients (34%) had significant disability (mRS > 2, Table 2).
Table 2.
Modified Rankin scores in 67 operations
| Score | Baseline | Post-Surgery Discharge |
Long-Term Follow-Up |
|---|---|---|---|
| Rankin 0–1 | 14 (20%) | 30 (45%) | 39 (58%) |
| Rankin 2 | 30 (45%) | 20 (30%) | 16 (24%) |
| Rankin 3 | 16 (25%) | 12 (18%) | 7 (10%) |
| Rankin 4 | 7 (10%) | 5 (7%) | 2 (3%) |
| Rankin 5 | 0 | 0 | 1 (2%) |
| Death | 0 | 0 | 2 (3%)* |
One patient had decline in mRS due to comorbidities unrelated to moyamoya disease progression
The majority of patients presented with Suzuki and Takaku Angiographic stage 3 (77%) and 86% of patients had bilateral disease. All patients with unilateral disease demonstrated formation of moyamoya collateral vessels in conjunction with occlusion or stenosis of an intracranial portion of an internal carotid artery. Sixteen percent of patients had aneurysms on diagnostic angiography of which 57% were in the posterior circulation. All patients had hypoperfusion on baseline SPECT scans and decreased reserve on during cerebral blood flow challenge.
The median long-term follow-up was 41 months (range 4 to 126). In 67 operations there were a total of 12 complications in 10 patients occurring prior to hospital discharge (Table 3). Five patients experienced new neurological deficits prior to discharge of which 4 were transient and 1 persisted on follow-up. There were two (3%) infarctions prior to discharge, both <48 hours from the time of surgery).
Table 3.
Patient outcomes
| Outcome | Number of Operations (n=67) |
|---|---|
| Post-surgical complications* | |
| New focal deficit due to surgery | 5 (7%) |
| Persistent focal deficit due to surgery on follow-up | 1 (1.5%) |
| Post-surgical infarctionΔ | 2 (3%) |
| Wound infection/granuloma | 2 (3%) |
| Hygroma€ | 3 (4%) |
| CSF leak | 2 (3%) |
| Follow-up Events^ | |
| TIA | 10 (7%) |
| Infarction | 6 (9%) |
| Infarction ipsilateral to surgery | 2 (3%) |
| Infarction within 1 yr surgery | 0 |
| Intracranial hemorrhage | 1 (1.5%) |
| Seizure | 7 (10%) |
| Perfusion | |
| Increased | 41 of 50 (82%) |
| Decreased | 4 (8%) |
| No change | 5 (10%) |
| Collateral Formation | 50 of 52 (96%) |
| Modified Rankin | |
| Improved independence or no change | 39 of 43 (88%) |
| Decreased independence | 5 (12%)^ |
Median follow-up duration of 41 months (range 4 to 126)
Less than 48 hours after surgery
Post-surgical infarctions account for 2 of 5 of the new focal deficits due to surgery. Only 1 patient had persistent deficit on long-term follow-up
1 of 2 Hygromas account for 1 of the 5 new focal deficits due to surgery
Greater than 48 hours after surgery
Functional outcome according to the modified Rankin Scale
One patient had decline in independence due to unrelated comorbidities
Following 67 EDAS operations, 50 patients received at least 1 SPECT study 3 months after surgery. On follow-up, 41 patients (82%) had increased perfusion to previously hypoperfused areas, 5 (10%) had no change in perfusion, and 4 (8%) had decreased perfusion. In patients with available follow-up imaging, 50 of 52 (96%) had collateral formation in the surgical territory.
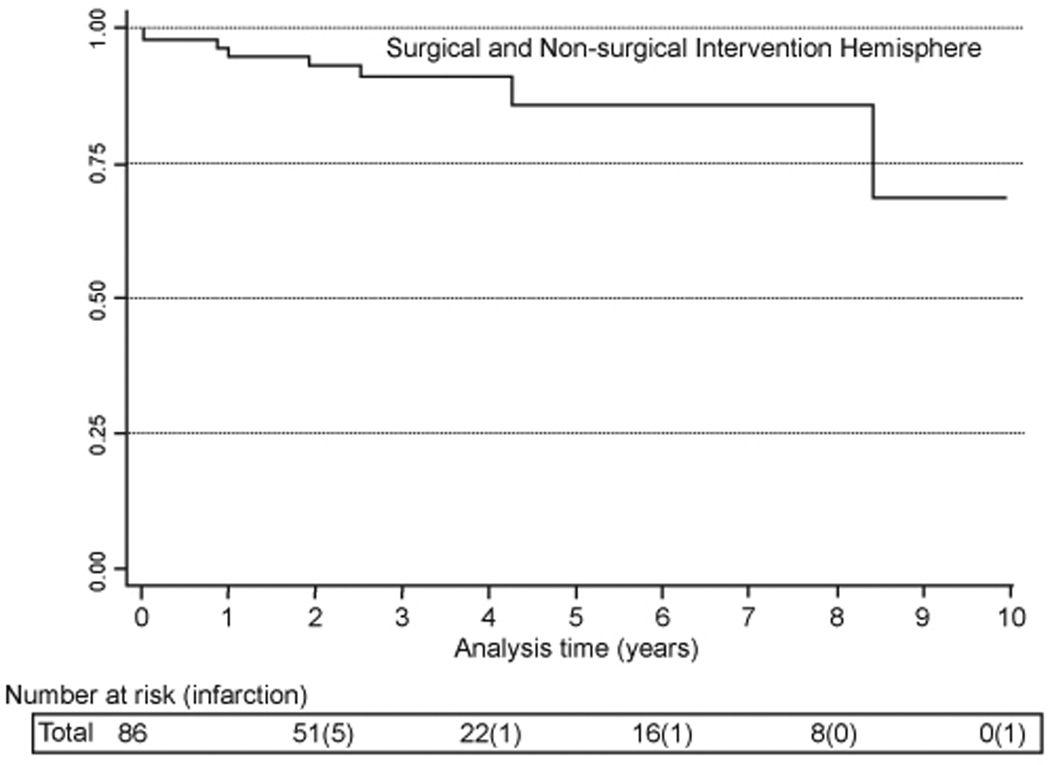
Seven patients experienced seizures after surgery. One patient experienced 1 seizure after a meningeal wound infection, and 3 patients with a prior history of seizures had a single seizure during the follow-up period. In the follow-up period (hospital discharge to time of event of censor), there was 1 intracranial hemorrhage, 10 TIAs, and 6 infarctions. There were no new infarcts from the time of discharge to the end of the first year of follow-up. Two infarctions occurred ipsilateral to EDAS operations and 4 infarctions occurred in the contralateral hemisphere. The 5-year infarction free survival rate, including post-operative and follow-up infarctions, was 70% (Figure 1).
Figure 1.
Cumulative hazard for infarction
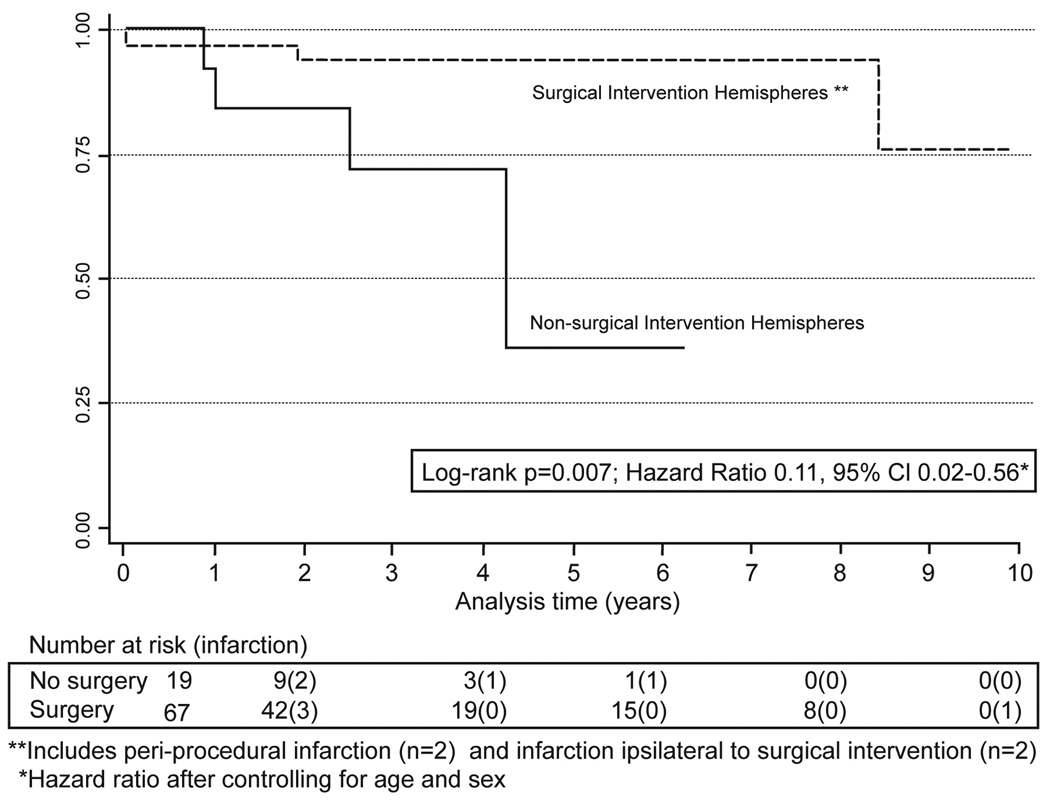
There was a significant difference between cumulative infarction curves when comparing operative and contralateral hemispheres (p=0.007, Figure 2). The 5-year infarction free survival rate was 94% (95% CI 0.84–0.98) in operative hemispheres (including all peri-operative infarcts) versus less than 36% (95% CI 0.15–0.78) in contralateral hemispheres. After controlling for age and sex, operative hemispheres were 89% less likely to experience infarction than contralateral hemispheres (hazard ratio: 0.11; 95% CI 0.02–0.56).
Figure 2.
Cumulative hazard for infarction by intervention
Following 67 surgical procedures, 17 patients (25%) had significant disability (mRS >2) on discharge and 13 patients (18%) had significant disability on long-term follow-up (Table 2). From the time of admission to follow-up, 4 of 43 patients (9%) had a decline in their functional independence according to the modified Rankin scale due to moyamoya disease progression. One died as a result of a stroke and 1 patient died due to unrelated comorbidities. Thirty-eight of 43 patients (88%) had improvement or no change in functional independence.
DISCUSSION
Moyamoya disease is rare outside of Asia, but increased awareness regarding this condition has led to its characterization in many other countries. In this study we have found that: (1) indirect bypass promotes adequate pial collateral development and increased perfusion in adult patients; and (2) patients undergoing indirect bypass have acceptably low rates of neurological deficits, TIAs, stroke, infarction, and hemorrhage, with the majority of patients preserving or improving functional independence.
The racial demographics of our cohort were roughly representative of the northeastern United States, with 65% of patients being Caucasian, 5% black, 9% Latino, and 21% Asian. The majority of patients in our cohort were women between the ages of 30 and 50, which is consistent with prior studies.9,15,30 Although a bimodal age distribution has been demonstrated in Asia, with children primarily suffering from ischemic symptoms and adults experiencing intracranial hemorrhage,9 the majority of our adult cohort of surgically treated patients presented with ischemic symptoms (98%) and only a small number of patients had a history of intracranial hemorrhage (12%). Other studies have reported similar discrepancies, with only 13–20% of adult moyamoya patients in the United States presenting with intracranial hemorrhage 2,8 versus more than 60% in Asian populations.9,30 This may be the result of delayed arterial narrowing and collateral circulation formation in patients outside of the Eastern hemisphere. Although possibly due to referral and/or selection bias, our patient demographics support the hypothesis of a separate disease phenotype in North American moyamoya disease patients.2,4
Although moyamoya patients frequently undergo revascularization procedures, the safety and efficacy of these techniques remain largely unproven. While many studies recommend direct bypass or a combination of indirect and direct bypass in adult moyamoya patients,4,8,22,24 EDAS is the preferred surgical revascularization procedure at our institution. Currently, there is no evidence to support the notion that there is a superior method of bypass in adult moyamoya disease patients.28 Unfortunately, there have been no randomized clinical trials and only a few small case series regarding the role of EDAS in adult moyamoya patients.5,21
Despite the fact that our patients often had more severe presentations (Table 2), the incidence of new neurological deficit and persistent deficit on long-term follow-up was 7% and 1.5% respectively. This is favorable in comparison to other studies where the rates of periprocedural ischemia range from 4–31%, but may be due to limited follow-up in some studies.2,4,5,8,10,18,20,24 Some studies have noticed increased early ischemic injury in patients receiving indirect versus direct surgery, possibly the result of delayed revascularization and collateral growth. We did not appreciate this in our study and further believe that reversal of flow in critical perforator segments due to direct or combination of direct and indirect bypass procedures may result in ischemia, poor collateral growth, and worse outcome.
In our cohort there were low rates of new focal neurological deficits, TIA’s, strokes, infarction, intracranial hemorrhages, and seizures. Moreover, these conditions resolved in the majority of patients. The 5-year infarction free rate was greater than 94% in operative hemispheres versus less than 50% in contralateral hemispheres. This translates into an 89% infarction risk reduction following surgical intervention (hazard ratio 0.11 95% CI 0.02–0.56). It is important to note that the operative side is based on patient symptoms, cerebral blood flow studies, and angiographic findings. Thus, patients underwent surgery on the side with more severe symptoms and higher risk of future cerebrovascular accidents. Despite the increased number of patients with bilateral disease (86%) and worse clinical grades of patients on presentation, demonstrated outcomes are more favorable than those reported in the literature. For comparison, a previous study4 involving medical management of moyamoya disease stated 5-year stroke free survival rates of only 35% and 18% in patients with unilateral and bilateral disease, respectively. Only randomized clinical trials can determine whether surgery or medical therapy is optimally, but such trials are likely difficult to perform in patients with a rare condition like moyamoya disease.
In our study, 82% percent of patients with available imaging had increased perfusion and 96% had augmented collaterals following surgery. These favorable results may be attributable to a large craniotomy planned according to preoperative CBF pattern, as well as wide and extensive opening of the dura and arachnoid. We also believe that a CBF pattern indicating misery perfusion is a prerequisite for excellent revascularization with indirect bypass. Other small case series in adults have reported similar results.5 Previous studies have noted that EDAS is limited in comparison to direct or combination direct and indirect bypass in its ability to reperfuse the anterior or posterior circulation.24 For these patients we placed additional burr holes with extensive dural and arachnoid dissection to increase collateral blood flow, a maneuver which has been supported by prior investigations.3
Moyamoya patients appear to readily form collaterals and augment perfusion following indirect bypass. In contrast, we have found that EDAS is not beneficial in inducing collateral vessel growth in non-moyamoya patients with symptomatic intracranial athero-occlusive disease.13 Patients with atherosclerotic disease may have impaired angiogenesis secondary to reduced endothelial repair capacity. It is known, for example, that endothelial progenitor cells (EPCs), which have been characterized as (KDR+)/CD133+ cells,1 are reduced in patients with atherosclerotic risk factors and cardiovascular disease.7 For these reasons, patients should receive a thorough work-up to exclude alternative diagnoses of intracranial vascular occlusion.
A number of researchers have noted that it is particularly difficult to judge outcome following surgery in patients with moyamoya disease, as surgery is a form of palliative care in a disease with poor natural history.30 Furthermore, studies have not used standardized outcome measures to assess patients on presentation and on long-term follow-up. Despite the poor clinical state of patients in this study, only 4 patients had a decline in their functional state (mRS) due to disease progression over a median follow-up period of 41 months. This provides further evidence regarding the benefits of surgery in this patient population.
It is important to note inherent limitations of our outcome data. The modified Rankin scale does not measure higher cortical function and postoperative cognitive decline. This must be taken into account when considering operative morbidity.17,23 Studies have noted subtle changes in neurocognitive decline in moyamoya disease patients even in the absence of obvious ischemic or hemorrhagic events. Neuropsychological testing following surgery in moyamoya disease is critical to accurate outcome assessment and should be integrated into future clinical trials. Importantly, referral and selection bias cannot be excluded in this single institution, single surgeon series.
CONCLUSION
This study provides evidence for a different moyamoya disease phenotype in North American adults. Patients are primarily middle-aged women presenting with ischemic symptoms and ethnicities reflect those of the regional referral base. In this population, indirect bypass promotes adequate pial collateral development and increased perfusion. Surgery leads to a decreased incidence TIAs, infarction, and hemorrhage, with the majority of patients have preserved or improved functional independence.
Acknowledgement
Mr. Starke’s efforts were partially supported by the CTSA Grant UL1 RR025750 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. Dr. Hickman's efforts were partially supported by the AHA Student Scholarship in Cerebrovascular Disease and Stroke. We would like to thank the Department of Nuclear Medicine and Radiology at Columbia University for their expertise in imaging and Nancy Heim for her artistic assistance.
Footnotes
Conflict of Interest Disclosure: None. No grants or funding sources are pertinent to this paper
REFERENCES
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Chiu D, Shedden P, Bratina P, Grotta JC. Clinical features of moyamoya disease in the United States. Stroke. 1998;29:1347–1351. doi: 10.1161/01.str.29.7.1347. [DOI] [PubMed] [Google Scholar]
- 3.Endo M, Kawano N, Miyaska Y, Yada K. Cranial burr hole for revascularization in moyamoya disease. J Neurosurg. 1989;71:180–185. doi: 10.3171/jns.1989.71.2.0180. [DOI] [PubMed] [Google Scholar]
- 4.Hallemeier CL, Rich KM, Grubb RL, Chicoine MR, Moran CJ, Cross DT, et al. Clinical features and outcome in North American adults with moyamoya phenomenon. Stroke. 2006;37:1490–1496. doi: 10.1161/01.STR.0000221787.70503.ca. [DOI] [PubMed] [Google Scholar]
- 5.Han DH, Nam DH, Oh CW. Moyamoya disease in adults: characteristics of clinical presentation and outcome after encephalo-duro-arterio-synangiosis. Clin Neurol Neurosurg. 1997;99(Suppl 2):S151–S155. doi: 10.1016/s0303-8467(97)00058-9. [DOI] [PubMed] [Google Scholar]
- 6.Hartmann A, Mast H, Mohr JP, Pile-Spellman J, Connolly ES, Sciacca RR, et al. Determinants of staged endovascular and surgical treatment outcome of brain arteriovenous malformations. Stroke. 2005;36:2431–2435. doi: 10.1161/01.STR.0000185723.98111.75. [DOI] [PubMed] [Google Scholar]
- 7.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 8.Houkin K, Ishikawa T, Yoshimoto T, Abe H. Direct and indirect revascularization for moyamoya disease surgical techniques and peri-operative complications. Clin Neurol Neurosurg. 1997;99(Suppl 2):S142–S145. doi: 10.1016/s0303-8467(97)00075-9. [DOI] [PubMed] [Google Scholar]
- 9.Ikezaki K, Han DH, Kawano T, Kinukawa N, Fukui M. A clinical comparison of definite moyamoya disease between South Korea and Japan. Stroke. 1997;28:2513–2517. doi: 10.1161/01.str.28.12.2513. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa T, Houkin K, Kamiyama H, Abe H. Effects of surgical revascularization on outcome of patients with pediatric moyamoya disease. Stroke. 1997;28:1170–1173. doi: 10.1161/01.str.28.6.1170. [DOI] [PubMed] [Google Scholar]
- 11.Kappelle LJ, Klijn CJ, Tulleken CA. Management of patients with symptomatic carotid artery occlusion. Clin Exp Hypertens. 2002;24:631–637. doi: 10.1081/ceh-120015339. [DOI] [PubMed] [Google Scholar]
- 12.Karasawa J, Touho H, Ohnishi H, Miyamoto S, Kikuchi H. Long-term follow-up study after extracranial-intracranial bypass surgery for anterior circulation ischemia in childhood moyamoya disease. J Neurosurg. 1992;77:84–89. doi: 10.3171/jns.1992.77.1.0084. [DOI] [PubMed] [Google Scholar]
- 13.Komotar RJ, Starke RM, Otten ML, Merkow MB, Garrett MC, Marshal RS, et al. The Role of Indirect Extracranial-Intracranial Bypass for Symptomatic Intracranial Athero-occlusive Disease. Journal of Neurosurgery. 2008 doi: 10.3171/2008.9.JNS17658. (under revision) [DOI] [PubMed] [Google Scholar]
- 14.Krayenbuhl HA. The Moyamoya syndrome and the neurosurgeon. Surg Neurol. 1975;4:353–360. [PubMed] [Google Scholar]
- 15.Kuriyama S, Kusaka Y, Fujimura M, Wakai K, Tamakoshi A, Hashimoto S, et al. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. 2008;39:42–47. doi: 10.1161/STROKEAHA.107.490714. [DOI] [PubMed] [Google Scholar]
- 16.Lee SK, Kim DI, Jeong EK, Kim SY, Kim SH, In YK, et al. Postoperative evaluation of moyamoya disease with perfusion-weighted MR imaging: initial experience. AJNR Am J Neuroradiol. 2003;24:741–747. [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall GA, Jonker BP, Morgan MK, Taylor AJ. Prospective study of neuropsychological and psychosocial outcome following surgical excision of intracerebral arteriovenous malformations. J Clin Neurosci. 2003;10:42–47. doi: 10.1016/s0967-5868(02)00217-5. [DOI] [PubMed] [Google Scholar]
- 18.Matsushima T, Inoue T, Suzuki SO, Fujii K, Fukui M, Hasuo K. Surgical treatment of moyamoya disease in pediatric patients--comparison between the results of indirect and direct revascularization procedures. Neurosurgery. 1992;31:401–405. doi: 10.1227/00006123-199209000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Matsushima Y, Aoyagi M, Koumo Y, Takasato Y, Yamaguchi T, Masaoka H, et al. Effects of encephalo-duro-arterio-synangiosis on childhood moyamoya patients--swift disappearance of ischemic attacks and maintenance of mental capacity. Neurol Med Chir (Tokyo) 1991;31:708–714. doi: 10.2176/nmc.31.708. [DOI] [PubMed] [Google Scholar]
- 20.Matsushima Y, Aoyagi M, Suzuki R, Tabata H, Ohno K. Perioperative complications of encephalo-duro-arterio-synangiosis: prevention and treatment. Surg Neurol. 1991;36:343–353. doi: 10.1016/0090-3019(91)90022-2. [DOI] [PubMed] [Google Scholar]
- 21.Matsushima Y, Suzuki R, Yamaguchi T, Tabata H, Inaba Y. Effects of indirect EC/IC bypass operations on adult moyamoya patients. No Shinkei Geka. 1986;14:1559–1566. [PubMed] [Google Scholar]
- 22.Mizoi K, Kayama T, Yoshimoto T, Nagamine Y. Indirect revascularization for moyamoya disease: is there a beneficial effect for adult patients? Surg Neurol. 1996;45:541–548. doi: 10.1016/0090-3019(95)00475-0. discussion 548–549. [DOI] [PubMed] [Google Scholar]
- 23.Mohr JP. Thomas Willis Lecture. Acute clinical trials: An expression of concern. Cerebrovasc Dis. 1999;9(Suppl 3):45–50. doi: 10.1159/000047554. [DOI] [PubMed] [Google Scholar]
- 24.Nakashima H, Meguro T, Kawada S, Hirotsune N, Ohmoto T. Long-term results of surgically treated moyamoya disease. Clin Neurol Neurosurg. 1997;99(Suppl 2):S156–S161. doi: 10.1016/s0303-8467(97)00056-5. [DOI] [PubMed] [Google Scholar]
- 25.Nishimoto A. Moyamoya disease(author's transl) Neurol Med Chir (Tokyo) 1979;19:221–228. doi: 10.2176/nmc.19.221. [DOI] [PubMed] [Google Scholar]
- 26.Rankin J. Cerebral vascular accidents in patients over the age of 60. III. Diagnosis and treatment. Scott Med J. 1957;2:254–268. [PubMed] [Google Scholar]
- 27.Saeki N, Yamaura A, Hoshi S, Sunami K, Ishige N, Hosoi Y. Hemorrhagic type of moyamoya disease. No Shinkei Geka. 1991;19:705–712. [PubMed] [Google Scholar]
- 28.Starke RM, Komotar RJ, Hickman ZL, Paz YE, Pugliese AG, Otten ML, et al. Clinical features, surgical treatment, and long-term outcome in adult patients with moyamoya disease. J Neurosurg. 2009 doi: 10.3171/2009.3.JNS08837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki J, Takaku A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20:288–299. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- 30.Ueki K, Meyer FB, Mellinger JF. Moyamoya disease: the disorder and surgical treatment. Mayo Clin Proc. 1994;69:749–757. doi: 10.1016/s0025-6196(12)61094-5. [DOI] [PubMed] [Google Scholar]