Abstract
Leukocytes play a central role in vein graft neointimal hyperplasia, which is significantly augmented under low shear conditions. The current concept is that shear force regulates leukocyte adhesion predominately through up-regulation of chemokines and growth factors within the graft wall. Using rabbit and murine vein graft models, we demonstrate that CCR2/MCP-1 mediated monocyte recruitment and a low shear environment act synergistically to augment neointimal hyperplasia development and removal of either of the conditions leads to a significant reduction in neointimal thickening. We propose a novel concept that the shear stress response element phenotypically stems from the complex interplay of the biological and physical microenvironments.
Keywords: CCR2, Shear Stress, Leukocyte, Vein Graft, Neointimal Hyperplasia
1. Introduction
Although vein bypass grafting remains a mainstay for clinical revascularization, accelerated neointimal hyperplasia development leading to poor performance or complete luminal occlusion occurs in up to 40% of grafts within one year of implantation.[1] While a variety of biologic processes have been implicated in pathologic vein graft remodeling, invasion of inflammatory cells has increasingly been recognized as one of the dominant components in these events.[2, 3] CC chemokines, particularly monocyte chemoattractant protein (MCP)-1 binding to CC chemokine receptor 2 (CCR2), appears to be central to the process of monocyte recruitment and macrophage accumulation within developing lesions.[4]
Beyond biologic factors, the local hemodynamic environment stands among the important regulators of neointimal hyperplasia development, where reductions in wall shear lead to substantial augmentation in the rate of neointimal growth.[5] Grounded in the pioneering work of Gimbrone and his identification of positive and negative shear stress response elements, the effect of alterations in shear have been thought to induce local changes in chemokine and growth factor transcription within the wall, leading to differential growth of the neointima. In particular, leukocyte adhesion has been demonstrated to be highly shear responsive secondary to the regulation of shear stress responsive element (SSRE)-dependent, vascular adhesion molecules expressed on the endothelial surface.[6]
The current study attempts to define the relative importance of leukocyte recruitment and wall shear in vein graft failure. Our initial hypothesis was that modification of MCP-1/CCR2-dependent leukocyte recruitment would be the dominant regulator of vein graft remodeling, with alterations in wall shear forces providing a secondary, modulatory effect on the final graft morphology. Interestingly, the results suggest that there appears to be substantial interplay between the physical and biologic systems for the complex process of neointimal hyperplasia. Specifically, while reductions in flow lead to enhanced leukocyte accumulation and advanced neointimal hyperplasia, this is not accomplished through an up-regulation of cell adhesion molecules or augmentation of MCP-1/CCR2 signaling. These data suggest that while increased chemokine expression may be important in the early recruitment of leukocytes into the vein graft wall, the differential accumulation of these cells is mediated by the local effect of physical forces. Further investigations using a murine CCR2 -/- vein graft model confirm an important interaction between biologic mediators and physical forces, where accelerated neointimal hyperplasia is observed only in the presence of intact leukocyte recruitment pathways combined with a favorable (low shear) hemodynamic environment for leukocyte binding and transmigration.
2. Materials and Methods
2.1 Animal models of bypass vein grafting
This study conforms to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23). Male New Zealand White rabbits underwent bilateral jugular vein interposition grafting followed by immediate unilateral distal carotid artery branch ligation to create two distinct flow environments within the same animal.[7] Vein grafts harvested at 1, 7 or 28 days postoperatively were either snap frozen for biochemical analysis or perfusion fixed in situ (10% neutral buffered formalin) for immunohistochemical and morphometric evaluation.
Murine vein grafts were created via interposition of an inferior vena cava from a donor animal into the right common carotid artery of another animal. The right common carotid artery was divided and each free end everted over a cuff and secured with a 10-0 suture. An inferior vena cava harvested from a separate animal was sleeved over the cuffed arterial ends and secured with another 10-0 suture. Flow reduction was achieved through ligation of the external carotid artery. Vein grafts were collected at 7 or 28 days postoperatively for detection of macrophages biochemical evaluation or morphometric analysis.
2.2 MCP-1, CCR2, and E-selectin expression
The levels of MCP-1, CCR2, and E-selectin mRNA in rabbit and murine vein grafts were measured using quantitative polymerase chain reaction (RT-PCR) as detailed previously.[8]
Murine vein grafts (harvested at 7 and 28 days) were stained with monoclonal antibodies against MCP-1 and CCR2, using a double label technique with confocal microscopy to identify specific localization to endothelial or smooth muscle cells, as previously described.[9]
2.3 Leukocyte trafficking to the vein graft wall
Early homing of circulating leukocytes to the vein graft wall was evaluated with the bilateral rabbit vein graft model. Prior to vein graft implantation, a total of 30ml of peripheral blood was collected. Using a Percoll gradient (density: 1.096 and 1.076) centrifugation, total leukocytes were isolated and labeled with CM-DiI per manufacturer's instructions. Total leukocyte recovery rates averaged 25%, with no difference in the proportion of leukocyte subpopulations before and after cell processing. The viability of the labeled leukocytes averaged 95% by trypan blue measurements. The labeled cells (∼34 million cells) were then delivered intravenously to the same animal immediately after graft implantation. Twenty-four hours later, vein grafts were perfusion fixed in situ, incised longitudinally, and mounted on glass slides for immediate analysis. A two-photon confocal microscope, with an effective depth of penetration of 500μm, was used for quantification of CM-DiI positive cells throughout the entire depth of the vein graft wall. Cell counts were normalized to the concentration of positive cells in the blood and expressed as cells per luminal surface area. Total number of cells counted per graft averaged approximately 3100 and 1400 for low and high shear grafts, respectively.
2.4 Vein graft morphology and macrophage localization
Murine wild type and CCR2-/- vein grafts harvested at Day 28 were used for morphologic evaluation. Two cross-sections from the mid-portion of each graft underwent Masson staining for neointimal area measurements, with the average used for statistical analyses.
Murine wild type and CCR2 -/- vein grafts were harvested at 7 days following exposure to high or low flow. Histologic sections (n=10 per graft) were stained with an F4/80 specific antibody to determine the macrophage content within the wall.
2.5 Statistical analyses
Data are expressed as mean ± standard error of the mean (SEM), and statistically meaningful differences determined using two-way ANOVA or unpaired t-tests.
3. Results
3.1 Flow regulation of MCP-1 and CCR2 expression
To assess the role of wall shear stress in modulating chemokine and chemokine receptor transcription, rabbit vein grafts exposed to reduced and elevated flow rates were harvested 1, 7, and 28 days following implantation. In Day 1 grafts, MCP-1 mRNA content was significantly up-regulated (P=0.02), with an approximate 20-fold increase above those levels present in vein segments pre-implantation (Figure 1A). This early increase in MCP-1 declined to near baseline by Day 28. Neither low nor high flow grafts demonstrated a difference in mRNA expression patterns in response to the differential flow conditions (P=NS, low vs. high flow).
Figure 1.
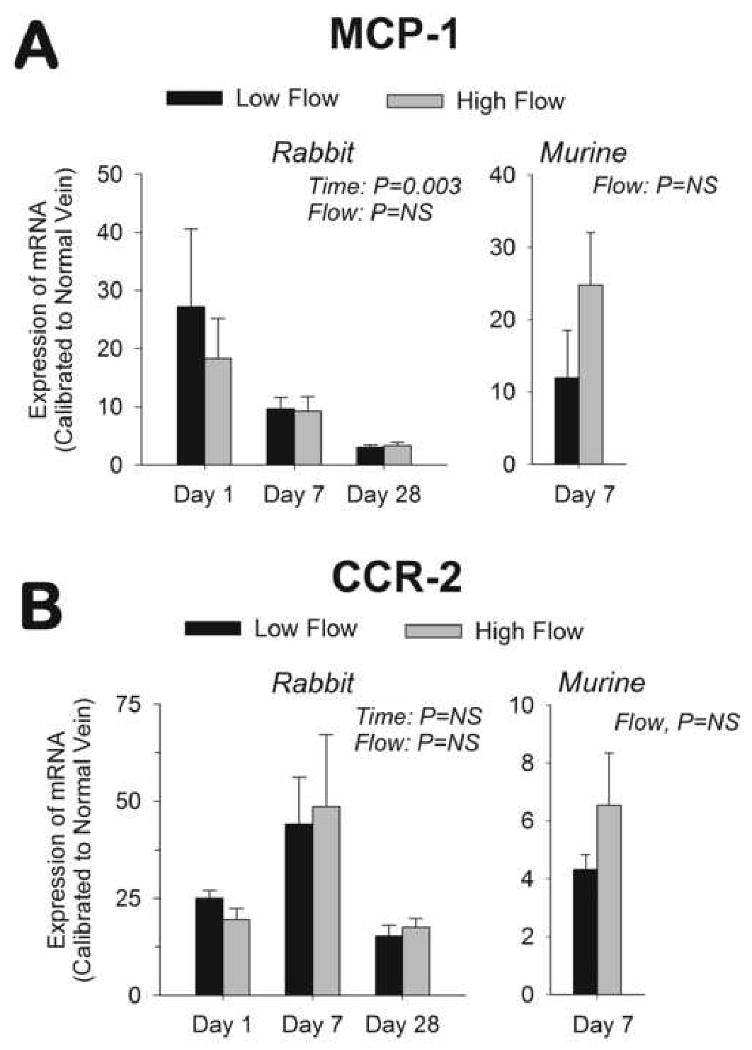
mRNA expression of MCP-1 (A) and CCR2 (B). Significant up-regulation of MCP-1 (P<0.001) and CCR2 (P<0.001) was observed in rabbit vein grafts following implantation. Rabbit MCP-1 demonstrated a return towards baseline (P=0.003) but CCR2 remained elevated (P=NS) throughout the 28 days. No difference in rabbit MCP-1 or CCR2 mRNA content was observed between low and high flow grafts. (n=4 to 9 per group) Significant increases in MCP-1 (P=0.004) and CCR2 murine, 7-day vein grafts were also observed, with this increase independent of the imposed flow. (n=4 per group)
CCR2 transcription was also significantly enhanced in Day 1 rabbit vein grafts (P<0.001, Figure 1B), but in contrast to MCP-1, the augmentation of CCR2 remained elevated 28 days following implantation (P<0.001). Similar to MCP-1, no notable difference in CCR2 mRNA content was observed between low and high flow grafts. Comparable flow-independent elevations in MCP-1 and CCR2 transcription were confirmed in murine vein grafts 7 days after implantation (Figures 1A and 1B).
The distributions of MCP-1 and CCR2 within the murine vein graft are shown in Figure 2. Although highest near the luminal surface, broad expression of both of MCP-1 and CCR2 are noted throughout the wall.
Figure 2.
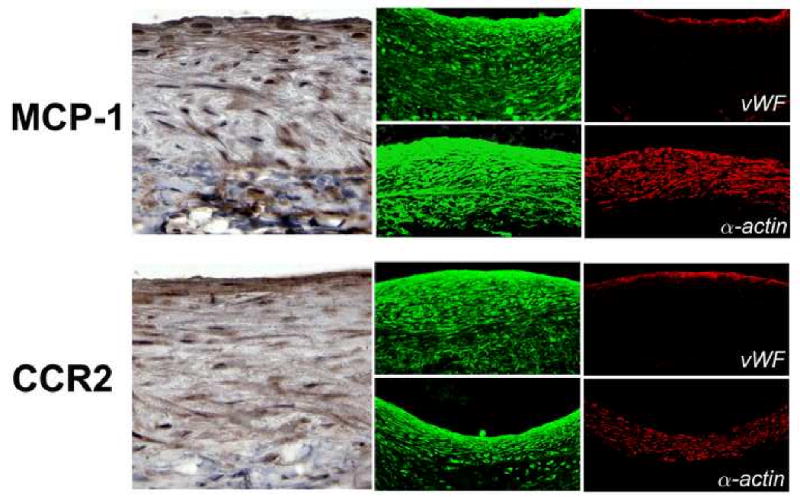
Distribution of MCP-1 and CCR2 in murine vein grafts 28 days following implantation. Von Willebrand Factor (vWF) and α-actin staining, for identification of endothelial and smooth muscle cells respectively, demonstrate a broad distribution of MCP-1 and CCR throughout the wall without cell-specific localization.
3.2 Effect of Wall Shear on Leukocyte Binding and Transmigration
With an evolving understanding of the role of cell mediated inflammation in the progression of neointimal hyperplasia, the binding and transmigration of leukocytes into low and high flow rabbit vein grafts was investigated. Following the CM-DiI labeling of leukocytes at the time of graft implantation, Day 1 vein grafts were assayed using two-photon confocal microscopy (Figure 3A). Vein grafts subjected to low blood flow rates demonstrated a 2-fold increase in the density of surface adherent or transmigrated leukocytes, compared to high flow grafts (Figure 3B).
Figure 3.
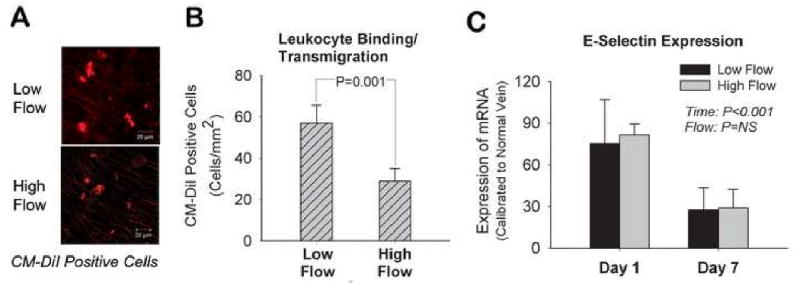
Shear regulated leukocyte recruitment and infiltration in rabbit vein grafts. Two-photon confocal laser scan images demonstrating CM-DiI labeled leukocytes transmigrated into the graft wall 24-hours after graft implantation (A). Quantitatively, leukocytes binding and transmigration was increased 2-fold in low flow versus high flow vein grafts (B). (n=4 per group) This flow-dependent difference in leukocyte binding was independent of E-selectin mRNA expression (C). (n=4-5 per group)
To examine the potential role of endothelial surface adhesion molecules as the underlying mechanism for the difference in leukocyte recruitment, E-selectin gene expression in high and low flow rabbit vein grafts was assayed (Figure 3C). Although a clear time-dependent effect was evident, with an early up-regulation of E-selectin (P<0.001), no differences in E-selectin expression were observed between high and low flow grafts.
3.3 Effect of CCR2 Deletion in Shear-Mediated Neointimal Hyperplasia
To directly assess the interaction between CCR2-mediated macrophage recruitment and wall shear stress, a mouse vein graft model was utilized. CCR -/- and wild type murine vein grafts exposed to reduced and elevated blood flow rates were harvested at 7 and 28 days following implantation. Consistent with the rabbit grafts, neointimal hyperplasia in 28 day wild type murine vein grafts was augmented under low conditions (P=0.04, Figure 4). In contrast, vein grafts lacking CCR2 demonstrated no difference in neointimal area under low and high flow conditions, with the magnitude of neointimal thickening in both high and low flow CCR-/- grafts similar to high flow, wild type controls (P=NS). Examination of F4/80 staining in Day 7 vein grafts supported the importance of the MCP-1/CCR2 axis in macrophage recruitment under low flow conditions, as evidenced by a 39% and 44% reduction in macrophage content in high flow, wild type and low flow, CCR2 -/- grafts, compared to low flow, wild type controls (Figure 5). These data demonstrate that both a favorable (low shear) hemodynamic condition and intact macrophage chemotactic pathways are required for the recruitment of macrophages and the accelerated development of neointimal hyperplasia. The absence of either of these conditions leads to a notable reduction in neointimal thickening.
Figure 4.
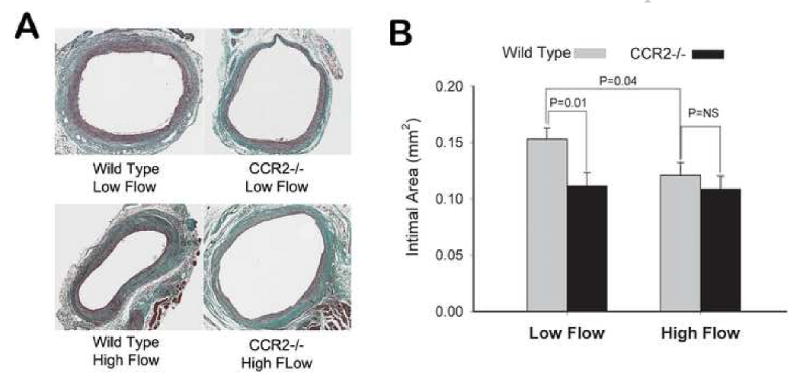
Flow regulated neointimal hyperplasia in CCR2 -/- and wild type vein grafts. While reduction in flow accelerated neointimal hyperplasia in wild type vein grafts (P=0.01), this augmentation was abolished when CCR2 was absent. Absence of the CCR2 receptor had an influence only under low flow conditions, where significant attenuation in neointimal hyperplasia was observed with loss of this signaling pathway (P=0.04).
Figure 5.
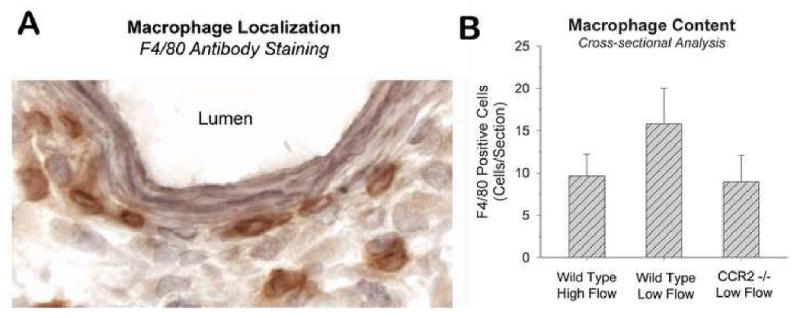
F4/80 antibody staining of Day 7, murine vein grafts (10 sections per graft). Low flow, wild type grafts demonstrated a notable increase in macrophage content compared to high flow, wild type grafts or low flow grafts lacking the CCR2 receptor. (n=3 per group)
4. Discussion
Vein grafts adapt to arterial environment by neointimal thickening. Medial smooth muscle cells undergo a conversion from a contractile to synthetic phenotype and migrate to the neointimal layer where these cells proliferate and lay down extracellular matrix proteins. Vital to understanding these events, however, is the clinical observation that vein graft occlusive disease is segmental, with clear regional variability in the processes that drive the wall towards an adaptive or pathologic phenotype.[10, 11] Local wall shearing forces have been postulated as a major regulator of these events where reductions in wall shear have been demonstrated to be critical components leading to accelerated neointimal hyperplasia and vein graft failure.
Evidence for the direct action of shear stress on endothelial function comes primarily from in vitro studies, where it has been demonstrated that genes important to vascular endothelial physiology are all influenced by this stimulus[12, 13] Several candidate shear stress sensors in endothelial cells have been postulated, which include integrins, ion channels, G proteins, and MAPK coupled receptors. Acting through a shear stress responsive element, consisting of a 6-bp core element (GAGACC) in many promoter regions, NF-κB, AP-1, Sp-1, and Erg-1 can be activated by changes in shear.[14] Despite these significant insights into the interaction of the endothelium with the flow environment, in vivo evidence of their biologic significance is limited. Several strong lines of evidence suggest an important role for monocytes in the development of neointimal hyperplasia following vascular injury.[15, 16] Among the seminal works in this area was completed by Richter et al.,[17] who demonstrated monocyte transmigration to be enhanced in regions of low shear following stent placement within an arterial bifurcation. Subsequent histologic analysis confirmed these areas to be the sites with advanced neointimal thickening. To further validate this critical link between the circulating inflammatory cell and the physical microenvironment in vein graft adaptation, we utilized a transgenic mouse lacking the CCR2 chemokine receptor, a vital link in the homing of leukocytes to sites of vascular injury. Using a partial distal ligation approach in this murine model, low shear CCR-2 -/- vein grafts demonstrated significantly less neointimal thickening than low shear controls, yet CCR2 -/- neointimal thickening was not different from wild-type controls in the high shear setting (Figure 3). These data support the conclusion that CCR2/MCP-1 monocyte recruitment and a low shear environment act synergistically to augment neointimal hyperplasia development and removal of either of the conditions leads to a significant reduction in neointimal thickening. Taken together, these experiments indicate a critical interaction between local hemodynamics and monocyte recruitment, and suggest a novel concept for the “shear stress response element”. Rather than a response based largely on biologic perturbations induced by shear (e.g. changes in gene expression), we theorize that the SSRE phenotypically stems from the complex interplay between the biologic and physical microenvironments that modulate the process of vascular adaptation.
In summary, the interaction between shear force and biologic mediators suggest that the impact of shear stress on neointimal hyperplasia is more complex than can be described through the expression and production of a limited set of key cytokines or signaling mediators. We theorize that the physical and biologic environments are implicitly linked and lead to specific vein graft phenotypes. Fundamental to this study is the concept that leukocyte entry into the wall is shear-dependent and that once entering the wall, these activated inflammatory cells have a critical role in vascular adaptation. Focusing on the critical process of monocyte entry into wall, we have identified that both chemokine up-regulation and reduced shear (with increased leukocyte binding and entry into the wall) are necessary to drive the neointimal hyperplastic response.
Acknowledgments
This work was supported by NIH Grants R01HL079135 and K08 HL04070, Lifeline Foundation, American Heart Association, and James & Esther King Biomedical Research Program
Abbreviations
- CCR2
CC chemokine receptor 2
- MCP-1
monocyte chemoattractant protein-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conte MS. Results of PREVENT III: a multi-center, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. 2005 doi: 10.1016/j.jvs.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 2.Westerband A, Mills JL, Marek JM, Heimark RL, Hunter GC, Williams SK. Immunocytochemical determination of cell type and proliferation rate in human vein graft stenoses. J Vasc Surg. 1997;25:64–73. doi: 10.1016/s0741-5214(97)70322-7. [DOI] [PubMed] [Google Scholar]
- 3.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 4.Schober A, Weber C. Mechanisms of monocyte recruitment in vascular repair after injury. Antioxid Redox Signal. 2005;7:1249–1257. doi: 10.1089/ars.2005.7.1249. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Z, Wu L, Miller BL, Goldman DR, Fernandez CM, Abouhamze ZS, Ozaki CK, Bercel SA. A novel vein graft model: adaptation to differential flow environments. Am J Physiol Heart Circ Physiol. 2004;286:H240–H245. doi: 10.1152/ajpheart.00760.2003. [DOI] [PubMed] [Google Scholar]
- 6.Gimbrone-MA J, Nagel T, Topper JN. Biomechanical activation: an emerging paradigm in endothelial adhesion biology. J Clin Invest. 1997;99:1809–1813. doi: 10.1172/JCI119346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Z, Wu L, Miller BL, Goldman DR, Fernandez CM, Abouhamze ZS, Ozaki CK, Berceli SA. A novel vein graft model: adaptation to differential flow environments. Am J Physiol Heart Circ Physiol. 2004;286:H240–H245. doi: 10.1152/ajpheart.00760.2003. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Z, Berceli SA, Pfahnl CL, Wu L, Goldman D, Tao M, Kagayama M, Matsukawa A, Ozaki CK. Wall shear modulation of cytokines in early vein grafts. J Vasc Surg. 2004;40:345–350. doi: 10.1016/j.jvs.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 9.Berceli SA, Jiang Z, Klingman NV, Schultz GS, Ozaki CK. Early differential MMP-2 and -9 dynamics during flow-induced arterial and vein graft adaptations. J Surg Res. 2006;134:327–334. doi: 10.1016/j.jss.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 10.Mills JL, Sr, Wixon CL, James DC, Devine J, Westerband A, Hughes JD. The natural history of intermediate and critical vein graft stenosis: recommendations for continued surveillance or repair. J Vasc Surg. 2001;33:273–278. doi: 10.1067/mva.2001.112701. [DOI] [PubMed] [Google Scholar]
- 11.Vesti BR, Primozich J, Bergelin RO, Strandness E., Jr Follow-up of valves in saphenous vein bypass grafts with duplex ultrasonography. J Vasc Surg. 2001;33:369–374. doi: 10.1067/mva.2001.111744. [DOI] [PubMed] [Google Scholar]
- 12.Chiu JJ, Wung BS, Hsieh HJ, Lo LW, Wang DL. Nitric oxide regulates shear stress-induced early growth response-1. Expression via the extracellular signal-regulated kinase pathway in endothelial cells. Circ Res. 1999;85:238–246. doi: 10.1161/01.res.85.3.238. [DOI] [PubMed] [Google Scholar]
- 13.Bao X, Lu C, Frangos JA. Temporal gradient in shear but not steady shear stress induces PDGF-A and MCP-1 expression in endothelial cells: role of NO, NF kappa B, and egr-1. Arterioscler Thromb Vasc Biol. 1999;19:996–1003. doi: 10.1161/01.atv.19.4.996. [DOI] [PubMed] [Google Scholar]
- 14.Resnick N, Gimbrone-MA J. Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J. 1995;9:874–882. doi: 10.1096/fasebj.9.10.7615157. [DOI] [PubMed] [Google Scholar]
- 15.Hoch JR, Stark VK, van Rooijen N, Kim JL, Nutt MP, Warner TF. Macrophage depletion alters vein graft intimal hyperplasia. Surgery. 1999;126:428–437. [PubMed] [Google Scholar]
- 16.Miyake T, Aoki M, Shiraya S, Tanemoto K, Ogihara T, Kaneda Y, Morishita R. Inhibitory effects of NFkappaB decoy oligodeoxynucleotides on neointimal hyperplasia in a rabbit vein graft model. J Mol Cell Cardiol. 2006;41:431–440. doi: 10.1016/j.yjmcc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Richter Y, Groothuis A, Seifert P, Edelman ER. Dynamic flow alterations dictate leukocyte adhesion and response to endovascular interventions. J Clin Invest. 2004;113:1607–1614. doi: 10.1172/JCI21007. [DOI] [PMC free article] [PubMed] [Google Scholar]


