Abstract
Bone morphogenetic protein 2 (BMP-2) is used clinically to stimulate bone formation and accelerate fracture repair. Adding prostaglandin (PG) E2 or PGE2 receptor agonists to BMP-2 has been proposed to improve BMP-2 efficacy. However, this may enhance bone resorption, since PGE2 can increase receptor activator of NF-κB ligand (RANKL) expression and decrease osteoprotegerin (OPG) expression in osteoblasts, and the RANKL: OPG ratio is critical for osteoclast formation. We used bone marrow (BM) cultures and BM macrophage (BMM) cultures from outbred CD-1 mice to examine effects on osteoclast formation of BMP-2 and PGE2. In BM cultures, which contain both osteoblastic and osteoclastic lineage cells, BMP-2 (100 ng/ml) alone did not increase osteoclast formation but enhanced the peak response to PGE2 by 1.6- to 9.6-fold. In BMM cultures, which must be treated with RANKL because they do not contain osteoblastic cells, BMP-2 did not increase osteoclast formation, with or without PGE2. Our results suggest that BMP-2 can increase osteoclast formation in response to PGE2 by increasing the RANKL:OPG ratio in osteoblasts, which may have therapeutic implications for the use of BMP-2.
Keywords: prostaglandin, cyclooxygenase 2 (COX-2), bone morphogenetic proteins, osteoclasts, mice, receptor activator of NF-kappa B ligand (RANKL), osteoprotegerin (OPG), macrophage colony-stimulating factor (M-CSF), bone marrow macrophage (BMM)
Introduction
Bone morphogenetic protein 2 (BMP-2) increases bone formation both in vitro and in vivo1,2. BMP-2 is used clinically as an alternative to bone grafting3. We have previously shown in vitro that BMP-2 requires cyclooxygenase (COX)-2 dependent prostaglandins (PGs) for full activity in stimulating osteoblastic differentiation2. Futhermore, combining BMP-2 with a PGE2 receptor agonist increases ectopic mineralization in vivo 4–6. These data suggest that PGE2 can enhance BMP-2 anabolic activity in bone.
Changes in bone mass are due to inequalities in formation of bone by osteoblasts and resorption of bone by osteoclasts. BMP-2 and PGE2 increase bone formation by increasing the differentiation of osteoblasts. PGE2 also increases osteoclast formation by stimulating osteoblasts to increase expression of receptor activator of nuclear factor κB ligand (RANKL), which, with macrophage-colony stimulating factor (M-CSF), is required for the differentiation of monocytic cells into active osteoclasts7,8. In addition, PGE2 can decrease osteoblastic expression of osteoprotegerin (OPG), a decoy receptor for RANKL, which inhibits osteoclast formation9.
A number of reports describe stimulatory effects of BMP-2 on osteoclast formation10–15. On the other hand there are reports that BMP-2 may suppress osteoclast formation, possibly by increasing OPG expression16–18. We previously found that BMP-2 increased osteoclast formation in murine bone marrow (BM) cultures from wild type but not COX-2 knockout mice2. Our data also suggested that the combination of BMP-2 with PGE2 resulted in greater osteoclast formation compared to BMP-2 or PGE2 alone. The goal of the present study was this determine if the BMP-2 enhancement of PGE2-stimulated osteoclast formation was due to effects of BMP-2 on RANKL and OPG expression in osteoblastic cells or due to direct effects of BMP-2 on osteoclastic precursors. We found that BMP-2 enhanced PGE2-stimulated osteoclast formation and RANKL:OPG ratio in BM cultures, but had no stimulatory effect on osteoblast-independent osteoclast formation in BM macrophage (BMM) cultures.
Materials and Methods
Animals
CD1 mice, aged 6–12 weeks, were used in all experiments. Mice were euthanized with carbon dioxide followed by cervical dislocation. All protocols were approved by the animal care committee of the University of Connecticut Health Center.
Materials
Unless stated otherwise, all reagents were obtained from Sigma-Aldrich (St. Louis, MO). PGE2 was purchased from Cayman Chemical (Ann Arbor, MI). Recombinant human BMP-2 was produced in E. coli using a maltose-binding protein (MBP)-fusion expression vector and the MBP-BMP-2 fusion protein was dimerized and cleaved, as described previously19. Consistent with our previous study, we found that 100 ng/ml BMP-2 maximally activated the COX-2 promoter in MC3T3-E1 cells2. Therefore, for the present study, we selected the same dose of BMP-2 (100 ng/ml) as we had used in the previous study2.
Cell culture
Whole BM for culture was obtained as previously described2,20,21. Briefly, femora and tibiae were dissected free of connective tissue. Using aseptic technique, the ends of the bones were cut and BM was flushed with complete cell culture media using a 21-gauge needle. The marrow was resuspended in cell culture media. An aliquot was hemolyzed with 2% acetic acid and counted by hemocytometer. Media for flushing and for all cultures was αMEM with 10% heat-inactivated fetal calf serum (HIFCS), penicillin (100 U/ml), and streptomycin (50 µg/ml). For BM cultures, marrow was plated at 106 cells/cm2 in 12-well dishes. Media and treatments were changed every 3 days. For BMM cultures, 5×106 cells were plated in Petri dishes and expanded with M-CSF (100 ng/ml; R&D Systems, Minneapolis, MN) using the method described by Epple et al.22. When cells reached confluence, they were replated at 5000 cells/well in a 96-well dish in media with RANKL (30 ng/ml; R&D Systems, Minneapolis, MN), M-CSF (30 ng/ml; R&D Systems) and treatments. Media were changed every 3 days.
Tartrate-resistant acid phosphatase (TRAP) stain
Osteoclasts were defined as TRAP+ multi-nucleated cells (MNCs). At the end of culture, cells were fixed with 2.5% gluteraldehyde for 30 minutes at room temperature and stained using a leukocyte acid phosphatase A kit (Sigma-Aldrich). Cells staining for TRAP with 3 or more nuclei were counted as osteoclasts.
Quantitative polymerase chain reaction (qPCR)
Total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA) and reverse transcribed with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). qPCR was performed using TaqMan Gene Expression Assays on the Applied Biosystems ABI Prism 7300 Sequence Detection System instrument utilizing universal thermal cycling parameters. Each sample was run in duplicate and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was the endogenous control, in parallel reactions. Each primer had equal efficiency with GAPDH over a range of target gene concentrations. The ΔΔCT method was used to analyze the relative quantity of target mRNA.
Statistics
Data are presented as means ± SEM. All experiments were performed with 3–4 replicates. Statistical analysis was performed using two-way analysis of variance, followed by Bonferroni post-hoc test. A p-value of less than 0.05 was considered statistically significant.
Results
BMP-2 enhanced PGE2-stimulated osteoclast formation in BM cultures
In 8 experiments, the average peak number of osteoclasts formed with BMP-2 (100 ng/ml) alone was 4.7±1.6 osteoclasts/well (range: 0–12), which was slightly greater, but not significantly, than control treated cultures, which formed 1.3±0.4 osteoclasts/well (range: 0–4). Similar to previous reports20,23, PGE2 (1 µM) increased osteoclast formation compared with control in 3 of 5 experiments (average peak: 320±183; range 12–965).
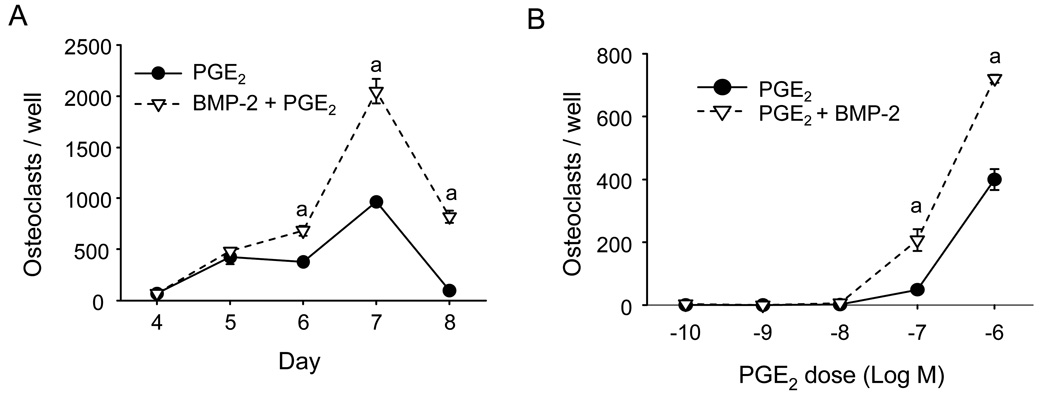
Addition of BMP-2 to PGE2 increased PGE2-stimulated osteoclast formation 4.5±0.9 fold in 8 experiments (range: 1.6–9.6 fold; Fig 1A). The average peak osteoclast formation in 8 experiments was 543±236 (range: 34–2047). Osteoclast number peaked on days 5 to 8, and there was no consistent change in the time course of osteoclast formation between PGE2 alone and PGE2 plus BMP-2. PGE2 dose-dependently increased osteoclast formation at 100 nM and 1 µM (Fig 1B). BMP-2 increased the number of osteoclasts formed at these doses but did not decrease the minimum dose of PGE2 required to stimulate osteoclastogenesis.
Figure 1.
Effect of PGE2 or the combination of BMP-2 and PGE2 on osteoclast formation in BM cultures. A) Cultures treated with PGE2 (1 µM), or PGE2 (1 µM) and BMP-2 (100 ng/ml). Osteoclasts per well were counted on the indicated days. B) Osteoclast numbers in cultures treated with increasing doses of PGE2 alone or in combination with BMP-2 (100 ng/ml) for 5 days. Data are presented as mean ± SEM for 3 wells/group. aSignificantly different from PGE2 alone, p<0.05.
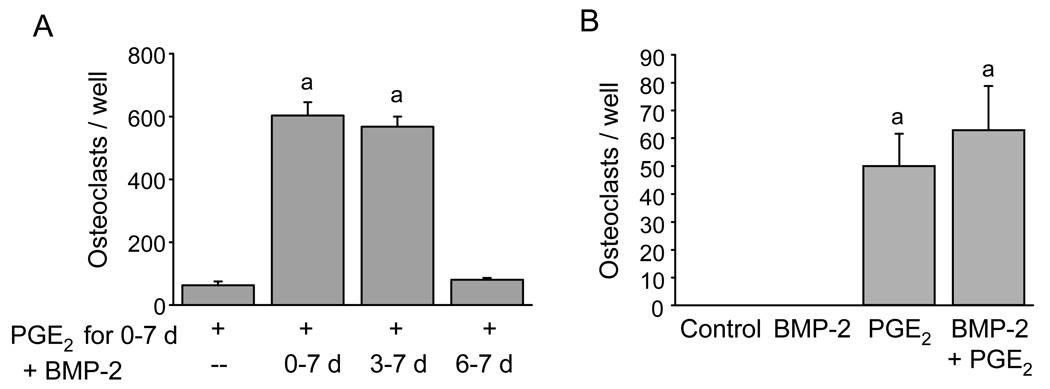
To determine the period of culture during which BMP-2 had its effects, we treated BM cultures with PGE2 (1 µM) for 9 days and added BMP-2 (100 ng/ml) for days 3–9, days 6–9, or continuously during cell culture. Osteoclast formation peaked on day 7 for all groups (data not shown). Comparison of groups on day 7 is shown in Fig 2A. BMP-2 added to PGE2 increased PGE2-stimulated osteoclast formation similarly when added from day 0–7 or from day 3–7. However, BMP-2 added from day 6–7 had no effect on peak PGE2-stimulated osteoclast formation.
Figure 2.
Timing of BMP-2 treatment on enhancement of osteoclast formation stimulated by PGE2. A) Osteoclast number on day 7 of culture. Cells were treated throughout with PGE2 (1 µM). BMP-2 (100 ng/ml) was added on day 0, 3, or 6 of the culture period. B) Acute effects of BMP-2. BM was cultured for 7 days before adding BMP-2 (100 ng/ml), PGE2 (1 µM), or the combination. Osteoclasts were counted 48 hours after treatment. Data are presented as mean ± SEM for 3 wells/group. aSignificantly different from PGE2 alone, p<0.05.
We also cultured BM for 7 days in basal media before beginning treatment with PGE2 (1 µM), BMP-2 (100 ng/ml), or the combination. Using this acute, delayed treatment protocol, we found that PGE2 stimulated osteoclast formation within 48 hours of treatment (Fig 2B). Treatment with BMP-2 alone did not stimulate osteoclast formation. There was no significant difference between treatment with PGE2 alone and BMP-2 and PGE2. Together, these results suggest that BMP-2 acts early in culture to increase osteoclast formation.
BMP-2 did not increase osteoclast formation in BMM cultures
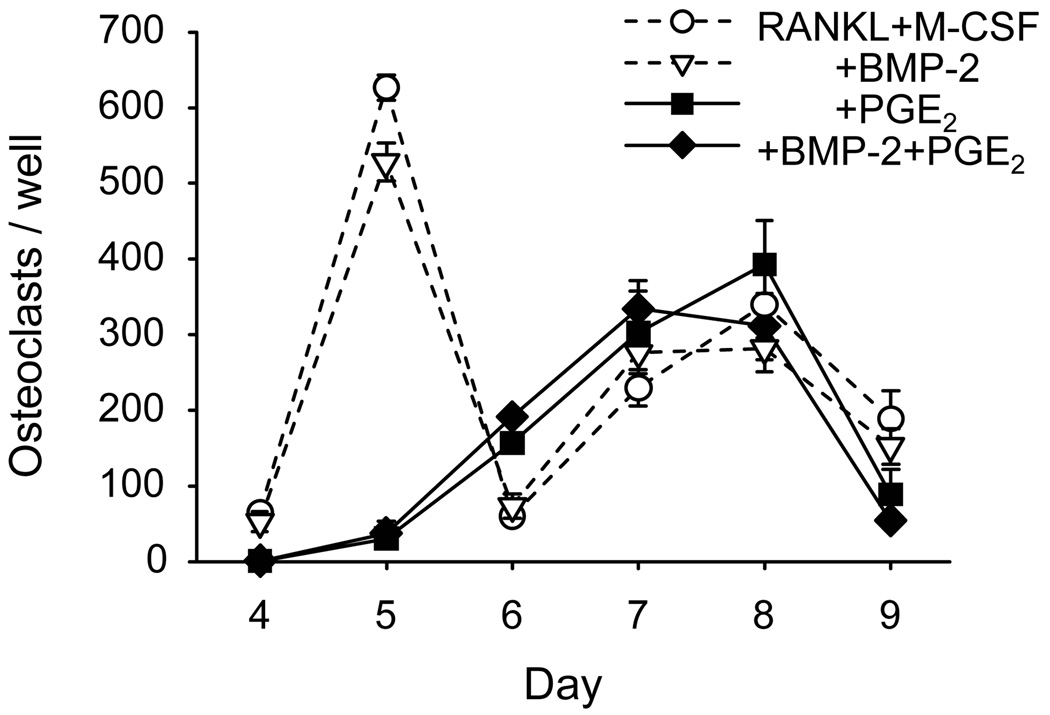
It is possible that BMP-2 increases osteoclast formation by increasing the number of osteoclast precursors or the ability of the precursors to fully differentiate. To test for effects of BMP-2 on osteoclast precursors in the absence of osteoblasts, we stimulated osteoclast formation in BMM cultures with RANKL (30 ng/ml) and M-CSF (30 ng/ml) and treated with BMP-2 (100 ng/ml), PGE2 (1 µM) or vehicle for 9 days (Fig 3). There were 2 waves of osteoclast formation. PGE2 inhibited osteoclast formation during the first wave, but not the second. Treatment with BMP-2 did not increase osteoclast formation, either alone or in the presence of PGE2, suggesting that BMP-2 is not acting on osteoclast precursors directly to increase osteoclast formation. Similarly, in spleen cell cultures, which also form osteoclasts with RANKL and M-CSF treatment, BMP-2 had no effect on the number of osteoclasts formed24.
Figure 3.
Effect of BMP-2 on RANKL and M-CSF stimulated osteoclast formation in BMMs. BMM cultures were stimulated to form osteoclasts with RANKL (30 ng/ml) and M-CSF (30 ng/ml), in the presence of BMP-2 (100 ng/ml), PGE2 (1 µM), or the combination. Osteoclasts per well were count on the indicated days. Data are presented as mean ± SEM for 4 wells/group.
Effect of BMP-2 and PGE2 on regulators of osteoclast formation
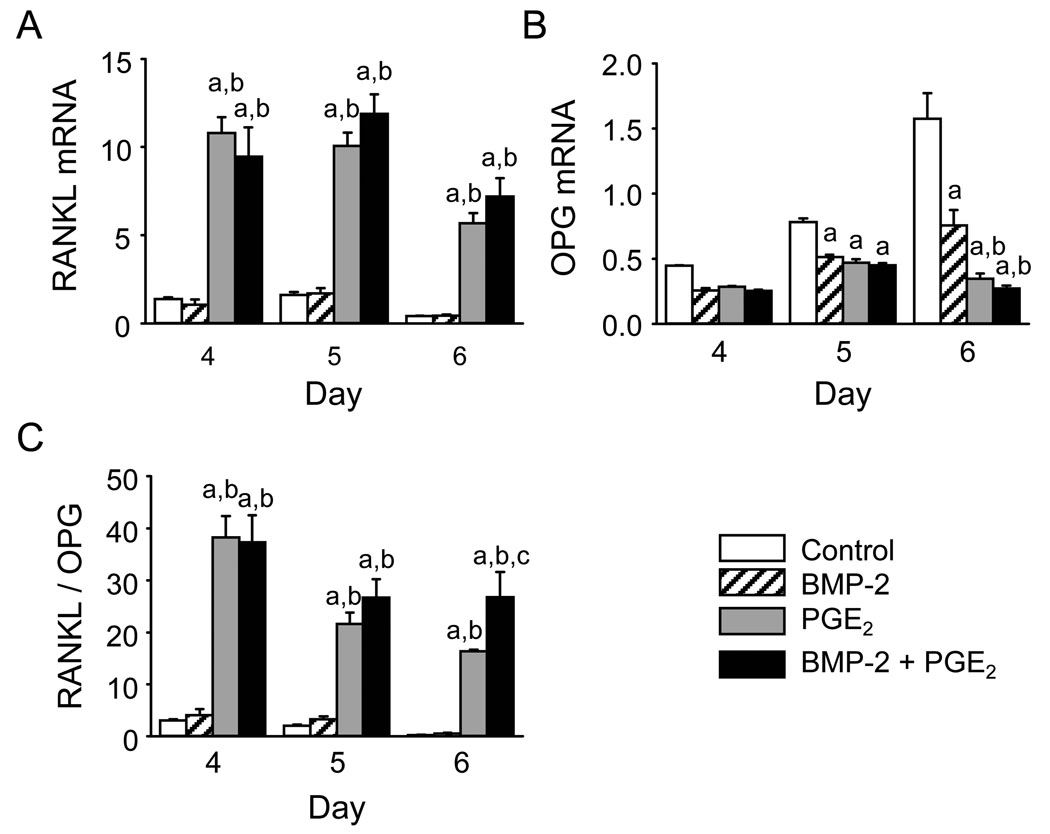
BM cultures contain precursors not only for osteoclasts but also for osteoblasts. Cells of the osteoblast lineage can express RANKL, OPG, and M-CSF, all of which regulate osteoclast differentiation. We measured the effects of BMP-2 and PGE2 on the expression of RANKL and OPG mRNA in BM cultures on days 4–6, prior to peak osteoclast formation, consistent with the early effect of BMP-2 (Fig 4). BMP-2 (100 ng/ml) alone had no effect on RANKL mRNA. Although BMP-2 decreased OPG mRNA 32–54% compared with control, the RANKL:OPG ratio was not significantly increased. Treatment with PGE2 (1 µM) both increased RANKL mRNA and decreased OPG mRNA, compared with control, resulting in an increased RANKL to OPG ratio. BMP-2 in combination with PGE2 resulted in small, non-significant increases in RANKL and decreases in OPG, compared with PGE2 alone. However, the RANKL to OPG ratio was increased by BMP-2 and PGE2 compared with PGE2 alone by 64±29% on day 6 in the experiment shown in Fig. 4. In a second experiment, the RANKL to OPG ratio was increased 64±8% on day 5.
Figure 4.
Effect of BMP-2, PGE2, or the combination on RANKL and OPG mRNA expression. BM cultures were treated with BMP-2 (100 ng/ml), PGE2 (1 µM), or the combination. On the indicated days, total RNA was extracted and the mRNA was reverse transcribed. mRNA levels of A) RANKL and B) OPG measured by qPCR. C) RANKL:OPG ratios calculated from the mRNA expression data. Data are presented as mean ± SEM for 3 samples/group. aSignificantly different from control, p<0.05. bSignificantly different from BMP-2 alone, p<0.05. cSignificantly different from PGE2 alone, p<0.05.
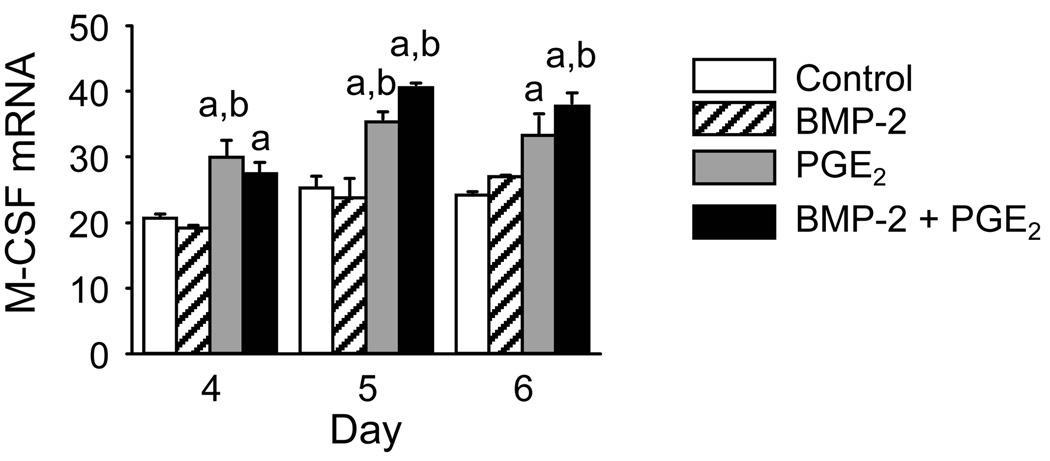
BMP-2 (100 ng/ml) had no effect on M-CSF mRNA compared with control (Fig 5). PGE2 (1 µM) increased M-CSF mRNA. Treatment with the combination of BMP-2 and PGE2 had no further effect on M-CSF mRNA.
Figure 5.
Effect of BMP-2, PGE2, or the combination on M-CSF mRNA expression. BM cultures were treated with BMP-2 (100 ng/ml), PGE2 (1 µM), or the combination. On the indicated days, total RNA was extracted and the mRNA was reverse transcribed. mRNA levels of M-CSF were measured by qPCR. Data are presented as mean ± SEM for 3 samples/group. aSignificantly different from control, p<0.05. bSignificantly different from BMP-2 alone, p<0.05.
Discussion
We and others have previously reported that BMP-2 alone increased osteoclast formation2,10. However, in the current study, BMP-2 alone had no significant effect on osteoclast formation in murine BM cultures. It is possible that differences in the effects of BMP-2 on osteoclast formation are due to differences in mouse strains used in the experiments. In this study, we used outbred CD1 mice, whereas in our previous study, the mice were in a mixed C57Bl/6 × 129/sv background. BMP-2 treatment did not increase osteoclast formation in BMM cultures stimulated with RANKL and M-CSF but did increase the RANKL to OPG ratio in PGE2-treated BM cultures. These results suggest that the major mechanism by which BMP-2 increases osteoclast formation is through an interaction with PGE2 in cells of the osteoblast lineage.
It is possible that the predominant role of BMP-2 in osteoclast formation is to enhance the effects of other osteoclastogenic stimuli. Similar to our results, BMP-2 has been reported to enhance interleukin 1 (IL-1), 1,25-dihydroxyvitamin D, and parathyroid hormone (PTH) stimulated formation of osteoclasts10–12,14. The BMP-2 enhancement of IL-1 stimulated osteoclastogenesis was associated with increased COX-2 and inhibited by a COX-2 selective inhibitor, NS-398. Since 1,25-dihydroxyvitamin D and PTH can stimulate COX-2 expression and PG synthesis, it is possible that BMP-2 enhancement of osteoclast formation by these hormones is also mediated in part by PGs25.
Others have found a variable effect of BMP-2 on RANKL-dependent osteoclast formation13,17,26,27. In our model of osteoclast precursors, BMMs, we found no effect of BMP-2 on RANKL stimulated osteoclast formation. Treatment with RANKL and M-CSF stimulated osteoclast formation with peaks at two different time points. Multiple peaks have been described in other models of osteoclast formation28. PGE2 inhibited osteoclast formation at the first peak and later had no effect. We have observed similar biphasic effects of PGE2 on osteoclast formation in spleen cells treated with M-CSF and RANKL29. These results suggest that data obtained at one time point in osteoclast cultures can be misleading and that it is essential to perform a time course.
BMP-2 had small, non-significant effects on RANKL and OPG mRNA in the presence of PGE2, but these effects combined to increase the RANKL:OPG ratio compared with PGE2 alone. Koide, et. al., reported that in co-cultures of primary calvarial osteoblasts and BM, BMP-2 and IL-1 individually increased RANKL mRNA and together there was a further increase in RANKL mRNA, which was dependent on endogenous PGs11. BMP-2 has also been reported to increase RANKL expression in C2C12 myoblastic cell and chondrocytes30,31. However, we found no effect of BMP-2 alone on RANKL expression. BMP-2 has been reported to increase expression of M-CSF in C2C12 myoblastic cells15,16. However there was no change in M-CSF mRNA in our BM cultures with BMP-2 treatment. As our effect on osteoclast formation is large and the effect on RANKL and OPG is modest, it is possible that BMP-2 also acts on other osteoblastic factors, such as ITAM-stimulated signaling32.
BMP-2 has been reported to increase OPG expression. Treatment of immortalized human fetal osteoblasts with BMP-2 increased OPG mRNA and protein, but treatment of other human cell lines with BMP-2 did not have any effect on OPG mRNA33. Additionally, BMP-2 has been found to increase the expression of OPG in the mesenchymal cell line C3H10T1/2 and the myoblastic cell line C2C1216,18. In contrast, we observed a small decrease in OPG mRNA with BMP-2 treatment alone on day 5 and 6, but this effect was not sufficient to alter the RANKL:OPG ratio. It is likely that BMP-2 effects on OPG expression depends the cell type or culture conditions.
BMP-2 could otherwise change the phenotype of osteoblastic cells. This would be consistent with our observation that BMP-2 acts early in culture to enhance PGE2-stimulated osteoclast formation. This may also account for the variability of published observation of the effect of BMP-2 on OPG expression.
In conclusion, we found that while BMP-2 alone had minimal effects on osteoclast formation, it enhanced osteoclast formation that was stimulated by PGE2. Thus, endogenous and exogenous PGs likely increase both osteoblast and osteoclast differentiation in response to BMP-2. Modulating PGs may have important implications in BMP-2 based therapies. For example, in spinal fusion, resorption would likely be deleterious and co-administration of BMP-2 with a COX inhibitor might improve the outcome34–36. On the other hand, treatment with PGE2 agonists might enhance the response to or decrease the dose of BMP-2 required for conditions in which both bone formation and resorption are critical, such as fracture repair or distraction osteogenesis. In vivo inhibition of PGs may have deleterious effects, as we have found that BMP-2 has a diminished capacity to mineralize collagen pellets implanted in COX-2 knockout mice2. Future in vivo studies of the modulatory effect of PGs on BMP-2 induced bone formation and resorption could help to tailor BMP-2 therapy for improved outcomes.
Acknowledgments
This work was supported by NIH grants F30AG034013 to KAB, R01DK048361 to CCP, and R01AR018063 to LGR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang EA, Rosen V, D'Alessandro JS, et al. Recombinant Human Bone Morphogenetic Protein Induces Bone Formation. Proc Natl Acad Sci U S A. 1990;87:2220. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chikazu D, Li X, Kawaguchi H, et al. Bone Morphogenetic Protein 2 Induces Cyclo-Oxygenase 2 in Osteoblasts Via a Cbfal Binding Site: Role in Effects of Bone Morphogenetic Protein 2 in Vitro and in Vivo. J Bone Miner Res. 2002;17:1430. doi: 10.1359/jbmr.2002.17.8.1430. [DOI] [PubMed] [Google Scholar]
- 3.Schmidmaier G, Wildemann B. The Role of BMPs in Current Orthopedic Practice. IBMS BoneKEy. 2009;6:244. [Google Scholar]
- 4.Sasaoka R, Terai H, Toyoda H, Imai Y, Sugama R, Takaoka K. A Prostanoid Receptor EP4 Agonist Enhances Ectopic Bone Formation Induced by Recombinant Human Bone Morphogenetic Protein-2. Biochem Biophys Res Commun. 2004;318:704. doi: 10.1016/j.bbrc.2004.04.080. [DOI] [PubMed] [Google Scholar]
- 5.Toyoda H, Terai H, Sasaoka R, Oda K, Takaoka K. Augmentation of Bone Morphogenetic Protein-Induced Bone Mass by Local Delivery of a Prostaglandin E EP4 Receptor Agonist. Bone. 2005;37:555. doi: 10.1016/j.bone.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 6.Namikawa T, Terai H, Hoshino M, et al. Enhancing Effects of a Prostaglandin EP4 Receptor Agonist on Recombinant Human Bone Morphogenetic Protein-2 Mediated Spine Fusion in a Rabbit Model. Spine. 2007;32:2294. doi: 10.1097/BRS.0b013e318154c5b6. [DOI] [PubMed] [Google Scholar]
- 7.Tsukii K, Shima N, Mochizuki S, et al. Osteoclast Differentiation Factor Mediates an Essential Signal for Bone Resorption Induced by 1 Alpha,25-Dihydroxyvitamin D3, Prostaglandin E2, or Parathyroid Hormone in the Microenvironment of Bone. Biochem Biophys Res Commun. 1998;246:337. doi: 10.1006/bbrc.1998.8610. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Pilbeam CC, Pan L, Breyer RM, Raisz LG. Effects of Prostaglandin E2 on Gene Expression in Primary Osteoblastic Cells From Prostaglandin Receptor Knockout Mice. Bone. 2002;30:567. doi: 10.1016/s8756-3282(02)00683-x. [DOI] [PubMed] [Google Scholar]
- 9.Suda K, Udagawa N, Sato N, et al. Suppression of Osteoprotegerin Expression by Prostaglandin E2 Is Crucially Involved in Lipopolysaccharide-Induced Osteoclast Formation. J Immunol. 2004;172:2504. doi: 10.4049/jimmunol.172.4.2504. [DOI] [PubMed] [Google Scholar]
- 10.Otsuka E, Notoya M, Hagiwara H. Treatment of Myoblastic C2C12 Cells With BMP-2 Stimulates Vitamin D-Induced Formation of Osteoclasts. Calcif Tissue Int. 2003;73:72. doi: 10.1007/s00223-002-1071-0. [DOI] [PubMed] [Google Scholar]
- 11.Koide M, Murase Y, Yamato K, Noguchi T, Okahashi N, Nishihara T. Bone Morphogenetic Protein-2 Enhances Osteoclast Formation Mediated by Interleukin-1alpha Through Upregulation of Osteoclast Differentiation Factor and Cyclooxygenase-2. Biochem Biophys Res Commun. 1999;259:97. doi: 10.1006/bbrc.1999.0715. [DOI] [PubMed] [Google Scholar]
- 12.Abe E, Yamamoto M, Taguchi Y, et al. Essential Requirement of BMPs-2/4 for Both Osteoblast and Osteoclast Formation in Murine Bone Marrow Cultures From Adult Mice: Antagonism by Noggin. J Bone Miner Res. 2000;15:663. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- 13.Itoh K, Udagawa N, Katagiri T, et al. Bone Morphogenetic Protein 2 Stimulates Osteoclast Differentiation and Survival Supported by Receptor Activator of Nuclear Factor-KappaB Ligand. Endocrinology. 2001;142:3656. doi: 10.1210/endo.142.8.8300. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto M, Murai J, Yoshikawa H, Tsumaki N. Bone Morphogenetic Proteins in Bone Stimulate Osteoclasts and Osteoblasts During Bone Development. J Bone Miner Res. 2006;21:1022. doi: 10.1359/jbmr.060411. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh-Choudhury N, Singha PK, Woodruff K, et al. Concerted Action of Smad and CREB-Binding Protein Regulates Bone Morphogenetic Protein-2-Stimulated Osteoblastic Colony-Stimulating Factor-1 Expression. J Biol Chem. 2006;281:20160. doi: 10.1074/jbc.M511071200. [DOI] [PubMed] [Google Scholar]
- 16.Susperregui AR, Vinals F, Ho PW, Gillespie MT, Martin TJ, Ventura F. BMP-2 Regulation of PTHrP and Osteoclastogenic Factors During Osteoblast Differentiation of C2C12 Cells. J Cell Physiol. 2008;216:144. doi: 10.1002/jcp.21389. [DOI] [PubMed] [Google Scholar]
- 17.Wan C, He Q, Li G. Osteoclastogenesis in the Nonadherent Cell Population of Human Bone Marrow Is Inhibited by RhBMP-2 Alone or Together With RhVEGF. J Orthop Res. 2006;24:29. doi: 10.1002/jor.20010. [DOI] [PubMed] [Google Scholar]
- 18.Wan M, Shi X, Feng X, Cao X. Transcriptional Mechanisms of Bone Morphogenetic Protein-Induced Osteoprotegrin Gene Expression. J Biol Chem. 2001;276:10119. doi: 10.1074/jbc.M006918200. [DOI] [PubMed] [Google Scholar]
- 19.Sachse A, Wagner A, Keller M, et al. Osteointegration of Hydroxyapatite-Titanium Implants Coated With Nonglycosylated Recombinant Human Bone Morphogenetic Protein-2 (BMP-2) in Aged Sheep. Bone. 2005;37:699. doi: 10.1016/j.bone.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Tomita M, Li X, Okada Y, et al. Effects of Selective Prostaglandin EP4 Receptor Antagonist on Osteoclast Formation and Bone Resorption in Vitro. Bone. 30;159:2002. doi: 10.1016/s8756-3282(01)00688-3. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Okada Y, Pilbeam CC, et al. Knockout of the Murine Prostaglandin EP2 Receptor Impairs Osteoclastogenesis in Vitro. Endocrinology. 2000;141:2054. doi: 10.1210/endo.141.6.7518. [DOI] [PubMed] [Google Scholar]
- 22.Epple H, Cremasco V, Zhang K, Mao D, Longmore GD, Faccio R. Phospholipase Cgamma2 Modulates Integrin Signaling in the Osteoclast by Affecting the Localization and Activation of Src Kinase. Mol Cell Biol. 2008;28:3610. doi: 10.1128/MCB.00259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins DA, Chambers TJ. Effect of Prostaglandins E1, E2, and F2α on Osteoclast Formation in Mouse Bone Marrow Cultures. J Bone Miner Res. 1991;6:157. doi: 10.1002/jbmr.5650060209. [DOI] [PubMed] [Google Scholar]
- 24.Blackwell KA, Hortschansky P, Sanovic S, et al. Mechanism of BMP-2 Enhancement of PGE2 Stimulated Osteoclastogenesis. J Bone Miner Res. 2008;23:S263. [Google Scholar]
- 25.Okada Y, Lorenzo JA, Freeman AM, et al. Prostaglandin G/H Synthase-2 Is Required for Maximal Formation of Osteoclast-Like Cells in Culture. J Clin Invest. 2000;105:823. doi: 10.1172/JCI8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez JS, Mansky KC, Jensen ED, et al. Enhanced Osteoclastogenesis Causes Osteopenia in Twisted Gastrulation-Deficient Mice Through Increased BMP Signaling. J Bone Miner Res. 2009 doi: 10.1359/JBMR.090507. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koseki T, Gao Y, Okahashi N, et al. Role of TGF-Beta Family in Osteoclastogenesis Induced by RANKL. Cell Signal. 2002;14:31. doi: 10.1016/s0898-6568(01)00221-2. [DOI] [PubMed] [Google Scholar]
- 28.Akchurin T, Aissiou T, Kemeny N, Prosk E, Nigam N, Komarova SV. Complex Dynamics of Osteoclast Formation and Death in Long-Term Cultures. PLoS One. 2008;3:e2104. doi: 10.1371/journal.pone.0002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ono K, Kaneko H, Choudhary S, et al. Biphasic Effect of Prostaglandin E2 on Osteoclast Formation in Spleen Cell Cultures: Role of the EP2 Receptor. J Bone Miner Res. 2005;20:23. doi: 10.1080/14041040510033842. [DOI] [PubMed] [Google Scholar]
- 30.Fujita K, Janz S. Attenuation of WNT Signaling by DKK-1 and -2 Regulates BMP2-Induced Osteoblast Differentiation and Expression of OPG, RANKL and M-CSF. Mol Cancer. 2007;6:71. doi: 10.1186/1476-4598-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usui M, Xing L, Drissi H, et al. Murine and Chicken Chondrocytes Regulate Osteoclastogenesis by Producing RANKL in Response to BMP2. J Bone Miner Res. 2008;23:314. doi: 10.1359/JBMR.071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koga T, Inui M, Inoue K, et al. Costimulatory Signals Mediated by the ITAM Motif Cooperate With RANKL for Bone Homeostasis. Nature. 2004;428:758. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 33.Hofbauer LC, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Osteoprotegerin Production by Human Osteoblast Lineage Cells Is Stimulated by Vitamin D, Bone Morphogenetic Protein-2, and Cytokines. Biochem Biophys Res Commun. 1998;250:776. doi: 10.1006/bbrc.1998.9394. [DOI] [PubMed] [Google Scholar]
- 34.Poynton AR, Lane JM. Safety Profile for the Clinical Use of Bone Morphogenetic Proteins in the Spine. Spine. 2002;27:S40. doi: 10.1097/00007632-200208151-00010. [DOI] [PubMed] [Google Scholar]
- 35.Hansen SM, Sasso RC. Resorptive Response of RhBMP2 Simulating Infection in an Anterior Lumbar Interbody Fusion With a Femoral Ring. J Spinal Disord Tech. 2006;19:130. doi: 10.1097/01.bsd.0000168512.61351.3a. [DOI] [PubMed] [Google Scholar]
- 36.Lewandrowski KU, Nanson C, Calderon R. Vertebral Osteolysis After Posterior Interbody Lumbar Fusion With Recombinant Human Bone Morphogenetic Protein 2: a Report of Five Cases. Spine J. 2007;7:609. doi: 10.1016/j.spinee.2007.01.011. [DOI] [PubMed] [Google Scholar]