Abstract
Rationale
Voltage-dependent L-type (CaV1.2) Ca2+ channels are a heteromeric complex formed from pore forming α1 and auxiliary α2δ and β subunits. CaV1.2 channels are the principal Ca2+ influx pathway in arterial myocytes and regulate multiple physiological functions, including contraction. The macromolecular composition of arterial myocyte CaV1.2 channels remains poorly understood, with no studies having examined the molecular identity or physiological functions of α2δ subunits.
Objective
Investigate the functional significance of α2δ subunits in myocytes of resistance-size (100–200 μm diameter) cerebral arteries.
Methods and Results
α2δ-1 was the only α2δ isoform expressed in cerebral artery myocytes. Pregabalin, an α2δ-1/-2 ligand, and an α2δ-1 antibody, inhibited CaV1.2 currents in isolated myocytes. Acute pregabalin application reversibly dilated pressurized arteries. Using a novel application of surface biotinylation, data indicated that >95 % of CaV1.2 α1 and α2δ-1 subunits are present in the arterial myocyte plasma membrane. α2δ-1 knockdown using shRNA reduced plasma membrane-localized CaV1.2 α1 subunits, caused a corresponding elevation in cytosolic CaV1.2 α1 subunits, decreased intracellular Ca2+ concentration, inhibited pressure-induced vasoconstriction (“myogenic tone”), and attenuated pregabalin-induced vasodilation. Prolonged (24 hour) pregabalin exposure did not alter total α2δ-1 or CaV1.2 α1 proteins, but decreased plasma membrane expression of each subunit, which reduced myogenic tone.
Conclusions
α2δ-1 is essential for plasma membrane expression of arterial myocyte CaV1.2 α1 subunits. α2δ-1 targeting can block CaV1.2 channels directly and inhibit surface expression of CaV1.2 α1 subunits, leading to vasodilation. These data identify α2δ-1 as a novel molecular target in arterial myocytes, manipulation of which regulates contractility.
Keywords: L-type Ca2+ channels, arterial contractility
Introduction
Voltage-dependent calcium (Ca2+) channels are expressed in multiple cell types, including neurons, cardiac myocytes, skeletal muscle, and smooth muscle. 1 Voltage-dependent Ca2+ channels are heteromeric complexes comprising a pore forming α1 subunit and auxiliary α2δ, β and γ subunits. 1 Four major β subunit isoforms (β1 - β4) have been described, which are the products of different genes.1, 2 Four genes that encode different α2δ isoforms (α2δ-1 - α2δ-4) have also been cloned.3, 4 Each α2δ isoform is the product of a single gene, which is post-translationally cleaved into a highly glycosylated extracellular α2 and a smaller membrane-spanning δ. α2 and δ subunits re-associate via a disulfide bond to form a single functional protein. 3, 5, 6
In arterial smooth muscle cells, voltage-dependent Ca2+ channels are the major Ca2+ entry pathway and regulate numerous cellular functions, including contractility and gene expression. 7–9 Cav1.2 α1 is generally considered to be the principal pore-forming Cav subunit that is expressed in arterial smooth muscle cells. 9–11 In contrast, expression and physiological functions of Cav auxiliary subunits in the vasculature are poorly understood. Several β subunit isoforms (β1b, β2 and β3), have been identified in smooth muscle cells, although physiological functions of these subunits are uncertain. 7, 12–15 Furthermore, there are no studies that have examined the molecular identity and physiological functions of α2δ subunits that are expressed in arterial smooth muscle cells. Investigating Cav channel auxiliary subunits in arterial smooth muscle cells is important given the relevance of these channels to vascular physiology.
Cellular functions of α2δ subunits have previously been studied primarily through heterologous overexpression of recombinant proteins. α2δ subunits promote plasma membrane trafficking of recombinant Cav channel α1 subunits and modify the biophysical properties of currents generated by recombinant Cav channels.3, 6, 14, 16 However, recent studies performed in native cell types have suggested that physiological functions of α2δ subunits may differ from those in heterologous expression systems. In dysgenic myotubes, α2δ-1 did not alter membrane targeting of Cav1.1 α1 subunits or modify Ca2+ current amplitude and was not essential for excitation-contraction, but knockdown accelerated current activation. 17, 18 In cardiac myocytes, α2δ-1 knockdown did not alter Cav1.2 α1 subunit membrane targeting, but modified current voltage-dependence and inhibited excitation-contraction coupling. 19 Thus, evidence suggests that physiological functions of native α2δ subunits can differ from those of recombinant proteins.
Here, we investigated the molecular identity and physiological functions of α2δ subunits that are expressed in arterial smooth muscle cells. Our study was performed using resistance-size cerebral arteries that regulate brain regional blood pressure and flow. Our data indicate that α2δ-1 is the only α2δ isoform that is expressed in arterial smooth muscle cells. Targeting of membrane resident α2δ-1 subunits inhibits CaV1.2 currents and causes vasodilation. We also show that α2δ-1 stimulates membrane insertion of Cav1.2 α1 subunits and that inhibition of this process causes prolonged vasodilation. These data indicate that α2δ-1 subunits are an essential regulator of arterial contractility and identify α2δ-1 targeting as a novel approach to cause vasodilation.
Methods
Tissue preparation
Male Sprague-Dawley rats (~250g) were euthanized by intra-peritoneal injection of sodium pentobarbital solution (150 mg/kg). The brain was removed and placed into physiological saline solution (PSS) containing (in mM): KCl 6, NaCl2 112, NaHCO3 24, MgSO4 1.2, KH2PO4 1.2, CaCl2 1.8, and glucose 10. Middle cerebral, posterior cerebral, and cerebellar arteries (100–200 μm diameter), were dissected from the brain and used for this study. Smooth muscle cells were enzymatically dissociated from cerebral arteries, as previously described. 20
Reverse Transcription and Polymerase Chain Reaction
For RT-PCR, acutely isolated arterial smooth muscle cells were manually collected under a microscope using an enlarged patch-clamp pipette to prevent message contamination from other vascular wall cell types, as we have done previously. 21 RT-PCR was also performed on whole brain and intact cerebral arteries.
α2δ-1 knockdown
Three gene specific shRNA sequences were designed to the α2 and δ-1 regions of the α2δ-1 gene and inserted into the pRNA-U6.1/Neo vector (Genscript). All three α2δ-1 suppression vectors (α2δ-1shV) were inserted (10 μg/ml) into rat cerebral arteries using reverse permeabilization. 22 Suppression vectors that encode scrambled sequences (α2δ-1scrm) were used as a control. Arteries were then placed into serum-free DMEM F12 supplemented with 1% penicillin-streptomycin (Sigma) for 4 days at 37°C in a sterile incubator (21 % O2/5 % CO2), as we have done previously.23
Biotinylation
Arteries were incubated with EZ-Link Sulfo-NHS-LC-LC-Biotin and Maleimide-PEG2-Biotin reagents (Pierce). Unbound biotin was removed by quenching and washing. For fluorescence measurements, biotinylated arteries or control arteries were incubated with Texas red-conjugated streptavidin. Texas red was excited at 561 nm and emission collected at 575–632 nm using a Zeiss 5-live laser-scanning confocal microscope.
For protein determination, biotinylated arteries were homogenized in RIPA buffer (Sigma) and cellular debris removed by centrifugation. Total protein was determined 24 to allow normalization for Avidin (Monomeric Avidin, Pierce) pull-down of biotinylated surface proteins. Following pull down, the supernatant comprised the non-biotinylated (cytosolic) protein fraction, while surface proteins remained bound to the Avidin beads. Proteins were eluted from beads by boiling in Laemmli-buffer containing 2-mercaptoethanol. Total, surface, and cytosolic proteins were analyzed using Western blotting. Band intensity was determined using Quantity One software (BioRad). Surface and cytosolic proteins were calculated as a percentage of total protein.
Patch Clamp Electrophysiology
Whole cell patch-clamp recordings were carried out on isolated cerebral artery smooth muscle cells, as previously described. 14
Pressurized artery myography
Where required, endothelium was denuded as previously described. 21 Pressurized cerebral artery diameter was measured using edge-detection myography. Myogenic tone (%) was calculated as 100 ×(1 – active diameter/passive diameter).
Statistical Analysis
Summary data are presented as mean ± SEM. Significance was determined using ANOVA for multiple groups, or paired or unpaired t-tests with Welsh correction. P<0.05 was considered significant. Power analysis was carried out where P>0.05 to verify that sample size was sufficient to give a value >0.8.
Expanded Materials and Methods are provided as Supplemental Documentation.
Results
Rat cerebral artery smooth muscle cells express only one α2δ isoform, α2δ-1
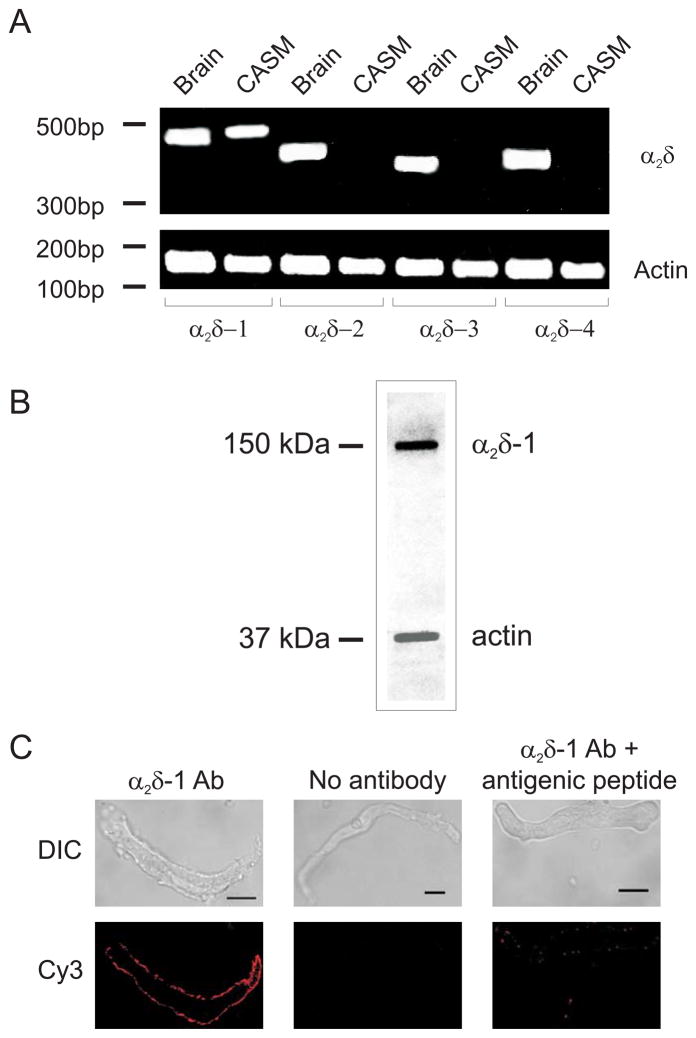
To measure α2δ isoform expression, RT-PCR was performed on RNA extracted from rat brain, cerebral arteries, and pure isolated cerebral artery smooth muscle cells. RT-PCR amplified transcripts for all four α2δ isoforms in brain and cerebral arteries, which contain multiple cell types (Figs. 1A, Supplemental Fig. 1). In contrast, only α2δ-1 message was detected in isolated arterial smooth muscle cells (Fig. 1A). Western blotting indicated that α2δ-1 protein was expressed in cerebral arteries (Fig. 1B). The α2δ-1 antibody used for Western blotting recognizes an extracellular epitope on α2. Immunofluorescence performed by applying the α2δ-1 primary antibody to live isolated smooth muscle cells via the bath solution, produced plasma membrane-localized fluorescence that was abolished by antigenic peptide or removal of the primary antibody (Fig. 1C). These data indicate that cerebral artery smooth muscle cells express only the α2δ-1 isoform of α2δ, and that α2δ-1 subunits are present in the smooth muscle cell plasma membrane.
Figure 1.
Cerebral artery smooth muscle cells express only α2δ-1 subunits. A. RT-PCR amplified transcripts for all α2δ isoforms in rat brain, but only amplified transcripts for α2δ-1 in isolated myocytes. The image is representative of 5 separate experiments. CASM: cerebral artery smooth muscle cells. B. Representative Western blot of rat cerebral artery lysate probed first with α2δ-1 antibody (~150 kDa) and subsequently with anti-actin antibody. C. Immunofluorescence (Cy3-conjugated secondary antibody) images of isolated cerebral artery smooth muscle cells illustrating α2δ-1 localization at the plasma membrane. Scale bars = 10 μm.
Pregabalin, an α2δ ligand, and an α2δ-1 antibody inhibit arterial smooth muscle cell CaV1.2 currents
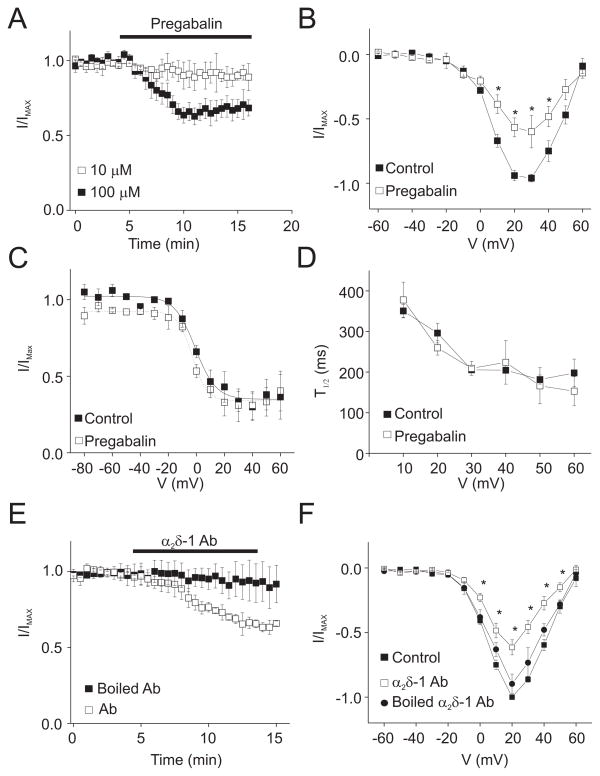
Regulation of arterial smooth muscle cell Cav1.2 currents by α2δ-1 was examined using patch-clamp electrophysiology. Pregabalin, an α2δ-1/2 ligand 25, 26, at 10 and 100 μM reduced mean voltage-dependent Ba2+ currents by ~10 and ~33 %, respectively (at +20 mV; Fig. 2A, B, Supplemental Fig. 2A). In contrast, pregabalin did not shift the current-voltage (I-V) relationship, alter steady-state inactivation, or modify the rate of current inactivation (Fig. 2B, C, D, Supplemental Fig. 2B).
Figure 2.
Pregabalin and α2δ-1 antibody inhibit voltage-dependent Ba2+ currents in isolated myocytes. A. Time course illustrating mean time-dependent effect of 10 (n=5) and 100 μM (n=5) pregabalin on voltage-dependent Ba2+ (20 mM as charge carrier) currents elicited by repetitive step depolarization from −80 to +20 mV (P<0.05 for each concentration). B. I-V plot for mean data obtained from the same cells in the absence and presence of pregabalin (100 μM, n=5). C. Voltage-dependence of inactivation in the same cells in the presence or absence of pregabalin (100 μM, n=3). D. Mean time to half peak for voltage-dependent inactivation in the same cells in the absence and presence of pregabalin (100 μM) (n=3). E. Time course illustrating the mean effect of boiled α2δ-1 antibody (1:500, n=4) or α2δ-1 antibody (1:500, n=4) on currents elicited by repetitive step depolarizations from −80 to +20 mV. F. Mean I-V plot in control (no antibody, n=4), boiled α2δ-1 antibody (n=4), and active α2δ-1 antibody (n=4). * indicates P<0.05.
Bath application of the α2δ-1 antibody that was used for Western blotting and immunofluorescence experiments also reduced arterial smooth muscle cell voltage-dependent Ba2+ currents to ~65% of control (at +20 mV) (Fig. 2E). Similarly to pregabalin, α2δ-1 antibody did not shift the I-V relationship (Fig. 2F). Boiled (95°C for 15 min) antibody did not inhibit voltage-dependent Ba2+ currents, when compared to untreated control (Fig. 2E, F). These data indicate that α2δ-1 ligands target membrane-resident α2δ-1 subunits in arterial smooth muscle cells, leading to Cav1.2 current inhibition.
Pregabalin dilates pressurized cerebral arteries
Cav1.2 channels are essential for pressure-induced vasoconstriction; termed the “myogenic response”. 7–9,11 To examine physiological functions of smooth muscle cell α2δ-1 subunits, we studied diameter responses to α2δ-1 manipulation in pressurized cerebral arteries.
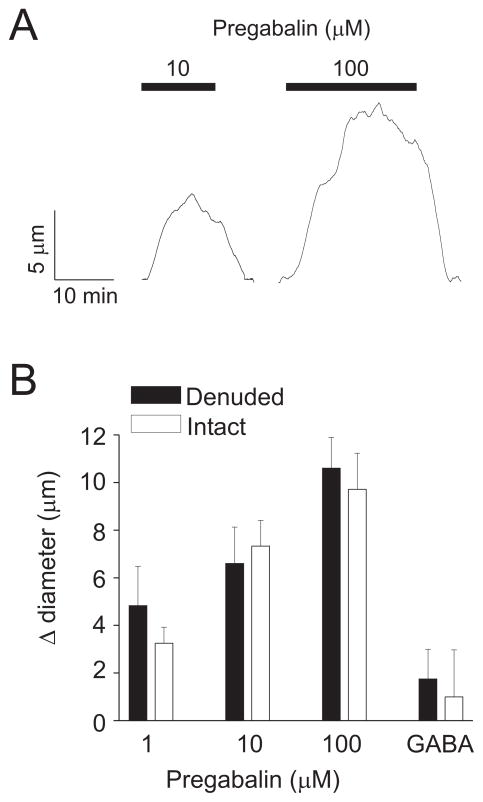
Acute pregabalin caused a concentration-dependent, reversible vasodilation in arteries pressurized to 60 mmHg (Fig. 3A, B). Endothelium-denudation did not alter pregabalin-induced vasodilation (Fig. 3B). GABA, which is molecularly similar to pregabalin but does not bind to α2δ subunits 27, did not alter the diameter of endothelium-intact or -denuded pressurized arteries (Fig. 3B).
Figure 3.
Pregabalin dilates cerebral arteries. A. Exemplary traces illustrating changes in diameter in response to acute pregabalin application. B. Bar graph summarizing mean data for acute pregabalin-induced vasodilation in endothelium-intact (n=5–8 for each) or -denuded arteries (n=7 for each). GABA concentration was 100 μM (n=4 for each). Mean myogenic tone in endothelium-intact and -denuded arteries was 28.0±3.2 % and 29.2 ± 5.3 %, respectively (P>0.05).
α2δ-1 knockdown reduces surface expression of Cav1.2 α1 subunits
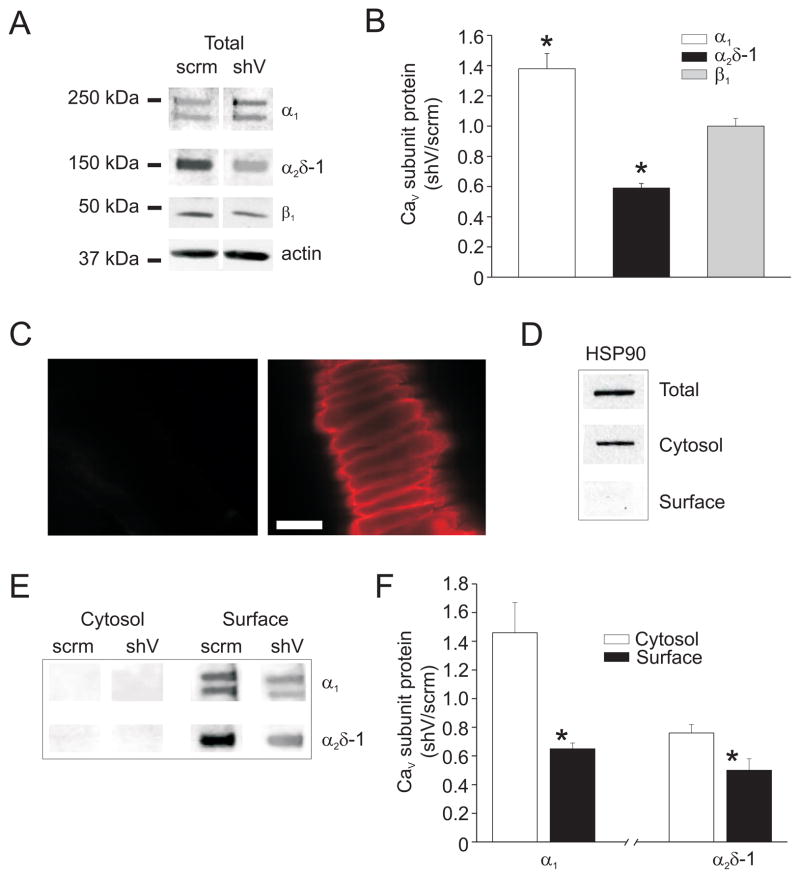
To further examine physiological functions of α2δ-1 subunits in cerebral artery smooth muscle cells, vectors encoding shRNA (α2δ-1shV) were constructed and inserted intracellularly into cerebral arteries using reverse permeabilization. Vectors encoding scrambled shRNA (α2δ-1scrm) were used as a control. Total protein for α2δ-1, Cav1.2 α1, and β1 subunits were quantified using Western blotting. α2δ-1shV reduced cerebral artery α2δ-1 protein to ~57 % of α2δ-1scrm (Fig. 3A, B). α2δ-1 knockdown did not alter β1 subunit protein, but elevated CaV1.2 α1 protein to ~134 % of α2δ-1scrm (Fig. 4A, B).
Figure 4.
α2δ-1 knockdown reduces surface expression of Cav1.2 α1 and α2δ-1 subunits in cerebral arteries. A. Representative blot illustrating the effect of α2δ-1shV and α2δ-1scrm on Cav1.2 α1, α2δ-1, and β1 subunit total protein. B. Mean effect of α2δ-1shV on total Cav1.2 α1 (n=9), α2δ-1 (n=7), and β1 (n=5) subunit protein. C. Fluorescent images of control arteries (left) or arteries treated with biotin reagents (right) followed by exposure to Texas red-conjugated streptavidin. Scale bar = 20 μm. D. Representative Western blot illustrating detection of HSP90 in arterial lysate (total), or in the non-biotinylated (cytosolic) or biotinylated (surface) fractions isolated from biotin-treated arteries. E. Representative Western blots showing cytosolic and surface protein levels of α2δ-1 and α1 subunits in arteries treated with α2δ-1shV or α2δ-1scrm. F. Mean relative cytosolic and surface Cav1.2 α1 and α2δ-1 protein in arteries treated with α2δ-1shV (n=4–5) relative to α2δ-1scrm (n=4–5) control. * indicates P<0.05 versus cytosol.
Surface biotinylation was used to study the regulation of CaV1.2 subunit membrane expression by α2δ-1 in cerebral arteries. To our knowledge, this is the first time that biotinylation has been used to measure the proportion of plasma membrane-localized ion channel proteins in native arterial smooth muscle cells. We confirmed that biotin effectively binds to extracellular proteins on smooth muscle cells by imaging biotinylated arteries that had been exposed to Texas red-conjugated streptavidin (Fig. 4C). Surface biotinylation followed by Western blotting was used to measure the proportion of surface to cytosolic protein in cerebral arteries. The cytosolic marker protein HSP90 was detected in the total and cytosolic fractions, but not in the surface fraction, indicating that this procedure distinguishes between plasma membrane and intracellular proteins (Fig. 4D). Data indicated that >95% of both α2δ-1 and CaV1.2 α1 subunits reside within the plasma membrane in cerebral arteries (Fig. 4E). Importantly, α2δ-1 knockdown using α2δ-1shV reduced the proportion of plasma membrane-localized α2δ-1 and Cav1.2 α1 subunits to ~50 % and 65 % of α2δ-1scrm control, respectively (Figs. 4E, F). These data indicate that α2δ-1 is essential for plasma membrane expression of Cav1.2 α1 subunits in arterial smooth muscle cells.
α2δ-1 knockdown reduces [Ca2+]i and dilates cerebral arteries
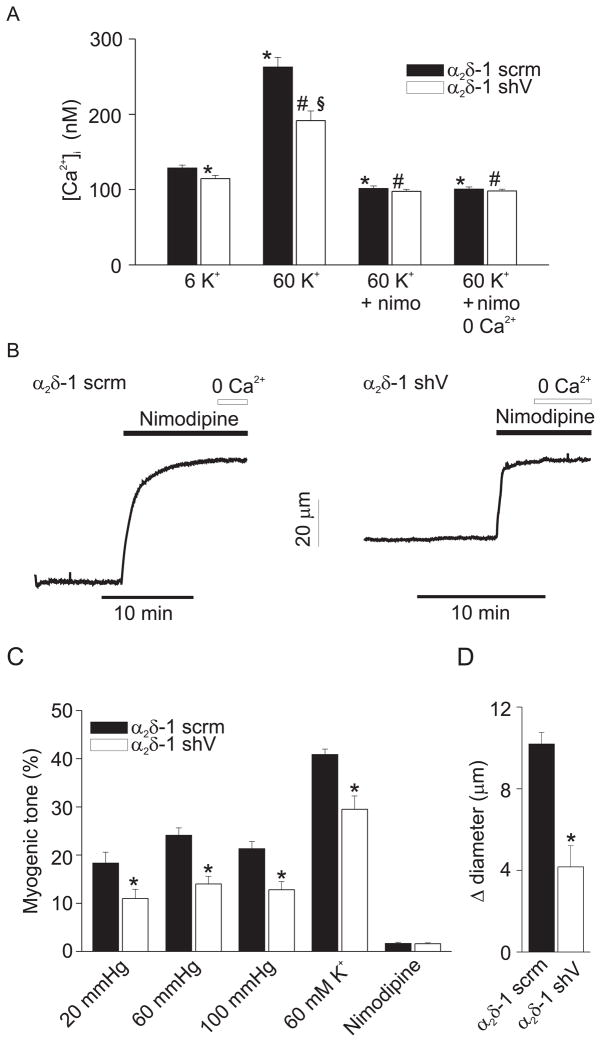
α2δ-1 knockdown reduced mean [Ca2+]i in endothelium-denuded arteries from ~129 to 115 nM (Fig. 5A). α2δ-1 knockdown also attenuated depolarization (60 mM K+)-induced [Ca2+]i from ~263 to 192 nM (Fig. 5A). Nimodipine, a voltage-dependent Ca2+ channel blocker, reduced [Ca2+]i to a similar level in arteries treated with α2δ-1shV or α2δ-1scrm, and removal of extracellular Ca2+ in the presence of nimodipine had no further effect on [Ca2+]i.
Figure 5.
α2δ-1 knockdown reduces [Ca2+]i and dilates cerebral arteries. A, Mean [Ca2+]i in endothelium-denuded arteries treated with α2δ-1scrm (n=5) or α2δ-1shV (n=5) and normalization of differences with nimodipine (1 μM) and Ca2+ removal. B. Exemplary traces illustrating that myogenic tone at 60 mmHg is attenuated in arteries treated with α2δ-1shV versus α2δ-1scrm. Nimodipine (1 μM) fully dilates both α2δ-1scrm- and α2δ-1shV-treated arteries. C. Mean myogenic tone in endothelium-intact arteries treated with α2δ-1shV or α2δ-1 scrm at 20 (α2δ-1shV, n=7, α2δ-1scrm, n=10), 60 (α2δ-1shV, n=13, α2δ-1scrm, n=16), and 100 (α2δ-1shV, n=7, α2δ-1scrm, n=10) mmHg in PSS, and in response to 60 mM K+ at 60 mmHg (α2δ-1shV, n=10, α2δ-1scrm, n=13), or nimodipine at 60 mmHg (1 μM; α2δ-1shV, n=9, α2δ-1scrm, n=8). At 60 mmHg, tone in endothelium-intact non-cultured arteries (see Fig. 3) and arteries treated with α2δ-1scrm were similar (P>0.05). D. Pregabalin-induced vasodilation is attenuated in endothelium-intact arteries treated with α2δ-1shV (n=6) versus α2δ-1scrm (n=5). * indicates P<0.05 versus α2δ-1scrm in 6 K+, # versus α2δ-1shV in 6 K+, and § versus α2δ-1scrm in 60 K+.
α2δ-1 knockdown reduced myogenic tone to between 43 and 63 % of α2δ-1scrm over a range of intravascular pressures between 20 and 100 mmHg (Fig. 5B, C). α2δ-1 knockdown also reduced 60 mM K+-induced vasoconstriction (at 60 mmHg) to ~72 % of α2δ-1scrm (Fig. 5C). Nimodipine fully dilated pressurized arteries that had been treated with α2δ-1shV or α2δ-1scrm, indicating that myogenic tone in these vessels occurred due to Cav1.2 channel activation (Fig. 5B, C). α2δ-1 knockdown also reduced pregabalin-induced vasodilation to ~41 % of that in α2δ-1scrm control arteries (Fig. 5D). These data indicate that α2δ-1 controls Ca2+ influx through CaV1.2 channels in myocytes, thereby regulating arterial diameter.
Pregabalin inhibits plasma membrane expression of α2δ-1 and Cav1.2 α1 subunits, leading to vasodilation
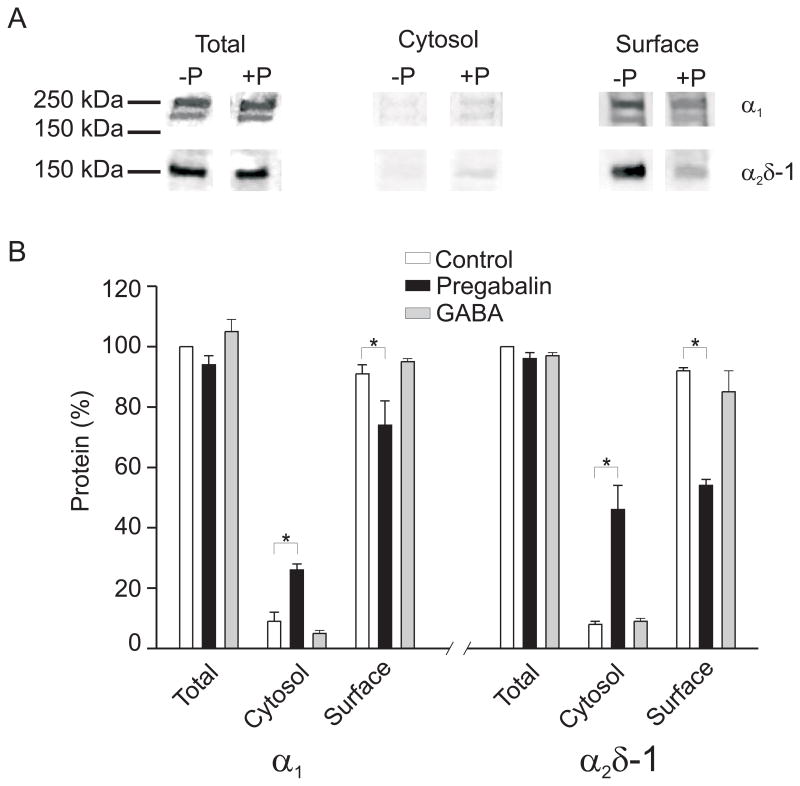
Chronic application of gabapentinoids inhibits surface expression of recombinant α2δ-1, α2δ-2, and Cav2 α1 subunits, and reduces membrane trafficking of α2δ-1 in dorsal root ganglion neurons. 28, 29 Therefore, the regulation of CaV1.2 subunit membrane expression by chronic exposure to pregabalin was studied in cerebral arteries. Pregabalin (24 hr) did not alter total α2δ-1 or CaV1.2 α1 subunit protein (Figs. 6A, B). However, pregabalin (24 hr) decreased plasma membrane-localized α2δ-1 and CaV1.2 α1 proteins to ~55 and 75 % of untreated (24 hr) controls, respectively (Figs. 6A, B). In accordance, pregabalin elevated cytosolic α2δ-1 and CaV1.2 α1 subunit protein (Figs. 6A, B). GABA (24 hr) did not alter α2δ-1 or Cav1.2 α1 subunit surface expression (Fig. 6B). These data indicate that prolonged exposure to pregabalin reduces plasma membrane localization of α2δ-1 and Cav1.2 α1 subunits in cerebral artery smooth muscle cells.
Figure 6.
Pregabalin reduces plasma membrane expression of α2δ-1 and Cav1.2 α1 subunits in cerebral arteries. A. Representative blots illustrating the effects of a 24 h exposure to pregabalin (+P, 100 μM) on total, cytosolic, and surface protein levels of α2δ-1 and Cav1.2 α1 subunits. B. Bar graph indicating the percentage of total, cytosolic, and surface protein for Cav1.2 α1 and α2δ-1 in untreated controls (white bars, n=3–4), and following 24 h exposure to pregabalin (100 μM, black bars, n=3–4) or GABA (100 μM, gray bars, n=3–4). * indicates P<0.05 versus control.
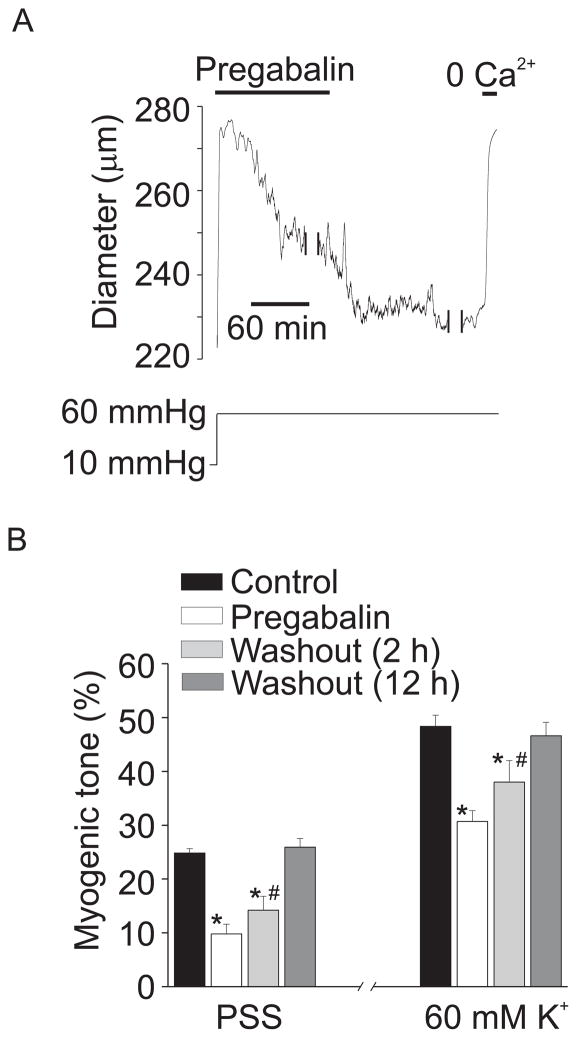
The regulation of arterial diameter by chronic exposure to pregabalin was studied. Arteries were exposed to pregabalin for 24 hours and then pressurized. Pregabalin (24 hr) reduced myogenic tone at 60 mmHg to ~40 % of untreated controls (Fig. 7A, B). Pregabalin also reduced 60 mM K+-induced vasoconstriction to ~63% of control (Fig. 7B). Removal of pregabalin (2 hr wash) from the bath solution caused an immediate partial recovery of myogenic tone, consistent with washout of the acute pregabalin-induced vasodilation (see Figs. 3A, B; 7A, B). Two hours following pregabalin washout, myogenic tone and 60 mM K+-induced vasoconstriction partially recovered but was still attenuated, consistent with prolonged CaV1.2 inhibition (Fig. 7A, B). Twelve hours after pregabalin washout, myogenic tone and 60 mM K+-induced vasoconstriction fully recovered (Fig. 7B).
Figure 7.
Prolonged pregabalin exposure attenuates myogenic tone. A. Representative trace illustrating diameter at 10 and 60 mmHg in an artery treated for 24 hours with pregabalin (100 μM). Following the development of stable myogenic tone at 60 mmHg in the continuous presence of pregabalin, pregabalin washout caused a further contraction. Two breaks in the record are each 20 minutes. B. Mean data indicating myogenic tone at 60 mmHg and response to 60 mM K+ at 60 mmHg in untreated arteries (black bars), 24 hour pregabalin treatment (100 μM, white), and 2 (light gray) or 12 hour pregabalin washout (dark gray, n=4–8). * indicates P<0.05 when compared to control under the same condition (PSS or 60 K+), # indicates P<0.05 versus pregabalin under the same condition (PSS or 60 K+).
These data indicate that α2δ-1 subunits are an essential regulator of cerebral artery contractility by promoting Cav1.2 α1 subunit plasma membrane insertion. These results also demonstrate that α2δ-1 can be targeted either to cause acute vasodilation by modulating membrane-resident subunits or prolonged vasodilation by altering plasma membrane expression of CaV1.2 α1 subunits.
Discussion
We have examined the molecular identity and physiological functions of α2δ subunits in arterial smooth muscle cells. Our data show that cerebral artery smooth muscle cells express one α2δ isoform, α2δ-1. α2δ-1 ligands inhibited CaV1.2 currents in isolated arterial smooth muscle cells. α2δ-1 knockdown reduced plasma membrane-localized α2δ-1 and Cav1.2 α1 subunits and [Ca2+]i. Prolonged (24 h) pregabalin exposure reduced plasma membrane insertion of α2δ-1 and CaV1.2 α1 subunits. α2δ-1 knockdown, and acute and prolonged pregabalin, all caused vasodilation. Taken together, these data indicate that α2δ-1 stimulates plasma membrane insertion of smooth muscle cell Cav1.2 α1 subunits and that α2δ-1 is a viable therapeutic target for causing both acute and prolonged vasodilation.
Cav1.2 channels regulate a variety of physiological functions in arterial smooth muscle cells, including contraction and gene expression. 7, 9 However, the macromolecular composition of vascular Cav1.2 channels was unclear. The present investigation was designed to determine physiological functions of arterial smooth muscle cell α2δ subunits. The few previous studies that have investigated physiological functions of α2δ subunits in other native cell types generated different findings. 17–19 One explanation for these diverse findings is that physiological functions of α2δ-1 subunits may depend on native Cav channel isoforms and splice variants. Skeletal muscle cells express Cav1.1 α1 subunits, whereas cardiac and smooth muscle cells both express Cav1.2 α1 subunits. 1, 11 Cardiac and smooth muscle cells express different Cav1.2 α1 subunit splice variants.14, 30, 31 For instance, resistance-size cerebral artery smooth muscle cell Cav1.2 α1 subunits primarily contain exon 1c, whereas most cardiac myocyte Cav1.2 α1 subunit mRNA contains exon 1b. 14 Exon 1 splicing modified membrane insertion and voltage-dependent regulation of recombinant Cav1.2 α1 subunits by α2δ-1. 14 One possibility is that exon splicing underlies different physiological functions of α2δ-1 in smooth versus cardiac muscle. Another possibility is that cell-specific auxiliary subunits, including different β isoforms, may modify physiological functions of α2δ subunits. Regardless of the molecular mechanisms involved, our data indicate that α2δ-1 subunits modulate arterial contractility by regulating plasma membrane insertion of Cav1.2 α1 subunits in smooth muscle cells. This is stark contrast to the apparent lack of effect of α2δ-1 on membrane targeting of CaV subunits in skeletal and cardiac muscle cells. 17–19
Gabapentinoid drugs, including gabapentin and pregabalin, a higher affinity analogue, are antiallodynic and antihyperalgesic, and are useful in the treatment of neuropathic pain that can result from nerve damage. 32–34 Gabapentinoid drugs were originally developed as GABA receptor ligands, but were subsequently found not to bind to GABA receptors. 3, 35, 36 Rather, binding of gabapentinoid drugs to α2δ-1 and α2δ-2 subunits were discovered to underlie their therapeutic effects. 25, 26 Acute effects of gabapentin, the most widely studied gabapentinoid drug, and pregabalin on CaV currents produced variable results. Gabapentin inhibits a variety of Cav currents, including L-, N-, and P/Q-type. 37 Gabapentin has also been reported to have no acute effect on Cav currents. 29 These different observations may occur due to CaV channel isoform, cell culture conditions, auxiliary subunits present in the cells studied, or the affinity for α2δ subunits of the gabapentinoid ligand that was used. 38 Gabapentinoid-regulation of arterial smooth muscle cell Cav currents and arterial contractility had not previously been examined. Our data indicate that acute pregabalin application inhibits cerebral artery smooth muscle cell Cav1.2 currents and dilates pressurized cerebral arteries. We show that pregabalin similarly dilated endothelium-intact and -denuded arteries, α2δ-1 knockdown reduced pregabalin-induced vasodilation, and GABA had no effect, consistent with a previous report. 39 These data indicate that pregabalin causes vasodilation by binding to smooth muscle cell α2δ-1 subunits. Acute application of pregabalin inhibits CaV currents in cultured dorsal root ganglion neurons without causing a shift in current voltage-dependence 40, which is similar to the results shown here. Voltage-independent CaV current inhibition is consistent with pregabalin acting as a pore blocker, an effect that may be facilitated by prior binding to an α2δ-1 extracellular domain. Supporting this conclusion, we show that α2δ-1 antibody also inhibited arterial myocyte Cav1.2 currents without shifting the I-V relationship. Taken together, our data indicate that α2δ-1 ligands inhibit arterial smooth muscle cell Cav1.2 currents, leading to vasodilation.
Chronic exposure to gabapentin disrupted membrane trafficking of both recombinant and dorsal root ganglion neuron α2δ and CaV2 α1 subunits, reduced α2δ-1 trafficking in the spinal cord, and alleviated allodynia. 29 α2δ subunits modify Cav channel trafficking through the Von Willebrand factor-A domain in the α2 subunit. 41 It has been proposed that gabapentinoid drugs may displace an endogenous ligand that is necessary for α2δ subunit trafficking. 28, 29 To measure membrane expression of Cav1.2 α1 and α2δ-1 subunits, we used membrane biotinylation. Our successful implementation of this technique provides a basis from which future studies can measure the proportion and localization of a wide variety of native vascular proteins. Biotinylation indicated that >95 % of both Cav1.2 α1 and α2δ-1 subunits are present in the cerebral artery smooth muscle cell plasma membrane. These data indicate that there is essentially no intracellular reserve of α2δ-1 and Cav1.2 α1 subunits that could be triggered to traffic to the plasma membrane, for example in response to stimuli. We show that α2δ-1 knockdown and 24 hour pregabalin treatment both reduced plasma membrane expression of α2δ-1 and Cav1.2 α1 subunits in arterial smooth muscle cells, leading to vasodilation. In contrast, GABA had no effect on CaV subunit trafficking, indicating that pregabalin prevents α2δ-1 and Cav1.2 α1 membrane expression by binding to α2δ-1 subunits in smooth muscle cells. α2δ-1 knockdown did not alter β1 subunit expression, but elevated CaV1.2 α1 subunit protein. One explanation for this effect is that the decrease in plasma membrane CaV1.2 channels caused by α2δ-1 knockdown, and the subsequent reduction in voltage-dependent Ca2+ influx, leads to a compensatory mechanism that stimulates CaV1.2 α1 expression. Inhibition of α2δ-1 and Cav1.2 α1 membrane expression by knockdown and pregabalin treatment blocked pressure-induced vasoconstriction by reducing voltage-dependent Ca2+ activity. This was measured as a reduction in nimodipine-sensitive Ca2+ influx and tone. Pregabalin washout after a 24 hour exposure caused an immediate vasoconstriction that was consistent with removal of acute pregabalin-induced CaV1.2 inhibition. Reversal of the chronic functional effect of pregabalin on α2δ-1 membrane expression took between 2 and 12 hours, consistent with protein trafficking being slower than washout of a pharmacological channel inhibitor. Since α2δ-1 knockdown did not alter β subunit expression, these data indicate that α2δ-1 can function in a β-subunit-independent manner. Thus, α2δ-1 expression and functionality is essential for plasma membrane insertion of Cav1.2 α1 subunits in arterial smooth muscle cells and the regulation of arterial contractility.
In summary, we identify α2δ-1 as a vasoregulatory protein and show that this subunit promotes Cav1.2 α1 subunit membrane insertion in cerebral artery smooth muscle cells. Our data also indicate that molecular and pharmacological targeting of α2δ-1 may be used as novel therapeutic mechanism to regulate arterial contractility.
Supplementary Material
Acknowledgments
We thank Dr. Judith Pachuau for critical reading of the manuscript. Pregabalin was a gift from Pfizer Inc, Groton CT, USA.
Sources of Funding
HL67061, HL77678, and HL094378 to J.H.J and NIH K01 grant HL096411 to A.A. from the NHLBI of the NIH. D.N. is a recipient of a Predoctoral Fellowship from the AHA Greater Southeast Affiliate. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- α2δ-1shV
vectors encoding α2δ-1 shRNA
- α2δ-1scrm
vectors encoding scrambled shRNA
Footnotes
Disclosures
None.
References
- 1.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–55. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 2.Dolphin AC. β subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35(6):599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- 3.Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the α2δ subunits of voltage-gated calcium channels. Trends Pharmacol Sci. 2007;28(5):220–8. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Cole RL, Lechner SM, Williams ME, Prodanovich P, Bleicher L, Varney MA, Gu G. Differential distribution of voltage-gated calcium channel alpha-2 delta (α2δ) subunit mRNA-containing cells in the rat central nervous system and the dorsal root ganglia. J Comp Neurol. 2005;24;491(3):246–69. doi: 10.1002/cne.20693. [DOI] [PubMed] [Google Scholar]
- 5.Andrade A, Sandoval A, Oviedo N, De WM, Elias D, Felix R. Proteolytic cleavage of the voltage-gated Ca2+ channel α2δ subunit: structural and functional features. Eur J Neurosci. 2007;25(6):1705–10. doi: 10.1111/j.1460-9568.2007.05454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13(3):298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 7.Sonkusare S, Palade PT, Marsh JD, Telemaque S, Pesic A, Rusch NJ. Vascular calcium channels and high blood pressure: pathophysiology and therapeutic implications. Vascul Pharmacol. 2006;44(3):131–42. doi: 10.1016/j.vph.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res. 2006;14;98(7):868–78. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- 9.Gollasch M, Nelson MT. Voltage-dependent Ca2+ channels in arterial smooth muscle cells. Kidney Blood Press Res. 2000;20:355–71. doi: 10.1159/000174250. [DOI] [PubMed] [Google Scholar]
- 10.Navedo MF, Amberg GC, Westenbroek RE, Sinnegger-Brauns MJ, Catterall WA, Striessnig J, Santana LF. CaV1.3 channels produce persistent calcium sparklets, but CaV1.2 channels are responsible for sparklets in mouse arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2007;293(3):H1359–H1370. doi: 10.1152/ajpheart.00450.2007. [DOI] [PubMed] [Google Scholar]
- 11.Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel CaV1.2 for blood pressure regulation. EMBO J. 2003;17;22(22):6027–34. doi: 10.1093/emboj/cdg583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bielefeldt K. Molecular diversity of voltage-sensitive calcium channels in smooth muscle cells. J Lab Clin Med. 1999;133(5):469–77. doi: 10.1016/s0022-2143(99)90024-0. [DOI] [PubMed] [Google Scholar]
- 13.Reimer D, Huber IG, Garcia ML, Haase H, Striessnig J. beta subunit heterogeneity of L-type Ca2+ channels in smooth muscle tissues. FEBS Lett. 2000;4;467(1):65–9. doi: 10.1016/s0014-5793(00)01124-8. [DOI] [PubMed] [Google Scholar]
- 14.Cheng X, Liu J, Asuncion-Chin M, Blaskova E, Bannister JP, Dopico AM, Jaggar JH. A novel CaV1.2 N terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits. J Biol Chem. 2007;282(40):29211–21. doi: 10.1074/jbc.M610623200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsh JD, Telemaque S, Rhee SW, Stimers JR, Rusch NJ. Delivery of ion channel genes to treat cardiovascular diseases. Trans Am Clin Climatol Assoc. 2008;119:171–82. [PMC free article] [PubMed] [Google Scholar]
- 16.Yasuda T, Chen L, Barr W, McRory JE, Lewis RJ, Adams DJ, Zamponi GW. Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur J Neurosci. 2004;20(1):1–13. doi: 10.1111/j.1460-9568.2004.03434.x. [DOI] [PubMed] [Google Scholar]
- 17.Gach MP, Cherednichenko G, Haarmann C, Lopez JR, Beam KG, Pessah IN, Franzini-Armstrong C, Allen PD. α2δ1 dihydropyridine receptor subunit is a critical element for excitation-coupled calcium entry but not for formation of tetrads in skeletal myotubes. Biophys J. 2008;15;94(8):3023–34. doi: 10.1529/biophysj.107.118893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obermair GJ, Kugler G, Baumgartner S, Tuluc P, Grabner M, Flucher BE. The Ca2+ channel α2δ-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of α1S or excitation-contraction coupling. J Biol Chem. 2005;21;280(3):2229–37. doi: 10.1074/jbc.M411501200. [DOI] [PubMed] [Google Scholar]
- 19.Tuluc P, Kern G, Obermair GJ, Flucher BE. Computer modeling of siRNA knockdown effects indicates an essential role of the Ca2+ channel α2δ-1 subunit in cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2007;104(26):11091–6. doi: 10.1073/pnas.0700577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaggar JH. Intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol. 2001;281(2):C439–C448. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- 21.Adebiyi A, McNally EM, Jaggar JH. Sulfonylurea Receptor-Dependent and -Independent Pathways Mediate Vasodilation Induced by KATP Channel Openers. Mol Pharmacol. 2008;29(74):736–43. doi: 10.1124/mol.108.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesh RE, Somlyo AP, Owens GK, Somlyo AV. Reversible permeabilization. A novel technique for the intracellular introduction of antisense oligodeoxynucleotides into intact smooth muscle. Circ Res. 1995;77(2):220–30. doi: 10.1161/01.res.77.2.220. [DOI] [PubMed] [Google Scholar]
- 23.Zhao G, Adebiyi A, Blaskova E, Xi Q, Jaggar JH. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Am J Physiol Cell Physiol. 2008;295(5):C1376–C1384. doi: 10.1152/ajpcell.00362.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henkel AW, Bieger SC. Quantification of proteins dissolved in an electrophoresis sample buffer. Anal Biochem. 1994 December;223(2):329–31. doi: 10.1006/abio.1994.1595. [DOI] [PubMed] [Google Scholar]
- 25.Melrose HL, Kinloch RA, Cox PJ, Field MJ, Collins D, Williams D. [3H] pregabalin binding is increased in ipsilateral dorsal horn following chronic constriction injury. Neurosci Lett. 2007;417(2):187–92. doi: 10.1016/j.neulet.2007.02.068. [DOI] [PubMed] [Google Scholar]
- 26.Bian F, Li Z, Offord J, Davis MD, McCormick J, Taylor CP, Walker LC. Calcium channel alpha2-delta type 1 subunit is the major binding protein for pregabalin in neocortex, hippocampus, amygdala, and spinal cord: an ex vivo autoradiographic study in a2-d type 1 genetically modified mice. Brain Res. 2006;1075(1):68–80. doi: 10.1016/j.brainres.2005.12.084. [DOI] [PubMed] [Google Scholar]
- 27.Brown JP, Dissanayake VU, Briggs AR, Milic MR, Gee NS. Isolation of the [3H]gabapentin-binding protein/α2δ Ca2+ channel subunit from porcine brain: development of a radioligand binding assay for α2δ subunits using [3H]leucine. Anal Biochem. 1998;255(2):236–43. doi: 10.1006/abio.1997.2447. [DOI] [PubMed] [Google Scholar]
- 28.Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri RY, Fernandez-Alacid L, Millar NS, Dickenson AH, Lujan R, Dolphin AC. The increased trafficking of the calcium channel subunit α2δ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2δ ligand pregabalin. J Neurosci. 2009;29(13):4076–88. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the α2δ ligand gabapentin. Proc Natl Acad Sci U S A. 2008;105(9):3628–33. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang ZZ, Liang MC, Lu S, Yu D, Yu CY, Yue DT, Soong TW. Transcript scanning reveals novel and extensive splice variations in human l-type voltage-gated calcium channel, CaV1.2 α1 subunit. J Biol Chem. 2004;279(43):44335–43. doi: 10.1074/jbc.M407023200. [DOI] [PubMed] [Google Scholar]
- 31.Tang ZZ, Hong X, Wang J, Soong TW. Signature combinatorial splicing profiles of rat cardiac-and smooth-muscle CaV1.2 channels with distinct biophysical properties. Cell Calcium. 2007;41(5):417–28. doi: 10.1016/j.ceca.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell S, Corradini L, England S, Winks J, Kinloch RA, Hendrich J, Dolphin AC, Webb T, Williams D. Identification of the α2-δ-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A. 2006;103(46):17537–42. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzellos TG, Papazisis G, Amaniti E, Kouvelas D. Efficacy of pregabalin and gabapentin for neuropathic pain in spinal-cord injury: an evidence-based evaluation of the literature. Eur J Clin Pharmacol. 2008;64(9):851–8. doi: 10.1007/s00228-008-0523-5. [DOI] [PubMed] [Google Scholar]
- 34.Stacey BR, Swift JN. Pregabalin for neuropathic pain based on recent clinical trials. Curr Pain Headache Rep. 2006;10(3):179–84. doi: 10.1007/s11916-006-0043-x. [DOI] [PubMed] [Google Scholar]
- 35.Taylor CP. Mechanisms of analgesia by gabapentin and pregabalin--calcium channel α2-δ [CaVα2-δ] ligands. Pain. 2009;142(1–2):13–6. doi: 10.1016/j.pain.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the α2δ subunit of a calcium channel. J Biol Chem. 1996;271(10):5768–76. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 37.Cheng JK, Chiou LC. Mechanisms of the antinociceptive action of gabapentin. J Pharmacol Sci. 2006;100(5):471–86. doi: 10.1254/jphs.cr0050020. [DOI] [PubMed] [Google Scholar]
- 38.Martin DJ, McClelland D, Herd MB, Sutton KG, Hall MD, Lee K, Pinnock RD, Scott RH. Gabapentin-mediated inhibition of voltage-activated Ca2+ channel currents in cultured sensory neurones is dependent on culture conditions and channel subunit expression. Neuropharmacology. 2002;42(3):353–66. doi: 10.1016/s0028-3908(01)00181-2. [DOI] [PubMed] [Google Scholar]
- 39.Takayasu M, Dacey RG., Jr Effects of inhibitory and excitatory amino acid neurotransmitters on isolated cerebral parenchymal arterioles. Brain Res. 1989;482(2):393–6. doi: 10.1016/0006-8993(89)91207-9. [DOI] [PubMed] [Google Scholar]
- 40.McClelland D, Evans RM, Barkworth L, Martin DJ, Scott RH. A study comparing the actions of gabapentin and pregabalin on the electrophysiological properties of cultured DRG neurones from neonatal rats. BMC Pharmacol. 2004;4:14. doi: 10.1186/1471-2210-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canti C, Nieto-Rostro M, Foucault I, Heblich F, Wratten J, Richards MW, Hendrich J, Douglas L, Page KM, Davies A, Dolphin AC. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of α2δ subunits is key to trafficking voltage-gated Ca2+ channels. Proc Natl Acad Sci U S A. 2005;102(32):11230–5. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.