Abstract
We describe novel TDP-43 (trans-activation response [TAR] DNA-binding protein of 43 kDa)-positive structures in the brains of 3 patients with frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U) and a case of familial Lewy body disease. TDP-43 immunohistochemistry revealed small round structures closely associated with small blood vessels. By immunoelectron microscopy, these TDP-43-positive structures were unmyelinated cell processes located adjacent to and sometimes enclosed by the capillary basal lamina (BL). Some processes protruded from outside of the vascular BL to a position beneath the BL. The processes contained 10- to 17-nm-diameter straight filaments or filaments coated with granular material, similar to those described in neurites in FTLD-U and other disorders. In some of the abnormal structures, electron dense material formed paracrystalline arrays composed of TDP-43. The inclusions were variably positive by immunostaining for the small heat shock protein αB-crystallin and less often glial fibrillary acidic protein. Bundles of astrocytic glial fibrils characteristic of reactive astrocytes were often found in proximity but glial fibrils were negative for TDP-43. These data suggest that these processes are astrocytic end-feet with abnormal TDP-43 fibrillary inclusions. The significance of this novel TDP-43 microvasculopathy on blood-brain barrier integrity warrants further investigation.
Keywords: αB-Crystallin, Astrocyte, Capillary basal lamina, Frontotemporal lobar degeneration, Immunoelectron microscopy, Lewy body disease, TDP-43
INTRODUCTION
Trans-activation response (TAR) DNA-binding protein of 43 kDa (TDP-43) was first demonstrated in neuronal cytoplasmic inclusions (NCIs) that are immunoreactive for ubiquitin, but not tau or α-synuclein in cases of frontotemporal lobar degeneration and in amyotrophic lateral sclerosis (ALS) (1, 2). In addition to NCIs, abnormal TDP-43 immunoreactivity is also present in dystrophic neurites (DNs) and in neuronal intranuclear inclusions in the cerebral cortex, amygdala, hippocampus and striatum, as well as skein-like and Lewy-like NCIs in motor neurons of the brainstem and spinal cord (3). In addition to abnormal neuronal inclusions, TDP-43-positive inclusions have also been reported in glial cells in frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U), ALS, Guam Parkinson dementia complex and corticobasal degeneration (CBD) (4-9). The glial cells were considered most likely oligodendrocytes by light microscopic morphologic criteria and were found in white matter (9) or in superficial cortex (5). To our knowledge, there are no reports of TDP-43-immunoreactive inclusions in astrocytes.
During a recent study on ultrastructural localization of TDP-43 in brains of different neurodegenerative diseases (10), we noted TDP-43-positive inclusions in cell processes located outside and inside of the basal lamina of capillaries in brains of cases of FTLD-U and familial diffuse Lewy body disease (DLBD). The purpose of this report is to describe in greater detail this novel “TDP-43 microvasculopathy.”
MATERIALS AND METHODS
Immunohistochemistry
This study focused on the brains of 3 FTLD-U cases with mutations in the gene for progranulin and a case of familial DLBD due to A53T mutation in the gene for α-synuclein. Methods employed were similar to those reported previously (11). For double labeling immunohistochemistry, deparaffinized and glass mounted sections were pretreated by heating in a steamer for 30 min prior to immunostaining using a DAKO Autostainer (DAKO, Carpinteria, CA) and EnVision G/2 Doublestain kit with HRP polymer with 3, 3′-diaminobenzidine as the chromogen for TDP-43 and alkaline phosphatase with VectaBlue (Vector Labs, Burlingame, CA) as the chromogen for collagen IV. The sections were lightly counterstained with hematoxylin. The primary antibodies were a rabbit polyclonal to TDP-43 (ProteinTech Group, Inc., Chicago, IL; 1:3000) and a mouse monoclonal antibody to collagen type IV (MP Biomedicals, Solon, OH; 1:1000).
Immunoelectron Microscopy
Small pieces of tissues (1.5 × 1.5 mm) were collected from the hippocampus and parahippocampal gyrus of FTLD-U brains and the amygdala of the familial DLBD brain. They were processed as previously reported (10). Briefly, tissues were dehydrated in alcohols, infiltrated and embedded in London White resin (LR White, medium grade; Polysciences, Warrington, PA), and polymerized in a vacuum oven at 50°C. We used the following antibodies: TDP-43 (polyclonal, ProteinTech Group; monoclonal, Abnova, Taipei, Taiwan); glial fibrillary acidic protein ([GFAP], polyclonal and monoclonal, BioGenex, San Ramon, CA); ubiquitin (polyclonal and monoclonal, Chemicon, Temecula, CA); αB-crystallin (polyclonal [12]); α smooth muscle actin (monoclonal; clone 1A4, Sigma, St. Louis, MO).
In the 3 cases of FTLD-U studied by immunoelectron microscopy (IEM), at least 1 pericapillary TDP-43 inclusion was detected in 14 of the 30 blocks examined; TDP-43-positive neurites not associated with vessels were detected more frequently (at least 1 DN in 16 of 30 blocks). For familial DLBD, 4 blocks of amygdala were studied and perivascular TDP-43 inclusions and DNs were each detected in 4 of the 6 blocks.
RESULTS
Immunohistochemistry
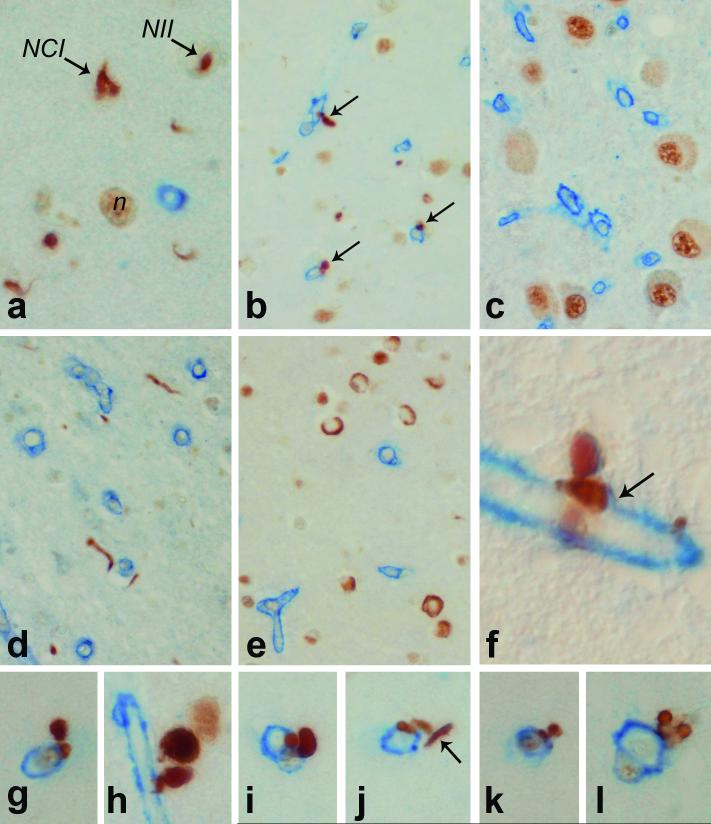
Double labeling immunohistochemistry for TDP-43 and type IV collagen (which immunolabels the basal lamina [BL] of blood vessels) was initially performed on hippocampal and medial temporal lobe sections taken at the level of the lateral geniculate nucleus of 16 cases of FTLD-U, including 6 cases of Type 1, 5 cases of Type 2 and 5 cases of Type 3 FTLD-U, using the classification scheme proposed by Mackenzie (13). We recently found this scheme to detect reliable clinical and pathological differences when multiple subcortical brain regions were evaluated (14). Mackenzie Type 1 is similar to Type 3 in the scheme proposed by Cairns et al (15). We found small globular, dense TDP-43-positive structures in close proximity to small but not large blood vessels in Type 1 cases (Fig. 1). They were rare in Type 2 cases (Fig. 1j) and not detected in Type 3 cases. They followed the distribution of neuronal loss and TDP-43 pathology and were most dense in superficial cortical layers in regions of laminar spongiosis. They were not detected in regions unaffected by TDP-43 pathology, such as the lateral geniculate nucleus (Fig. 1c). In many cases their relationship to blood vessels was only apparent with double staining for collagen IV. In some cases a thin rim of collagen IV immunoreactivity was found at the base of the TDP-43 inclusion (Fig. 1f). In most regions the inclusions were less numerous than NCIs or DNs (Fig. 1a), but in select areas they were the predominant type of TDP-43 pathology (Fig. 1b). They were clearly different from DNs that by chance were adjacent to small vessels in that they often had a bi- or multi-lobed appearance that neurites do not have. In addition, 1 of the lobes was adherent to or embedded in the basal lamina and the other lobe or lobes projecting away from the basal lamina (Figs. 1f-l). In many cases the density of the immunoreactivity was greater than in nearby DNs; this probably corresponds to the high density of TDP-43 epitopes in the densely packed filaments or paracrystalline arrays noted at the ultrastructural level.
Figure 1.
Double immunohistochemistry of frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U) cases for TDP-43 (brown) and type IV collagen (blue) shows a range of TDP-43-immunoreactive structures. In FTLD-U Type 1 (a) TDP-43 is present in normal nuclei (n), neuronal cytoplasmic inclusions (NCIs), neuronal intranuclear inclusions (NIIs) and dystrophic neurites. In brain regions with neuronal loss and gliosis such as superficial cortical layers (b) TDP-43 immunoreactivity is present in globular structures (arrows) associated with capillaries, while areas that do not have TDP-43 staining, such as the lateral geniculate nucleus (c) have only normal nuclear TDP-43 without vascular lesions. In an FTLD-U Type 2 case in which neurites predominate (d), and in an FTLD-U Type 3 case in which NCIs predominate (e), there was little or no TDP-43 microvasculopathy. A rare vascular lesion in Type 2 is illustrated in (j). At high magnification some of the TDP-43 vessel-associated lesions can be shown to have collagen IV immunoreactivity at their base (arrow) (f). TDP-43 vascular lesions are often bilobed (a, h-k) or multilobe structures (l) with one lobe adherent to the basal lamina and the other protruding away. They can be distinguished from neurites that by chance are near the vessel (arrow in j). (a, g-l = X1,000, b-f = x200, f = x4,000, Nomarski optics).
Immunoelectron Microscopy
Ultrastructurally, TDP-43-positive perivascular dense globular structures were located adjacent to, or enclosed partially or completely by, the BL of capillaries (Figs. 2-7). The structures were swollen cell processes filled with filaments or granulofilamentous structures that were immunolabeled by anti-TDP-43 antibody. The TDP-43-positive filaments were similar in size and morphology to those reported in neurites not associated with blood vessels (10). Sometimes, TDP-43-positive masses of electron dense material were also found inside these processes in contiguity with the filamentous structures (Figs. 5, 7, 8).
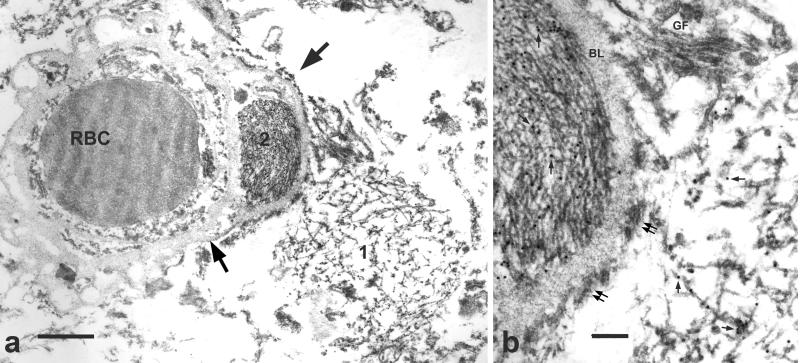
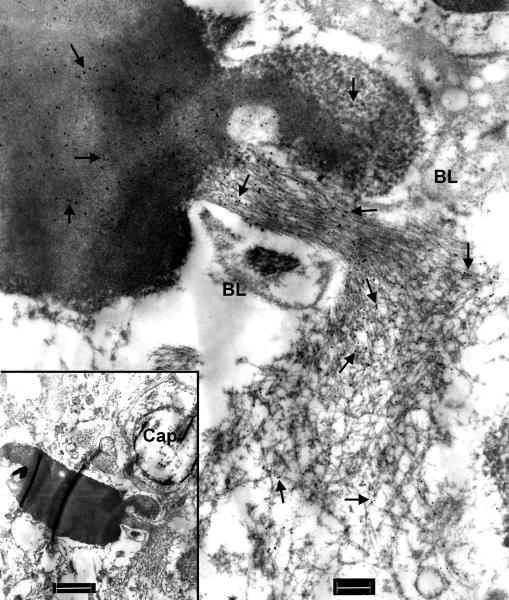
Figure 2.
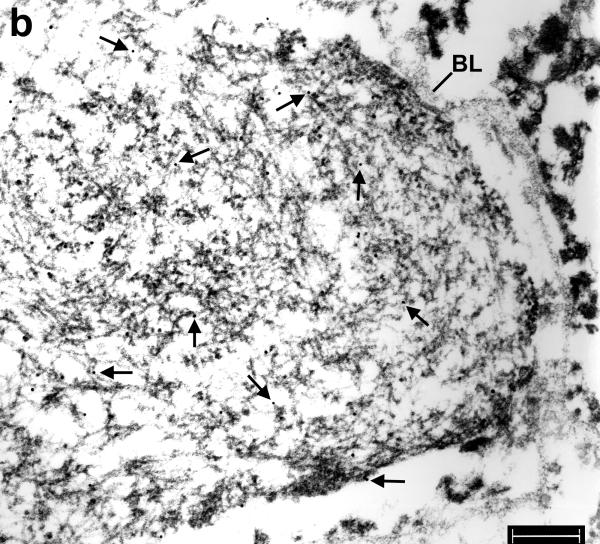
Immunoelectron microscopy of hippocampus in frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U). (a) A filamentous aggregate [1] abuts a capillary while another aggregate [2] is enclosed by the capillary basal lamina (arrows). RBC = red blood cell in the capillary lumen. Bar = 1 μm. (b) At higher magnification, aggregate 1 on the right side of the figure has loosely arranged filaments partially coated with granular material, whereas aggregate 2 on the left side of the figure has tightly packed uncoated filaments. The diameter of the filaments ranges from 10 to 17 nm. TDP-43 is localized to both filaments and associated granular material (arrows point to gold particles). Astrocytic fibrils (GF, note their parallel arrangement) and hemi-desmosomes (double arrows) and the vascular basal lamina (BL) are unlabeled. Bar, = 0.3 μm.
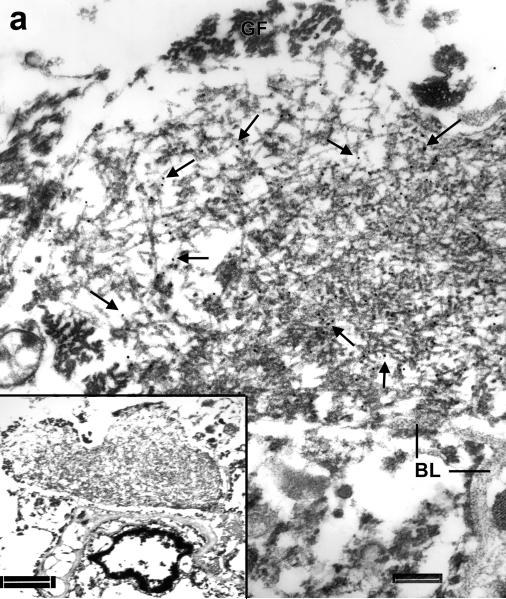
Figure 7.
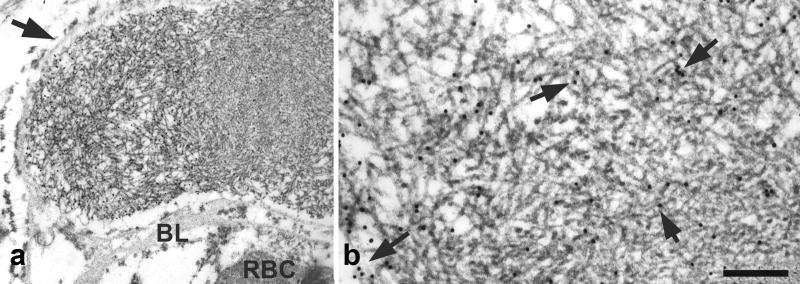
Immunoelectron microscopy of amygdala in familial diffuse Lewy body disease. (Inset) A peculiar process located next to a capillary (Cap) has a filamentous bundle and a dense mass connected by a stalk of filaments. Note the resemblance of this figure to Figure 5 of a case of frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U). Bar = 1 μm. At higher magnification it is clear that the entire structure is heavily labeled with anti-TDP-43 antibody (arrows point to gold particles). Note the contiguity of the labeled filaments in loose bundles in the stalk (surrounded by thin BL) and those in the dense aggregate. Also note the absence of labeling over the vascular basal lamina (BL). Bar, 0.3 μm.
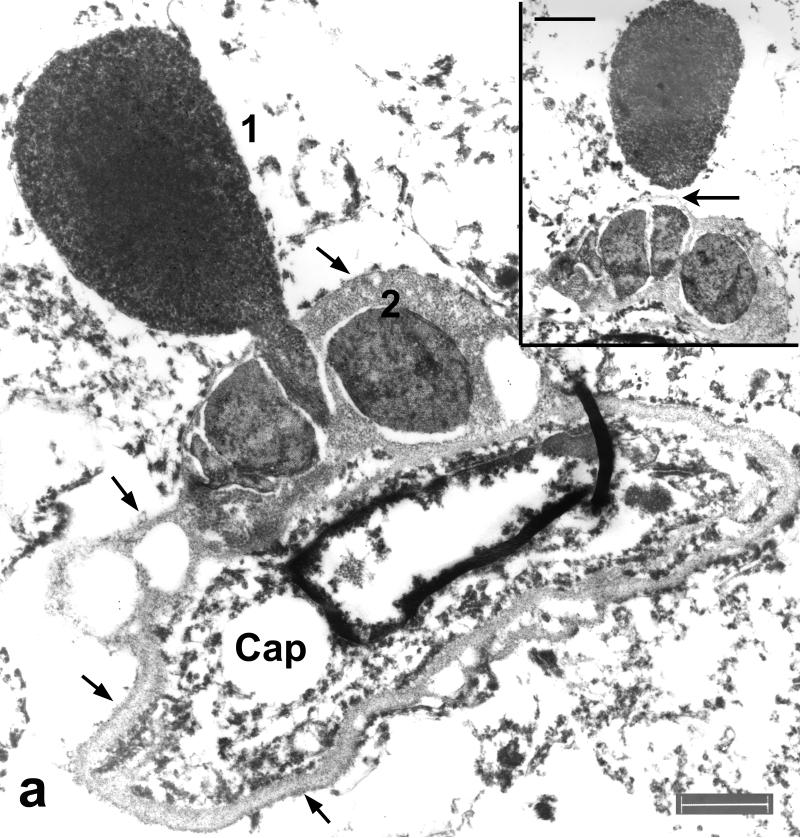
Figure 5.
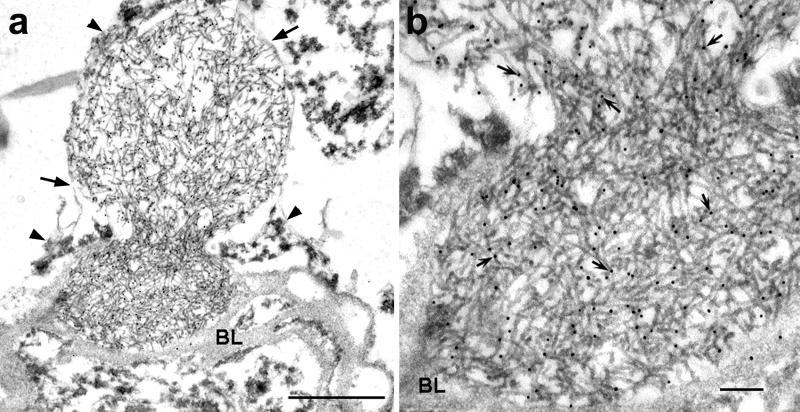
Immunoelectron microscopy of hippocampus in frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U). (a) A cell process with an electron dense core (1) and a filamentous aggregate located respectively on the outside and inside of the capillary (Cap) basal lamina (arrows), which also encloses 2 other inclusions with patchy densities (2). Note the similarity of this figure to Figure 7. Bar = 1 μm. (Inset) A serial section shows aggregate 1 is bisected by the capillary BL (arrow). Bar = 0.5 μm. (b) At higher magnification, both aggregates have TDP-43 immunolabeling (arrows point to gold particles). The image is slightly bleached to show the presence of gold particles in the dense core. Also note in aggregate 1 the continuity of labeled filaments from the dense core to those inside the basal lamina. Bar = 0.3 μm. (c) Aggregate 2 shows highly compact and ordered paracrystalline arrays of filaments and patchy dense material labeled with anti-TDP-43 antibody (arrows point to gold particles). Bar = 0.1 μm.
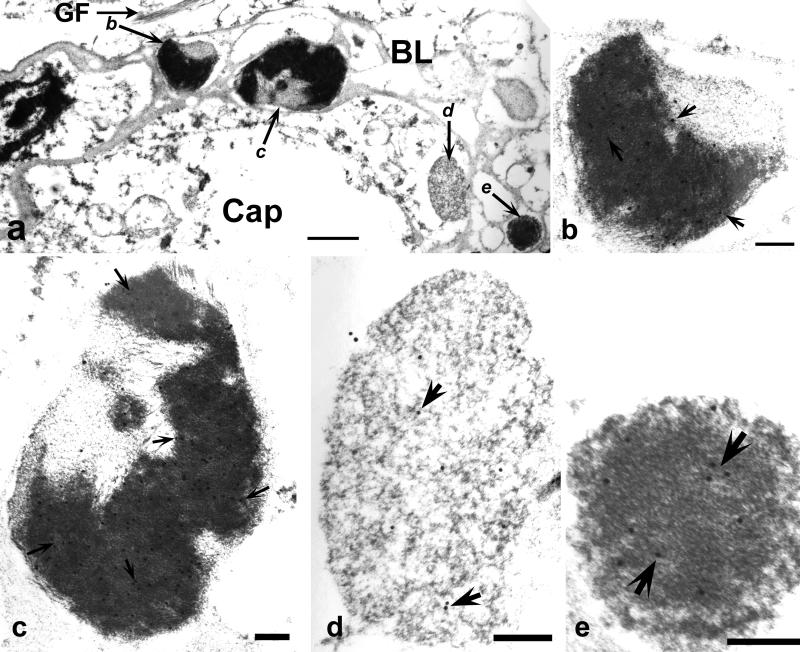
Figure 8.
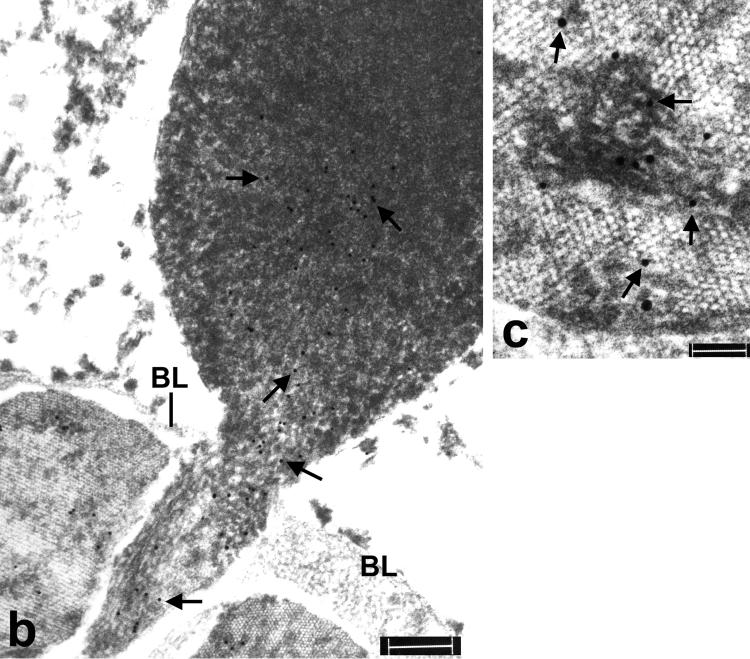
Immunoelectron microscopy of amygdala in familial diffuse Lewy body disease. (a) Multiple TDP-43 positive structures with dense and/or pale regions (lettered arrows at higher magnifications in b-e) enclosed completely by the capillary (Cap); basal laminas (BL); astrocytic fibrils (GF). Bar, 1 μm. (b-e) Higher magnifications of structures in serial sections stained for with anti-αB-crystallin antibody reveal heavy labeling (arrows point to gold particles) over areas with dense material. Images are slightly bleached to show gold particles. Bars, 0.3 um.
Filaments found on the outside of BL tended to be loosely and disorderly arranged granulofilamentous structures (Fig. 2), whereas filaments inside of BL were often more tightly packed (Figs. 2-4, 6, 7). Sometimes they were associated with electron dense amorphous material (Fig. 7) or, less often, a dense paracrystalline array (Fig. 5). These dense paracrystalline arrays were never detected in TDP-43-positive dystrophic neurites or in neuronal cytoplasmic inclusions.
Figure 4.
Immunoelectron microscopy of hippocampus in frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U). (a) A filamentous aggregate enclosed by thick basal lamina (BL) and thin vascular basal lamina (arrows) with the neck surrounded by astrocytic processes (arrowheads). The aggregate surrounded by thick BL is more compact than that enclosed by the thin BL. Bar = 1 μm. (b) At higher magnification the uncoated filaments have dense labeling with the anti-TDP-43 antibody (arrows point to gold particles). Bars = 0.2 μm.
Figure 6.
Immunoelectron microscopy of hippocampus in frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U). Serial sections of a filamentous aggregate (inset) abutting a capillary is labeled with anti-TDP-43 (a) and anti-αB-crystallin (b) antibodies, respectively. Arrows point to gold particles. Astrocytic fibrils (GF) were unlabeled by either antibody. Note the partial covering of the aggregate by a thin basal lamina (BL) (C). Bars in a = 2 μm; b, 0.3 μm.
In fortuitous sections, contiguous processes could be traced from outside the BL to structures that were encased in BL (Figs. 4, 5, 7). Upon closer examination, the outer BL that surrounded the filamentous aggregates was thinner than the BL underneath the vascular endothelium (Figs. 3, 4, 6). Furthermore, thin BL was found partially enclosing the filamentous aggregates (Figs. 6, 7). The filamentous aggregates appeared to be cordoned off by the BL (Figs. 4, 5, 7). Some filamentous aggregates formed a dense mass or a highly ordered structure (Figs. 5, 7, 8).
Figure 3.
Immunoelectron microscopy of parahippocampal gyrus in frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U). (a) A filamentous aggregate containing a denser core (right side) is enclosed by a thin, perhaps newly formed, basal lamina (arrow) that is contiguous to the thicker BL of a capillary. Note the astrocytic hemi-desmosomes attached to the outside, but not the inside, of the thin BL. RBC = red blood cell in the capillary lumen. (b) The uncoated filaments are contiguous from the core to the periphery and are heavily labeled with the anti-TDP-43 antibody (arrows point to gold particles). Bar = 1 μm in (a) and 0.3 μm in (b).
Astrocytic intermediate filament bundles were sometimes in close proximity to cell processes outside of the BL that contained loosely aggregated filaments and these typical glial filaments were unlabeled by anti-TDP-43 antibody (Figs. 2, 6, 8), but heavily labeled with an antibody to GFAP (Fig. 9). Occasionally, GFAP-positive fibrillary bundles were also found enclosed by vascular BL but these structures were TDP-43-negative (Figs. 9c, d). Another feature of reactive astrocytes is the attachment of hemi-desmosomes to the vascular BL (16). Hemi-desmosomes were present on the outside, but not inside, of thin BL that surrounded the TDP-43-positive aggregates (Figs. 2, 3).
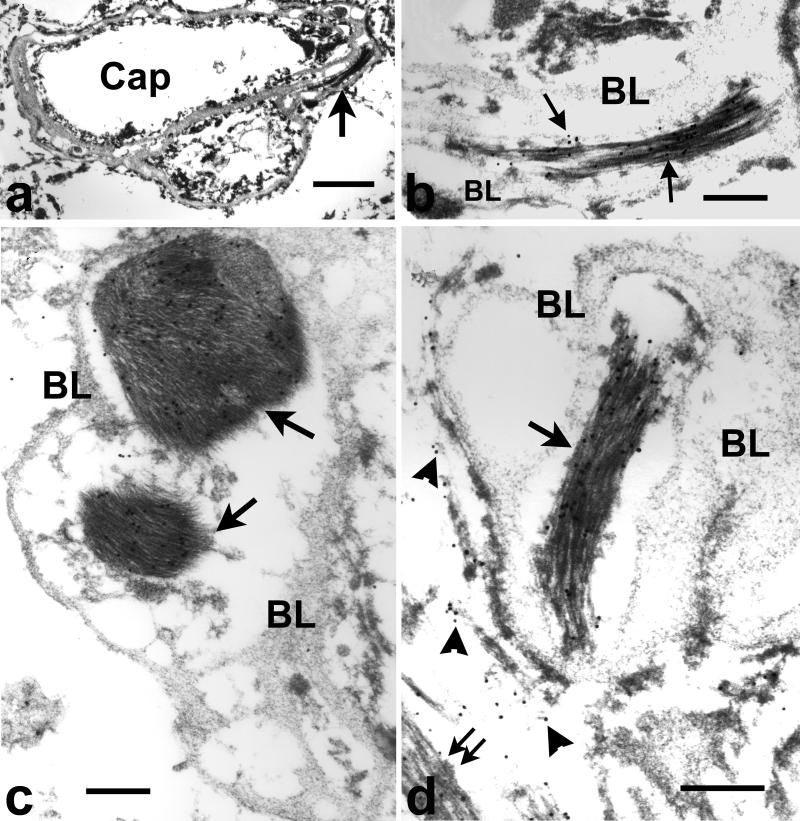
Figure 9.
Immunoelectron microscopy of hippocampus frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U). (a) A capillary (cap) with duplicated basal lamina. Arrow points to a structure shown at higher magnification in (b) showing immunogold labeling for glial fibrillary acidic protein (GFAP) over densely packed bundles of astroglial fibrils (arrows point to gold particles) surrounded by the vascular basal lamina (BL). Bar = 1μm in a, 0.3 μm in b. Additional examples of glial fibrils inside capillary basal lamina at higher magnification. (c) Amygdala in familial diffuse Lewy body disease. Two GFAP-positive glial bundles (arrows) are enclosed by vascular basal lamina (BL). Note the thinner BL on the outermost surface of the vessel. Bar, 0.3 μm. (d) Parahippocampal gyrus, FTLD-U. GFAP-positive glial fibrils are inside (arrow) and outside (double arrow) of the vascular basal lamina (BL). Loose fibrils outside of the BL (arrowheads) are almost invisible due to bleaching of the image to show gold particles in densely packed fibrils. Bar, 0.3 μm.
Serial sections of the same intra- and extra-BL filaments were labeled with anti-TDP-43 and anti-αB-crystallin antibodies, respectively (Figs. 6, 8). The characteristic astrocytic glial filament bundles were not labeled by either antibody.
The filaments were also immunopositive for ubiquitin (data not shown). No specific labeling was observed when primary antibodies were omitted. Other structures, such as neurofilaments and astrocytic glial fibrils, served as negative internal controls.
DISCUSSION
We report abnormal TDP-43-positive structures associated with capillaries in FTLD-U and familial DLBD cases. We have also previously reported similar structures in a case of autosomal dominant Parkinsonism associated with TDP-43 pathology (17). These structures appear to be perivascular astrocytic processes filled with filaments that are immunolabeled for TDP-43 and αB-crystallin. The TDP-43-positive filaments are morphologically identical to those found in swollen neuritic processes not associated with blood vessels in FTLD-U, DLBD, ALS and Alzheimer disease (10). A unique feature in the perivascular TDP-43 inclusion not previously reported in dystrophic neurites or neuronal cytoplasmic inclusions are the paracrystalline arrays of TDP-43. Similar paracrystalline arrays are formed by actin filaments in Hirano bodies (18).
These structures may not have been noted in previous reports of TDP-43 proteinopathy because of their small sizes and the failure to appreciate their association with capillaries. Cytoplasmic inclusions have been reported in glial cells with light microscopic features consistent with oligodendrocytes in FTLD-U, ALS and CBD (5, 7-9, 19, 20) but this study is the first report of TDP-43-positive filamentous aggregates in astrocytic end-feet of FTLD-U and familial DLBD cases. Interestingly, TDP-43-positive structures were found by immunohistochemistry in association with small blood vessels in the superficial layer of entorhinal cortex in a Guam parkinsonism-dementia complex brain (8). These structures were not labeled immunolabeled for GFAP and the authors suggested that they might be degenerating nuclei or swollen processes (8). Further studies are needed to determine whether the latter structures are similar to those we describe.
Although they were not labeled by GFAP antibody, the structures we describe were most likely inclusions in astrocytic processes. An analogous situation exists for αB-crystallin-positive Rosenthal fibers in astrocytes but which show little or no GFAP immunoreactivity in their dense granular component; there is GFAP immunoreactivity in other portions of astrocytes that contain Rosenthal fibers (21).
Normal brain capillaries are almost completely surrounded by astrocytic end-feet that are attached to the vascular BL (22). Paired helical filaments have been reported in astrocytic processes enclosed by basal lamina of small vessels in Alzheimer disease, indicating that abnormal proteins can accumulate in these processes (23). This might explain the presence of the small heat shock protein αB-crystallin in these structures; αB-crystallin is not a component of astrocytic fibrils. In contrast, there was little or no αB-crystallin immunoreactivity in TDP-43-positive NCIs (not shown). Thus, the presence of TDP-43 and αB-crystallin in dense material and filaments in the same processes suggests upregulation of the stress protein αB-crystallin in association with filament pathology (24).
It is possible, although unlikely, that the TDP-43-positive cell processes might be neuronal. Neurovascular contacts are present mostly on large vessels in humans but in other mammals, some brain capillaries are innervated. For example, Tong and Hamel showed by IEM nerve terminals abutting directly on capillary basal lamina in the rat frontoparietal cortex (25), and early studies also showed vesicle-laden axon terminals surrounded by capillary BL in the cat and rat hypothalamus (26, 27). Similar axon terminals were found directly on the capillary BL in the cat supraoptic nucleus (28). To our knowledge, however, the only region in the human brain where neurons and their processes are found closely associated with blood vessels is the substantia nigra; this close contact may be lost due to infiltration of glia in Parkinson disease (29).
It is very unlikely that these processes are in oligodendroglia, although oligodendroglia have been shown to develop TDP-43 inclusions in FTLD-U, ALS and CBD (4-7, 9). On the other hand, oligodendroglial inclusions have not been reported in DLBD (5, 20). There are no ultrastructural studies showing oligodendroglial processes adjacent to capillary BL.
Pericytes and their processes normally reside within the capillary basal lamina. They contain α-smooth muscle actin filaments that are less than 10 μm in diameter, clearly smaller than the TDP-43-positive filaments identified. There are no reports of TDP-43 in pericytes in normal or diseased brains. Moreover, TDP-43 inclusions inside capillary BL were negative for α-smooth muscle actin by IEM (data not shown).
Definitive identification of these cell processes in our study is difficult due to postmortem changes of autopsy tissues and limitations of post-embedding IEM using LR White resin. The IEM method used avoids osmium tetroxide, a good fixative for membrane preservation but poor for antigen preservation. In addition, LR White is a strong solvent, which tends to severely disrupt membranes, making it difficult to use membrane markers, such as CD44 for astrocytic membranes (30), to identify cell the processes.
In conclusion, we demonstrate a new feature of TDP-43 proteinopathy characterized by astrocytic TDP-43 associated with microvasculopathy. These structures are not uncommon and have most likely been overlooked in previous studies or misinterpreted as dystrophic neurites or “extracellular” NCIs. The tendency for the processes to be associated with capillaries and their enclosure by vascular BL is intriguing, particularly the formation of new basal lamina that appears to compress and pinch off the end-feet. In view of the role of astrocytes as critical components of the blood-brain barrier (BBB) (22, 31, 32), the presence of TDP-43 pathology in astrocytic end-feet may indicate that TDP-43 proteinopathies are associated with loss of BBB integrity. It remains to be determined whether there is a correlation between the severity of TDP-43 microvasculopathy and plasma or cerebrospinal fluid levels of TDP-43 (33, 34). Increasing recognition of TDP-43 microvasculopathy and its functional significance with respect to the BBB warrants further investigation.
Figure 10.
Figure 11.
ACKNOWLEDGMENTS
We thank Dr. Larry Golbe for the arrangement of brain donation of the familial DLBD case, Dr. Jack Liang (Harvard University) for his generous gift of αB-crystallin antibody, and Virginia Phillips and Linda Rousseau for their histologic expertise. Presented at annual meeting of American Association of Neuropathologists in 2008.
Supported by NIH grants P50 AG25711, P50 AG16574, P50 NS40256, P01 AG17216 and P01 AG03949.
REFERENCES
- 1.Arai T, Hasegawa M, Akiyama H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–11. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 2.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–33. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 3.Dickson DW, Josephs KA, Amador-Ortiz C. TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol. 2007;114:71–79. doi: 10.1007/s00401-007-0234-5. [DOI] [PubMed] [Google Scholar]
- 4.Brandmeir NJ, Geser F, Kwong LK, et al. Severe subcortical TDP-43 pathology in sporadic frontotemporal lobar degeneration with motor neuron disease. Acta Neuropathol. 2008;115:123–31. doi: 10.1007/s00401-007-0315-5. [DOI] [PubMed] [Google Scholar]
- 5.Higashi S, Iseki E, Yamamoto R, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–94. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 6.Mackenzie IR, Bigio EH, Ince PG, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Annals of neurology. 2007;61:427–34. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- 7.Seelaar H, Schelhaas HJ, Azmani A, et al. TDP-43 pathology in familial frontotemporal dementia and motor neuron disease without Progranulin mutations. Brain. 2007;130:1375–85. doi: 10.1093/brain/awm024. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa M, Arai T, Akiyama H, et al. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain. 2007;130:1386–94. doi: 10.1093/brain/awm065. [DOI] [PubMed] [Google Scholar]
- 9.Neumann M, Kwong LK, Truax AC, et al. TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J Neuropathol Exp Neurol. 2007;66:177–83. doi: 10.1097/01.jnen.0000248554.45456.58. [DOI] [PubMed] [Google Scholar]
- 10.Lin WL, Dickson DW. Ultrastructural localization of TDP-43 in filamentous neuronal inclusions in various neurodegenerative diseases. Acta Neuropathol. 2008;116:205–13. doi: 10.1007/s00401-008-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujishiro H, Tsuboi Y, Lin WL, et al. Co-localization of tau and alpha-synuclein in the olfactory bulb in Alzheimer’s disease with amygdala Lewy bodies. Acta Neuropathol. 2008;116:17–24. doi: 10.1007/s00401-008-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang JJ. Interaction between beta-amyloid and lens alphaB-crystallin. FEBS letters. 2000;484:98–101. doi: 10.1016/s0014-5793(00)02136-0. [DOI] [PubMed] [Google Scholar]
- 13.Mackenzie IR, Baborie A, Pickering-Brown S, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: Classification and relation to clinical phenotype. Acta Neuropathol. 2006;112:539–49. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josephs KA, Stroh A, Dugger B, et al. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol. 2009;118:349–58. doi: 10.1007/s00401-009-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano I, Kato S, Yazawa I, et al. Anchorage densities associated with hemidesmosome-like structures in perivascular reactive astrocytes. Acta Neuropathol. 1992;84:85–88. doi: 10.1007/BF00427219. [DOI] [PubMed] [Google Scholar]
- 17.Wider C, Dickson DW, Stoessl AJ, et al. Pallidonigral TDP-43 pathology in Perry syndrome. Parkinsonism & related disorders. 2009;15:281–86. doi: 10.1016/j.parkreldis.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldman JE. The association of actin with Hirano bodies. J Neuropathol Exp Neurol. 1983;42:146–52. doi: 10.1097/00005072-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Geser F, Winton MJ, Kwong LK, et al. Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol. 2008;115:133–45. doi: 10.1007/s00401-007-0257-y. [DOI] [PubMed] [Google Scholar]
- 20.Nakashima-Yasuda H, Uryu K, Robinson J, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007;114:221–29. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 21.Tomokane N, Iwaki T, Tateishi J, et al. Rosenthal fibers share epitopes with alpha B-crystallin, glial fibrillary acidic protein, and ubiquitin, but not with vimentin. Immunoelectron microscopy with colloidal gold. The American journal of pathology. 1991;138:875–85. [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer H, Bauer H-C, Haseloff RF, et al. The role of glia in the formation and function of the blood-brain barrier. In: Kettenmann H, Ransom BD, editors. Neuroglia. Oxford University Press; Oxford: 2005. pp. 325–33. [Google Scholar]
- 23.Yamazaki M, Nakano I, Imazu O, et al. Paired helical filaments and straight tubules in astrocytes: an electron microscopic study in dementia of the Alzheimer type. Acta Neuropathol. 1995;90:31–36. doi: 10.1007/BF00294456. [DOI] [PubMed] [Google Scholar]
- 24.Quinlan RA, Brenner M, Goldman JE, et al. GFAP and its role in Alexander disease. Exp Cell Research. 2007;313:2077–87. doi: 10.1016/j.yexcr.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong XK, Hamel E. Basal forebrain nitric oxide synthase (NOS)-containing neurons project to microvessels and NOS neurons in the rat neocortex: Cellular basis for cortical blood flow regulation. Eur J Neurosci. 2000;12:2769–80. doi: 10.1046/j.1460-9568.2000.00158.x. [DOI] [PubMed] [Google Scholar]
- 26.Rennels ML, Nelson E. Capillary innervation in the mammalian central nervous system: An electron microscopic demonstration. Am J Anat. 1975;144:233–41. doi: 10.1002/aja.1001440208. [DOI] [PubMed] [Google Scholar]
- 27.Swanson LW, Connelly MA, Hartman BK. Ultrastructural evidence for central monoaminergic innervation of blood vessels in the paraventricular nucleus of the hypothalamus. Brain Res. 1977;136:166–73. doi: 10.1016/0006-8993(77)90142-1. [DOI] [PubMed] [Google Scholar]
- 28.Bargmann W, Oksche A, Fix JD, et al. Meninges, choroid plexuses, ependyma and their reactions. In: Haymaker W, Adams RD, editors. Histology and histopathology of the nervous system. Thomas; Springfield: 1982. pp. 582–83. [Google Scholar]
- 29.Issidorides MR. Neuronal vascular relationships in the zona compacta of normal and parkinsonian substantia nigra. Brain Res. 1971;25:289–99. doi: 10.1016/0006-8993(71)90439-2. [DOI] [PubMed] [Google Scholar]
- 30.Feany MB, Dickson DW. Widespread cytoskeletal pathology characterizes corticobasal degeneration. The American journal of pathology. 1995;146:1388–96. [PMC free article] [PubMed] [Google Scholar]
- 31.Wolburg H, Noell S, Wolburg-Buchholz K, et al. Agrin, aquaporin-4, and astrocyte polarity as an important feature of the blood-brain barrier. Neuroscientist. 2009;15:180–93. doi: 10.1177/1073858408329509. [DOI] [PubMed] [Google Scholar]
- 32.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Foulds P, McAuley E, Gibbons L, et al. TDP-43 protein in plasma may index TDP-43 brain pathology in Alzheimer’s disease and frontotemporal lobar degeneration. Acta Neuropathol. 2008;116:141–46. doi: 10.1007/s00401-008-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinacker P, Hendrich C, Sperfeld AD, et al. TDP-43 in cerebrospinal fluid of patients with frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Archives of neurology. 2008;65:1481–87. doi: 10.1001/archneur.65.11.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]