Abstract
Background
Monocyte chemoattractant protein-1 (MCP-1) is an inflammatory chemokine known to induce adipocyte dedifferentiation and insulin resistance. Inflammation, insulin resistance, and obesity have been implicated in the pathogenesis of non-alcoholic fatty liver disease (NAFLD).
Methods
Fasting plasma from 43 baboons were assayed for MCP-1, insulin, glucose, alanine aminotransferase (ALT), and aspartate aminotransferase (AST). Adipocyte number and volume were measured via biopsies of omental adipose tissue. The homeostatic model assessment method (HOMA) was used to estimate systemic insulin resistance.
Results
Sex and age adjusted correlations were significant for MCP-1 with adipocyte number (r = −0.42; P = 0.01), adipocyte volume (r = 0.38; P = 0.02), HOMA (r = 0.45; P = 0.004), ALT (r = 0.46; P = 0.03) and AST (r = 0.45; P = 0.03).
Conclusions
These results suggest that MCP-1 is related with adipocyte dedifferentiation and systemic insulin resistance, thereby potentially contributing to the development of NAFLD.
Keywords: adipocyte number, adipocyte volume, AST and ALT, insulin resistance, liver function marker, MCP-1, NAFLD
Introduction
In obesity adipocytes expand in size because of the deposition of triglyceride [14]. However, this expansion may be limited. Chen et al. hypothesized that excessive hypertrophy of the fat cells might create a hypoxic environment within the adipose tissue [6]. Consequently, this reduced availability of oxygen could lead to adipocyte death [31], which may then trigger macrophage infiltration to clear the cellular debris [8]. It is believed that monocyte chemoattractant protein-1 (MCP-1), a chemokine released by adipocytes, is responsible for attracting monocytes into these injured fat depots [7].
The expression and secretion of MCP-1 is known to be elevated during obesity in both mice [44] and humans [4]. MCP-1 mediates its action by binding to its specific receptor CC chemokine receptor 2 (CCR2) [5]. In a recent study, obese mice lacking CCR2 had low macrophage concentrations in adipose tissue [45]. In addition, MCP-1 is believed to induce the dedifferentiation of adipocytes, which might result in obesity-related disorders because of ectopic fat deposition. The expression of MCP-1 is higher in visceral, as compared with subcutaneous, adipose tissue [4]. This protein impairs insulin-stimulated glucose uptake in adipocytes, resulting in insulin resistance [34]. Thus, it is postulated that MCP-1 may play an integral role in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) [19].
Two major risk factors of NAFLD are visceral obesity and insulin resistance [1]. In the pathogenesis of NAFLD in overweight and obese individuals, insulin, and excessive free fatty acids from insulin-resistant omental adipose tissue drain directly into the liver via the portal vein. In the liver, insulin up-regulates the conversion of unesterified fatty acids to triacylgycerols by the activation of sterol regulatory binding protein-1C [23]. In insulin-resistant states there is a sustained upregulation of microsomal TG transfer protein (MTP) expression and protein levels as a result of resistance to insulin’s inhibitory effect on MTP. This results in increased Very-low-density lipoprotein (VLDL) production and secretion. When TG production exceeds FA oxidation and VLDL production [42, 43], this result in lipid accumulation in the hepatocytes, which might triggers a cascade of necroinflammatory changes in the liver [11].
At present, the gold standard for diagnosing fatty liver disease is a biopsy [3]. However, two biochemical markers of hepatic injury, plasma alanine aminotransferase (ALT), and aspartate aminotransferase (AST) will be used in this study as non-invasive surrogates for NAFLD [41].
The purpose of this study is to evaluate the importance of inflammation in the pathogenesis of NAFLD, using overweight and obese baboons as a model of chronic inflammation. We hypothesize that the increased circulating levels of MCP-1 are associated with fewer mature adipocytes. This inflammatory molecule might also result in insulin resistance and elevate circulating levels of markers of liver dysfunction because of subsequent liver injury caused by fat deposition.
Materials and methods
Animals
Forty-three (31 females and 12 males) unrelated baboons (Papio hamadrayas cynocephalus) were included in this study. The baboon colony is maintained at the Southwest National Primate Research Center located at the Southwest Foundation for Biomedical Research (SFBR) in San Antonio, TX, USA. These animals are gang-housed and fed a low fat, standard monkey chow diet ad libitum (Harlan Teklad 15% monkey diet, 8715, Indianapolis, IN, USA).
Sampling and analyses
The Institutional Animal Care and Use Committee of the SFBR approved all procedures. Animals were fasted overnight (12 hours) and sedated with ketamine before collection of blood samples. Body weights were measured on a calibrated electronic scale (GSE, Chicago, IL, USA). A total of 10 ml of blood was drawn from the antecubital vein in heparin tubes. Plasma was obtained by centrifugation at 2000 g for 10 minutes and was stored in aliquots at −80°C for future analysis. Assays for ALT and AST were conducted by standard laboratory techniques using an Alfa Wasserman ACE clinical chemistry instrument (West Cladwell, NJ, USA). Glucose was analyzed on an Analox spectrophotometer by the glucose oxidase method. Insulin and MCP-1 were measured by chemiluminescence in a Luminex 100 with endocrine multiplex immunoassay (Linco Research Inc., St. Charles, MO, USA). Insulin resistance was calculated by the homeostatic model assessment (HOMA) method [26].
Biopsies of omental adipose tissue were taken from anesthetized baboons. One centimeter incision was made in the abdominal midline a centimeter above the umbilicus through the abdominal wall. The omentum was retrieved using a grasping forceps. Approximately 1 gm of omental fat was sequestered by ligature, excised, and immediately frozen in liquid nitrogen, for storage at −80°C and/or in liquid nitrogen. The facia and skin were sutured.
Adipocyte number and volume per gram of tissue was analyzed by methods previously described by Lewis et al. [24]. All samples whose replicates varied >5% variations were reanalyzed. Wet weight, triglyceride mass, fat cell volume, and fat cell number were measured in the omental, fat depots. The mean diameter of adipocytes was measured by the method of DiGirolamo et al. [12] as modified by Stiles et al. [40]. The fat pads from the omental adipose depots of baboons were minced, gassed for 15 s (O2–CO2, 95:5%) and incubated at 37°C in a shaking water bath for 60 minutes with Krebs ringer bicarbonate buffer (KRB) at pH 7.4 containing 1.5 mg/ml collagnease. The freed adipocytes were filtered through a 200 μm mesh silk, washed three times with KRB buffer. Mean adipocyte volume i.e. cell diameters were measured microscopically assuming the adipocytes are spherical. Triglyceride was extracted and triglyceride content was measured from the omental tissue sample. Fat cell number was calculated by dividing the total volume of triglyceride per sample by mean adipocyte volume.
Percent body fat was obtained by bioimpedance using a Xitron multi-frequency bioimpedance analyzer (Xitron Technologies Corp., San Diego, CA, USA). The animals were placed on their backs on a rubber mat and electrodes were attached to shaved areas on both wrists and ankles.
Statistical analysis
Descriptive statistics and other analyses were conducted using spss (version 10.0; SPSS Inc., Chicago, IL, USA). Results are expressed as mean (standard error of mean). Student’s t-test was used to evaluate the differences between sexes. All variables, other than sex and age, were log transformed to obtain a Gaussian distribution. Linear regression was performed, controlling for the effect of age and sex to estimate the partial correlation of plasma MCP-1 with omental adipocyte number and volume, HOMA scores, and plasma ALT and AST. Residual analysis, using Cook’s distance and leverage coefficients, was conducted to analyze the effect of any point on the estimated regression [39].
Results
Table 1 provides sex-specific descriptive statistics for all traits analyzed in this study. The study population was comprised of more than twice as many females as males. The mean body weight of females was lower than that of males with female body weights ranging from 14 kg to 32 kg and that of males from 28 kg to 47 kg. Females were significantly older and had reduced concentrations of plasma MCP-1 as compared with males. Adipocyte number and volume, HOMA and plasma levels of ALT were not substantially different between the sexes. However, differences between the circulating concentrations of AST approached statistical significance.
Table 1.
Descriptive statistics of baboons*
| Trait | Male | Female | P-value |
|---|---|---|---|
| Number | 12 | 31 | |
| Weight (kg) | 32.42 ± 1.6 | 19.22 ± 0.8 | 0.0001 |
| Age (years) | 14.35 ± 1.06 | 20.67 ± 1.0 | 0.001 |
| Monocyte chemoattractant protein 1 (pg/ml) | 229.68 ± 19.1 | 152.89 ± 8.9 | 0.0001 |
| Adipocyte number (× 106 cell) | 5 × 106 ± 1 × 106 | 4 × 106 ± 9 × 105 | 0.60 |
| Adipocyte volume (nl) | 0.33 ± 0.1 | 0.55 ± 0.1 | 0.09 |
| Percent body fat (%) | 15.06 ± 2.3 | 20.06 ± 1.8 | 0.14 |
| Glucose (mg/dl) | 77.29 ± 2.9 | 75.17 ± 4.6 | 0.7 |
| Insulin (μU/dl) | 47.87 ± 10.7 | 56.94 ± 6.9 | 0.49 |
| HOMA | 1.37 ± 0.4 | 1.59 ± 0.2 | 0.62 |
| Alanine aminotransferase (IU/l) | 32.41 ± 3.7 | 28.40 ± 3.1 | 0.47 |
| Aspartate aminotransferase (IU/l) | 29.25 ± 2.9 | 23.51 ± 1.5 | 0.06 |
HOMA, Homeostasis model assessment method.
Mean (SEM).
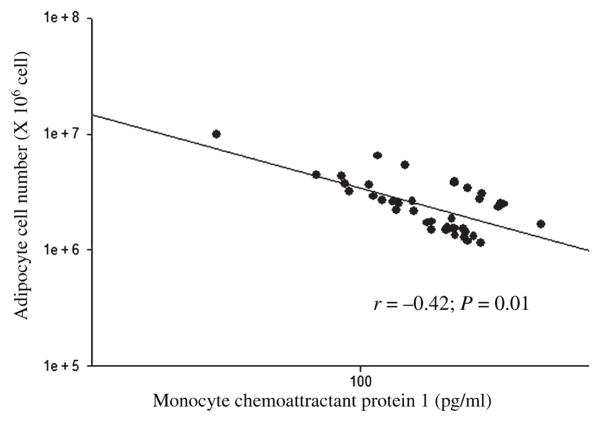
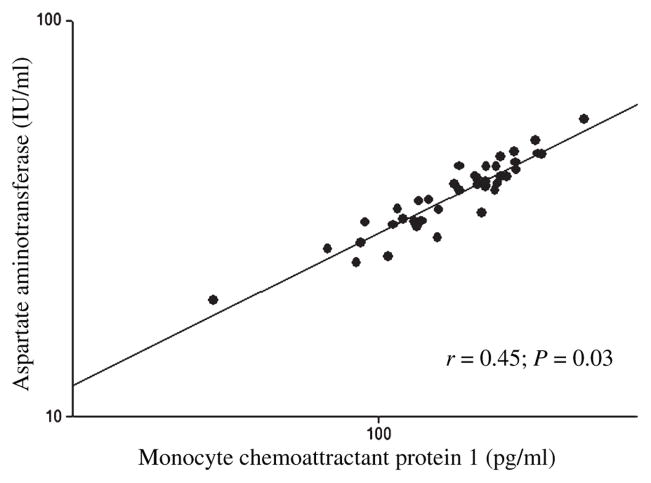
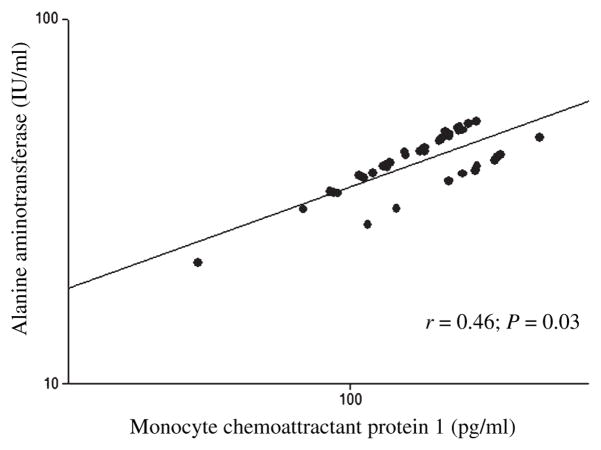
After controlling for age and sex, plasma MCP-1 was negatively associated with adipocyte number (Fig. 1) and positively associated with adipocyte volume (r = 0.38; P = 0.02), HOMA (Fig. 2), ALT (Fig. 3), and AST (Fig. 4).
Fig. 1.
The association between the plasma monocyte chemoattractant protein 1 and adipocyte cell number in baboons. *Age and sex adjusted.
Fig. 2.
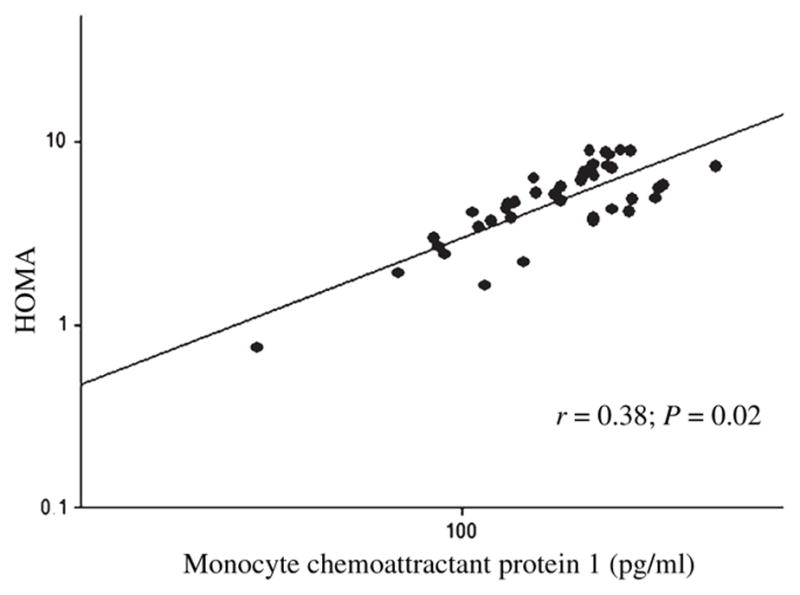
The association between the plasma monocyte chemoattractant protein 1 and HOMA insulin resistance index in baboons. *Age and sex adjusted.
Fig. 3.
The association between the plasma monocyte chemoattractant protein 1 and aspartate aminotransferase in baboons. *Age and sex adjusted.
Fig. 4.
The association between the plasma monocyte chemoattractant protein 1 and alanine aminotransferase in baboons. *Age and sex adjusted.
Discussion
This is the first study to demonstrate that plasma levels of MCP-1 are negatively associated with omental adipocyte number and positively associated with HOMA and markers of liver dysfunction in baboons.
In adipocyte dedifferentiation loss of mature adipocyte phenotype occurs through the suppression of two transcription factors, CCAAT/enhancer binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) [36]. These factors act in concert to regulate the expression of adipogenic genes required to maintain mature fat cells in a differentiated state [22]. Sartipy and Loskutoff have suggested that MCP-1 may cause a reduction in the number of fat cells by reducing the mRNA expression of PPARγ [34]. In addition, Nadler et al. have reported that adipose tissue from obese mice exhibit a decreased number of C/EBPα and PPARγ transcripts [29]. The down regulation of these adipogenic genes might be because of obesityrelated inflammation.
An alternate explanation for the relationship between adipocyte size and MCP-1 is that enlarged adipocytes secrete more MCP-1. In obesity, white adipose tissue becomes enlarged because of increased adipocyte size (hypertrophy) and/or number (hyperplasia) [16]. Adipocyte size is a function of the balance between lipogenesis and lipolysis [37]. However, fat cell number is controlled by the equilibrium between proliferation or differentiation and apoptosis [2]. It is postulated that failure of pre-adipocytes to differentiate into mature lipid storage cells expands the existing adipocytes during periods of surplus energy intake [17]. The resultant hypertrophic cells are known to have dysfunctional lipid and glucose metabolism [38], leading to insulin resistance [27] and ectopic fat accumulation in tissues other than adipose depots [10]. However, it is hypothesized that fat cells have a limited capacity to expand [20]. Once the enlarged cells reach a critical mean volume they are liable to rupture because of stress [28].
The dead adipocytes activate inflammatory signaling pathways. These pathways could compromise the insulin sensitivity of the remaining fat cells [8]. In addition, dead cells attract macrophages into the adipose tissue to clear cellular debris [45]. These immune cells release cytokines into the milieu, which further exacerbates insulin resistance within the adipose tissue [46]. The impairment of insulin signaling, in turn, stimulates lipolysis and increases the circulating concentrations of unesterified fatty acids [32]. These free fatty acids that are released, particularly from the visceral fat depots, are transported to the nearby organs such as the liver and may cause organ damage by initiating hepatic triglyceride accumulation [13]. Our data suggests that obesity-related inflammation may lead to an abnormal adipocyte life cycle, which reduces the number of lipid-bearing mature adipocytes and thereby potentially leading to other metabolic complications.
A positive relationship between plasma levels of MCP-1 and the insulin resistance index (HOMA) was observed in this study. This association has also been shown in mice and humans. For example, Kamei et al. demonstrated that over expression of MCP-1 in transgenic mice resulted in a systemic insulin resistance [18]. In a study by Weisberg et al. insulin sensitivity improved in obese mice treated with an antagonist of CCR2, the MCP-1 receptor [45]. In overweight and obese humans who are prone to having low insulin sensitivity, the circulating levels of MCP-1 were positively related to HOMA scores [21]. Thus, it is likely that MCP-1 may participate in the pathogenesis of insulin resistance by impairing insulin signaling and reducing insulin-mediated glucose uptake [18].
We also found that circulating concentrations of MCP-1 were positively related to markers of liver dysfunction, plasma ALT, and AST. The levels of these liver enzymes are known to be elevated in obese and insulin-resistant individuals [9]. MCP-1 may be associated with elevated levels of ALT and AST through its contribution to both insulin resistance and hepatic inflammation. Thus, the disruption of insulin signaling in adipocytes because of inflammatory molecules may lead to ectopic fat deposition [33]. This triglyceride accumulation in the liver may then increase the susceptibility to hepatic inflammation and apoptosis. Subsequently, these events may lead to a release of hepatocyte constituents into the circulation and raising the blood levels of liver injury markers.
The serum levels of MCP-1 are elevated in individuals with steatosis, with the highest concentrations in subjects with non-alcoholic steatohepatitis [15]. The association between MCP-1 and NAFLD is strengthened further by the findings of Kanda et al. who showed that insulin resistance and hepatic steatosis were ameliorated as a result the deletion of the MCP-1 gene in mice that were fed a high fat diet [19].
Although baboons have been used extensively for the study of alcoholic fatty liver disease [25, 30, 35], this is the first time that this primate has been used as a model for obesity-related, hepatic pathology. Briefly, this article suggests that MCP-1 and adipocyte cell number are inversely related. This endogenous, immunomodulatory chemokine might promote adipocyte dedifferentiation (reduced cell number) events that may interfere with insulin-mediated glucose uptake and subsequent insulin resistance. Ultimately, obesityrelated inflammation, in conjunction with insulin resistance, may lead to the pathogenesis associated with NAFLD.
Acknowledgments
This study was supported in part by NIH Grants HL28972, MH59490, and RR013986. This investigation was conducted in facilities constructed with support form the Research Facilities Improvement Program under grant numbers C06 RR014578, C06 RR013556, C06 RR015456, and C06 RR017515 from the National Center for Research Resources of the National Institutes of Health.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Avram MM, Avram AS, James WD. Subcutaneous fat in normal and diseased states: 1. Introduction. J Am Acad Dermatol. 2005;53:663–70. doi: 10.1016/j.jaad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Brunt EM. Nonalcoholic steatohepatitis: pathologic features and differential diagnosis. Semin Diagn Pathol. 2005;22:330–8. doi: 10.1053/j.semdp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90:2282–9. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- 5.Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA. 1994;91:2752–6. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen B, Lam KS, Wang Y, Wu D, Lam MC, Shen J, Wong L, Hoo RL, Zhang J, Xu A. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem Biophys Res Commun. 2006;341:549–56. doi: 10.1016/j.bbrc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes. 2005;29:146–50. doi: 10.1038/sj.ijo.0802839. [DOI] [PubMed] [Google Scholar]
- 8.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Colicchio P, Tarantino G, del Genio F, Sorrentino P, Saldalamacchia G, Finelli C, Conca P, Contaldo F, Pasanisi F. Non-alcoholic fatty liver disease in young adult severely obese non-diabetic patients in South Italy. Ann Nutr Metab. 2005;49:289–95. doi: 10.1159/000087295. [DOI] [PubMed] [Google Scholar]
- 10.Danforth E., Jr Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet. 2000;26:13. doi: 10.1038/79111. [DOI] [PubMed] [Google Scholar]
- 11.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–5. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 12.DiGirolamo M, Mendlinger S, Fertig W. A simple method to determine fat cell size and number in four mammalian species. Am J Physio. 1971;227:850–8. doi: 10.1152/ajplegacy.1971.221.3.850. [DOI] [PubMed] [Google Scholar]
- 13.Eguchi Y, Eguchi T, Mizuta T, Ide Y, Yasutake T, Iwakiri R, Hisatomi A, Ozaki I, Yamamoto K, Kitajima Y, Kawaguchi Y, Kuroki S, Ono N. Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J Gastroenterol. 2006;41:462–9. doi: 10.1007/s00535-006-1790-5. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–5S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 15.Haukeland JW, Damas JK, Konopski Z, Loberg EM, Haaland T, Goverud I, Torjesen PA, Birkeland K, Bjoro K, Aukrust P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167–74. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–54. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 17.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord Suppl. 2004;28:S12–21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- 18.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–14. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 19.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawada T, Takahashi N, Fushiki T. Biochemical and physiological characteristics of fat cell. J Nutr Sci Vitaminol. 2001;47:1–12. doi: 10.3177/jnsv.47.1. [DOI] [PubMed] [Google Scholar]
- 21.Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, Kwon BS, Erickson KL, Yu R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes. 2006;30:1347–55. doi: 10.1038/sj.ijo.0803259. [DOI] [PubMed] [Google Scholar]
- 22.Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol. 2002;37:757– 67. doi: 10.1016/s0531-5565(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 23.Kotani K, Peroni OD, Minokoshi Y, Boss O, Kahn BB. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J Clin Invest. 2004;114:1666–75. doi: 10.1172/JCI21341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis DS, Bertrand HA, McMahan CA, McGill HC, Jr, Carey KD, Masoro EJ. Preweaning food intake influences the adiposity of young adult baboons. J Clin Invest. 1986;78:899–905. doi: 10.1172/JCI112678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieber CS, Leo MA, Mak KM, DeCarli LM, Sato S. Choline fails to prevent liver fibrosis in ethanol-fed baboons but causes toxicity. Hepatology. 1985;5:561–72. doi: 10.1002/hep.1840050407. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin rsistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.McGarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia. 1999;42:128–38. doi: 10.1007/s001250051130. [DOI] [PubMed] [Google Scholar]
- 28.Monteiro R, de Castro PM, Calhau C, Azevedo I. Adipocyte size and liability to cell death. Obes Surg. 2006;16:804–6. doi: 10.1381/096089206777346600. [DOI] [PubMed] [Google Scholar]
- 29.Nadler ST, Stoehr JP, Schueler KL, Tanimoto G, Yandell BS, Attie AD. The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc Natl Acad Sci USA. 2000;97:11371–6. doi: 10.1073/pnas.97.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navder KP, Baraona E, Leo MA, Lieber CS. Oxidation of LDL in baboons is increased by alcohol and attenuated by polyenylphosphatidylcholine. J Lipid Res. 1999;40:983–7. [PubMed] [Google Scholar]
- 31.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006;116:33–5. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Permana PA, Menge C, Reaven PD. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Commun. 2006;341:507–14. doi: 10.1016/j.bbrc.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Raz I, Eldor R, Cernea S, Shafrir E. Diabetes: insulin resistance and derangements in lipid metabolism Cure through intervention in fat transport and storage. Diabetes Metab Res Rev. 2005;21:3–14. doi: 10.1002/dmrr.493. [DOI] [PubMed] [Google Scholar]
- 34.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–70. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savolainen MJ, Baraona E, Leo MA, Lieber CS. Pathogenesis of the hypertriglyceridemia at early stages of alcoholic liver injury in the baboon. J Lipid Res. 1986;2710:1073–83. [PubMed] [Google Scholar]
- 36.Schaffier A, Muller-Ladner U, Scholmerich J, Buchler C. Role of adipose tissue as an inflammatory organ in human diseases. Endocr Rev. 2006;27:449–67. doi: 10.1210/er.2005-0022. [DOI] [PubMed] [Google Scholar]
- 37.Schling P, Loffier G. Cross talk between adipose tissue cells: impact on pathophysiology. News Physiol Sci. 2002;17:99–104. doi: 10.1152/nips.01349.2001. [DOI] [PubMed] [Google Scholar]
- 38.Smith J, Al-Amri M, Dorairaj P, Sniderman A. The adipocyte life cycle hypothesis. Clin Sci. 2006;110:1–9. doi: 10.1042/CS20050110. [DOI] [PubMed] [Google Scholar]
- 39.Sokal RR, Rohlf FJ. Biometry; the Principles and Practice of Statistics in Biological research. W. H. Freeman; 1994. [Google Scholar]
- 40.Stiles JW, Francendese AA, Masoro EJ. Influence of age on size and number of fat cells in the epididymal depot. Am J Physiol. 1975;229:1561–8. doi: 10.1152/ajplegacy.1975.229.6.1561. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki A, Lymp J, Sauver JS, Angulo P, Lindor K. Values and limitations of serum aminotransferases in clinical trials of nonalcoholic steatohepatitis. Liver Int. 2006;26:1209–16. doi: 10.1111/j.1478-3231.2006.01362.x. [DOI] [PubMed] [Google Scholar]
- 42.Taghibiglou C, Carpentier A, Van Iderstine SC, Chen B, Rudy D, Aiton A, Lewis GF, Adeli K. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular apo B degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J Biol Chem. 2000;275:8416–25. doi: 10.1074/jbc.275.12.8416. [DOI] [PubMed] [Google Scholar]
- 43.Taghibiglou C, Van-Inderstine S, Letien H, Fantus G, Lewis GF, Adeli K. Hepatic VLDL-apo B overproduction is associated with attenuated hepatic insulin signaling in a fructose-fed hamster model of insulin resistance: evidence for increased expression of PTP-1B and decreased abundance of ER-60 protease. J Biol Chem. 2002;277:793–803. doi: 10.1074/jbc.M106737200. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi K, Mizuarai S, Araki H, Mashiko S, Ishihara A, Kanatani A, Itadani H, Kotani H. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J Biol Chem. 2003;278:46654–60. doi: 10.1074/jbc.M309895200. [DOI] [PubMed] [Google Scholar]
- 45.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115– 24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]