Abstract
Depressive symptoms, poor sleep quality, and systemic markers of inflammation (e.g. interleukin (IL)-6) are frequently associated. Interferon-alpha (IFN-α) therapy results in major depressive disorder (MDD) in some people, offering the possibility to elucidate the relationship of MDD to sleep and inflammation during treatment. In particular, delineating the temporal relations among these factors could help inform their causal relationships. To this end, a cohort of 95 non-depressed hepatitis C patients was followed prospectively for four consecutive months during IFN-α therapy. We found that higher pre-treatment levels of circulating IL-6 predicted incidence of MDD (X2(1)=7.7; p<0.05). Time-lagged mixed-effect analyses supported uni-directional associations in which IL-6 predicted next month’s PSQI scores (F(47, 11.6) = 78.4; p<0.0005), and PSQI scores predicted next month’s depressive Beck Depression Inventory-II (BDI) scores (F(16,22.6) = 3.4; p<0.005). In addition, on any given month of treatment, IL-6 levels predicted BDI symptoms the following month (F(16,97.5) = 7.3; p<0.0005), and conversely BDI predicted next month’s IL-6 (F(14,7.4) = 5.2; p<0.05) – providing evidence for a positive feedback relationship between depressive symptoms and systemic inflammation. These data provide further evidence that high levels of inflammation and poor sleep quality may be risk factors for IFN-α induced depression. Furthermore, these findings highlight the complex temporal relationships that exist among sleep, depression, and inflammation, and support the need for further prospective investigations to elucidate the dynamics that underlie depression during IFN-α treatment.
Introduction
Major Depressive Disorder (MDD) is associated with the onset and progression of several medical conditions (Eaton et al., 1996; Everson-Rose and Lewis, 2005; Frasure-Smith et al., 1993; Zautra et al., 1994). One link between MDD and illness is inflammation, which plays an essential role in the pathogenesis of a number of chronic diseases, including cardiovascular disease, diabetes, and cancer (Antoni et al., 2006; Black, 2003; Papanicolaou et al., 1998; Pradhan et al., 2001; Pradhan and Ridker, 2002; Ridker et al., 2000). Relatedly, elevations in systemic inflammation have been observed in patients with MDD (Howren et al., 2009; Raison et al., 2006; Zorrilla et al., 2001), as evinced by increases in circulating levels of interleukin (IL)-6, when compared to non-depressives (Frommberger et al., 1997; Maes et al., 1995; Musselman et al., 2001b; Pike and Irwin, 2006; Sluzewska et al., 1996).
An emerging literature suggests that proinflammatory cytokines may contribute to the development of depressive symptoms including depressed mood, anhedonia, fatigue, and impaired sleep (Dantzer et al., 2008; Raison et al., 2006). IL-6 and its receptor are synthesized in the brain (Schobitz et al., 1994), with high densities in the hippocampus and hypothalamus (Hopkins and Rothwell, 1995). Healthy humans treated with an acute inflammatory stimulus (e.g. Salmonella abortis equi endotoxin or typhoid vaccination) exhibit significant increases in depressive symptoms, decreases in cognitive functioning, and alterations in sleep electrophysiology (Haack et al., 2001; Mullington et al., 2000; Reichenberg et al., 2001; Wright et al., 2005). Furthermore, there is growing evidence that cytokine antagonists mitigate these behavioral changes in rodents and have been shown to reduce depressive symptoms among patients with inflammatory conditions (Cohen et al., 2006; Dantzer et al., 2008; Tyring et al., 2006). Nevertheless, it is also plausible that the converse may be true. In other words, depression may lead to changes in inflammatory cytokine levels. For instance, there is laboratory evidence that people with depression display exaggerated stress-related increases in inflammatory responses (Miller et al., 2005; Pace et al., 2006). In addition, perturbations in depressed mood have been associated with subsequent increases of circulating IL-6 (Rohleder and Miller, 2008), raising the possibility that changes in negative mood may contribute to elevations in inflammatory activity.
To prospectively explore the bi-directional relations between depression and proinflammatory cytokines in humans, patients undergoing interferon (IFN)-α therapy offer a unique opportunity. IFN-α is a cytokine of the early innate immune system released in response to viral infection and induces cellular release of other proinflammatory mediators, namely IL-1β and IL-6, into systemic circulation (Dantzer et al., 2008; Shimizu et al., 1995). In combination with ribavirin, IFN-α is the primary treatment for patients with chronic hepatitis C viral (HCV) infection. But while efficacious, a substantial portion of patients (10–40%) develop major depression during treatment (Capuron et al., 2002; Capuron and Miller, 2004; Musselman et al., 2001a). Several studies have demonstrated elevations in peripheral levels of IL-6 among patients undergoing IFN-α therapy (Bonaccorso et al., 2001; Wichers et al., 2007). Recent findings indicate that levels of IL-6 in the CSF of patients receiving IFN-α may negatively correlate with serotonin metabolite levels, which in turn may negatively correlate with depression symptoms (Raison et al., 2009). To date, however, whether increased IL-6 precedes, follows, or simply co-occurs with depression remains unresolved.
Sleep disturbance may be another variable that is related to both MDD and inflammation (Motivala et al., 2005; Opp et al., 2007). Controlling for co-existing depressive symptoms, poor sleep quality increases risk for subsequent IFN-α induced depression (Franzen et al., in press), and poor sleep has been cross-sectionally associated with inflammation (Bryant et al., 2004; Suarez, 2008). But again, the direction of causation between sleep and inflammation is not definitively known. In experimental settings, sleep architecture is readily modulated with cytokines or cytokine inducers (e.g. endotoxin) (Haack et al., 2001; Spath-Schwalbe et al., 1998; Spath-Schwalbe et al., 2000). Thus changes in inflammation may plausibly contribute to sleep abnormalities, and thereby increase depression risk. Conversely, full and partial sleep deprivation results in increased circulating levels of IL-6, TNF-α, and C-reactive protein when compared to periods of undisturbed sleep (Meier-Ewert et al., 2004; Vgontzas et al., 1999; Vgontzas et al., 2004). For instance, Vgontzas and colleagues (2004) demonstrated that one week of a 2 hour sleep reduction (from 8 to 6 hours/night) in normal non-depressed sleepers results in deeper sleep (i.e. increased slow wave sleep), increased daytime sleepiness, and elevated circulating levels of IL-6 and TNF-α. Thus sleep disturbances could plausibly contribute to increased systemic inflammation.
There is a need to delineate the temporal relationships among systemic inflammation, sleep, and depression. Towards this end, we prospectively measured depression, global sleep quality, and circulating levels of IL-6 for four consecutive months in 95 HCV patients undergoing IFN-α therapy. This design enabled us to test whether depressive symptoms preceded elevated systemic inflammation or vice versa, and whether poor sleep quality preceded or followed inflammatory activity. Using time-lagged analyses, we examined the temporal relationships of sleep quality, depressive symptoms, and the inflammatory cytokine IL-6 with the goal of disentangling these dynamic associations.
Materials and Methods
Participants
Ninety-five non-depressed patients were started on pegylated (PEG) IFN-α2 (PEG-IFN-α2a: 135 µg/week or PEG-IFN-α2b: 120 or 150 µg/week) and oral ribavirin for treatment of HCV and followed for 4 months of treatment. We used this time frame because depression incidence during IFN-α treatment most typically occurs by week eight (Dieperink et al., 2003; Reichenberg et al., 2005) or twelve (Castera et al., 2006; Hauser et al., 2002; Schaefer et al., 2004). Using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), patients were excluded from this study if they had active mood, anxiety, psychotic, or drug/alcohol use disorders within 6 months prior to starting IFN-α treatment, known neurologic disease, or known active inflammatory disorders other than HCV. In addition, prior to beginning IFN-α therapy study participants were free from medications known to influence the immune system including corticosteroids, antidepressants, anticonvulsants, and/or antipsychotics (although they could be taking as-needed sleeping medications). The study was approved by the University of Pittsburgh Institutional Review Board.
Procedures
Prior to initiating IFN-α therapy, participants completed a battery of psychosocial questionnaires including measures of sleep quality and depressive symptoms (see below). Once IFN-α therapy was initiated, subjective measures of depression symptoms and sleep quality were obtained monthly. In addition, categorical MDD (via an abbreviated SCID-I) was assessed every two months, if Beck Depression Inventory-II (BDI)>15, or sooner if requested by either the treating heptalogist or subject. Participants who developed MDD during the course of treatment, or where concerns about lethality arose, were immediately recommended for clinical intervention (typically starting an antidepressant or discontinuing IFN-α treatment). Blood samples used for assessment of circulating IL-6 were obtained at these monthly appointments. For those missing an appointment for any reason, BDI scores could be mailed in. As antidepressant treatment may affect cytokine levels (Castanon et al., 2002; Szelenyi and Selmeczy, 2002), potentially confounding the results, cytokine levels were not included after antidepressant treatment was initiated or IFN-α treatment discontinued.
Quantitative Measures
The BDI was used to assess depressive symptoms during each monthly visit (but could be returned by mail if the participant was unable to attend the scheduled appointment). Sleep quality was measured monthly using the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989). The PSQI is a widely used and reliable measure of global sleep quality (Cronbach α= .83). Higher PSQI global sleep quality scores are indicative of poorer overall sleep.
Plasma samples used for determination of circulating daytime levels of IL-6 were collected in red-top vacutainer tubes between 10AM and 4PM (mean= 12:50 PM +/− 2.1 hours), when levels are low but least variable (Haack et al., 2002). In this very limited time window, we did not find a correlation between time of blood draw and IL-6 (r=0.015; p=0.82). After centrifugation of clotted blood, plasma was stored at −80°C until assaying in batches. IL-6 levels were determined using a high-sensitivity quantitative enzyme immunoassay (Diaclone, Besancon, France). The assay standard range is 1.56–50 pg/ml with an assay sensitivity of 0.8 pg/ml. Briefly, standards, controls, and samples were added to a 96-well microplate coated with IL-6 monoclonal antibodies and then washed. Biotinylated IL-6 antibodies were added and incubation performed with Streptavidin-bound horse radish peroxidase (HRP). The product of the subsequent substrate for HRP was measured at 450nm. All samples were run in duplicate and the average intra-assay and inter-assay coefficients of variation were below 5% and 10%, respectively.
Statistical Analyses
All statistics employed SPSS 16.0. Repeated-measure mixed-effect analyses with an ante-dependence model (Kenward, 1987) were used to compare changes in either subjective symptoms or IL-6 levels over time (i.e., assessing an interaction with time). We used Kaplan-Meier with Mantel-Cox log rank comparisons to assess the incidence of categorical MDD over time. For these survival analyses, baseline measures of either IL-6 or BDI were dichotomized using the median value. For multivariate exams of MDD incidence, Cox regression analyses were subsequently used.
We employed hierarchical multi-level models (Raudenbush and Bryk, 2002) to determine time-lagged associations of depressive symptoms, sleep quality, and IL-6 levels within subjects. In this regard, multi-level models are used to conduct time-lagged analyses wherein outcomes can be predicted by variables occurring earlier in time (e.g. IL-6 levels at month 2 predicting BDI scores at month 3).(Abela et al., 2007; Vidal et al., 2006) An advantage of these time-lagged analyses is the capacity to co-vary for the outcome measured at the time of the predictor (e.g. controlling for BDI scores at month 2 when examining BDI at month 3). For these models, we first examined repeated covariance structures, selecting analyses which provided the smallest -2Log Likelihood (and typically the smallest AIC and BIC as well), noting that first order ante-dependence and/or first-order heterogeneous autoregressive covariance provided the best fits. Therefore, using models with ante-dependent covariance, we examined the temporal relationship between IL-6 and BDI, BDI and sleep, IL-6 and sleep, and then all three together.
Because age and weight correlated with IL-6 levels, these were included as fixed-effect covariates in all analyses unless otherwise indicated. In all analyses, square root transformation was applied to normalize raw score distributions of IL-6.
Results
Demographic characteristics of the sample and correlations to baseline levels of circulating IL-6 are displayed in Table 1. Baseline IL-6 levels were higher among older and heavier patients; however, basal pre-treatment levels of IL-6 were unrelated to gender, race, and nicotine usage. In addition, pre-treatment IL-6 levels were not correlated with pre-treatment BDI or PSQI scores. Baseline IL-6 levels were also not correlated with initial viral load (r=0.02; p=0.85), gamma-glutamyl transpeptidase levels (r=0.24; p=0.08), alanine aminotransferase levels (r=0.02: p=0.89), or albumin (r=0.17, p=0.18). In contrast, baseline IL-6 was related to baseline C-reactive protein (r=0.30, p<0.001), indicating that elevated IL-6 could be likely related to systemic inflammation.
Table 1.
Demographics, pre-treatment characteristics, and relationship to circulating IL-6.
| IL-6 levels (pg/mL) – square root transformed (Mean +/− S.D). | |||
|---|---|---|---|
| Gender | Male (67%) 0.95 +/− 0.89 | Female (33%) 1.12 +/− 0.65 | r<0.01; p=0.36 |
| Race | Caucasian (87%) 1.02 +/− 0.85 | African-American (13%) 0.93 +/− 0.64 | r<0.01; p=0.73 |
| Nicotine use | Yes (53%) 0.88 +/− 0.99 | No (47%) 1.09 +/− 0.67 | r=0.01; p=0.91 |
| Mean +/− S.D. | Regression with square-root of IL-6 | ||
| BDI | 7.2 +/− 6.9 | B = −0.009 +/− 0.013 | r=0.08; p=0.49 |
| PSQI | 6.1 +/− 3.7 | B = 0.007 +/− 0.027 | r=0.03; p=0.80 |
| Weight (kg) | 84.9 +/− 16.7 | B = 0.015 +/− 0.005 | r=0.30; p<0.01 |
| Age (years) | 47.3 +/− 11.8 | B = 0.018 +/− 0.008 | r=0.25; p<0.05 |
IFN-α therapy increases incidence of MDD, depressive symptoms, and circulating IL-6
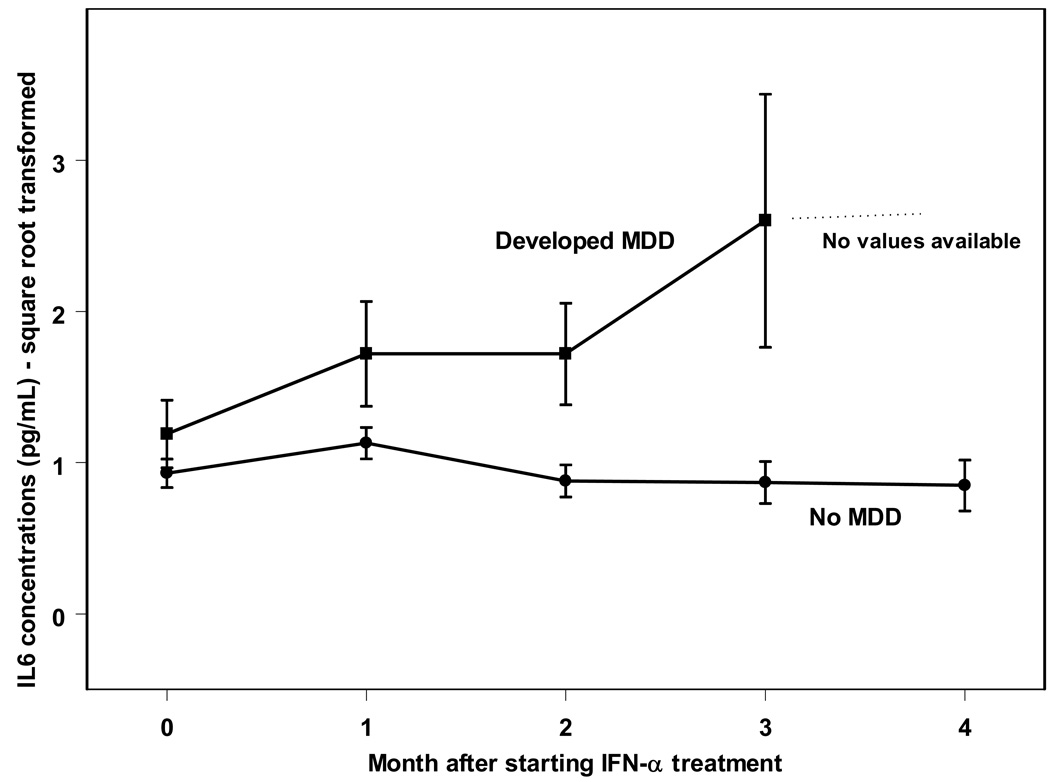
In the present sample, 21 of 95 participants (22%) developed MDD within 3 months of treatment as determined by SCID-I interviews. Table 2 displays demographic characteristics for patients who did and did not develop MDD in this study. Patients who went on to develop MDD during treatment had higher BDI scores at baseline and were more likely to have a history of a mood disorder than those who did not develop MDD. Using repeated-measure mixed-effects analyses, IFN-α therapy was associated with a significant time related increase in BDI depression scores (F(4,60.8) = 6.7; p<0.0005), as well as increases in circulating IL-6 (F(4, 39.5) = 3.4; p<0.05). As displayed in Figure 1, patients who developed MDD displayed higher levels of IL-6 throughout treatment, including at baseline, when compared to patients who did not develop MDD (F(1, 70.2)= 7.8, p<0.01). All patients who developed MDD did so by month 3, and therefore no IL-6 levels are available for this group at month four. The patients not developing MDD appeared to have unchanging IL-6 concentrations throughout treatment. That said, the interaction between depression status (MDD vs. no MDD) by time failed to meet statistical significance (F(4, 39.5) = 1.4, p=0.25). Table 3 displays the raw IL-6 values for patients at each month of treatment.
Table 2.
Baseline characteristics (means and standard deviations) of patients who developed and did not develop MDD during IFN-α treatment.
| MDD (n=21) | no MDD (n=74) | p-value | |
|---|---|---|---|
| BDI-II score | 10.2+/− 1.8 | 6.4 +/− 0.7 | p<.05 |
| Gender | 71% male | 66% male | p=0.66 |
| Ethnicity | 76% Caucasian | 90% Caucasian | p=0.16 |
| Age (years) | 47.4+/− 1.5 | 47.3+/− 1.5 | p=0.98 |
| Weight (kg) | 86.8+/− 2.9 | 84.4+/−2.1 | p=0.59 |
| History of mood disorder | 55% | 22% | p<0.005 |
| History of drug or alcohol disorder | 50% | 57% | p=0.55 |
Figure 1.
Serum IL-6 concentrations in those who develop MDD and those who do not develop MDD during treatment with IFN-α.
Table 3.
Raw mean and standard deviations for unadjusted IL-6 values (pg/ml) for patients who did and did not develop MDD during treatment and the combined sample.
| Month | MDD | no MDD | Combined |
|---|---|---|---|
| Baseline | 2.45 +/− 3.51 | 1.44 +/− 1.69 | 1.68 +/− 2.28 |
| 1 | 4.32 +/− 6.59 | 1.79 +/− 1.82 | 2.33 +/− 3.53 |
| 2 | 3.76 +/− 3.42 | 1.19 +/− 1.15 | 1.65 +/− 1.99 |
| 3 | 8.17 +/− 8.39 | 1.28 +/− 1.33 | 1.97 +/− 3.30 |
| 4 | na | 1.32 +/− 1.45 | 1.32 +/− 1.45 |
Does IL-6 predict subsequent depression or does depression predict subsequent IL-6?
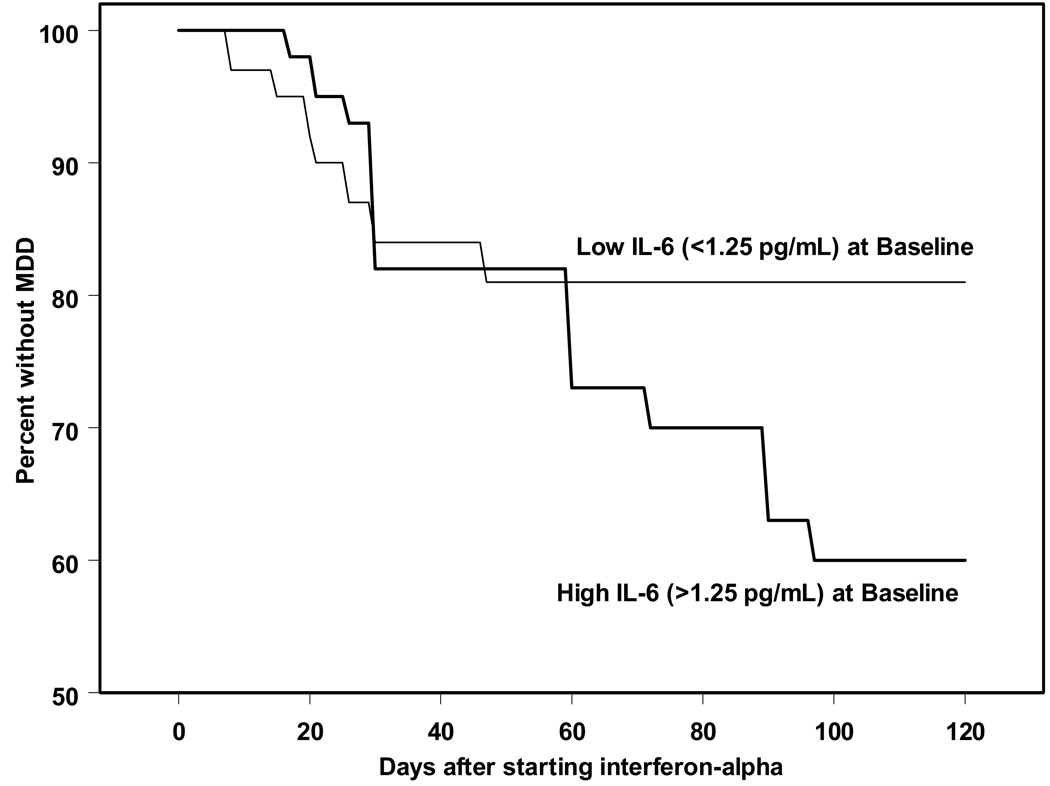
To determine whether pre-treatment levels of IL-6 predicted incidence of MDD during IFN-α therapy, we defined patients with high and low levels of IL-6 at baseline using a median split. Kaplan-Meier analyses revealed that those with higher pre-treatment IL-6 levels (>1.25 pg/ml) had an increased incidence of MDD during IFN-α treatment (Figure 2; X2(1)=7.7; p<0.05) when compared to those with lower pre-treatment IL-6 (<1.25 pg/ml). Cox regression analyses, controlling for the effects of weight and age, supported the association between pre-treatment IL-6 and subsequent MDD incidence (B=0.6 +/− 0.2; Wald=7.8; p<0.005). Pre-treatment BDI scores were 8.4 +/− 1.1 vs. 5.8 +/− 0.9 for patients above and below the IL-6 median, respectively, which trended towards a relationship (p=0.08). However, co-varying for baseline BDI scores did not significantly affect these Cox regression results. Also, because IL-6 levels are at the lower limits of assay detection, we compared those who had baseline levels <0.8 pg/mL with those >0.8 pg/mL. 45% of patients who did not develop MDD had these very low levels, while only 28% of patients who went on to develop MDD fell below this threshold. Further supporting these findings, higher levels of pre-treatment IL-6 (> 1.25 pg/mL) predicted higher BDI scores over time during IFN-α therapy (F(4, 290) = 2.9; p<0.05).
Figure 2.
Pre-treatment levels of circulating IL-6 predicts MDD incidence
We then conducted time-lagged hierarchical analyses to test whether IL-6 at any given month predicted BDI scores the following month – controlling for age, weight, and BDI scores from the preceding month. Indeed, higher peripheral levels of IL-6 significantly predicted the next month’s BDI scores (F(16,97.5) = 7.3; p<0.0005). This finding is consistent with the hypothesis that IL-6 precedes, and may plausibly contribute to, the manifestation of depressive symptoms.
We then tested the opposite relation – namely whether BDI could predict subsequent IL-6 –using a parallel set of analyses. There was a trend for higher pre-treatment BDI scores, via median split, to predict elevated IL-6 levels over time (F(4, 48.4) = 2.5, p=0.051). After controlling for age, weight, and IL-6 levels, BDI scores did predict peripheral levels of IL-6 in the subsequent month (F(14,7.4) = 5.2; p<0.05). Taken together, these data suggest that during IFN-α treatment, not only do systemic levels of IL-6 predicts depressive symptoms, but the converse may also be true. That is, elevated depression can be associated with subsequent increases in IL-6. This implicates a potential positive feedback loop between depressive symptoms and peripheral IL-6.
Does sleep quality predict subsequent depression or does depression predict changes in sleep quality?
We have previously found that poor pre-treatment sleep quality is a very strong predictor of subsequent IFN-induced MDD (Franzen et al., in press), even when controlling for other pre-treatment depression symptoms. To further examine this, we now employed time-lagged hierarchical analyses, examining the association between PSQI scores at any given month and BDI scores the following month – controlling for age, weight and BDI scores from the preceding month. Poor sleep quality predicted depressive symptoms one month later (F(16,22.6) = 3.4; p<0.005). However, the converse was not true. BDI scores failed to predict PSQI scores one month later (F(25,26.5) = 1.3; p=0.24) -- controlling for age, weight, and PSQI scores from the preceding month. Furthermore, higher than median values of pre-treatment BDI failed to predict changes in PSQI during IFN-α treatment (F(4,44.8)=1.6; p=0.19) when compared with those who had lower than median values of pre-treatment BDI. Taken together, these finding suggest that poor sleep quality is associated with subsequent depression during IFN-α therapy, but not vice versa, indicating a possible unidirectional relationship.
Does IL-6 predict sleep quality or does sleep quality predict changes in IL-6 levels?
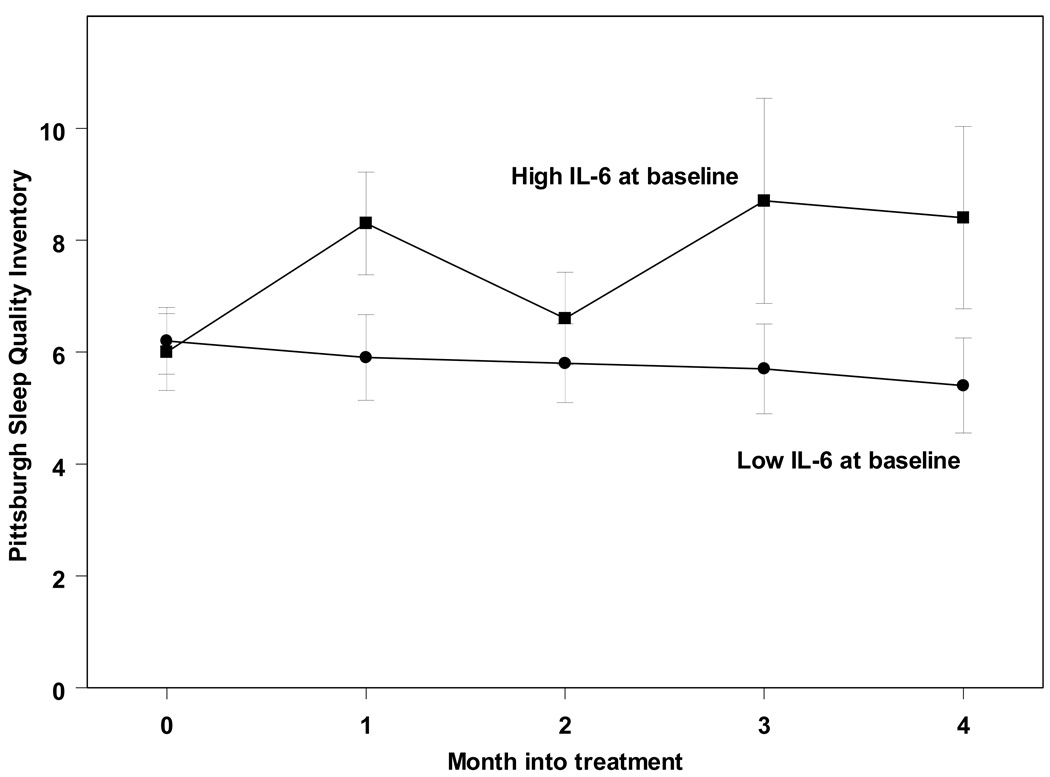
Pre-treatment levels of IL-6, when split at the median, significantly predicted higher PSQI scores over time (F(4, 42.9) = 2.91; p<0.05; Figure 3). In time-lagged analyses, IL-6 levels also predicted the following month’s PSQI scores – after controlling for age, weight, and PSQI scores from the previous month (F(47, 11.6) = 78.4; p<0.0005). However, the converse was not demonstrable. When split at the median, pre-treatment PSQI scores failed to predict systemic IL-6 over time (F(4,40.5) = 0.62; p=0.65). Similarly, time-lagged analyses demonstrated that PSQI scores at any given month did not predict IL-6 levels the following month – controlling for age, weight, and IL-6 levels the previous month (F(10, 5.8) = 1.5; p=0.33). Thus, increased IL-6 was associated with subsequent poor sleep, but not vice versa, suggesting a unidirectional relationship during IFN-α therapy.
Figure 3.
Sleep quality (PSQI) worsens in patients with higher pre-treatment levels of IL-6 (>1.25 pg/ml).
Because IL-6 and poor sleep quality both appear to predict MDD incidence, and IL-6 uni-directionally predicts sleep quality, it is plausible that PSQI may mediate the association between IL-6 and MDD incidence. We therefore assessed the association of pre-treatment PSQI and IL-6 with MDD incidence using Cox-regression, again using a median split for both IL-6 and PSQI – and again controlling for age, weight, and pre-treatment BDI. When co-entered together, PSQI continued to predict MDD incidence (B = 2.7 +/− 0.4; Wald = 60.1; p<0.001), while the association with IL-6 was lost (going from B = 0.6 +/− 0.2 when entered alone to B = 0.31 +/− 0.29; p=0.28 when co-entered with PSQI). Thus, in this sample poor sleep quality may have partially accounted for the association between high levels of circulating IL-6 and MDD incidence.
Discussion
IFN-α administration has been associated with elevated systemic IL-6 in a subset of individuals (Bonacccorso et al., 2001; Wichers et al., 2007). In 17 patients, the increased IL-6 was only statistically elevated at week 8 (Wichers et al., 2007). We also found that IFN-α treatment resulted in both modulation of circulating daytime IL-6 levels and concomitant changes in depression scores. Notably, IL-6 levels were only higher in those who ultimately developed MDD. Moreover, replicating an earlier study of 16 patients (Wichers et al., 2006), we found that circulating IL-6 levels prior to IFN-α treatment predicted MDD incidence, an association that remained after adjustment for age, gender, weight, and baseline depressive symptoms.
Not only did baseline IL-6 levels predict MDD, but time-lagged hierarchical analyses revealed that circulating IL-6 was associated with following month’s BDI scores. Importantly, this relationship held even after controlling for BDI scores in the prior month. Conversely, BDI scores predicted the following month’s IL-6 levels, leading to the conclusion that a bi-directional relationship exists. This conclusion is consistent with the emergence of a positive feedback system during IFN-α treatment. That is, when exogenous IFN-α is given, the emergent positive feedback loop between depressive symptoms and IL-6 may escalate into the development of MDD in a vulnerable set of individuals. In the absence of IFN-α injections, there must be an interruption or brake in this positive feedback system.
Interestingly, IL-6 was associated with the next month’s self-report of poor sleep quality but not vice versa, suggesting more of a unidirectional relationship between these two variables during IFN-α treatment. There also appeared to be a unidirectional association between poor sleep quality and subsequent depression. Other studies have reported that subjective sleep disturbance can account for the association between depression and nocturnal IL-6 levels (Motivala et al., 2005). Similarly, we found that poor sleep partially accounted for the relationship between elevated IL-6 and incidence of MDD, supporting the possibility that good sleep quality may interrupt the dynamics between elevated IL-6 and depression. However, to truly conclude that sleep is a mediator between IL-6 and depression, it would be necessary to experimentally improve sleep in patients with elevated IL-6 and examine whether this mitigates the development of MDD. At this point, because we did not find a correlation between sleep and IL-6 at pre-treatment baseline, we cannot definitively conclude that poor sleep mediates the relationship between elevated IL-6 and MDD.
There are several potential physiologic mechanisms that may moderate the relationship between inflammation and depressive symptoms. Impaired hypothamalic-pituitary-adrenal axis feedback and hyper-reactivity are some of the biological hallmarks of MDD (Pace et al., 2007; Pariante and Miller, 2001), including MDD secondary to IFN-α (Capuron et al., 2003). Patients treated with IFN-α show flattened diurnal cortisol slopes and increased evening cortisol, which is correlated with depressive symptoms (Raison et al., 2008). It is possible that failure of cortisol to regulate inflammatory processes, in those vulnerable to depression, may contribute to unleashing the bi-directional feedback loop observed in the present study. Consistent with this possibility, growing evidence supports glucocorticoid receptor (GR) dysfunction among depressives including disrupted GR expression, translocation, and concomitant resistance to cortisol (Pace et al., 2007).
The parasympathetic arm of the autonomic nervous system can also regulate inflammation via the vagus nerve (Pavlov and Tracey, 2005; Tracey, 2002). In this regard, cross-sectional evidence shows that heart rate variability (HRV), an index of sympatho-vagal balance, is inversely related to systemic IL-6 (Sloan et al., 2007). Heart rate variability has been shown to be reduced in depressed patients (Krittayaphong et al., 1997; Rottenberg et al., 2007). Whether this source of regulation is lost in vulnerable patients undergoing IFN-α therapy remains unknown. Thus far, the research exploring the effects of IFN-α treatment on HRV has been equivocal (Kadayifci et al., 1997; Takase et al., 2005).
The relationship between IL-6 and depression may also be influenced by the serotonergic system. Indeed, reduced serotonin metabolites (e.g. 5-HIAA) have been observed in CSF of patients treated with IFN-α (Raison et al., 2009), which correlated both with CSF IL-6 and depression. Similarly, converging evidence in animals and humans demonstrate that antidepressant medications reduce systemic inflammation in some contexts (Castanon et al., 2002; Kenis and Maes, 2002). With the aim of identifying genetic vulnerability, we reported previously that individuals with the L/L genotype for the serotonin transporter polymorphism (5-HTTLPR) are resilient to developing MDD during IFN-a treatment (Lotrich et al., 2009). 5-HTTLPR may interact with IL-6 polymorphisms to influence MDD risk (Bull et al., 2008). That is, the G/G genotype in the promoter region of the IL-6 gene (rs1800795), which has been previously associated with higher levels of IL-6 (Di Renzo et al., 2008), was also associated with increased risk of MDD during IFN-α treatment (Bull et al., 2008).
The current findings should be interpreted in the context of several limitations. First, while IFN-α treatment provides a distinct advantage for prospective investigations, our findings may not generalize to medically healthy populations and other instances of MDD. Indeed, not all cross-sectional studies have found elevations of circulating IL-6 in MDD patients compared to controls (Brambilla and Maggioni, 1998; Haack et al., 1999; Mikova et al., 2001; O’Connor et al., 2007). Whether our findings in patients receiving IFN-α can be extended to MDD more generally, or only a subtype of MDD requires determination. Increased systemic inflammatory activity may be present in a specific group of depressed patients, associated with antidepressant response (Lanquillon et al., 2000; Maes et al., 1997). That said, emerging evidence suggests that there is considerable overlap in symptom profiles of those with cytokine-induced depression and depression observed in medically healthy individuals, including on sleep measures (Capuron et al., 2009). This study excluded subjects who currently were experiencing an MDD episode, which may impact the generalizability of our findings. It also did not include a non-IFN-α treated control group, and thus we cannot definitively state that MDD during treatment was the result if IFN-α. Second, there were likely unobserved variables (i.e. third factors) that may independently or interactively influence depressive symptoms, sleep quality, and/or systemic IL-6. Statistical associations, even when temporal, are not proof of causation. Also, several factors, including HCV severity, genetic variation in IL-6, and psychological stress may have contributed to IL-6 levels, sleep quality, and depressive symptoms at baseline and across time. Third, potential influences on poor sleep quality, such as obstructive sleep apnea (OSA) or pain, were not assessed. That said, evidence suggests the PSQI scores may not be associated with apnea status. For instance, in a study of 435 individuals, nearly 60% of whom had OSA, PSQI scores failed to distinguish those with OSA from non-sleep disordered individuals (Gliklich et al., 2000).
Finally, the source of IL-6 elevations could not be determined. While we adjusted for weight in these analyses, given that adipose tissue can produce IL-6 (Mohamed-Ali et al., 1997), we did not directly measure CSF IL-6. In this regard, Raison et al (2009) found elevated CSF IL-6 without an increase in systemic IL-6.
Despite these limitations, we were nonetheless able to examine the temporal dynamics between depressive symptoms and evidence of systemic inflammation. In sum, we found that IFN-α treatment is associated with time-related increases in depressive symptoms, poor sleep quality, and systemic IL-6 in a subset of patients. Moreover, pre-treatment levels of circulating IL-6 predicted the subsequent development of MDD, a relationship that may have been partially accounted for by pre-treatment PSQI scores. Although this prospective design did not allow for causal inference, it did inform our understanding of temporal relations. Uni-directional associations indicated that IL-6 could predict next month’s PSQI, and PSQI could predict next month’s depressive symptoms. Moreover, time-lagged analyses supported a bi-directional positive feedback loop between depressive symptoms and circulating IL-6, plausibly contributing to emergence of MDD in those patients for whom this feedback dynamic was undeterred. Further prospective research is warranted to examine this possibility, and to further identify the mechanistic paths relating systemic cytokines to sleep, mood, and MDD.
Acknowledgments
Funding for this study was provided by NIMH grant K23 MH074012 (FEL); the NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abela JR, McGirr A, Skitch SA. Depressogenic inferential styles, negative events, and depressive symptoms in youth: an attempt to reconcile past inconsistent findings. Behaviour Research & Therapy. 2007;45:2397–2406. doi: 10.1016/j.brat.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran AS, Richert AC. Obstructive sleep apnea and depression. CNS Spectr. 2003;8:128–134. doi: 10.1017/s1092852900018356. [DOI] [PubMed] [Google Scholar]
- Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Puzella A, Marino V, Pasquini M, Biondi M, Artini M, Almerighi C, Levrero M, Egyed B, Bosmans E, Meltzer HY, Maes M. Immunotherapy with interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry Res. 2001;105:45–55. doi: 10.1016/s0165-1781(01)00315-8. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Maggioni M. Blood levels of cytokines in elderly patients with major depressive disorder. Acta Psychiatr Scand. 1998;97:309–313. doi: 10.1111/j.1600-0447.1998.tb10005.x. [DOI] [PubMed] [Google Scholar]
- Bryant PA, Trinder J, Curtis N. Sick and tired: Does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4:457–467. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, Miyazaki C, Alexander N, Hotopf M, Cleare AJ, Norris S, Cassidy E, Aitchison KJ, Miller AH, Pariante CM. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009 doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- Castanon N, Leonard BE, Neveu PJ, Yirmiya R. Effects of antidepressants on cytokine production and actions. Brain Behav Immun. 2002;16:569–574. doi: 10.1016/s0889-1591(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Castera L, Constant A, Henry C, Champbenoit P, Bernard PH, De Ledinghen V, Demotes-Mainard J, Couzigou P. Impact on adherence and sustained virological response of psychiatric side effects during peginterferon and ribavirin therapy for chronic hepatitis C. Aliment Pharmacol Ther. 2006;24:1223–1230. doi: 10.1111/j.1365-2036.2006.03107.x. [DOI] [PubMed] [Google Scholar]
- Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, Keystone EC, Loveless JE, Burmester GR, Cravets MW, Hessey EW, Shaw T, Totoritis MC. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-alpha-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44:104–112. doi: 10.1176/appi.psy.44.2.104. [DOI] [PubMed] [Google Scholar]
- Di Renzo L, Bertoli A, Bigioni M, et al. Body composition and −174G/C interleukin-6 promoter gene polymorphism: association with progression of insulin resistance in normal weight obese syndrome. Current Pharmaceutical Design. 2008;14:2699–2706. doi: 10.2174/138161208786264061. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes. A prospective population-based study. Diabetes Care. 1996;19:1097–1102. doi: 10.2337/diacare.19.10.1097. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- Franzen P, Buysse D, Rabinovitz M, Pollock B, Lotrich F. Poor sleep quality predicts onset of either major depression or subsyndromal depression with irratibility during interferonalpha treatment. Psychiatry Res. doi: 10.1016/j.psychres.2009.02.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270:1819–1825. [PubMed] [Google Scholar]
- Frommberger UH, Bauer J, Haselbauer P, Fraulin A, Riemann D, Berger M. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci. 1997;247:228–233. doi: 10.1007/BF02900219. [DOI] [PubMed] [Google Scholar]
- Gliklich RE, Taghizadeh F, Winkelman JW. Health status in patients with disturbed sleep and obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;122:542–546. doi: 10.1067/mhn.2000.102579. [DOI] [PubMed] [Google Scholar]
- Haack M, Hinze-Selch D, Fenzel T, Kraus T, Kuhn M, Schuld A, Pollmacher T. Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis. J Psychiatr Res. 1999;33:407–418. doi: 10.1016/s0022-3956(99)00021-7. [DOI] [PubMed] [Google Scholar]
- Haack M, Schuld A, Kraus T, Pollmacher T. Effects of sleep on endotoxin-induced host responses in healthy men. Psychosom Med. 2001;63:568–578. doi: 10.1097/00006842-200107000-00008. [DOI] [PubMed] [Google Scholar]
- Haack M, Kraus T, Schuld A, et al. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinol. 2002;27:921–931. doi: 10.1016/s0306-4530(02)00006-9. [DOI] [PubMed] [Google Scholar]
- Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, Gulati M, Thornton AJ, Schultz RL, Valentine AD, Meyers CA, Howell CD. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- Hopkins SJ, Rothwell NJ. Cytokines and the nervous system. I: Expression and recognition. Trends Neurosci. 1995;18:83–88. [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Kadayifici A, Aytemir K, Arslan M, Aksoyek S, Sivri B, Kabakci G. Interferon-alpha does not cause significant cardiac dysfunction in patients with chronic active hepatitis. Liver. 1997;17:99–102. doi: 10.1111/j.1600-0676.1997.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- Kenward M. A method for comparing profiles of repeated measurements. Applied Statistics. 1987;36:296–308. [Google Scholar]
- Krittayaphong R, Cascio WE, Light KC, Sheffield D, Golden RN, Finkel JB, Glekas G, Koch GG, Sheps DS. Heart rate variability in patients with coronary artery disease: differences in patients with higher and lower depression scores. Psychosom Med. 1997;59:231–235. doi: 10.1097/00006842-199705000-00004. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Risk for depression during interferon-alpha treatment is affected by the serotonin transporter polymorphism. Biol Psychiatry. 2009;65:344–348. doi: 10.1016/j.biopsych.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, Desnyder R. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 1995;34:301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- Mikova O, Yakimova R, Bosmans E, Kenis G, Maes M. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur Neuropsychopharmacol. 2001;11:203–208. doi: 10.1016/s0924-977x(01)00081-5. [DOI] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med. 2005;67:187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- Mullington J, Korth C, Hermann DM, Orth A, Galanos C, Holsboer F, Pollmacher T. Dose-dependent effects of endotoxin on human sleep. Am J Physiol Regul Integr Comp Physiol. 2000;278:R947–955. doi: 10.1152/ajpregu.2000.278.4.R947. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001a;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, Pearce BD, Landry J, Glover S, McDaniel JS, Nemeroff CB. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001b;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Irwin MR, Seldon J, Kwan L, Ganz PA. Pro-inflammatory cytokines and depression in a familial cancer registry. Psychooncology. 2007;16:499–501. doi: 10.1002/pon.1108. [DOI] [PubMed] [Google Scholar]
- Opp MR, Born J, Irwin MR. Sleep and the immune system. In: Ader R, editor. Psychoneuroimmunology. New York, NY: Academic Press; 2007. pp. 570–618. [Google Scholar]
- Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Pike JL, Irwin MR. Dissociation of inflammatory markers and natural killer cell activity in major depressive disorder. Brain Behav Immun. 2006;20:169–174. doi: 10.1016/j.bbi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J. 2002;23:831–834. doi: 10.1053/euhj.2001.3052. [DOI] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GJ, Massung B, Miller AH. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A. Hierarchical Linear Models: Applications and Data Analysis Methods. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Reichenberg A, Gorman JM, Dieterich DT. Interferon-induced depression and cognitive impairment in hepatitis C virus patients: a 72 week prospective study. AIDS. 2005;19 Suppl 3:S174–S178. doi: 10.1097/01.aids.0000192087.64432.ae. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Miller GE. Acute deviations from long-term trait depressive symptoms predict systemic inflammatory activity. Brain Behav Immun. 2008;22:709–716. doi: 10.1016/j.bbi.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007;44:450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Schmidt F, Horn M, Schmid-Wendtner MH, Volkenandt M. Depression during treatment with interferon alpha. Psychosomatics. 2004;45:176. doi: 10.1176/appi.psy.45.2.176. [DOI] [PubMed] [Google Scholar]
- Schobitz B, De Kloet ER, Holsboer F. Gene expression and function of interleukin 1, interleukin 6 and tumor necrosis factor in the brain. Prog Neurobiol. 1994;44:397–432. doi: 10.1016/0301-0082(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Ohtani K, Sato N, Nagamine T, Mori M. Increase in serum interleukin-6, plasma ACTH and serum cortisol levels after systemic interferon-alpha administration. Endocr J. 1995;42:551–556. doi: 10.1507/endocrj.42.551. [DOI] [PubMed] [Google Scholar]
- Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med. 2007;13:178–184. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluzewska A, Rybakowski J, Bosmans E, Sobieska M, Berghmans R, Maes M, Wiktorowicz K. Indicators of immune activation in major depression. Psychiatry Res. 1996;64:161–167. doi: 10.1016/s0165-1781(96)02783-7. [DOI] [PubMed] [Google Scholar]
- Spath-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, Fehm HL, Born J. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998;83:1573–1579. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- Spath-Schwalbe E, Lange T, Perras B, Fehm HL, Born J. Interferon-alpha acutely impairs sleep in healthy humans. Cytokine. 2000;12:518–521. doi: 10.1006/cyto.1999.0587. [DOI] [PubMed] [Google Scholar]
- Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun. 2008;22:960–968. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szelenyi J, Selmeczy Z. Immunomodulatory effect of antidepressants. Curr Opin Pharmacol. 2002;2:428–432. doi: 10.1016/s1471-4892(02)00173-x. [DOI] [PubMed] [Google Scholar]
- Takase B, Hamabe A, Uehata A, Hujioka T, Kondo T, Matsui T, Ohsuzu F, Ishihara M. Recombinant interferon alpha treatment decreases heart rate variability indices and impairs exercise tolerance in patients with chronic hepatitis. Biomed Pharmacother. 2005;59:S163–168. doi: 10.1016/s0753-3322(05)80025-3. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, Prolo P, Wong ML, Licinio J, Gold PW, Hermida RC, Mastorakos G, Chrousos GP. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Vidal A, Gomez-Gil E, Sans M, Portella MJ, Salamero M, Pique JM, Panes J. Life events and inflammatory bowel disease relapse: a prospective study of patients enrolled in remission. American Journal of Gastroenterology. 2006;101:775–781. doi: 10.1111/j.1572-0241.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Kenis G, Koek GH, Robaeys G, Nicolson NA, Maes M. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. J Psychosom Res. 2007;62:207–214. doi: 10.1016/j.jpsychores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Kenis G, Leue C, Koek G, Robaeys G, Maes M. Baseline immune activation as a risk factor for the onset of depression during interferon-alpha treatment. Biol Psychiatry. 2006;60:77–79. doi: 10.1016/j.biopsych.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, Hirano T, Adachi M. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–1134. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Burleson MH, Matt KS, Roth S, Burrows L. Interpersonal stress, depression, and disease activity in rheumatoid arthritis and osteoarthritis patients. Health Psychol. 1994;13:139–148. doi: 10.1037//0278-6133.13.2.139. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]