Summary
While there have been dramatic increases in the range and quality of information available from non-invasive imaging methods, their application in clinical trials have been limited. One promising approach is to apply imaging techniques in pre-clinical studies designed to mimic a corresponding clinical trial so as to inform that trial.
In this issue of Clinical Cancer Research, O’Connor and colleagues (1) contribute a valuable paper on the application of multi-modal imaging to quantify the anti-vascular effects of an anti-VEGF therapy in colorectal cancer. Their report is of particular interest because they performed their studies in both pre-clinical and clinical settings. In the pre-clinical studies, the authors employed the HM-7 colorectal xenograft model and used in vivo microbubble contrast enhanced sonography (MCES, 2) to assess various haemodynamic parameters before and after treatment and then followed these with ex vivo studies using micro-computed tomography (μCT, 3) to generate vascular casts of the tumors post excision. The μCT experiments showed that the tumors displayed a reduction in perfused vessels while the MCES studies showed a decrease in tumor blood volume 24-48 hours after treatment. In the clinical study, the authors employed dynamic contrast enhanced magnetic resonance imaging (DCE-MRI, 4) and those data showed changes in blood volume and enhancement that were consistent with the pre-clinical data. This paper is of interest not only because of the particular results it reports on the primary and secondary aims (to assess the temporal evolution of the anti-vascular effects of G6-31 and use cross-species imaging to provide insight into drug mechanism, respectively), but also for the more general implications for translational imaging upon which we now focus.
Current methods for assessing treatment response are limited. For example, although serial biopsies can be obtained to monitor tumor status, these are invasive and therefore may be clinically impractical or available only infrequently. They also provide poor spatial sampling and may prove to be misleading. The current standard-of-care radiological assessment of treatment response is based on the response evaluation criteria in solid tumors (RECIST), which offers an overly simple method for evaluating tumor growth on cross-sectional CT or MR images (5). The sum of the longest diameter for all measurable lesions is computed and a post-treatment percent change in the baseline sum is then used to classify the degree of response into one of four categories: complete response, partial response, stable disease, and progressive disease. While RECIST is a practical tool, it is recognized that it often is inadequate as its assessment of clinical response is based on only uni-dimensional changes. However, intervention-induced changes in tumor viability and eventually its volume are manifestations of earlier and complex treatment-induced molecular and cellular effects. Thus, improved methods are needed to characterize those early molecular and cellular changes which may predict treatment outcome and dictate the course of therapy. These demand more quantitative, sensitive, and specific indices of response.
In recent years there have been dramatic increases in the range and quality of information available from non-invasive imaging methods so several imaging techniques are now potentially available to assess tumor status and predict treatment response quantitatively. These have often been used in pre-clinical studies of animal models, but with mixed results that have been confounded by lack of standardization of imaging protocols, inadequate understanding of the underlying mechanisms, and/or absence of appropriate validation to assist their interpretation. Consequently, there are legitimate obstacles that inhibit moving these methods from the laboratory to the clinic. However, there remains a compelling need for validated imaging biomarkers so that these methods can positively influence patient care. To this end, the cancer imaging community has begun to systematically evaluate clinically-viable imaging methods to establish which method (or combination of methods) is most appropriate for predicting response to specific treatments (see, e.g., 6). By testing the ability of emerging imaging techniques to characterize the effects of particular treatments, it may be possible to accelerate their application, acceptance, and incorporation into clinical trials and, ultimately, have a direct impact on personalized medicine.
One area in which cancer imaging provides unique insights is in vascular imaging (7). It is well known that a tumor cannot grow beyond a few cubic millimeters without recruiting a new vasculature. This process of tumor driven angiogenesis leads to tumor blood vessels that are fragile, leaky, tortuous, non-hierarchical, and therefore quite different from their normal physiological counterparts (8). As nearly all malignancies are thought to be dependent upon these pathologic vessels, a number of techniques have been developed as candidate imaging biomarkers, for imaging tumor associated blood vessels; examples include contrast enhanced DCE-MRI (4), contrast enhanced CT (3), 15O labeled H2O for PET studies (9), and MCES (2). Unfortunately, and as the authors correctly observe, given a particular anti-vascular or anti-angiogenic drug, the time course of its effect on vascular status (i.e., blood volume, blood flow, vessel permeability, etc.) is usually not well characterized and this translates into a lack of knowledge of when to apply the appropriate imaging technique(s) to assess response. Pre-clinical animal data can therefore help to design clinical trials by identifying the appropriate time points for performing correlative imaging studies. This is the approach used by O’Connor et al. They report a multi-modality, multi-parametric in vivo and ex vivo studies of the response of CRC tumors to anti-angiogenic therapy in both pre-clinical and clinical settings. Multi-modality data can provide insight into drug mechanism by reporting on complimentary aspects of drug action. They reported multiple MRI (Ktrans, vp, ve, and tumor volume), US (rBF, rBV, MTT), and CT (vascular volume) measurements (see the Figure). An interesting and important finding was that μCT and MCES revealed a significant reduction in perfused vessels and relative blood volume, respectively, in mice following treatment with G6-31 at the 48 hour time point. Similarly, reductions in the fractional plasma volume and the enhancing fraction were also seen at the 48 hour time point in human DCE-MRI data. This provides an exciting example of clinical data mirroring its pre-clinical counterpart.
Figure.
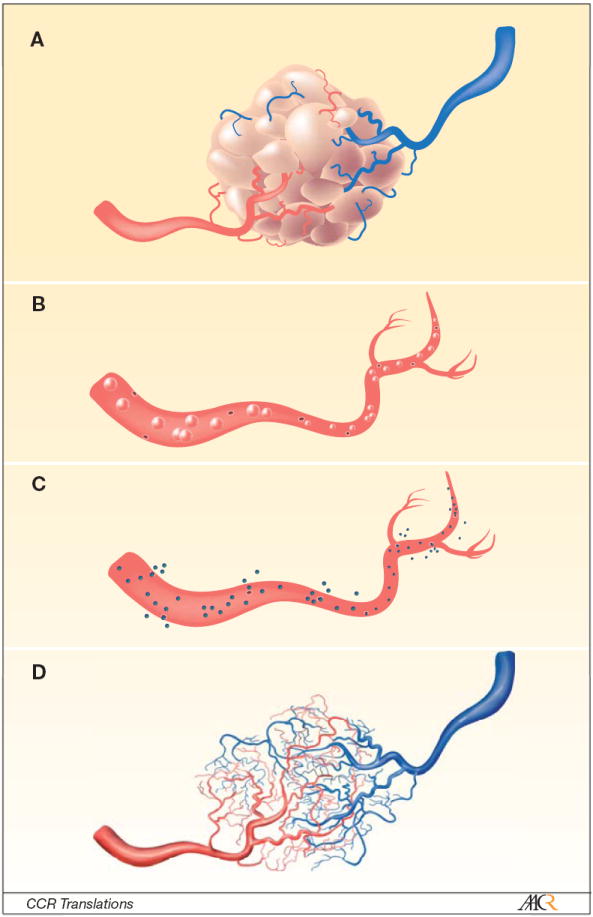
The Figure displays a cartoon depiction of methods to characterize the distinctively fragile and leaky blood vessels associated with tumors (panel a). In the O’Connor et al study, the authors made use of in vivo dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) and microbubble contrast enhanced sonography (MCES), as well as ex vivo micro computed tomography (μCT). The contrast agents they selected for their study allowed them to probe different aspects of the tumor vasculature. For example, since the contrast agent used in MCES is intravascular (panel b), it can report on relative blood flow (rBF), relative blood volume (rBV), and mean transit time (MTT), whereas the DCE-MRI contrast agent is an extravasacular agent and therefore reports on plasma volume (vp), extravascular extracellular volume fraction (ve), and a mixed measure of vessel perfusion and permeability (Ktrans, panel c). The μCT used an intravascular contrast agent that, when combined with the high-resolution ex vivo imaging, can display the vessels in 3D (panel d) and determine vascular volume. As these methods report on different, but related, aspects of tumor vessels, it is reasonable to hypothesize that more can be learned about, for example, a drug’s mechanism of action by studying all three. The O’Connor et al paper investigates the changes these techniques report during a longitudinal study of treatment response.
These results point to a number of possible future studies which could build upon and expand these results. For example, in order to more fully elucidate the temporal and spatial relationships between the parameters, it will be necessary to perform serial, in vivo, multimodality measurements that can be co-registered to a common imaging space (10). Indeed, the data presented in O’Connor et al show that there is a complex temporal evolution to the imaging parameters and it is not unreasonable to hypothesize that the spatial evolution is also complex and informative. Such data will be well suited to address the order in which various anti-cancer drugs work. It is important to note that access to this temporal and spatial evolution is only available through imaging as only imaging techniques can provide such physiologically meaningful data noninvasively, in 3D, and in vivo—and do so repeatedly in the same animal or human (11).
While there have been dramatic developments in quantitative imaging of cancer in the last several years, there is still much work to be done in validating the emerging techniques and determining when to use these tools. The investigation by O’Conner et al points the way to one possible direction to address these important issues and brings us one step closer to the acceptance of quantitative, multi-modality imaging in clinical trials.
Acknowledgments
T.E.Y. is supported, in part, by a Career Development Award from the National Institutes of Health (NIBIB 1K25 EB005936).
References
- 1.O’Connor JPB1, Carano RAD, Clamp AR, et al. Quantifying anti-vascular effects of monoclonal antibodies to VEGF: insights from multi-modality cross species imaging in colorectal cancer. Clin Cancer Res. 2009;15 doi: 10.1158/1078-0432.CCR-09-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin S, Caskey CF, Ferrara KW. Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering. Phys Med Biol. 2009;54:R27–57. doi: 10.1088/0031-9155/54/6/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulus MJ, Gleason SS, Kennel SJ, et al. High resolution X-ray computed tomography: an emerging tool for small animal cancer research. Neoplasia. 2000;2:62–70. doi: 10.1038/sj.neo.7900069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yankeelov TE, Gore JC. Dynamic Contrast Enhanced Magnetic Resonance Imaging in Oncology: Theory, Data Acquisition, Analysis, and Examples. Current Medical Imaging Reviews. 2007;3:91–107. doi: 10.2174/157340507780619179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Manning HC, Merchant NB, Foutch AC, et al. Molecular imaging of therapeutic response to epidermal growth factor receptor blockade in colorectal cancer. Clin Cancer Res. 2008;14:7413–22. doi: 10.1158/1078-0432.CCR-08-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49(Suppl 2):113S–28S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 8.Fukumura D, Jain RK. Imaging angiogenesis and the microenvironment. APMIS. 2008;116:695–715. doi: 10.1111/j.1600-0463.2008.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Langen AJ, van den Boogaart VE, Marcus JT, et al. Use of H2(15)O-PET and DCE-MRI to measure tumor blood flow. Oncologist. 2008;13:631–44. doi: 10.1634/theoncologist.2007-0235. [DOI] [PubMed] [Google Scholar]
- 10.Slomka PJ, Baum RP. Multimodality image registration with software: state-of-the-art. Eur J Nucl Med Mol Imaging. 2009;36(Suppl 1):S44–55. doi: 10.1007/s00259-008-0941-8. [DOI] [PubMed] [Google Scholar]
- 11.Giesel FL, Mehndiratta A, Locklin J, et al. Image fusion using CT, MRI and PET for treatment planning, navigation and follow up in percutaneous RFA. Exp Oncol. 2009;31:106–14. [PMC free article] [PubMed] [Google Scholar]


