Abstract
Skin-derived fibroblasts from long-lived mutant mice, including the Snell dwarf mice and mice defective in growth hormone receptor (“GHRKO”), are resistant to death induced by oxidative stresses or by UV light, but the molecular mechanism for their stress resistance is unknown. The present study showed that phosphorylation of the stress-activated protein kinases ERK1/2 induced by peroxide, cadmium, or paraquat was attenuated in cells from these mice. Induction of ERK phosphorylation by UV light was not altered in the Snell dwarf cells, and neither JNK nor p38 kinases showed increased phosphorylation in response to any of the stresses tested. Surprisingly, stress-induced elevation of mRNA for certain Immediate Early Genes (egr-1 and fos) was higher in Snell-derived cells than in control cells, despite the evidence of lower ERK phosphorylation. Thus cells from Snell dwarf mice differ from controls in two ways: (a) lower induction of ERK1/2 phosphorylation, and (b) increased expression of some ERK-dependent IEGs. These alterations in kinase pathways may contribute to the resistance of these cells to lethal injury.
Introduction
Loss-of-function mutations at the Prop1 (Ames dwarf) and Pit1 (Snell dwarf) loci, as well as targeted disruption of the growth hormone receptor/binding protein gene, delay aging and greatly prolong life span in the mouse. Ames and Snell dwarf mice models are the best-studied mutants in which altered GH/IGF-I signals produce dramatic increases in life span with concomitant delay of late life diseases and disabilities [1, 2]. Moreover, a number of aging-related phenotypes are also delayed in these mice, including collagen cross-linking, cataracts, kidney diseases, and fatal neoplastic disease, as well as declines in immune function, locomotor activity, learning and memory [2-4]. The recessive mutation of either Prop1 or Pit1 genes leads to abnormal development of the anterior pituitary, and thus to primary decline in production of growth hormone (GH), thyrotropin (TSH), and prolactin, with consequent declines in levels of circulating IGF-I and thyroxin. GH receptor/GH-binding protein knockout (GHRKO) mice were developed by targeted disruption of the Ghr/Ghrbp gene [5]. These mutant mice do not express the GH receptor, are GH resistant, and have profoundly suppressed circulating levels of IGF-I and insulin, markedly increased life span, and multiple indices of delayed aging [5-7].
Close association of enhanced stress resistance with extended longevity is well documented in lower species including worms, flies, and yeast [8-10]. Our group has previously reported that skin-derived fibroblast cell lines from adult mice of the Ames, Snell, and GHRKO stocks, for example, are resistant to cell death induced by exposure to oxidative (H2O2, paraquat), non-oxidative (UV, MMS) and mixed (heat, cadmium) stresses [11, 12]. Fibroblasts from two other long-lived mouse mutants, i.e. the IGF-IR knockout and the p66shc splice variant, are also resistant to oxidative stress in vitro [13, 14]. Fibroblasts from long-lived species also show unusually high resistance to multiple forms of lethal injury [15, 16]. However, the molecular basis of the cellular stress resistance remains to be discovered.
The mitogen activated protein kinases (MAPK) comprise a ubiquitous group of signaling proteins that play a prominent role in regulating cell proliferation, differentiation and adaptation. Members of each major MAPK subfamily, the extracellular signal regulated protein kinases (ERK), the c-Jun N-terminal kinases (JNK) and p38 MAPK, have been implicated in cell injury and disease [17, 18]. The MAPK signaling module is defined by a three-tiered kinase cascade, resulting in phosphorylation of a conserved Thr-X-Tyr activation motif by an upstream dual specificity MAPK kinase [17]. In particular, ERK1 and ERK2, which are activated by the MAPK/ERK kinase-1/2 (MEK1/2), are emerging as important regulators of cellular responses to various stimuli [19].
The goal of this research project was to test the hypothesis that the high stress resistance of cells from Snell dwarf and GHRKO mice was related to unusually fast or strong signals mediated by one or more members of the MAPK family.
Materials and Methods
Animals
Snell dwarf (homozygous dw/dw) animals (and heterozygote controls) were bred at Michigan as the progeny of (DW/J × C3H/HeJ)-dw/+ females and (DW/J × C3H/HeJ) F1-dw/dw males. The sires of the test mice had been treated with GH and thyroxine to increase body size and fertility. Littermates with the (+/dw) genotype were used as controls. Tail skin biopsies were taken from male mice 3–4 mo of age. Protocols were approved by the University Committee on the Use and Care of Animals. GH receptor knockout (GHRKO) mice and littermate controls were generated at Southern Illinois University (Springfield, IL), from breeding stock originally generated by Dr. John Kopchick's group at Ohio University. Tail skin biopsies from these mice were obtained from 3- to 6-mo-old males and sent overnight on ice to the University of Michigan in DMEM supplemented with 20% heat-inactivated fetal calf serum, antibiotics, fungizone, and 10 mM HEPES. Fibroblasts were prepared from the biopsy tissue as described [11]. GHRKO mice and normal littermate controls were produced by mating heterozygous (+/−) carriers of the disrupted GHR/GHBP gene or homozygous knockout (−/−) males with (+/−) females. The genetic background of these GHRKO animals is derived from 129/Ola embryonic stem cells and from BALB/c, C57BL/6, and C3H inbred strains [2].
Cell Culture and Treatment
Fibroblast cultures from all biopsies were isolated as previously reported [11]: tail skin biopsies were minced using a scalpel, and then placed into a 35-mm dish with 1.5 mL of complete medium (DMEM, 10% FCS, 100 U mL−1 penicillin, 100 U mL−1 streptomycin and 0.25 μg mL−1 fungizone) and 1 mL of collagenase solution (1000 U mL−1) in a humidified CO2 incubator at 37° containing room air with 20% O2. After 24 h the cells were pipetted vigorously to separate cells, passed through netting to remove large particles and centrifuged for 5 min at 200 g. The cell pellet was resuspended in 3 mL of complete medium, transferred to a 25- cm2 flask and cultured to 90% confluence. At this point 0.75 × 106 cells were subcultured into 75-cm2 flasks; this is considered ‘passage 1’. Each culture was passaged at 0.75 × 106 cells per 75- cm2 flask every 4–6 days, depending on the experiment. On the second or third day after each subculturing two-thirds of the medium was replaced with fresh complete medium. Cell numbers were determined by hemocytometer. Each set of experiments made use of cells grown from three or four different dw/dw mice and an equal number of littermate controls, with each cell line propagated and tested independently in parallel. Serum starvation was achieved by incubation in DMEM containing 2% BSA for at least 12 h prior to the direct addition of H2O2, paraquat (methyl viologen), or cadmium (Sigma) to this culture medium, after which cells were harvested at various times, as indicated, for biochemical assays. All measurements of stress-induced enzyme activity and gene expression were obtained from cells at passage 3.
Antibodies
Antibodies for immunoblotting were obtained as follows: p38 MAPK, phospho-p38 MAPK (Thr180/Tyr182), ERK, phospho-ERK (Thr202/Tyr204), JNK, and phospho-JNK (Thr183/Tyr185), phospho-Akt (Ser473), Akt (Thr308), from Cell Signaling Technology (Beverly, MA); β-actin from Sigma-Aldrich Corp. (St. Louis, MO); and goat anti-rabbit and goat anti-mouse antibodies from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The following secondary antibodies from Jackson ImmunoResearch (West Grove, PA) were used: fluorescein isothiocyanate (FITC)-conjugated donkey-mouse IgG; FITC-conjugated donkey-rabbit IgG ; rhodamine Red-Xconjugated donkey-goat IgG; rhodamine Red-X-conjugated donkey-mouse IgG; rhodamine Red-X-conjugated donkey-rat IgG; or biotin-conjugated donkey-rat IgG.
Real-time RT-PCR
Quantitative real-time PCR was performed using a Rotor-Gene 3000 system (Corbett Research) with a QuantiTect SYBR Green RT-PCR kit (Biorad) as described [20]. In brief, cells were homogenized with RNA extraction buffer (TRIZOL reagent; Invitrogen Life Technologies, Inc., Carlsbad, CA) to yield total RNA following the manufacturer's instructions. Total RNA was reverse transcribed with poly-dT oligodeoxynucleotide and SuperScript II (Invitrogen Life Technologies), using the manufacturer's recommended conditions. After an initial denaturation step (95 °C for 90 sec), amplification was performed over 40–45 cycles of denaturation (95 °C for 10 sec), annealing (60 °C for 5 sec), and elongation (72 °C for 13 sec). Amplification was monitored by measuring the fluorometric intensity of SYBR Green I at the end of each elongation phase. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or beta-actin expression was quantified to normalize the amount of cDNA in each sample. The change in threshold cycle number (ΔCt) was normalized to the GAPDH reference gene by subtracting ΔCtGAPDH from ΔCtgene. The effect of treatment (ΔΔCt) was calculated by subtracting ΔCtnormal from ΔCtTg. Fold induction was determined by calculating 2ΔΔCt.
Western Blot Analysis
After exposure to various forms of stress, cells were washed in ice-cold phosphate-buffered saline, then harvested in 1 ml of lysis buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton 100, with protease inhibitor cocktail and phosphatase inhibitor cocktails (Sigma-Aldrich Corp.)] and spun at 12,000 rpm for 40 min. The supernatants were removed and stored at −70 °C. Protein concentrations were determined using the bicinchoninic acid assay (Pierce Corp., Rockford, IL) according to the manufacturer's instructions. For Western blotting, protein extracts were mixed with Sample Buffer (Bio-Rad Laboratories, Inc., Hercules, CA) and boiled for 5 min. 40 micrograms of total protein were separated electrophoretically according to size by SDS-PAGE using Criterion XT Precast Gel (Bio-Rad Laboratories) for 60 min at 150 V. Proteins were then wet-transferred for 1 h at 100 V onto nitrocellulose membranes. Membranes were rinsed briefly in Tris-buffered saline (TBS; pH 7.6) and blocked with 5% dry milk (or 3% BSA for phosphorylated proteins) in TBS plus 0.05% Tween 20 (TBST) for 1 h at room temperature. Blots were washed with TBST and incubated with the primary antibody diluted in the appropriate blocking solution at 4 °C overnight with shaking. After incubation, blots were washed three times (15 min each) with TBST and incubated with an appropriate secondary antibody. For visualization of specific bands in the chemiluminescence assays, the membrane was exposed to X-OMAT film; for chemifluorescence the membrane was incubated with ECF (enhanced chemifluorescence) substrate and a digital image was generated with the Molecular Dynamics Storm system. Quantification of immunoblot signals was performed using the ImageQuant software package (Molecular Dynamics, Sunnyvale, CA).
Immunofluorescence Imaging
Fibroblasts were seeded at 5,000 cells per well and grown to subconfluence on 8-well LabTekII chamber glass slides. Staining was performed in baseline conditions and after overnight serum starvation followed by stress stimulation. Cells were washed in PBS and fixed in ice-cold methanol for 10 min at −20°C. After blocking with 20% normal goat serum in PBS for 1 hour at room temperature, cells were incubated with the primary antibody overnight at 4°C. The cells were washed in PBS and incubated with fluorescent goat anti-mouse or goat anti-rabbit IgG for one hour at room temperature. After washing the cells again in PBS, the nuclei were stained with DAPI, and the slides were mounted with Vectashield (Vector Laboratories). Negative controls were processed in a similar fashion, except incubation with the primary antibody was omitted. Cells were imaged using a confocal fluorescence microscope (Olympus IX SLA). Images were then imported into Photoshop (Adobe Systems, Inc.) for processing. Fluorescent mounting media were applied before placing coverslips onto the slides. Quantification of immunoreactive cells was performed following a reported procedure [21] with image analyzer software (Image-Pro Plus; Media Cybernetics).
Statistical Analyses
All bar graphics show mean values ± SEM. Differences between normal and dwarf cells in levels of protein phosphorylation were evaluated using a paired t-test, with each time point considered separately, using p = 0.05 as the criterion for significance. Data for induction of intermediate early gene expression were evaluated by analysis of variance (factors: genotype and treatment), followed by planned comparisons of peroxide vs. no treatment, peroxide vs peroxide with ERK inhibitor, and dwarf vs control cells for each exposure condition.
Results
1. Phosphorylation of MAPK (ERK1/2, JNK1/2 and P38) in skin-derived primary fibroblasts from Snell dwarf mice in response to multiple stresses (hydrogen peroxide, paraquat, cadmium and UV light)
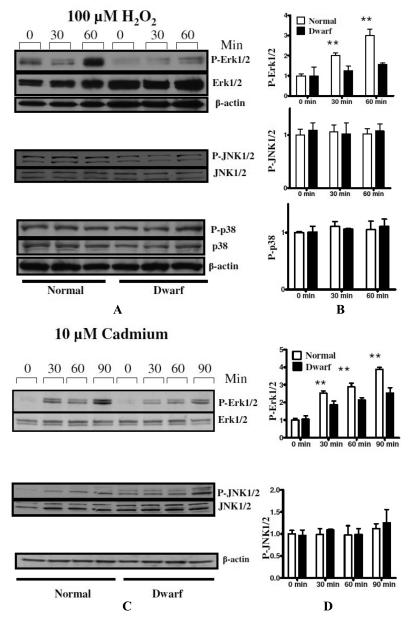
We speculated that differential activation of MAPK family members may contribute to the molecular mechanisms that confer resistance to stresses in fibroblasts from long-lived Snell dwarf mice. We examined the levels of the phosphorylation of various members of the MAPK family by western blotting. As shown in Figure 1A, we found that low doses of H2O2 led to significant increases in phosphorylation of ERK1/2 in both Snell dwarf cells and control cells. Interestingly, the phosphorylation of ERK1/2 by H2O2 was dramatically attenuated in dwarf fibroblasts compared with normal fibroblasts (Figure 1). By 60 minutes, phosphorylation levels had increased in control cells by approximately 3-fold, compared to a 50% increase in the dwarf cells. In contrast, phosphorylation of JNK1/2 or P38 was not increased by H2O2 exposure, and there was no difference between dwarf and control cells in the level of phosphorylation of JNK1/2 or P38 before or after exposure to H2O2 The graphic shows means and standard error of the mean for N = 6 pairs of mice, expressed as a ratio to the level of p-ERK1/2 measured in normal cells not exposed to H2O2. The differences between dwarf and control cells at 30 and 60 minutes are significant at p < 0.01.
Figure 1.
Phosphorylation of ERK1/2, JNK1/2 and P38 in skin-derived primary fibroblasts from Snell dwarf and normal mice in response to H2O2 and cadmium. After serum starvation for 12-24 h, cells were treated with H2O2 (100 μM, equivalent to 600 nanomoles of H2O2 per million cells) or cadmium (10 μM) for the indicated periods, then stained for p-ERK1/2, p-JNK1/2, or p-p38. The lefthand panels show representative autoradiographs of Western blots for phosphorylated and total forms of ERK1/2, JNK1/2 and P38 protein in dwarf and control cells. The righthand panels show means and sem (N=6 different pairs of dwarf and control mice), as percentages of unstressed control cells at 0 min. P < 0.01.
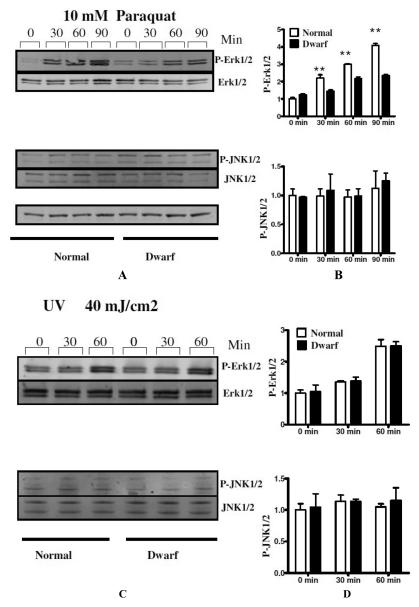
We also tested the activation of MAPK pathways by other stressors including paraquat and cadmium. Consistent with the data on H2O2 exposure, paraquat and cadmium both induced phosphorylation of ERK1/2, which was significantly attenuated in dwarf cells when compared with control cells (Figure 1 and Figure 2). JNK phosphorylation was not sensitive to cadmium or paraquat treatment in cells from mutant or control mice.
Figure 2.
Phosphorylation of ERK1/2and JNK1/2 in skin-derived primary fibroblasts from Snell dwarf and normal mice in response to paraquat and UV light. After serum starvation for at least 12-24 h, cells were treated with paraquat (10 mM) for the indicated period, or exposed to UV light (40 mJ/cm2) and further incubated for the time indicated. The lefthand panels show representative autoradiographs of Western blots for phosphorylated and total forms of ERK1/2and JNK1/2 protein, in dwarf and control cells. The righthand panels show means and sem (N=6 pairs), as percentages of unstressed control cells at 0 min **, P < 0.01).
We also evaluated the effect of UV light on the phosphorylation of ERK1/2 and JNK1/2. Cells were exposed to 40 mJ per cm2 UV irradiation and harvested at various times after treatment. As shown in figure 2C and 2D, UV induced a modest increase in ERK1/2 phosphorylation in both dwarf and normal cells, but this did not differ between the genotypes, and no significant phosphorylation of JNK1/2 could be observed in either dwarf or normal cells.
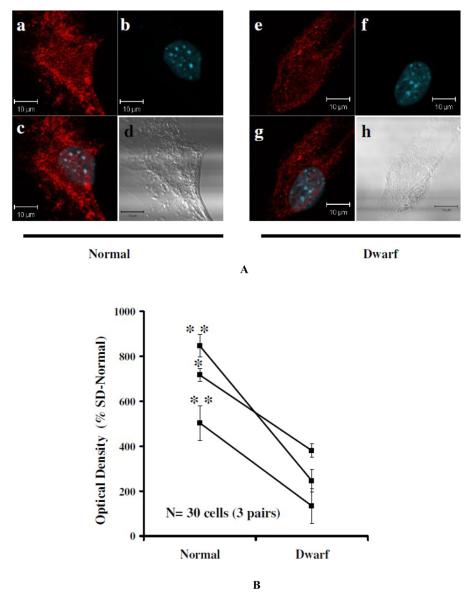
Differences (between dwarf and normal cells in phosphorylation of ERK1/2 in response to H2O2 stimulation were confirmed by an immunofluorescence study (Figure 3) using an antibody specific for phosphorylated ERK1/2. p-ERK1/2 signals were distributed throughout the cytoplasm and nucleus, with considerably higher fluorescent intensities in normal cells compared with dwarf cells after exposure to H2O2. The response in the normal cells was approximately 2-fold to 3-fold higher than the response in the dwarf cells evaluated in parallel. These results are in good agreement with the immunoblotting experiments illustrated in Fig. 1.
Figure 3.
Immunohistochemical detection of H2O2 induced ERK1/2 phosphorylation in fibroblasts from normal and dwarf mice. Each set of four images shows representative confocal images of a cell, treated with H2O2 (100 μM for 60 min), from normal and dwarf mice. Cells were labeled for phosphorylation of ERK1/2 (red; upper left in each set of panels: a and e) or DAPI staining (blue; upper right images: b and f). The merged images show both DAPI and p-ERK1/2 (lower left panels: c and g). DIC images of the cells are shown in the lower right panels (d and h). Scale bar, 10 μm. The graphic at bottom shows relative fluorescence intensities for 3 dwarf and 3 normal mice (10 cells from each of the six mice). Only cells giving fluorescence above a threshold determined from the control section (using Image-Pro Plus software) were counted. **, P < 0.01. Control experiments to measure nonspecific binding to tissue sections were performed by incubation in buffer overnight and omitting the primary antibody.
2. Increased H2O2-induced expression of ERK-dependent early growth response genes in cells from Snell dwarf mice
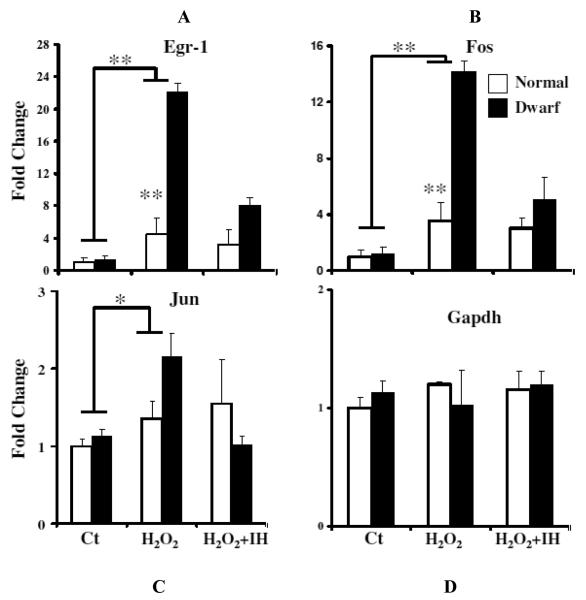
Early growth response gene (egr-1), encodes a zinc finger-containing DNA-binding protein, and levels of egr-1 mRNA rise very rapidly in response to various forms of stress [22]. It has been shown recently that the increase in expression of egr-1 is important for the resistance of cells from apoptotic stimuli [23]. We therefore investigated whether dwarf and normal cells differ in expression of this transcription factor in response to H2O2 exposure. Total mRNA prepared from cells exposed to 100 μM H2O2 for 1 h were subjected to Q-RT-PCR analysis. As shown in Figure 4A, egr-1 mRNA levels were robustly up-regulated in both normal and dwarf cells after H2O2 treatment. Surprisingly, the egr-1 response of dwarf cells to this oxidative stress was much stronger than the response of control cells. The increase of egr-1 gene expression induced by H2O2 was potently inhibited by exposure to PD98059, an inhibitor of MEK (a MAPK kinase), added before the H2O2 stress (Figure 4A). The results of this experiment indicate that ERK activation by MEK is required, in both normal and dwarf cells, for the induction of egr-1 expression by H2O2. The inhibitory effect of PD98059 on peroxide-induced egr-1 expression was at least as strong in cells from dwarf mice as in cells from normal mice. A similar pattern was seen for mRNA levels of another immediate early gene, fos (Figure 4): dramatic stimulation by H2O2 in dwarf compared to control cells, and attenuation by the MEK inhibitor. Peroxide-induced stimulation of Jun mRNA was less intense than the induction of egr-1 and fos, but showed a similar pattern. Figure 4 also shows mRNA levels for Gapdh, a peroxide-insensitive negative control. These results suggest that upregulation of egr-1, fos, and jun mRNA is mediated at least partially by ERK signals, and is substantially stronger in dwarf than in control fibroblasts.
Figure 4.
Expression of Egr-1, Fos and Jun mRNA levels in cultured Snell and control fibroblasts with or without exposure to H2O2. mRNA was measured using real-time RTPCR after 60 min 100 μM H2O2 stimulation in the presence or absence of the MEK inhibitor (IH) PD98059 (50 μM). Data are normalized to GAPDH values and were expressed as fold change of cDNA in dwarf cells relative to control (normal cells without any treatment). Each bar represents means ± SEM of 6 pairs of dwarf and normal donors. (*, P < 0.05; **, P < 0.01). Control (Ct) cells were incubated in serum-free medium without H2O2.
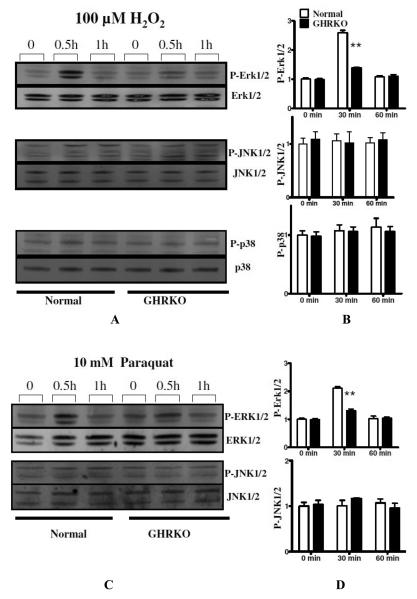
3. Skin-derived primary fibroblasts from GHRKO mice also show diminished ERK phosphorylation in response to H2O2 and paraquat
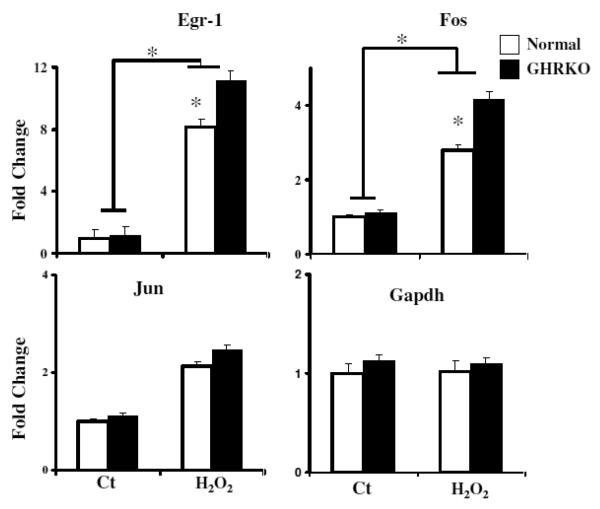
Mice with targeted disruption of the growth hormone receptor GH receptor/GH-binding protein knockout (GHRKO) have profoundly suppressed circulating levels of IGF-I and insulin, markedly increased life span, and multiple indices of delayed aging [6]. Previously, we have reported that fibroblasts from GHRKO mice were also resistant to death induced by H2O2, UV light, and paraquat compared to fibroblasts from littermate control mice [12]. To see if the defects in ERK1/2 phosphorylation noted in fibroblasts from Snell dwarf mice were also characteristic of GHRKO fibroblasts, we evaluated phosphorylation of several MAPK enzymes in these cells. We found that 100 μM H2O2 and 10 mM paraquat rapidly induced phosphorylation of ERK1/2, but not of JNK or p38 kinases, in GHRKO and control cells (Figure 5). Similar to the results with cells from Snell dwarf mice, stress-induced ERK1/2 activation was significantly attenuated in GHRKO cells compared with control cells (Figure 5). Despite the diminution of H2O2-mediated ERK1/2 phosphorylation, induction of transcription of egr-1 and fos mRNA was elevated in GHRKO compared to control fibroblasts (Figure 6), consistent with the results seen in cells from Snell dwarf mice. Induction of Jun mRNA was equivalent in cells from both mutant and control mice.
Figure 5.
Phosphorylation of ERK1/2, JNK1/2 and P38 signaling in skin-derived primary fibroblasts from GHRKO and normal mice in response to H2O2and paraquat. Top panels show representative autoradiographs of Western blots for phosphorylated and total forms of ERK1/2, JNK1/2 and P38 protein, respectively, in GHRKO and control cells after treatment with H2O2. Bottom panels show similar experiments using paraquat exposure. Graphics show phosphorylated/total ERK1/2, JNK and P38 as percentages of unstressed control cells. Means ± SEM are shown (n = 5 pairs; *, P < 0.05; **, P < 0.01).
Figure 6.
Expression of Egr-1, Fos and Jun mRNA in GHRKO and control fibroblasts with or without 30 min exposure to 100 μM H2O2. Data are normalized with GAPDH values and were expressed as fold change of cDNA in GHRKO cells relative to unstressed control cells. Each bar shows means ± SEM of 5 pairs of dwarf and mutant donors. (*, P < 0.05; **, P < 0.01).
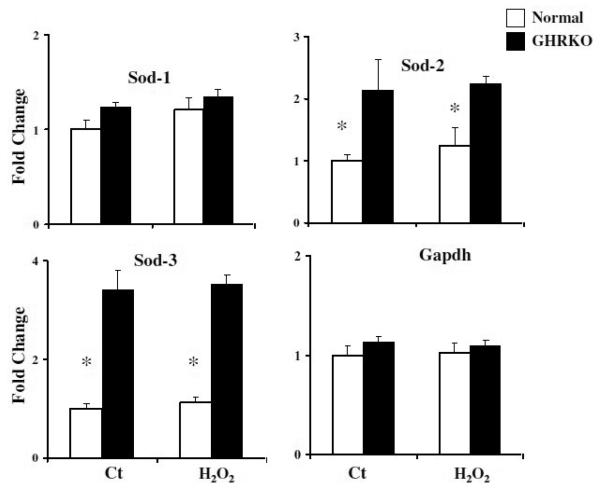
The SOD family of enzymes, which catalyze the dismutation of superoxide into H2O2 and oxygen, are thought to help defend against oxidative damage that might contribute to age-related pathology and cellular dysfunction. Many cell lines are highly dependent on SOD for survival and are sensitive to its suppression [24]. We found higher levels of sod2 and sod3 mRNA in GHRKO cell lines compared to controls. There was no alteration of transcription level of these two mRNAs after H2O2 stimulation (Figure 7) in control or mutant cells. These data support the idea that increased SOD expression might contribute to the resistance of GHRKO cells to death after oxidant treatment in vitro.
Figure 7.
Expression of Sod mRNAs in fibroblasts from GHRKO mice with or without 30 min exposure to 100 μM H2O2. Data are normalized with GAPDH values and expressed as fold change of cDNA in GHRKO cells relative to unstressed control cells. Each bar shows means ± SEM of 5 pairs of mutant and normal donors. (*, P < 0.05; **, P < 0.01).
Discussion
The present study showed that phosphorylation of the stress-activated ERK1/2 kinases induced by peroxide, cadmium, or paraquat was attenuated in cells from long-lived Snell dwarf mice. The Snell-derived cells did not differ from controls in ERK phosphorylation after UV treatment, and JNK and p38 did not show increased phosphorylation in response to any of the stresses tested in these cells. Immunofluorescence analysis of single cells showed that the attenuation of ERK1/2 phosphorylation in cells from dwarf mice represented a change in most of the cells in the cultures, rather than a change in the proportion of responsive to unresponsive cells. Paradoxically, the stress-induced elevation of mRNA of egr-1 and fos, members of the immediate early gene family, was higher in Snell dwarf cells than in control cells. Cells from long-lived GHRKO mice were, like Snell dwarf cells, relatively unresponsive to peroxide- and paraquat-mediated stimulation of ERK1/2 phosphorylation, and also hypersensitive in tests for egr-1 and fos gene expression. The disparity between the findings of ERK1/2 phosphorylation (lower in cells from mutant mice) and those for ERK-dependent gene activity (elevated in mutant-derived cells) could not be attributed to some ERK-independent signal pathway, because the increase in egr-1 and fos gene expression induced by peroxide was almost completely blunted by a MEK inhibitor.
These results have several implications concerning the mechanism of resistance, in GHRKO and Snell dwarf-derived skin fibroblasts, to lethal injury. It is not well understood how agents like cadmium, peroxide, and paraquat activate MEK and ERK, and it seems likely that the steps in these injury-responsive pathways may differ from agent to agent. Paraquat, for example, augments oxidative damage from free radicals produced, gradually, by intracellular mitochondria, while peroxide is likely to cause injury more rapidly by reactions with cellular membranes. The diminution of ERK1/2 phosphorylation (and thus presumably of MEK activity) in cells from Snell and GHRKO mice thus suggests that fibroblasts from the long-lived mutants are less susceptible to early steps in one or more of these damage detection pathways. It is possible, for example, that the damage initiated by H2O2 in cellular membranes might be repaired more quickly in Snell cells than in control cells, thus blunting the MEK-induction signal at an early stage. Experiments that evaluate the earliest stages of damage induction, and evaluate stages in the kinase cascade upstream from MEK, will be needed to address these ideas.
Despite the lower levels of stress-induced ERK1/2 phosphorylation, cells from the long-lived mutant mice show higher levels of mRNA production from at least two of the immediate early genes, i.e. egr-1 and fos. This surprising result suggests strongly that some of the immediate early genes are hyper-responsive to even blunted signals from ERK activation. Further work comparing these cells for cofactors that modulate transcription of immediate early genes as well as the maturation and stability of their mRNAs may be rewarding. It is plausible, though not at all certain, that improved activation of immediate early genes may contribute to the resistance of dwarf-derived fibroblasts to lethal injury in vitro.
The extracellular signal regulated protein kinases are integrally involved in regulating pivotal processes including proliferation, differentiation, adaptation (i.e., cell motility, long term potentiation), survival, and cell death. The ERK signaling module involves sequential activation of Raf (MAPKKK), MEK1/2 (MAPKK), and ERK1/2 (MAPK) [17, 19]. ERK1/2 is crucial for resistance to oxidative stress, and the cascade is known to be activated by oxidative injury [25-27]. Hydrogen peroxide stimulates extracellular signal-regulated protein kinases in pulmonary arterial smooth muscle cells [27], but these kinases can exhibit dual effects on cell death depending on the kinetics, duration, intensity, and context of activation [28]. ERK activation has been shown to promote cell survival under some conditions [29, 30], but it increases the sensitivity to oxidative stress under other conditions [31]. Inhibition of ERK abrogates cell death induced by H2O2 in pancreatic cancer cells [32] and protects against glutamate-induced neuronal death [33]. In the present context, it is noteworthy that fibroblasts from Snell dwarf mice, though resistant to many forms of lethal injury [11, 12], are actually more susceptible than control cells to the lethal effects of agents (tunicamycin and thapsigargin) that induce apoptosis through the ER (endoplasmic reticulum) stress pathway [34]. Whether the diminished levels of ERK phosphorylation, or the hypersensitivity of ERK-responsive mRNAs contribute to these cells' sensitivity to ER stress deserves further exploration. These biochemical abnormalities add to the growing list of properties that distinguish control fibroblasts from those of Snell dwarf mice. In addition to differences in responses to lethal stresses, Snell dwarf cells also show resistance to inhibition of the plasma membrane redox system induced either by low doses of rotenone or by culture in very low glucose concentrations [35]. They are also relatively resistant to the growth crisis induced when primary mouse fibroblasts are cultured in 20% oxygen, and have faster growth rates and higher levels of cell accumulation in long-term cultures compared to control cells [36]. Repair of UV-induced DNA damage, with restoration of RNA transcription, is also accelerated in cells from Snell dwarf animals [37]. It is not yet understood how these many cellular properties are coordinately induced, during early life development, in the skin tissue of GHRKO and Snell dwarf mice; nor is it understood how the properties are maintained, in vitro, during many rounds of replication in standardized growth conditions. The rationale for detailed analysis of the properties of fibroblasts derived from these long-lived mice depends on the assumption that similar traits may also arise, in vivo, in cell types that play a more prominent role in disease susceptibility and tissue maintenance. The association between whole-organism longevity, differences in insulin/IGF-I intracellular signals, and resistance to multiple forms of lethal stress was first noted in C. elegans [8], and has also been demonstrated in D. melanogaster [9], suggesting that this set of coordinated responses evolved at an early stage of metazoan diversity, a stage predating the splitting of nematode, arthropod, and vertebrate lineages.
The elucidation of differences in fibroblasts from long-lived and control mice should help to devise strategies for testing related hypotheses about stress resistance pathways in multiple cell types in vivo. Our findings would predict, for example, that some cell types of long-lived mice are likely to show both diminished ERK phosphorylation, and exaggerated responses of ERK-dependent genes, when exposed to stresses like those used in our in vitro work. A recent study, for example, shows that inhibition of Ras/ERK1/2 signaling is associated with resistance to oxidative damage rat cortical neurons in vitro [38]. ERK signaling has also been suggested to be involved in neurodegeneration [28, 39]. For example, in Parkinson's disease (PD), ERK is activated after application of the toxin 6-hydroxydopamine (6-OHDA). Under these conditions, cell death is decreased by PD98059, an inhibitor of ERK activation [40]. Activated ERK is also found in Lewy bodies, pathological hallmarks of PD that consist of aggregated alpha-synuclein in the degenerated neurons from PD patients [41]. It should be rewarding to explore protocols in which Snell dwarf and control mice are compared for resistance to various forms of in vivo injury, and to agents that induce varying forms of degenerative or neoplastic disease, to see if the long-lived mice are resistant, and, if so, to determine if alterations in ERK-dependent signals play a role in the altered disease susceptibility.
Acknowledgements
This work was supported by NIH grants AG023122, U19 AG023122 and AG019899. We thank John Kopchick for the gift of breeding pairs for the GHRKO mouse stock. We thank Maggie Lauderdale, William Kohler and Lisa Burmeister for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 2.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 4.Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001;39:277–284. doi: 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- 7.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen PL. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1993;90:8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- 10.Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat Genet. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- 11.Murakami S, Salmon A, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- 12.Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289:E23–29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- 13.Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 14.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 15.Harper JM, Salmon AB, Leiser SF, Galecki AT, Miller RA. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007;6:1–13. doi: 10.1111/j.1474-9726.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapahi P, Boulton ME, Kirkwood TB. Positive correlation between mammalian life span and cellular resistance to stress. Free Radic Biol Med. 1999;26:495–500. doi: 10.1016/s0891-5849(98)00323-2. [DOI] [PubMed] [Google Scholar]
- 17.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 18.Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- 19.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun LY, D'Ercole AJ. Insulin-like growth factor-I stimulates histone H3 and H4 acetylation in the brain in vivo. Endocrinology. 2006;147:5480–5490. doi: 10.1210/en.2006-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brami-Cherrier K, Valjent E, Herve D, Darragh J, Corvol JC, Pages C, Arthur SJ, Girault JA, Caboche J. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25:11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiel G, Cibelli G. Regulation of life and death by the zinc finger transcription factor Egr-1. J Cell Physiol. 2002;193:287–292. doi: 10.1002/jcp.10178. [DOI] [PubMed] [Google Scholar]
- 23.Pipaon C, Casado JA, Bueren JA, Fernandez-Luna JL. Jun N-terminal kinase activity and early growth-response factor-1 gene expression are down-regulated in Fanconi anemia group A lymphoblasts. Blood. 2004;103:128–132. doi: 10.1182/blood-2003-06-2091. [DOI] [PubMed] [Google Scholar]
- 24.Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 25.Gaitanaki C, Konstantina S, Chrysa S, Beis I. Oxidative stress stimulates multiple MAPK signalling pathways and phosphorylation of the small HSP27 in the perfused amphibian heart. J Exp Biol. 2003;206:2759–2769. doi: 10.1242/jeb.00483. [DOI] [PubMed] [Google Scholar]
- 26.Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Jin N, Liu Y, Rhoades RA. Hydrogen peroxide stimulates extracellular signal-regulated protein kinases in pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol. 1998;19:324–332. doi: 10.1165/ajrcmb.19.2.3209. [DOI] [PubMed] [Google Scholar]
- 28.Chu CT, Levinthal DJ, Kulich SM, Chalovich EM, DeFranco DB. Oxidative neuronal injury. The dark side of ERK1/2. Eur J Biochem. 2004;271:2060–2066. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 30.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 31.Subramaniam S, Zirrgiebel U, von Bohlen Und Halbach O, Strelau J, Laliberte C, Kaplan DR, Unsicker K. ERK activation promotes neuronal degeneration predominantly through plasma membrane damage and independently of caspase-3. J Cell Biol. 2004;165:357–369. doi: 10.1083/jcb.200403028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osada S, Sakashita F, Hosono Y, Nonaka K, Tokuyama Y, Tanaka H, Sasaki Y, Tomita H, Komori S, Matsui S, Takahashi T. Extracellular signal-regulated kinase phosphorylation due to menadione-induced arylation mediates growth inhibition of pancreas cancer cells. Cancer Chemother Pharmacol. 2008;62:315–320. doi: 10.1007/s00280-007-0610-9. [DOI] [PubMed] [Google Scholar]
- 33.Satoh T, Nakatsuka D, Watanabe Y, Nagata I, Kikuchi H, Namura S. Neuroprotection by MAPK/ERK kinase inhibition with U0126 against oxidative stress in a mouse neuronal cell line and rat primary cultured cortical neurons. Neurosci Lett. 2000;288:163–166. doi: 10.1016/s0304-3940(00)01229-5. [DOI] [PubMed] [Google Scholar]
- 34.Salmon AB, Sadighi Akha AA, Buffenstein R, Miller RA. Fibroblasts from naked mole-rats are resistant to multiple forms of cell injury, but sensitive to peroxide, ultraviolet light, and endoplasmic reticulum stress. J Gerontol A Biol Sci Med Sci. 2008;63:232–241. doi: 10.1093/gerona/63.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leiser SF, Salmon AB, Miller RA. Correlated resistance to glucose deprivation and cytotoxic agents in fibroblast cell lines from long-lived pituitary dwarf mice. Mech Ageing Dev. 2006;127:821–829. doi: 10.1016/j.mad.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Maynard SP, Miller RA. Fibroblasts from long-lived Snell dwarf mice are resistant to oxygen-induced in vitro growth arrest. Aging Cell. 2006;5:89–96. doi: 10.1111/j.1474-9726.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 37.Salmon AB, Ljungman M, Miller RA. Cells from long-lived mutant mice exhibit enhanced repair of ultraviolet lesions. J Gerontol A Biol Sci Med Sci. 2008;63:219–231. doi: 10.1093/gerona/63.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGFI/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22:631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Kulich SM, Chu CT. Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine: implications for Parkinson's disease. J Neurochem. 2001;77:1058–1066. doi: 10.1046/j.1471-4159.2001.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu JH, Kulich SM, Oury TD, Chu CT. Cytoplasmic aggregates of phosphorylated extracellular signal-regulated protein kinases in Lewy body diseases. Am J Pathol. 2002;161:2087–2098. doi: 10.1016/S0002-9440(10)64487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]