Abstract
Bacillus anthracis, the etiologic agent for anthrax, secretes edema factor (EF) to disrupt intracellular signaling pathways. Upon translocation into host cells and association with a calcium sensor, calmodulin (CaM), EF becomes a highly active adenylyl cyclase (AC) that raises the intracellular concentration of cyclic AMP (cAMP). Growing evidence shows that EF plays a key role in anthrax pathogenesis by affecting cellular functions vital for host defense. This strategy is also used by Bordetella pertussis, a bacterium that causes whooping cough. Pertussis bacteria secrete the bifunctional toxin CyaA which raises the intracellular cAMP. Here, we discuss recent advances from structural analyses that reveal the molecular basis of the conserved mechanism of activation and catalysis of EF and CyaA by CaM even though these two toxins use the completely different sequences to bind CaM. Comparison of the biochemical and structural characteristics of these two AC toxins with host ACs reveal that they have diverse strategies of catalytic activation, yet use the same two-metal-ion catalytic mechanism.
Keywords: anthrax edema factor, pertussis CyaA, class III adenylyl cyclase
Edema factor (EF) is an 89-kDa protein secreted by Bacillus anthracis, the Gram-positive bacterium that cause anthrax. EF belongs to a family of bacterial toxins that can specifically elevate the intracellular cyclic AMP (cAMP) level, which is the prototypic second messenger that regulates diverse cellular responses. The biological effects of cAMP are mediated by the binding of cAMP to three families of signal transducers: cAMP-dependent protein kinases, cyclic nucleotide gated channels (Biel and Michalakis 2009), and EPAC, the guanine nucleotide exchange factor for Ras GTPase homologs Rap1 and Rap2 (Borland, Smith, and Yarwood 2009). There are two major mechanisms by which bacterial toxins can raise the intracellular cAMP level. The first mechanism is by the action of the bacterial adenylyl cyclase (AC) toxins, which possess AC activity, the enzymatic activity that converts ATP to cAMP. Their AC activities are activated only upon their entrance into host cells and association with the specific cellular proteins that serve as the activator. In addition to the aforementioned EF from Bacillus anthracis, two other examples of AC toxins include CyaA from Bordetella pertussis and ExoY from Pseudomonas aeruginosa (Leppla 1984; Wolff et al. 1980; Yahr et al. 1998). EF and CyaA share CaM as a common activator while the activator of ExoY is still unknown. The second mechanism by which intracellular cAMP is increased by bacteria is the ADP-ribosylation of heterotrimeric G proteins by bacterial toxins, resulting in increased catalytic activity of host membrane-bound AC (mAC). Cholera toxin from Vibrio cholera and labile toxin from Escherichia coli can ADP-ribosylate Gsα, rendering it constitutively active to stimulate host mAC. Targeting a different G protein subunit, pertussis toxin from Bordetella pertussis ADP-ribosylates Giα, uncoupling G protein-coupled receptor from inhibiting host mACs. In this review, we discuss new insights from the structural studies of EF and compare the actions of EF with other ACs.
History of EF discovery and recent advances
EF was identified based on its ability to induce cutaneous edema and mortality of experimental animals when mixed with anthrax protective antigen (PA) (Smith and Keppie 1954; Stanley, Sargeant, and Smith 1960). EF together with PA is commonly referred to as edema toxin (EdTx). About 22 years after its discovery, EF was found to have adenylyl cyclase (AC) activity (Leppla 1982). EF was then found to be an inactive enzyme till activated by an eukaryotic calcium sensor, calmodulin (CaM) (Leppla 1984). The cloning of the EF gene permitted the generation of EF-defective anthrax bacteria, and such mutants had reduced virulence, indicating a key role for EF in anthrax pathogenesis (Mock et al. 1988; Pezard, Berche, and Mock 1991). Consistent with this notion, EdTx was also shown to inhibit the phagocytic activity of human neutrophils and alter cytokine production of human monocytes (O'Brien et al. 1985; Hoover et al. 1994).
Recently, significant advances have been made on EdTx. In order to enter into the targeted host cells, EF is required to bind PA. A series of experiments has offered the molecular details in the requisite steps for the delivery of EF by PA into host cells. These steps include the binding of PA to its cellular receptors (TEM8 or CMP2), the proteolytic activation and oligomerization of a PA heptamer, the association of three molecules of EF (or LF) to one PA heptamer, endocytosis and finally the PA-assisted translocation of EF from late endosomes into the cytoplasm (Gruenberg and van der Goot 2006; Young and Collier 2007; Dal Molin et al. 2006; Baldari et al. 2006). Furthermore, growing evidence shows that EdTx alone and together with LeTx could profoundly affect key functions of host cells involved in the defense against bacterial infection (Crawford et al. 2006; Hong et al. 2007; Paccani et al. 2005; Shen et al. 2004; Tournier et al. 2005; Szarowicz et al. 2009; Puhar et al. 2008; Rossi Paccani et al. 2007; Kim et al. 2008; Maldonado-Arocho et al. 2006; Tessier et al. 2007). These include the effects of EdTx on macrophages, dendritic cells, neutrophils, T cells, and endothelial cells. Furthermore, the molecular mechanisms behind such modulation also have begun to be elucidated.
The availability of highly purified recombinant EF allowed the demonstration that EdTx indeed could cause cardiovascular dysfunction, tissue damage, and mortality in mice (Firoved et al. 2005; Kuo et al. 2008; Watson, Kuo et al. 2007; Watson, Mock et al. 2007). In addition, EdTx can sensitize mice to the treatment of lethal toxin (Firoved et al. 2007). However, a high lethal dosage of EdTx (~2mg EdTx per kg mouse) is required to achieve lethality. Such high doses are also required for LeTx to cause the death of the experimental animals (Moayeri et al. 2003). This is consistent with the notion that the mortality of anthrax infection is caused by the combined effects of bacterial growth (bacteremia) and bacterial toxins (toxemia) so that the maximal production of bacterial progeny can be achieved.
The other major advance since 2001 deals with the molecular basis of catalysis and activation of EF, which is the focus of this review. This is achieved by the available X-ray structures of the AC domain of EF alone and in complex with CaM as well as CaM-bound EF (Drum et al. 2002; Shen et al. 2002; Shen et al. 2005). CaM is a calcium sensor and signal transducer that modulates the functions of over 100 effectors proteins. Combined with the biochemical characterization, these structures of EF offer understanding of how CaM binds to and activates its effectors (Hoeflich and Ikura 2002). This work has also showed that both EF and mACs utilize two-metal-ion catalysis for the phosphoryl transfer reaction during cAMP production, even though they both have the different structural folds and catalytic site sequences (Shen et al. 2005; Tesmer et al. 1999). Finally, an approved anti-viral drug targeted at the EF catalytic site has served as an experimental tool to address the function of EF (Kim et al. 2008; Paccani et al. 2005; Shen et al. 2004).
The molecular basis for the activation of anthrax EF and pertussis CyaA by CaM
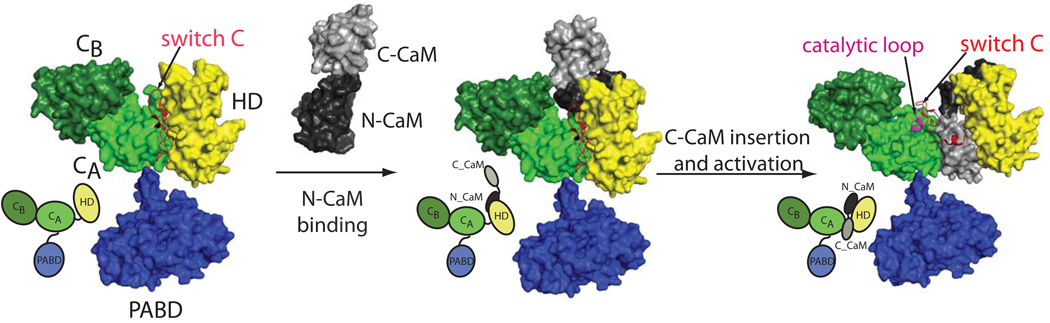
A series of structural studies have provided the molecular details of how EF is kept in the inactive state and how the binding of CaM triggers the activation of AC activity of this enzyme (Figure 1) (Drum et al. 2000; Drum et al. 2002; Shen et al. 2002; Shen et al. 2005; Ulmer et al. 2003). EF has three modular domains, a 30 kDa N-terminal PA binding domain (PABD), a 43 kDa AC domain, and a 17 kDa helical domain. The AC domain can be divided structurally into the CA and CB domains and the catalytic site is located at the interface of CA and CB. The comparison of the EF AC domain alone with CaM-bound EF and CaM-bound EF AC domain reveals that the disordered catalytic loop triggered by the interaction of the EF helical domain with the catalytic core domain is the key mechanism to keep EF in the inactive state in the absence of CaM (Drum et al. 2002). The NMR study indicates that CaM uses its N-terminal domain to bind the EF helical domain to initiate its binding with EF (Ulmer et al. 2003). The anchoring of N-CaM to the EF helical domain allows the insertion of C-CaM between the catalytic core and helical domains of EF. This initiates a conformational change of the switch C region to stabilize the critical catalytic loop of EF for the high rate AC activity (~2,000 per second). A series of structural and biochemical studies combined with computational simulations reveals that EF also has two conserved aspartate residues, which coordinate two magnesium ions required for AC activity; one magnesium ion deprotonates the 3’OH of ATP and the other stabilizes the penta-covalent transition state intermediate (Shen et al. 2005). This two-metal-ion catalytic mechanism is similar to those used by most DNR and RNA polymerases for phosphoryl transfer reactions (Steitz 1998).
Figure 1. Model for the catalytic activation of EF by CaM.
Catalytic core domains, CA and CB, helical domain (HD) and PA binding domain (PABD) of EF are colored in light green, dark green, yellow, and purple, respectively. The N- and C-terminal domains of CaM are colored black and grey, respectively. The key switch region and the activation catalytic loop are colored in red and magenta, respectively. EF alone structure is a model based on the AC domain of EF (pdb code 1k8t) with the insertion of the PABD domain from the CaM-bound EF structure (pdb code 1xfv) (Drum et al. 2002; Shen et al. 2005). The transition state for the initial contact of N-CaM to the helical domain of EF is modeled based on the NMR study (Ulmer et al. 2003). For better visualization of the domain organization, the cartoons depicting these three states are shown in the lower left panel.
Cyclic AMP and calcium are two key intracellular second messengers and have considerable crosstalk with each other (Willoughby and Cooper 2007; Cooper, Mons, and Karpen 1995). Interestingly, the calcium dependent response of EF is biphasic. Physiologically relevant intracellular calcium concentrations can enhance EF activity (Shen et al. 2002). At a resting or modestly elevated calcium concentration (0.1–0.4 µM), the AC activity of EF is stimulated by calcium ions. However, the AC activity of EF is inhibited at the higher concentrations of calcium. As opposed to the stimulation of EF by calcium-bound CaM, the AC activity of EF is inhibited by the competition of calcium ions with the magnesium ions at the catalytic site. Thus, the effects of EdTx on the targeted cells are likely to be dependent on the intracellular signaling context. Furthermore, other secreted factors from anthrax bacteria, such as the pore-forming toxin anthrolysin O, could raise the intracellular calcium level and would likely affect the functions of EdTx (Bourdeau et al. 2009).
CyaA, an 188 kDa adenylyl cyclase toxin, is secreted by a human pathogen that causes whooping cough, a severe childhood disease worldwide (Mattoo et al. 2001; Rogel, Meller, and Hanski 1991; Ladant and Ullmann 1999; Guermonprez et al. 2001). CyaA serves as an anti-inflammatory and anti-phagocytic factor, facilitating respiratory tract colonization by B. pertussis (Carbonetti et al. 2005; Mattoo et al. 2001). As opposed to EF, CyaA is a bifunctional toxin containing not only AC activity but also hemolytic activity. Upon binding to its cellular surface receptor (αMβ2 integrin), CyaA becomes a pore-forming integral membrane protein for its hemolytic activity. In addition, the N-terminal AC domain is translocated into the cytoplasm of the host cells. Similar to EF, CyaA is inactive without its activator, CaM. Upon binding to CaM, CyaA becomes a highly active enzyme with a catalytic rate similar to that of EF (Shen et al. 2002).
The AC domain of CyaA shares 25% sequence identity to that of EF. Thus, it is not surprising that these two toxins share the same domain organization and two-metal-ion catalytic mechanism (Figure 2A) (Guo et al. 2005; Shen et al. 2005). Furthermore, the activation mechanism of these two toxins by CaM is quite similar as well (Guo et al. 2005; Shen et al. 2005). However, even though the structures of these AC toxins are quite similar and the location where CaM binds to these two toxins is also identical, CyaA and EF have completely different CaM binding sequences (Guo et al. 2005; Guo et al. 2008). In addition, the specific roles of N- and C-CaM for the activation of these two toxins are also completely different (Guo et al. 2008; Shen et al. 2002).
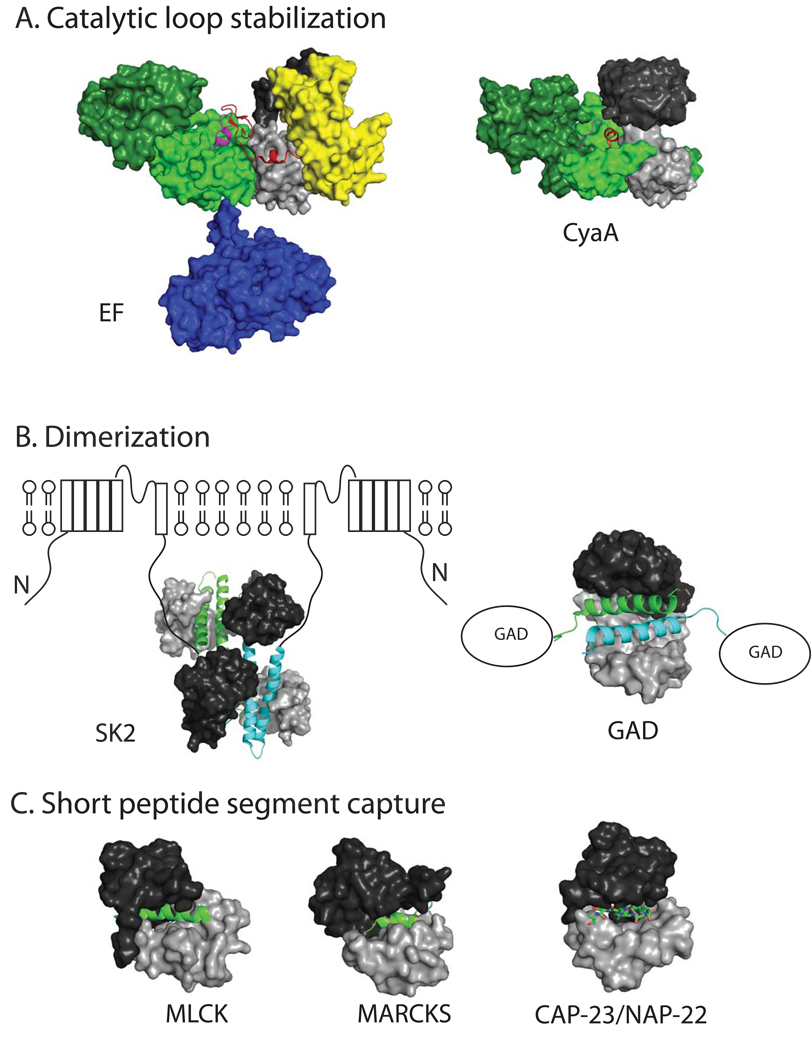
Figure 2. Three modes of CaM binding to its effectors.
The PDB accession codes for CaM bound anthrax EF (EF), C-CaM bound pertussis CyaA (CyaA), CaM bound rat small conductance potassium channel (SK2), CaM-bound C-terminal end of glutamic acid decarboxylase (GAD), and CaM-bound fragments of myosin light chain kinase (MLCK), myristoylated alanine-rich C-kinase substrate (MARCKS), and CAP-23/NAP-22 are 1xfv, 1yrt, 1g4y, 1nwd, 1cdl, 1iwq, and 1l7z, respectively. The N- and C-terminal CaM are colored black and grey, respectively and the color scheme of EF is same as figures 1. The domains in CyaA is colored according to those of EF. Specifically, CA and CB domain of CyaA is colored as light and dark green, respectively. The key switch region and the activation catalytic loop are colored in red and magenta, respectively. The N-terminal CaM is modeled in based on the biochemical analysis and computational simulation as described (Guo et al. 2008). The CaM binding segments that are involved in the dimerization of SK2 and GAD are colored in green and cyan, respectively.
The structural analysis of CaM bound AC toxins also revise our view of how CaM can interact with its effectors, leading to the desired biological outcomes. Structures of EF and CyaA in complex with CaM reveal how several discrete regions of EF and CyaA form a large surface to grab CaM, resulting in the stabilization of a catalytic loop for catalytic activation (Figure 2A) (Drum et al. 2002; Guo et al. 2008; Guo et al. 2005; Shen et al. 2005). CaM can grasp two or three helices to trigger the dimerization of its effectors such as the small conductance potassium channel (SK2) and glutamic acid decarboxylase (GAD) (Figure 2B) (Schumacher et al. 2001; Yap et al. 2003). In the case of the SK2 channel, the interaction between two dimers within the four subunits of SK2 triggers the channel opening to modulate the hyperpolarization phase of action potential of nerve cells (Maylie et al. 2004). In addition, outside of the conventional mode for CaM to bind an amphipathic α-helix such as that from myosin light chain kinase (MLCK), CaM can bind other short motifs such as the turn-helix peptides and the myristoylated peptides exemplified by CaM’s interaction with the major alanine-rich C-kinase substrate (MARCKS) and CAP-23/NAP-22, respectively (Figure 2C) (Matsubara et al. 2004; Yamauchi et al. 2003; Hoeflich and Ikura 2002). The CaM binding to these motifs can lead to the removal of auto-inhibitory region of CaM effectors from the active site, leading to catalytic activation.
Structural comparison of AC families and the development of selective EF inhibitor
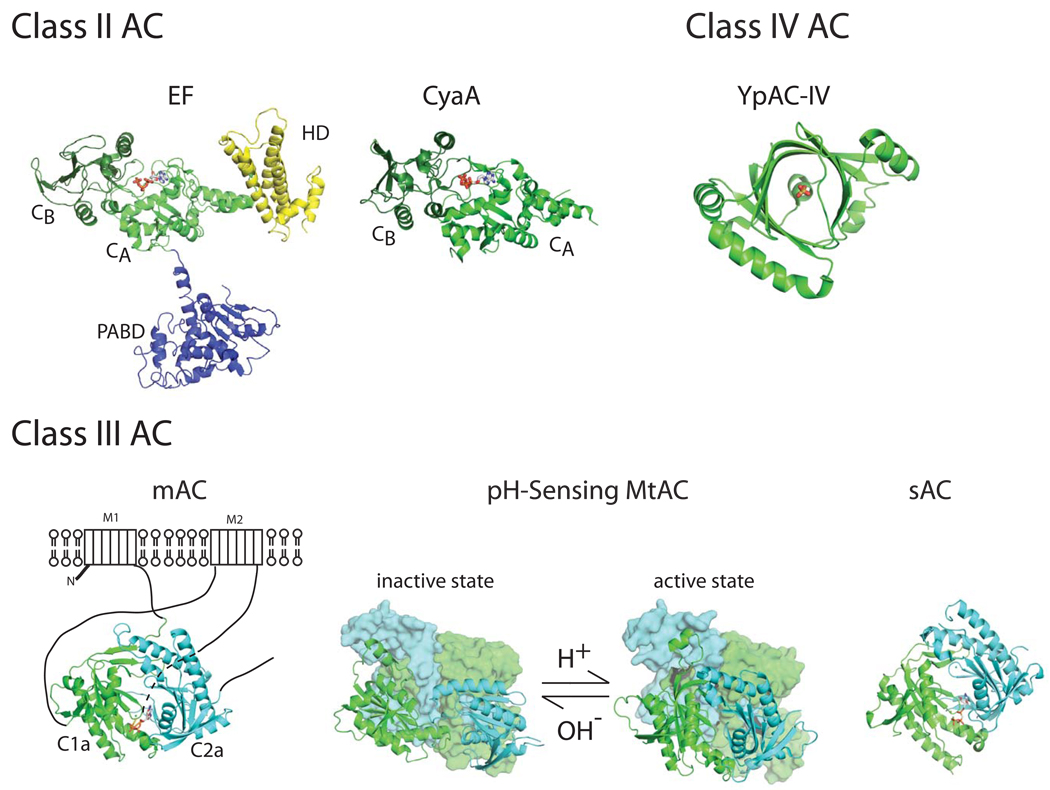
There are at least six classes of ACs based on their primary sequences (Linder 2006; Tang and Hurley 1998). To date, the structures of three of these classes are available (Figure 3) (Gallagher et al. 2006; Guo et al. 2005; Shen et al. 2005; Steegborn et al. 2005; Tesmer et al. 1997; Tews et al. 2005; Kamenetsky et al. 2006). Class II ACs are bacterial AC toxins, which include anthrax EF, pertussis CyaA, ExoY from P. aeruginosa, as well as a number of gene products from Yersinia pseudotuberculosis, Vibrio cholera, and Burkholderia thailandensis that may have AC activity. Class III ACs are the most abundant ACs and are found from bacteria to humans. Among them, the most studied member is the mammalian mAC (Linder 2006; Sunahara and Taussig 2002; Tang and Hurley 1998; Kamenetsky et al. 2006). At least 9 isoforms of mAC have been found and they are regulated by diverse intracellular cellular signaling proteins such as heterotrimeric G proteins, CaM, and kinases. The specific pattern of regulation for each mAC is dependent upon the particular isoform of the enzyme. All mAC isoforms have two transmembrane domains (M1 and M2), each followed by a conserved intracellular domain (C1a and C2a, respectively) (Figure 3). C1a and C2a are homologous to each other and they form a head-to-tail heterodimer. The catalytic site is formed between two conserved cytoplasmic domains and both domains contribute the residues critical for catalysis.
Figure 3. Structural comparisons of three classes (II–IV) of AC.
The PDB accession codes for anthrax EF and pertussis CyaA of class II AC, membrane bound mammalian AC (mAC), bicarbonate activated soluble AC from cyanobacteria (sAC), and low pH activated inactive and active forms of AC from Mycobacteria tuberculosis (MtAC) of class III AC, and Yersinia pestis AC of class IV AC are 1xfv, 1zot, 1cjk, 1wc5, 1y10, 1y11, and 2fjt, respectively. The color scheme of class II AC is same as figure 2. The heterodimeric catalytic domains of mAC and homodimeric catalytic domains of sAC and MtAC are depicted as ribbons colored in cyan and green. For the clarity, the regulatory domains of MtAC are shown as the surface representation and colored according to their catalytic domains. YpAC is a dimer and only monomer is depicted since the catalytic center is at the center of the protein. The ATP analogues in EF, CyaA, mAC, and sAC as well as the phosphate in YpAC denote the catalytic site of these enzymatic and are colored according to their atoms.
While the structure of inactive state mAC is not yet available, the conformational changes revealed by the structural studies of pH-sensing AC from Mycobacteria tuberculosis (MtAC) and bicarbonate-sensitive soluble AC from cyanobacteria (sAC) provide the possible activation mechanisms of class III ACs (Figure 3). M. tuberculosis, the etiological agent for tuberculosis in humans, has 15 putative class III AC. Of those, Rv1264 is activated by lower pH and the structures of Rv1264 at acidic and alkaline pH have been solved (Tews et al. 2005). The comparison of these structures reveals that the juxtaposition of two catalytic domains by rigid body movement is the key activation mechanism (Figure 3). Such juxtaposition of C1a and C2a of mAC is also inferred to be the mechanism of how mACs are activated by GTP-bound Gsα and forskolin, two potent activators of most mACs (Tesmer et al. 1997). The subtle conformational switch at the catalytic site is also postulated to play a key role in the activation of class III AC. Based on the structure of sAC from cyanobacteria, the treatment of bicarbonate, which is known to activate sAC, induces the conformational change at the triphosphate-binding region of the enzyme (Steegborn et al. 2005). This is postulated that such change could facilitate the separation of the reaction products, cAMP and pyrophosphate, from the penta-covalent transition intermediate to accelerate the catalytic rate of sAC.
Both EF and CyaA have at least 1,000 fold higher AC activity than those of class III AC (Shen et al. 2002; Tang and Hurley 1998; Kamenetsky et al. 2006; Linder 2006). Since these two classes of AC have completely different structural folds and sequences that make up the catalytic site, it was originally postulated that they use different catalytic mechanisms for their cyclization reaction (Drum et al. 2002). However, the follow-up structural and simulation studies reveal that both classes of AC share the same reaction mechanism, two-metal-ion catalysis (Shen et al. 2005; Tesmer et al. 1999). While it is difficult to pinpoint the molecular basis for the turn-over differences among these two classes of AC, the fundamental variation in domain movement for catalytic activation and the composition at the catalytic site likely provides the major contribution (Benkovic and Hammes-Schiffer 2003; Knowles 1991). It is known that the motion required for the activation of EF and CyaA is significantly smaller than that of mAC (Drum et al. 2002; Guo et al. 2005; Tesmer et al. 1997; Tews et al. 2005). In the case of EF and CyaA, the stabilization of the catalytic loop by switch C is the activation mechanism. To do so, no major domain movement between CA and AB is required. However, the juxtaposition or proper alignment of two cytoplasmic domains is the key step to activate class III AC, and a large conformational change by the rigid body movement of two catalytic domains is necessary. Thus, the ease in stabilizing the active state of the enzyme is likely to contribute to the overall rate of class II AC. Furthermore, there are fundamental differences at the catalytic site, which would dictate their ability to lower the transition state energy. For example, class III ACs have a basic residue (histidine or lysine) to deprotonate the water molecule to the hydroxyl ion, which, in turn, deprotonates 3’OH of ATP to the oxyanion for its nucleophilic attack to the α-phosphate of ATP (Shen et al. 2005).
Recently, the structure of class IV AC from Yersinia pestis, the etiologic agent for bubonic plague, was solved (Gallagher et al. 2006) (Figure 3). This family of ACs was first found based on its ability to complement the defects caused by the deletion of the AC gene in E. coli. Again, the class IV family has a structure distinct from class II and III ACs. It shares structural similarity to phosphonucleotide processing enzymes such thiamine triphosphatase, but currently the mechanism for the regulation and catalysis of this family is poorly characterized.
Distinct differences between AC toxin and class III AC catalytic site enabled us to search for small molecular inhibitor leads that can specifically inhibit the activation of AC toxins without affecting class III ACs. These inhibitors can be targeted to compete with the binding of EF to substrate or CaM (Lee et al. 2004; Shen et al. 2004; Soelaiman et al. 2003). Such inhibitors could be further developed as an anti-anthrax treatment to be administered in combination with traditional antibiotics. Among those, the most potent EF inhibitor is an approved drug, Adefovir, which can selectively inhibit the activity of EF both in the test tube (27 nM Ki) and in cultured cells (IC50 around 100 nM) with no inhibition of the activity of endogenous host AC (Shen et al. 2004). Adefovir is an acyclic nucleoside and it is approved to treat chronic hepatitis B virus infection. This drug is proven to be a powerful experimental tool to demonstrate the role of EdTx on the antigen presentation of T cells and enhanced migration of macrophages (Kim et al. 2008; Paccani et al. 2005). It is worth noting that other approved acyclic nucleoside such as Tenofovir, an approved drug against human immunodeficiency virus, also show high affinity to EF and can potently inhibit the AC activity of EdTx (Shen et al. 2004); owing to its more favorable bioavailability, it may serve as a better inhibitor of EdTx than Adefovir.
Evolution of the diverse CaM binding surfaces of EF and CyaA
A strong driving force must exist to select the two completely different, high affinity CaM-binding surfaces of EF and CyaA. It can be speculated that one such driving force is the affinity of the AC toxins for CaM. The affinity of CyaA for CaM is approximately 0.2 nM, two orders of magnitude higher that that of EF. The need to have such a different affinity may be due to the route of cell entry of these two toxins. The AC domain of CyaA translocates across the host cell surface and stays on the cytosolic side of the plasma membrane (Ladant and Ullmann 1999). EF enters the cytosol of host cells through endocytosis and low pH-driven membrane translocation (Abrami, Reig, and van der Goot 2005). Such high affinity would offer the competitive edge for the AC domain of CyaA to reach the plasma membrane associated CaM of host cells (Bahler and Rhoads 2002; Schumacher et al. 2001).
Conclusion remarks
Since the discovery of EF by Harry Smith nearly 50 years ago, significant progress were made in our understanding of the molecular basis of regulation and catalysis of the AC activity of this toxin. The comparison of EF with the other class II AC, CyaA from B. pertussis, and class III ACs reveal how diverse signaling inputs can lead to the activation of AC through various mechanisms, including the catalytic loop stabilization in class II ACs and the rigid body movement of the two catalytic core domains and the conformational switch at the catalytic site in class III ACs. Despite the difference in their activation mechanism, these two classes of AC use the same two-metal-ion mechanism, which is also shared by many DNA and RNA polymerases.
However, several questions regarding the structures and functions of EF remains to be answered. Little is known of the structural basis of the interaction of EF with PA and the translocation of EF into the host cell cytoplasm. Once entering into host cells, EF becomes membrane-associated (Dal Molin et al. 2006; Guidi-Rontani et al. 2000). It is unclear whether this is due to the association of EF with CaM or to its binding with other cellular factors. In addition, growing evidence indicates that EdTx plays a key role in anthrax pathogenesis by modulating the diverse functions of host cells vital for innate immunity and host defense (see accompaining review by Tournier et al in this volume). EdTx also is shown to functionally interact with other anthrax secreted factors (Kim et al. 2008; Paccani et al. 2005). Recent genomic and proteomic studies reveal that anthrax bacteria secrete approximately 400 factors (Chitlaru et al. 2007; Gat et al. 2006), and future studies will be necessary to address how EdTx works together with other secreted factors to contribute to the complex processes of anthrax pathogenesis.
Acknowledgment
This work is supported by National Institute of Health grants GM62548 and AI66503. We thank for the critical reading of Yao Bian and Brian Bishop.
Abbreviations
- cAMP
cyclic 3’5’ adenosine monophosphate
- AC
adenylyl cyclase
- EF
anthrax edema factor
- LF
anthrax lethal factor
- PA
anthrax protective antigen
- EdTx
edema toxin
- LeTx
lethal toxin
- CaM
calmodulin
- CA and CB
the conserved domain A and B of the catalytic core domain of class II AC
- PABD
PA binding domain
- HD
EF helical domain
- C1a and C2a
the conserved cytoplasmic domains of mAC
- MLCK
myosin light chain kinase
- MARCKS
major alanine-rich C-kinase substrate
- SK2
small conductance potassium channel
- GAD
glutamic acid decarboxylase
- sAC
bicarbonate-sensitive soluble AC
- YpAC
AC from Yersinia pestis
- MtAC
AC from Mycobacteria tuberculosis
References
- Abrami L, Reig N, van der Goot FG. Anthrax toxin: the long and winding road that leads to the kill. Trends Microbiol. 2005;13(2):72–78. doi: 10.1016/j.tim.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Bahler M, Rhoads A. Calmodulin signaling via the IQ motif. FEBS Lett. 2002;513(1):107–113. doi: 10.1016/s0014-5793(01)03239-2. [DOI] [PubMed] [Google Scholar]
- Baldari CT, Tonello F, Paccani SR, Montecucco C. Anthrax toxins: A paradigm of bacterial immune suppression. Trends lmmunol. 2006;27(9):434–440. doi: 10.1016/j.it.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Benkovic SJ, Hammes-Schiffer S. A perspective on enzyme catalysis. Science. 2003;301(5637):1196–1202. doi: 10.1126/science.1085515. [DOI] [PubMed] [Google Scholar]
- Biel M, Michalakis S. Cyclic nucleotide-gated channels. Handb Exp Pharmacol. 2009;(191):111–136. doi: 10.1007/978-3-540-68964-5_7. [DOI] [PubMed] [Google Scholar]
- Borland G, Smith BO, Yarwood SJ. EPAC proteins transduce diverse cellular actions of cAMP. Br J Pharmacol. 2009 doi: 10.1111/j.1476-5381.2008.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau RW, Malito E, Chenal A, Bishop BL, Musch MW, Villareal ML, Chang EB, Masser EM, Rest RF, Tang WJ. Cellular functions and X-ray structure of anthrolysin O, a cholesterol-dependent cytolysis secreted by Bacillus anthracis. J Biol Chem. 2009 doi: 10.1074/jbc.M807631200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetti NH, Artamonova GV, Andreasen C, Bushar N. Pertussis toxin and adenylate cyclase toxin provide a one-two punch for establishment of Bordetella pertussis infection of the respiratory tract. Infect lmmun. 2005;73(5):2698–2703. doi: 10.1128/IAI.73.5.2698-2703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitlaru T, Gat O, Grosfeld H, Inbar I, Gozlan Y, Shafferman A. Identification of in vivo-expressed immunogenic proteins by serological proteome analysis of the Bacillus anthracis secretome. Infect lmmun. 2007;75(6):2841–2852. doi: 10.1128/IAI.02029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DM, Mons N, Karpen JW. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature. 1995;374(6521):421–424. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- Crawford MA, Aylott CV, Bourdeau RW, Bokoch GM. Bacillus anthracis toxins inhibit human neutrophil NADPH oxidase activity. J lmmunol. 2006;176(12):7557–7565. doi: 10.4049/jimmunol.176.12.7557. [DOI] [PubMed] [Google Scholar]
- Dal Molin F, Tonello F, Ladant D, Zornetta I, Zamparo I, Di Benedetto G, Zaccolo M, Montecucco C. Cell entry and cAMP imaging of anthrax edema toxin. EMBO J. 2006;25(22):5405–5413. doi: 10.1038/sj.emboj.7601408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drum CL, Yan SZ, Bard J, Shen YQ, Lu D, Soelaiman S, Grabarek Z, Bohm A, Tang WJ. Structural basis for the activation of anthrax adenylyl cyclase exotoxin by calmodulin. Nature. 2002;415(6870):396–402. doi: 10.1038/415396a. [DOI] [PubMed] [Google Scholar]
- Drum CL, Yan SZ, Sarac R, Mabuchi Y, Beckingham K, Bohm A, Grabarek Z, Tang WJ. An extended conformation of calmodulin induces interactions between the structural domains of adenylyl cyclase from Bacillus anthracis to promote catalysis. J Biol Chem. 2000;275(46):36334–36340. doi: 10.1074/jbc.M004778200. [DOI] [PubMed] [Google Scholar]
- Firoved AM, Miller GF, Moayeri M, Kakkar R, Shen Y, Wiggins JF, McNally EM, Tang WJ, Leppla SH. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am J Pathol. 2005;167(5):1309–1320. doi: 10.1016/S0002-9440(10)61218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firoved AM, Moayeri M, Wiggins JF, Shen Y, Tang WJ, Leppla SH. Anthrax edema toxin sensitizes DBA/2J mice to lethal toxin. Infect Immun. 2007;75(5):2120–2125. doi: 10.1128/IAI.01781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher DT, Smith NN, Kim SK, Heroux A, Robinson H, Reddy PT. Structure of the class IV adenylyl cyclase reveals a novel fold. Journal of Molecular Biology. 2006;362(1):114–122. doi: 10.1016/j.jmb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Gat O, Grosfeld H, Ariel N, Inbar I, Zaide G, Broder Y, Zvi A, Chitlaru T, Altboum Z, Stein D, Cohen S, Shafferman A. Search for Bacillus anthracis potential vaccine candidates by a functional genomic-serologic screen. Infect Immun. 2006;74(7):3987–4001. doi: 10.1128/IAI.00174-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, van der Goot FG. Mechanisms of pathogen entry through the endosomal compartments. Nat Rev Mol Cell Biol. 2006;7(7):495–504. doi: 10.1038/nrm1959. [DOI] [PubMed] [Google Scholar]
- Guermonprez P, Khelef N, Blouin E, Rieu P, Ricciardi-Castagnoli P, Guiso N, Ladant D, Leclerc C. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18) J Exp Med. 2001;193(9):1035–1044. doi: 10.1084/jem.193.9.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi-Rontani C, Weber-Levy M, Mock M, Cabiaux V. Translocation of Bacillus anthracis lethal and oedema factors across endosome membranes. Cellular Microbiology. 2000;2(3):259–264. doi: 10.1046/j.1462-5822.2000.00057.x. [DOI] [PubMed] [Google Scholar]
- Guo Q, Jureller JE, Warren JT, Solomaha E, Florian J, Tang WJ. Protein-protein docking and analysis reveal that two homologous bacterial adenylyl cyclase toxins interact with calmodulin differently. J Biol Chem. 2008;283(35):23836–23845. doi: 10.1074/jbc.M802168200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Shen Y, Lee YS, Gibbs CS, Mrksich M, Tang WJ. Structural basis for the interaction of Bordetella pertussis adenylyl cyclase toxin with calmodulin. EMBO J. 2005;24(18):3190–3201. doi: 10.1038/sj.emboj.7600800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeflich KP, Ikura M. Calmodulin in action: diversity in target recognition and activation mechanisms. Cell. 2002;108(6):739–742. doi: 10.1016/s0092-8674(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Hong J, Doebele RC, Lingen MW, Quilliam LA, Tang WJ, Rosner MR. Anthrax edema toxin inhibits endothelial cell chemotaxis via Epac and Rap1. J Biol Chem. 2007;282(27):19781–19787. doi: 10.1074/jbc.M700128200. [DOI] [PubMed] [Google Scholar]
- Hoover DL, Friedlander AM, Rogers LC, Yoon IK, Warren RL, Cross AS. Anthrax edema toxin differentially regulates lipopolysaccharide-induced monocyte production of tumor necrosis factor α and interleukin-6 by increasing intracellular cyclic AMP. Infect Immun. 1994;62(10):4432–4439. doi: 10.1128/iai.62.10.4432-4439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, Steegborn C. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol. 2006;362(4):623–639. doi: 10.1016/j.jmb.2006.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Wilcox-Adelman S, Sano Y, Tang WJ, Collier RJ, Park JM. Antiinflammatory cAMP signaling and cell migration genes co-opted by the anthrax bacillus. Proc Natl Acad Sci USA. 2008;105(16):6150–6155. doi: 10.1073/pnas.0800105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles JR. Enzyme catalysis: not different, just better. Nature. 1991;350(6314):121–124. doi: 10.1038/350121a0. [DOI] [PubMed] [Google Scholar]
- Kuo SR, Willingham MC, Bour SH, Andreas EA, Park SK, Jackson C, Duesbery NS, Leppla SH, Tang WJ, Frankel AE. Anthrax toxin-induced shock in rats is associated with pulmonary edema and hemorrhage. Microb Pathog. 2008;44(6):467–472. doi: 10.1016/j.micpath.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Ladant D, Ullmann A. Bordetella pertussis adenylate cyclase: a toxin with multiple talents. Trends Microbiol. 1999;7(4):172–176. doi: 10.1016/s0966-842x(99)01468-7. [DOI] [PubMed] [Google Scholar]
- Lee YS, Bergson P, He WS, Mrksich M, Tang WJ. Discovery of a small molecule that inhibits the interaction of anthrax edema factor with its cellular activator, calmodulin. Chem Biol. 2004;11(8):1139–1146. doi: 10.1016/j.chembiol.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Leppla SH. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci USA. 1982;79(10):3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla SH. Bacillus anthracis calmodulin-dependent adenylate cyclase: chemical and enzymatic properties and interactions with eucaryotic cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:189–198. [PubMed] [Google Scholar]
- Linder JU. Class III adenylyl cyclases: molecular mechanisms of catalysis and regulation. Cell Mol Life Sci. 2006;63(15):1736–1751. doi: 10.1007/s00018-006-6072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Arocho FJ, Fulcher JA, Lee B, Bradley KA. Anthrax oedema toxin induces anthrax toxin receptor expression in monocyte-derived cells. Mol Microbial. 2006;61(2):324–337. doi: 10.1111/j.1365-2958.2006.05232.x. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Nakatsu T, Kato H, Taniguchi H. Crystal structure of a myristoylated CAP-23/NAP-22 N-terminal domain complexed with Ca2+/calmodulin. EMBO J. 2004;23:712–718. doi: 10.1038/sj.emboj.7600093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo S, Foreman-Wykert AK, Cotter PA, Miller JF. Mechanisms of Bordetella pathogenesis. Frontiers in Bioscience. 2001;6:e168–e186. doi: 10.2741/mattoo. [DOI] [PubMed] [Google Scholar]
- Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol. 2004;554(Pt 2):255–261. doi: 10.1113/jphysiol.2003.049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayeri M, Haines D, Young HA, Leppla SH. Bacillus anthracis lethal toxin induces TNF-alpha-independent hypoxia-mediated toxicity in mice. J Clin Invest. 2003;112(5):670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock M, Labruyere E, Glaser P, Danchin A, Ullmann A. Cloning and expression of the calmodulin-sensitive Bacillus anthracis adenylate cyclase in Escherichia coli. Gene. 1988;64(2):277–284. doi: 10.1016/0378-1119(88)90342-3. [DOI] [PubMed] [Google Scholar]
- O'Brien J, Friedlander A, Dreier T, Ezzell J, Leppla SH. Effects of anthrax toxin components on human neutrophils. Infect Immun. 1985;47(1):306–310. doi: 10.1128/iai.47.1.306-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paccani SR, Tonello F, Ghittoni R, Natale M, Muraro L, d'elios MM, Tang W-J, Montecucco C, Baldari CT. Anthrax toxins suppress T-lymphocyte activation by disrupting antigen receptor signaling. J. Exp. Med. 2005;201:325–331. doi: 10.1084/jem.20041557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezard C, Berche P, Mock M. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect lmmun. 1991;59(10):3472–3477. doi: 10.1128/iai.59.10.3472-3477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhar A, Dal Molin F, Horvath S, Ladants D, Montecucco C. Anthrax edema toxin modulates PKA- and CREB-dependent signaling in two phases. PLoS ONE. 2008;3(10):e3564. doi: 10.1371/journal.pone.0003564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel A, Meller R, Hanski E. Adenylate cyclase toxin from Bordetella pertussis. The relationship between induction of cAMP and hemolysis. J Biol Chem. 1991;266(5):3154–3161. [PubMed] [Google Scholar]
- Rossi Paccani S, Tonello F, Patrussi L, Capitani N, Simonato M, Montecucco C, Baldari CT. Anthrax toxins inhibit immune cell chemotaxis by perturbing chemokine receptor signalling. Cell Microbiol. 2007;9(4):924–929. doi: 10.1111/j.1462-5822.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- Shen Y, Lee YS, Soelaiman S, Bergson P, Lu D, Chen A, Beckingham K, Grabarek Z, Mrksich M, Tang WJ. Physiological calcium concentrations regulate calmodulin binding and catalysis of adenylyl cyclase exotoxins. EMBO J. 2002;21(24):6721–6732. doi: 10.1093/emboj/cdf681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Zhukovskaya NL, Guo Q, Florian J, Tang WJ. Calcium-independent calmodulin binding and two-metal-ion catalytic mechanism of anthrax edema factor. EMBO J. 2005;24(5):929–941. doi: 10.1038/sj.emboj.7600574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Zhukovskaya NL, Zimmer MI, Soelaiman S, Bergson P, Wang CR, Gibbs CS, Tang WJ. Selective inhibition of anthrax edema factor by adefovir, a drug for chronic hepatitis B virus infection. Proc Natl Acad Sci USA. 2004;101(9):3242–3247. doi: 10.1073/pnas.0306552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H, Keppie J. Observations on experimental anthrax; demonstration of a specific lethal factor produced in vivo by Bacillus anthracis. Nature. 1954;173(4410):869–870. doi: 10.1038/173869a0. [DOI] [PubMed] [Google Scholar]
- Soelaiman S, Wei BQ, Bergson P, Lee YS, Shen Y, Mrksich M, Shoichet BK, Tang WJ. Structure-based inhibitor discovery against adenylyl cyclase toxins from pathogenic bacteria that cause anthrax and whooping cough. J Biol Chem. 2003;278(28):25990–25997. doi: 10.1074/jbc.M301232200. [DOI] [PubMed] [Google Scholar]
- Stanley JL, Sargeant K, Smith H. Purification of factors I and II of the anthrax toxin produced in vivo. J Gen Microbiol. 1960;22:206–218. doi: 10.1099/00221287-22-1-206. [DOI] [PubMed] [Google Scholar]
- Steegborn C, Litvin TN, Levin LR, Buck J, Wu H. Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat Struct Mol Biol. 2005;12(1):32–37. doi: 10.1038/nsmb880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz TA. A mechanism for all polymerases. Nature. 1998;391(6664):231–232. doi: 10.1038/34542. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv. 2002;2(3):168–184. doi: 10.1124/mi.2.3.168. [DOI] [PubMed] [Google Scholar]
- Szarowicz SE, During RL, Li W, Quinn CP, Tang WJ, Southwick FS. Bacillus anthracis Edema Toxin Impairs Neutrophil Actin-based Motility. Infect Immun. 2009 doi: 10.1128/IAI.00839-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WJ, Hurley JH. Catalytic mechanism and regulation of mammalian adenylyl cyclases. Mol Pharmacol. 1998;54(2):231–240. doi: 10.1124/mol.54.2.231. [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTPgammaS. Science. 1997;278(5345):1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Sunahara RK, Johnson RA, Gosselin G, Gilman AG, Sprang SR. Two-metal-Ion catalysis in adenylyl cyclase. Science. 1999;285(5428):756–760. doi: 10.1126/science.285.5428.756. [DOI] [PubMed] [Google Scholar]
- Tessier J, Green C, Padgett D, Zhao W, Schwartz L, Hughes M, Hewlett E. Contributions of histamine, prostanoids, and neurokinins to edema elicited by edema toxin from Bacillus anthracis. Infect Immun. 2007;75(4):1895–1903. doi: 10.1128/IAI.01632-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tews I, Findeisen F, Sinning I, Schultz A, Schultz JE, Linder JU. The structure of a pH-sensing mycobacterial adenylyl cyclase holoenzyme. Science. 2005;308(5724):1020–1023. doi: 10.1126/science.1107642. [DOI] [PubMed] [Google Scholar]
- Tournier JN, Quesnel-Hellmann A, Mathieu J, Montecucco C, Tang WJ, Mock M, Vidal DR, Goossens PL. Anthrax edema toxin cooperates with lethal toxin to impair cytokine secretion during infection of dendritic cells. J lmmunol. 2005;174(8):4934–4941. doi: 10.4049/jimmunol.174.8.4934. [DOI] [PubMed] [Google Scholar]
- Ulmer TS, Soelaiman S, Li S, Klee CB, Tang WJ, Bax A. Calcium dependence of the interaction between calmodulin and anthrax edema factor. J Biol Chem. 2003;278(31):29261–29266. doi: 10.1074/jbc.M302837200. [DOI] [PubMed] [Google Scholar]
- Watson LE, Kuo SR, Katki K, Dang T, Park SK, Dostal DE, Tang WJ, Leppla SH, Frankel AE. Anthrax toxins induce shock in rats by depressed cardiac ventricular function. PLoS ONE. 2007;2(5):e466. doi: 10.1371/journal.pone.0000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson LE, Mock J, Lal H, Lu G, Bourdeau RW, Tang WJ, Leppla SH, Dostal DE, Frankel AE. Lethal and edema toxins of anthrax induce distinct hemodynamic dysfunction. Front Biosci. 2007;12:4670–4675. doi: 10.2741/2416. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87(3):965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- Wolff J, Cook GH, Goldhammer AR, Berkowitz SA. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci USA. 1980;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. USA. 1998;95(23):13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi E, Nakatsu T, Matsubara M, Kato H, Taniguchi H. Crystal structure of a MARCKS peptide containing the calmodulin-binding domain in complex with Ca2+-calmodulin. Nat Struct. Biol. 2003;10:226–231. doi: 10.1038/nsb900. [DOI] [PubMed] [Google Scholar]
- Yap KL, Yuan T, Mal TK, Vogel HJ, Ikura M. Structural basis for simultaneous binding of two carboxy-terminal peptides of plant glutamate decarboxylase to calmodulin. J. Mol. Biol. 2003;328:193–204. doi: 10.1016/s0022-2836(03)00271-7. [DOI] [PubMed] [Google Scholar]
- Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Anne Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]