Abstract
N′-nitrosonornicotine (NNN) is a strong carcinogen present in unburned tobacco and cigarette smoke. We here analyze data obtained in two studies, in which a biomarker of exposure to NNN – the sum of NNN and its pyridine-N-glucuronide, referred to as total NNN – was quantified in the urine of people who had stopped smoking and used various nicotine replacement therapy (NRT) products. In 13 out of 34 nicotine gum or lozenge users from both studies, total NNN at one or more time points after biochemically confirmed smoking cessation was comparable to, or considerably higher than, the baseline levels. For most of the subjects who used the nicotine patch as a smoking cessation aid, urinary total NNN at all post-quit time points was less than 37% of their mean baseline levels. These results indicate that endogenous formation of significant amounts of NNN may occur sporadically in some users of oral NRT. Given the carcinogenicity of NNN and the frequent use of nicotine gum as a smoking cessation aid, further studies are needed so that preventive measures can be developed.
Introduction
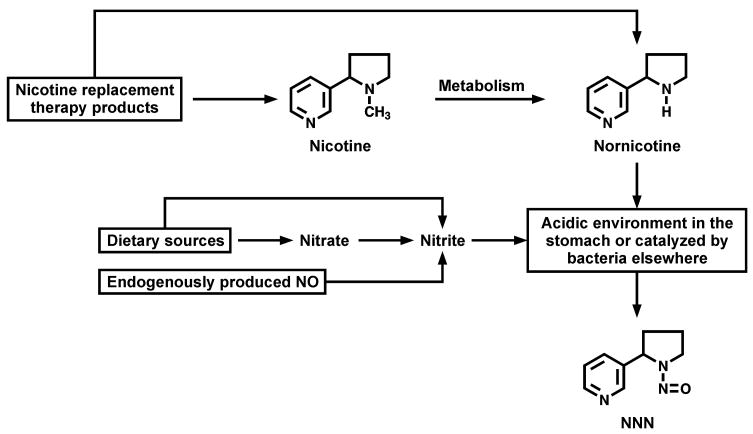
Nicotine replacement therapy (NRT) products were developed to assist smokers in quitting, and are virtually free of toxicants and carcinogens that are abundant in tobacco and cigarette smoke (1). However, there is a concern about possible endogenous nitrosation of nicotine, directly or via its metabolite nornicotine(2), to form N′-nitrosonornicotine (NNN) – a human carcinogen (3) that is believed to be important in the induction by tobacco products of cancers of the esophagus and oral cavity (4). Since endogenous formation of N-nitrosamines commonly occurs in humans via the reaction of dietary precursors with nitrosating agents (5), it is biologically plausible that endogenous formation of NNN can occur in users of oral NRT, either in the acidic stomach (5), or via bacteria-mediated nitrosation of the nicotine metabolite nornicotine elsewhere in the body (6) (Figure 1).
Figure 1.
Hypothesized pathways of endogenous NNN formation in oral NRT users a
We analyzed a biomarker of exposure to NNN – the sum of NNN and its pyridine-N-glucuronide, referred to as total NNN (7) – in the urine of people who had stopped smoking and used nicotine patch, nicotine gum, or nicotine lozenge. The sum of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its N- and O-glucuronides, referred to as total NNAL, was also analyzed, as this is the commonly measured urinary metabolite of the related nicotine-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (8).
Materials and Methods
Subjects and study design
Urine of subjects recruited for two separate studies was analyzed. In the first study, referred to as the Persistence of Biomarkers (POB) study (9), cigarette smokers continued smoking as usual over a two week period during which two baseline 24h urine samples were collected. After this period, subjects quit smoking and used either nicotine patch or nicotine gum or lozenge as a smoking cessation aid. On days 3, 7, 14, 21, 28, 42, and 56 after quitting, 24h urine samples were collected and analyzed for total NNN and total NNAL.
The second study is referred to as the Quit Nicotine (QuitNic) study (10). In this study, two baseline measurements were made during a two week period while the recruited subjects smoked ad libitum. After this period, in one of the three treatment conditions, subjects stopped smoking and used 4 mg nicotine lozenges for six weeks. Urine samples were collected at the end of weeks 2 and 6 of treatment, and total NNN and total NNAL were analyzed.
In both studies, the subjects were provided with the chosen or assigned cessation aid. Both studies were approved by the University of Minnesota Research Subjects’ Protection Programs Institutional Review Board: Human Subjects Committee.
Urine analyses
Total NNN was assayed essentially as previously described (11), except that β-glucuronidase was used to convert NNN-N-glucuronide to NNN (7), and liquid chromatography-electrospray ionization-tandem mass spectrometry was used for analysis. Urine samples with high total NNN after the quit date were re-analyzed by gas chromatography equipped with a thermal energy analyzer, to verify the identity and the amount of NNN (7). Total NNAL was analyzed as previously described (8). Negative controls (water blanks) were analyzed with each set of urine samples. Anatabine was analyzed as described elsewhere (12). Nitrate and nitrite content was assayed by ion chromatography (13). Creatinine was determined by using Vitros CREA slides.
Carbon monoxide
This was analyzed using the Bedfont Micro Smokerlyzer® (Bedfont Scientific Limited, Kent, UK). CO level < 6 was used to confirm abstinence.
Statistical analysis
For the POB study, both total NNN and total NNAL were analyzed on the natural log scale. The paired t-test compared the initial change from baseline to day 3 and the repeated measures analysis of variance evaluated the rate of change from day 3 to day 56. The percent of baseline was calculated for each time point and compared between total NNN and total NNAL using the paired t-test. Due to a high degree of variability in the QuitNic study, we used the Wilcoxon signed rank test to compare baseline to 2 and 6 weeks for total NNN and total NNAL. A p-value < 0.05 was considered statistically significant.
Results
Total NNN and total NNAL values in individual samples from both studies, as well as anatabine and nitrate and nitrite levels in selected samples from the POB study, are listed in Tables S1-S3 of Supporting Information.
POB study
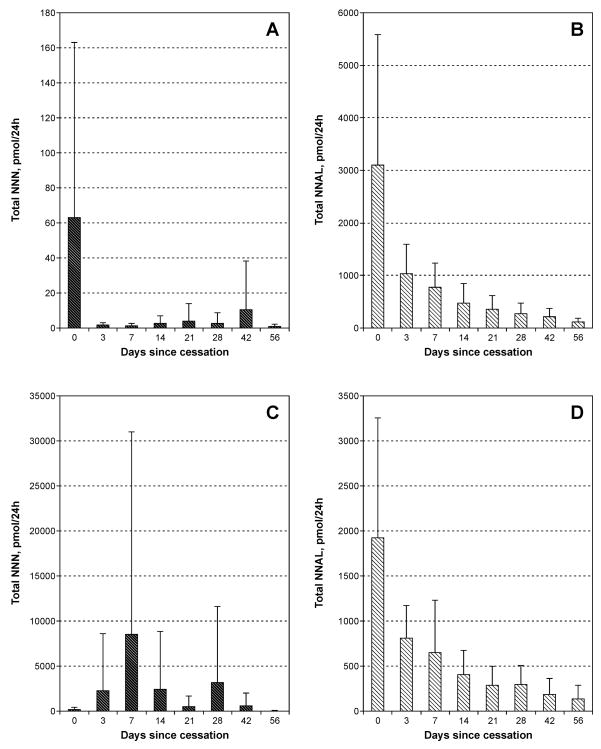
The results of total NNN and total NNAL analyses in 9 nicotine patch users and 8 oral NRT users are summarized in Figure 2.
Figure 2.
Total NNN and total NNAL in 9 nicotine patch users and 8 oral NRT users from the POB study: A, total NNN in nicotine patch users; B, total NNAL in nicotine patch users; C, total NNN in oral NRT users; D, total NNAL in oral NRT users. Each baseline value represents mean of two analyses. Bars, standard deviations.
In the nine nicotine patch users, after 3 days of abstinence from smoking, total NNN decreased from 63.1 (±99.8) pmol/24h (baseline level) to 1.69 (±1.56) pmol/24h (p=0.001, Figure 2A). These values for eight oral NRT users were 147 (±236) pmol/24h and 2280 (±6290) pmol/24h, respectively (p=0.759, Figure 2C). In nicotine patch users, the overall slight decrease in average urinary total NNN from day 3 to day 56 was not significant (p=0.159). Among oral NRT users, there was a large variation in urinary total NNN levels from day 3 to day 56; at one or more time points during this period, 6 out of 8 subjects had levels of NNN in their urine similar to, or higher than, their baseline levels (Figure 2C). For both nicotine patch and oral NRT users, the average decrease for total NNAL over this period was highly significant (p<0.001) (Figures 2B and 2D).
QuitNic study
The results of total NNN and total NNAL analyses in the urine of 26 QuitNic study subjects are summarized in Table 1. In 7 subjects, urinary total NNN at one or more time points following smoking cessation was similar to, or higher than, their mean baseline levels. The decrease in total NNN from baseline to week 2 and from baseline to week 6 in all QuitNic study subjects was not statistically significant (p=0.938 and p=0.844, respectively), but it was significant for total NNAL (p=0.016 and p=0.031, respectively).
Table 1.
Total NNN and total NNAL in the urine of QuitNic study participants
| Subjects | No | Pmol/mg creatinine | |||||
|---|---|---|---|---|---|---|---|
| Total NNN | Total NNAL | ||||||
| study week | study week | ||||||
| Baselinea | 2 | 6 | Baseline | 2 | 6 | ||
| All | 26 | 0.140 (±0.194) | 0.080 (±0.142) | 0.084 (±0.210) | 1.20 (±0.688) | 0.264 (±0.166) | 0.154 (±0.112) |
| Subjects with decreasing total NNN | 19 | 0.082 (±0.066) | 0.022 (±0.027) | 0.021 (±0.028) | 1.29 (±0.763) | 0.294 (±0.178) | 0.163 (±0.124) |
| Subjects with elevated total NNN b | 7 | 0.239 (±0.335) | 0.232 (±0.210) | 0.275 (±0.373) | 0.81 (±0.433) | 0.153 (±0.096) | 0.109 (±0.065) |
Two baseline urine samples were collected and analyzed; each value represents mean of the two analyses
Levels at week 2 or week 6 were similar to or higher than baseline levels.
Analysis of anatabine, nitrate and nitrite
Urine of those POB study subjects, who after smoking cessation had elevated urinary total NNN, was analyzed for anatabine, nitrate and nitrite (Supporting Information, Table S3). The levels of anatabine were either extremely low or non-detectable in samples collected after smoking cessation, and there was no overall correlation between urinary nitrite and total NNN in all samples.
Tests for artefactual NNN formation in urine
A set of ten 3-ml urine samples was selected to include both high- and low-total NNN samples from 4 different subjects. Neither addition of 500 ng nornicotine prior to overnight hydrolysis with β-glucuronidase, nor incubation of urine with 500 ng nornicotine for 24 hours at room temperature and subsequent hydrolysis with NaOH, had a significant effect on the measured total NNN in these samples.
Discussion
We report occasional significant increases in urinary biomarkers of the carcinogen NNN in some users of nicotine gum or lozenge, as compared to baseline smoking levels in the same subjects. We made these observations in the course of analyzing data from two separate studies designed to monitor changes in urinary biomarkers of a number of tobacco carcinogens in people who stopped smoking. Our findings suggest that significant amounts of NNN are formed occasionally in some users of oral NRT products, most likely via endogenous nitrosation of nornicotine that is metabolically formed from nicotine or originally present in NRT products. Given the carcinogenicity of NNN, this presents a possible cancer risk in long-term users.
In 13 out of 34 nicotine gum or lozenge users from both studies, total NNN at one or more time points after smoking cessation was comparable to, or considerably higher than, the baseline levels. Significant decreases in urinary total NNAL, exhaled CO, and urinary anatabine in these subjects confirmed their abstinence from tobacco products. NNN intake from NRT products could potentially contribute to the increase in urinary total NNN in our NRT users. Since these studies were not designed to specifically investigate the possible endogenous formation of NNN in users of oral NRT products, we did not analyze NNN in the nicotine gum or nicotine lozenges that were given to our subjects. However, our previous study demonstrated that NNN is virtually absent in this category of NRT products (1). Another potential contributor to the measured high total NNN levels could be artefactual NNN formation in the urine after its collection or during sample preparation, via nitrosation of nornicotine present in the urine. The lack of increase in total NNN in urine samples incubated with an excess amount of nornicotine, as compared to non-treated aliquots from the same urine sample, does not support this hypothesis. Moreover, nitrate and nitrite levels measured in selected urine samples (Table S3 of the Supporting Information) did not correlate with total NNN levels in the same samples. Given the design of the studies, we were not able to test urine samples for the presence of bacteria. However, it is unlikely that bacteria-mediated artefactual NNN formation occurred exclusively in the urine of oral NRT users: for most of the subjects who chose to use nicotine patch as a smoking cessation aid, urinary total NNN at all post-quit time points was less than 37% of their mean baseline levels (Figure 2 and Table S1).
Only one patch user demonstrated a sudden large increase in urinary total NNN 17.9 pmol/24h at day 28 of nicotine patch use, compared to 4.3 pmol/24h at baseline (subject P7, Table S1). This increase coincided with an increase in urinary nitrate, suggesting an overall increase in nitrosation potential at this time point. Subjects O2 and O6 (Table S1) stood out among oral NRT users. In subject O2, after 7 days of smoking cessation and oral NRT use, urinary total NNN was 700 times higher than baseline. This increase was not accompanied by an increase in either urinary nitrate or nitrite. Subject O6, who at several time-points after smoking cessation had ~ 30 times higher urinary total NNN than at baseline, also had elevated urinary total NNAL at the same time points, while anatabine was not detected. This is the first indication that NNK also can be formed endogenously in humans. The sporadic nature of high total NNN concentrations observed here most likely results from the multiple factors which influence endogenous nitrosation including different dietary catalysts and inhibitors of nitrosation, timing of their consumption, and infections. There are also indications that the extent of endogenous nitrosation in humans might be dependent on variations in the atmospheric concentrations of NO2 (14).
An interesting observation is that the QuitNic study participants with a sharp decline in urinary total NNN after smoking cessation also had lower average baseline total NNN levels when compared to the subjects whose urinary total NNN levels during nicotine lozenge use indicate endogenous nitrosation. These results suggest that some smokers, in addition to their exposure to NNN from cigarette smoke, probably form NNN endogenously, depending on host factors and/or dietary habits.
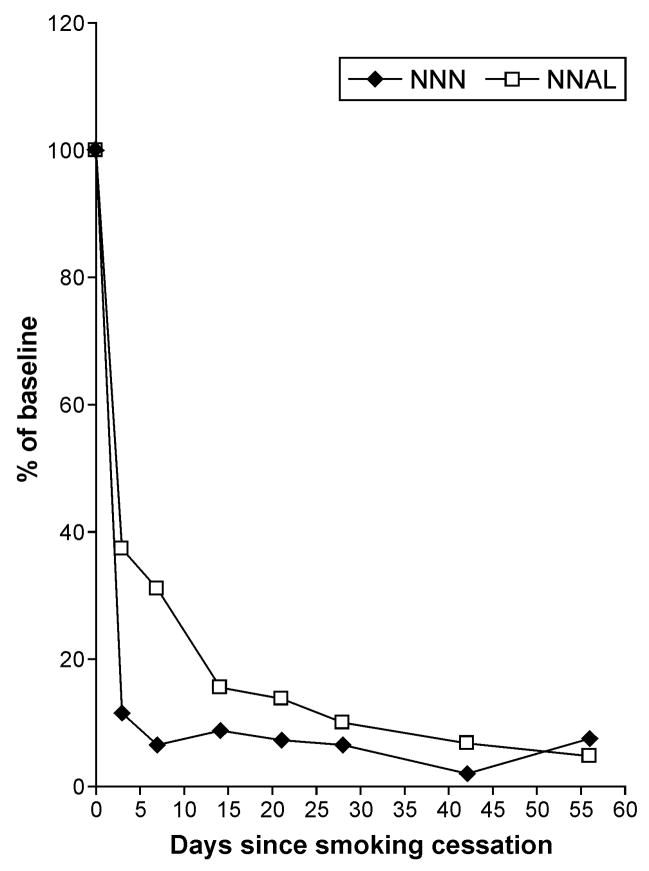
In subjects who did not have increases in urinary total NNN after smoking cessation, the levels of this biomarker dropped to 11% of the baseline value 3 days after quitting (p=0.015) (Figure 3). It took an average of 4 weeks for total NNAL to decrease to the same 11% of the baseline value. These results support the idea of NNAL retention in the body, followed by slow release and reconversion to NNK, which, in turn, is again metabolized to NNAL. The decrease in urinary NNAL upon NRT use observed for most of the subjects in this study is consistent with previous studies (8,15).
Figure 3.
Total NNN and total NNAL as % of mean baseline levels in eight POB subjects (7 used nicotine patch and 1 used oral NRT) who did not have increased urinary NNN excretion during NRT use.
Major limitations of this investigation include the fact that neither of the two studies was designed to investigate endogenous formation of NNN in NRT users, and the lack of information on NNN in the NRT products. We also lacked of control group in which subjects did not use any NRT product after smoking cessation.
Despite these limitations, the presence of significant amounts of NNN in the urine of some oral NRT users is an alarming sign, especially in view of the reported increased use of NRT products and their over-the-counter availability (16,17). In attempts to quit smoking, nicotine gum is one of the most frequently used NRT products (18), and some former smokers use these products for prolonged periods of time (19). These people, if susceptible to endogenous formation of NNN, can be continuously exposed to relatively high levels of this strong carcinogen and may eventually develop cancer.
In summary, we observed that significant amounts of NNN are excreted occasionally in some users of oral NRT, endogenous formation of this carcinogen being the most likely source. This presents a possible risk of cancer in long term users. Additional studies are urgently needed to understand the factors affecting endogenous NNN formation, and to develop preventive measures. The feasibility of preventing endogenous NNN formation in oral NRT users is supported by the sporadic nature of the increases in urinary total NNN, the significant reduction in urinary total NNN in some oral NRT users after smoking cessation, and the overall knowledge of the major factors affecting endogenous nitrosation in humans.
Supplementary Material
Acknowledgments
We thank Michael Lofgren, John Muzic, Aleksandar Knezevich, and Shaomei Han for technical assistance, and Bob Carlson for editorial assistance. Grant support: CA-81301 from The National Cancer Institute and DA-13333 from The National Institutes of Health.
Reference list
- 1.Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine Tob Res. 2006;8:309–13. doi: 10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- 2.Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 89. Lyon, FR: IARC; 2007. Smokeless tobacco and tobacco-specific nitrosamines; pp. 457–553. [PMC free article] [PubMed] [Google Scholar]
- 4.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 5.Mirvish SS. Formation of N-nitroso compounds: chemistry, kinetics, and in vivo occurrence. Toxicol Appl Pharmacol. 1975;31:325–51. doi: 10.1016/0041-008x(75)90255-0. [DOI] [PubMed] [Google Scholar]
- 6.Jiebarth D, Spiegelhalder B, Bartsch H. N-nitrosation of medicinal drugs catalysed by bacteria from human saliva and gastro-intestinal tract, including Helicobacter pylori. Carcinogenesis. 1997;18:383–9. doi: 10.1093/carcin/18.2.383. [DOI] [PubMed] [Google Scholar]
- 7.Stepanov I, Hecht SS. Tobacco-specific nitrosamines and their N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiol Biomarkers Prev. 2005;14:885–91. doi: 10.1158/1055-9965.EPI-04-0753. [DOI] [PubMed] [Google Scholar]
- 8.Hecht SS, Carmella SG, Chen M, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–6. [PubMed] [Google Scholar]
- 9.Carmella SG, Chen M, Han S, et al. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22:734–41. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2009 doi: 10.1111/j.1360-0443.2009.02780.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porubin D, Hecht SS, Li ZZ, Gonta M, Stepanov I. Endogenous formation of N′-nitrosonornicotine in F344 rats in the presence of some antioxidants and grape seed extract. J Agric Food Chem. 2007;55:7199–204. doi: 10.1021/jf0712191. [DOI] [PubMed] [Google Scholar]
- 12.Jacob P, III, Yu L, Liang G, Shulgin AT, Benowitz NL. Gas chromatographic mass spectrometric method for determination of anabasine, anatabine and other tobacco alkaloids in urine of smokers and smokeless tobacco users. J Chromatogr B Biomed Appl. 1993;619:49–61. doi: 10.1016/0378-4347(93)80445-a. [DOI] [PubMed] [Google Scholar]
- 13.Stepanov I, Hecht SS, Mirvish SS, Gonta M. Comparative analysis of tobacco-specific nitrosamines and total N-nitroso compounds in Moldovan cigarette tobacco. J Agric Food Chem. 2005;53:8082–6. doi: 10.1021/jf050747e. [DOI] [PubMed] [Google Scholar]
- 14.Garland WA, Kuenzig W, Rubio F, Kornychuk H, Norkus EP, Conney AH. Urinary excretion of nitrosodimethylamine and nitrosoproline in humans: interindividual and intraindividual differences and the effect of administered ascorbic acid and α-tocopherol. Cancer Res. 1986;46:5392–400. [PubMed] [Google Scholar]
- 15.Stepanov I, Carmella SG, Han S, et al. Evidence for endogenous formation of N′-nitrosonornicotine in some long term nicotine patch users. Nicotine Tob Res. 2009;11:95–105. doi: 10.1093/ntr/ntn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton SL, Gitchell JG, Shiffmann S. Use of FDA-approved pharmacologic treatments for tobacco dependence: United States, 1984–1998. MMWR Morb Mortal Wkly Rep. 2000;49:665–8. [PubMed] [Google Scholar]
- 17.Hyland A, Rezaishiraz H, Giovino G, Bauer JE, Cummings KM. Over-the-counter availability of nicotine replacement therapy and smoking cessation. Nicotine Tob Res. 2005;7:1–9. doi: 10.1080/14622200500185975. [DOI] [PubMed] [Google Scholar]
- 18.Bansal MA, Cummings KM, Hyland A, Giovino GA. Stop-smoking medications: Who uses them, who misuses them, and who is misinformed about them? Nicotine Tob Res. 2004;6:S303–10. doi: 10.1080/14622200412331320707. [DOI] [PubMed] [Google Scholar]
- 19.Hughes JR, Gust SW, Keenan R, Fenwick JW, Skog K, Higgins ST. Long-term use of nicotine vs. placebo gum. Arch Int Med. 1991:1993–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.