Abstract
Biventricular pacing (BiVP) can optimize cardiac output (CO) in patients after cardiac surgery, so devices that calculate continuous CO from arterial pressure may be a useful tool. We investigated PulseCO for measuring CO during optimization by comparison with aortic flow probe measurement. Seven patients in the Biventricular Pacing After Cardiac Surgery (BiPACS) trial were studied. Before weaning from cardiopulmonary bypass, BiVP was initiated. After bypass, CO was optimized by varying atrioventricular pacing delay, ventricular site, and interventricular pacing delay with a randomized protocol. Continuous CO was measured by PulseCO and aortic flow probe. Reliability was estimated by Fleiss’ method and agreement assessed by Bland-Altman analysis. Compared to flow probe, PulseCO reliably measured changes in CO (intraclass correlation coefficient=0.90), but underestimated the change (−4±17%). In contrast, changes in mean arterial pressure did not reflect changes in CO (intraclass correlation coefficient=0.02). Thus, PulseCO can measure continuous CO in open-chest patients after cardiac surgery, while underestimating changes occurring across 10 second pacemaker changes. Further studies in the closed chest are indicated.
Keywords: PulseCO, cardiac output, biventricular pacing, cardiac surgery
Introduction
Cardiac output (CO) is a clinically important measure of ventricular function, especially in cardiac surgery patients. Administration of intravenous inotropes and induction of temporary cardiac pacing, particularly biventricular pacing (BiVP), are therapies available to improve low CO states. With BiVP, CO can be maximized in real-time by varying left ventricular (LV) pacing site and atrioventricular (AVD) and interventricular (VVD) pacing delay by utilizing a continuous method of CO measurement after cardiopulmonary bypass (CPB).1–3 Aortic flow probes have the ability to provide accurate, instantaneous measurement of CO in the operative setting;4 however, such devices are invasive and must be removed prior to chest closure. Thus, less invasive devices that calculate CO from arterial pressure, such as PulseCO (LiDCO Ltd, London, UK), may be useful for optimizing CO with BiVP in the postoperative period by providing accurate beat-to-beat measurement.5–7 A recent study by our laboratory analyzed the accuracy and limitations of PulseCO as a means of monitoring acute changes in CO with a right heart bypass preparation in swine.8 The aim of the present study was to extend our analysis of the PulseCO system into the clinical setting by investigating its reliability and agreement compared to direct CO measurement by flow probe during optimization with BiVP immediately following cardiac surgery.
Methods and Materials
Patient Population
Seven patients (6 male, mean age 64±12 years) enrolled in the ongoing NIH-funded Biventricular Pacing After Cardiac Surgery (BiPACS) trial at Columbia-Presbyterian Medical Center were included in this study. Patients undergoing cardiac surgery using CPB with an LV ejection fraction≤40% and a QRS duration≥100 msec, or having concomitant mitral and aortic valve replacements, were enrolled with permission of the operating surgeon. Patients were excluded for intracardiac shunts, congenital heart disease, post-CPB heart rate>120 bpm, 2/3° heart block, or atrial fibrillation. All patients gave informed consent to participate in the trial, which is approved by the Columbia University Institutional Review Board and conducted under an Investigational Device Exemption from the Food and Drug Administration.
Anesthetic Regimen
A balanced general endotracheal anesthetic technique was utilized. This consisted of isoflurane, fentanyl, midazolam, and vecuronium, providing a platform of greater cardiovascular stability by causing less depression of cardiac contractility and systemic vascular resistance.9 Isoflurane was administered at 0.5–0.6 minimal alveolar concentration (1.0 MAC is defined as the concentration ofanesthetic at 1 atm that produces immobilityin 50% of subjects exposed to a surgical stimulus, usually askin incision10). Fentanyl and benzodiazepines were added in varying doses at the discretion of the anesthesiologist to achieve MACBAR (defined as the brain concentration of volatile anesthetic which blocks the adrenergic response to a surgical stimulus11). This attenuated the patient’s endogenous catecholamine release and autonomic responses, allowing precise control of hemodynamics using inotropic, chronotropic, and pressor medications. Vascular reactivity was also abolished (or at least latency of reflex response was increased to the point of being negligible). MACBAR for isoflurane alone is 1.3, which is reduced to 0.55 by balancing the anesthetic with at least 1.5 μg/kg of fentanyl; although the patients studied received much larger doses of fentanyl (30–50 μg/kg), there is a ceiling effect such that the larger doses of opiate produce no further decrease in MACBAR.12
Instrumentation
Before weaning from CPB, BiVP with standard settings (heart rate=90 bpm, AVD=150 msec, VVD=0 msec) was implemented using pairs of temporary epicardial pacing wires sewn to the right atrium, right ventricle, and one of two randomly selected LV sites (LV1, LV2). Possible LV sites were anterior, lateral, and posterior. A scissor-type electromagnetic flow probe (Carolina Medical Electronics, King, NC) was appropriately fitted and placed around the ascending aorta. The PulseCO system was connected to the radial artery pressure signal and calibrated with flow probe CO measurement. Surface electrocardiogram, arterial pressure, and instantaneous aortic volume flow were transferred by an analog-to-digital converter (ADInstruments Inc, Milford, MA) to a personal computer (Apple Computer, Cupertino, CA).
Optimization of CO with BiVP
Optimization of CO with BiVP began immediately after successful weaning from CPB with hemodynamic stability. AVD was varied from 90 to 270 msec (or the maximal value that still allowed ventricular capture) in 30 msec increments. Using the AVD that maximized CO, as measured by the flow probe, ventricular pacing site was varied between RV only, BiVP with LV1, and BiVP with LV2. Using the LV site that maximized CO, VVD was varied from +80 (RV first) to −80 msec in 20 msec increments. Values of each parameter were tested in random order for 10 sec intervals, which was then repeated with a new randomization, for a total of 38 settings tested in each patient. This defined the combination of pacing parameters that produced the maximum CO. The total time to complete the protocol was approximately eight minutes. As discussed above, by utilizing a general endotracheal anesthetic technique at MACBAR, the effects of vascular reactivity to anesthetic or surgical stimulus were mostly eliminated. In addition, vasoactive medications, inotropes, and intravascular volume were maintained at constant levels during testing so that the bulk of observed changes in CO would be due to changes in pacing settings.
Data Analysis
The aortic flow and arterial pressure signals were imported into Matlab (The MathWorks Inc, Natick, MA) and processed with custom routines13. In short, beat-to-beat CO and mean arterial pressure (MAP) were calculated by integrating the signals over each cardiac cycle. The PulseCO and Matlab signals were synchronized using artifacts in MAP caused by ectopic beats during weaning from CPB. PulseCO was recalibrated to flow probe CO for the first cardiac cycle during BiVP testing. Values of PulseCO and flow probe CO and MAP for each testing interval were obtained by averaging 3 consecutive cardiac cycles at end-expiration (determined from MAP). Cardiac cycles were chosen near the end of the interval to allow time for potential changes to take effect. Values of CO and MAP were expressed as a percentage of the first measured value.
Statistical Analysis
All values are presented as mean±standard deviation (SD). The intraclass correlation coefficient of reliability for CO measured by PulseCO and MAP was estimated by comparison with flow probe CO measurement using the method described by Fleiss, generalized to account for the replicate measures within each patient.14 This provides an index of ‘reproducibility’ or ‘consistency’ between measurements (i.e., between PulseCO and flow probe CO or between MAP and flow probe CO), accounting for intra- and inter-patient differences. In addition, Bland-Altman analysis was performed by calculating the bias (mean difference=(PulseCO+Flow Probe CO)/2) and limits of agreement (±2×SD of the bias).15 This provides an index of how accurately PulseCO measures changes in CO. Paired Student’s t-tests were used to compare percentage change from lowest to highest CO measured by PulseCO and the flow probe during optimization, with p<0.05 indicating significance. Statistical analysis was performed using the SAS System software (SAS Institute Inc, Cary, NC).
Results
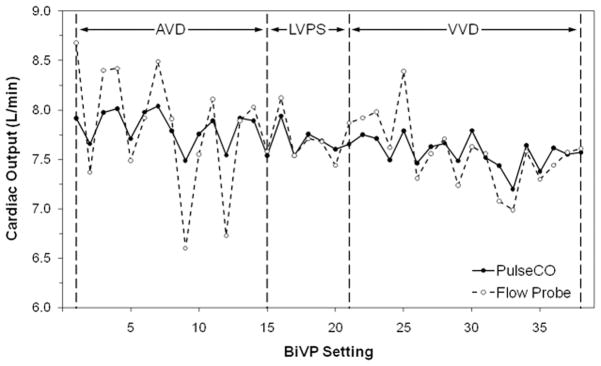
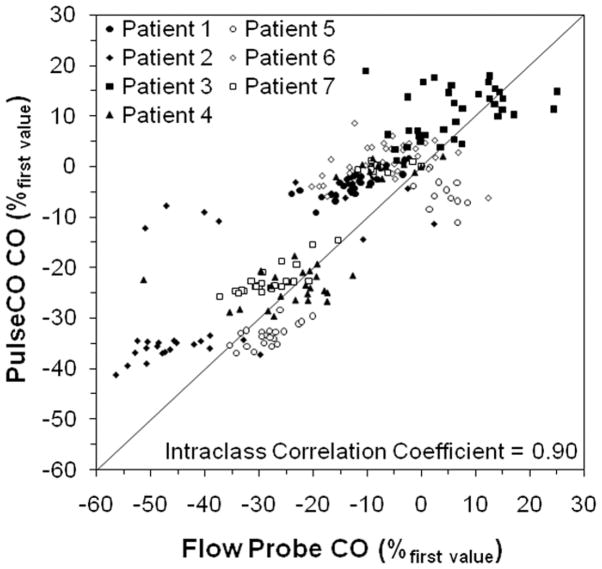
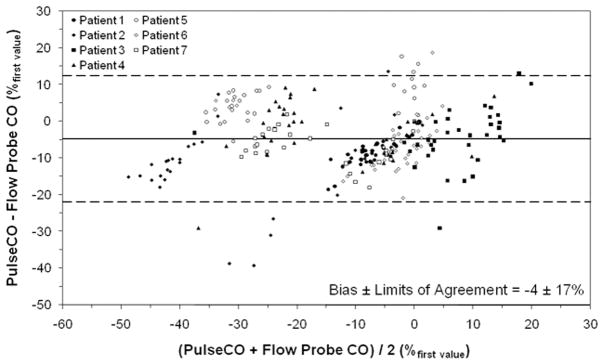
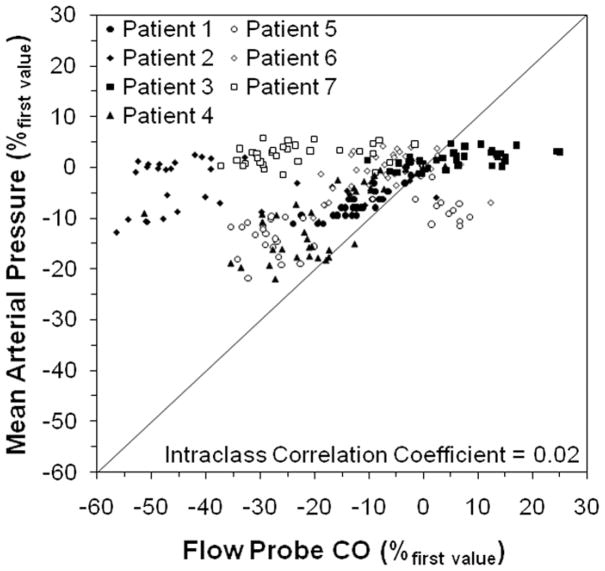
Figure 1 shows representative measurements of CO by PulseCO and the flow probe during optimization with BiVP in a single patient. Figure 2 shows PulseCO versus flow probe CO measurement during optimization with BiVP in each patient, expressed as a percentage of the first measured value. Across the patients, PulseCO showed excellent reliability compared to flow probe CO (intraclass correlation coefficient=0.90). Figure 3 shows a Bland-Altman plot of PulseCO and flow probe CO in each patient, expressed as a percentage of the first measured value. PulseCO underestimated the change in CO compared to the flow probe (bias±2×SD=−4±17%). This was also demonstrated by the percentage change from the lowest to highest CO measured during optimization with each parameter (Table 1). In contrast, as shown in Figure 4, MAP had extremely poor reliability compared to flow probe CO (intraclass correlation coefficient=0.02), although having a similar percentage change from the lowest to highest values as PulseCO (Table 1). While the reliability of CO measurement by PulseCO was good, BiVP settings determined by PulseCO to produce maximal CO agreed with those determined by the flow probe only 62% of the time (13 of 21 settings). The range of differences was ±1–3 settings (specifically, in the cases where there was disagreement: AVD differed by 1 setting in 1 patient, 2 settings in 1 patient, and 3 settings in 1 patient; LV site by 1 setting in 2 patients; VVD by 1 setting in 2 patients, and 3 settings in 1 patient), which resulted in 9±2% lower CO (as measured by flow probe) for settings identified by PulseCO compared to those identified by flow probe.
Figure 1.
Representative plot of cardiac output measured by PulseCO and the flow probe versus biventricular pacing (BiVP) setting in a patient during changes in atrioventricular pacing delay (AVD), left ventricular pacing site (LVPS), and interventricular pacing delay (VVD).
Figure 2.
Plot of cardiac output (CO) measured by PulseCO versus CO measured by the flow probe in each patient during changes in biventricular pacing settings, expressed as a percentage of the first measured value. The solid line represents the line of identity.
Figure 3.
Bland-Altman plot of cardiac output (CO) measured by PulseCO and the flow probe during changes in biventricular pacing settings, expressed as a percentage of the first measured value. Solid line indicates bias (mean difference=(PulseCO+Flow Probe CO)/2), dashed lines indicate limits of agreement (±2×standard deviation of the bias).
Table 1.
Percentage change from the lowest to highest measured cardiac output and mean arterial pressure during changes in biventricular pacing settings, averaged across the patients.
| BiVP Parameter | Flow Probe CO | PulseCO CO | MAP |
|---|---|---|---|
| AVD | 23 ± 15% | 8 ± 4%* | 9 ± 4% |
| VPS | 16 ± 9% | 5 ± 2%* | 4 ± 2% |
| VVD | 22 ± 8% | 8 ± 4%* | 7 ± 3% |
AVD, atrioventricular pacing delay; BiVP, biventricular pacing; CO, cardiac output; MAP, mean arterial pressure; VPS, ventricular pacing site; VVD, interventricular pacing delay.
Data are presented as mean ± standard deviation.
p<0.05 compared to Flow Probe CO.
Figure 4.
Plot of mean arterial pressure versus cardiac output (CO) measured by the flow probe in each patient during changes in biventricular pacing settings, expressed as a percentage of the first measured value. The solid line represents the line of identity.
Discussion
The present study demonstrates that during optimization of CO with BiVP following cardiac surgery, PulseCO successfully tracks changes in CO (indicated by strong reliability), but underestimates the magnitude of the change (indicated by the bias and difference in percentage change). As identification of settings that maximize CO is of primary importance, rather than absolute values, underestimation of changes may not preclude the use of PulseCO for optimization. However, following the current BiPACS protocol, in which pacemaker settings are changed every 10 sec, PulseCO was unsuccessful in selecting the best setting 38% of the time (despite the strong concordance seen with the flow probe). Possible reasons for this failure are examined below to help better understand the utility of PulseCO for optimization of CO with BiVP after cardiac surgery.
Literature dating back 30 years has suggested that CO could be calculated from arterial pressure.16 While arterial compliance is not constant at different pressures, the nonlinear relationship between pressure and volume is similar among different subjects.17 The PulseCO system takes advantage of this by using a nonlinear pressure-volume transformation to convert arterial pressure to stroke volume for calculating beat-to-beat CO.18 The PulseCO algorithm is limited however by its dependency on the arterial waveform, leaving it susceptible to acute hemodynamic changes that do not necessarily affect CO. The arterial waveform can be affected by changes in cardiac, aortic, and arterial compliance, secondary to changes in vasoactive drug administration, sympathetic activity, intrathoracic pressure, intravascular volume, body temperature, and position. It has been shown in patients undergoing off-pump coronary artery bypass surgery that PulseCO measurement is affected by alterations in compliance, even when MAP remains unchanged.19 In swine, PulseCO has been shown to erroneously indicate changes in CO with changes in heart rate, or following alterations in MAP secondary to vasodilation or constriction,8 presumably due to a change in wave reflection without significant changes in the mechanical properties of the aorta.20, 21 Similarly, PulseCO has been shown to overestimate CO after episodes of hemorrhage and hypotension, or after volume resuscitation in dogs.22, 23 These problems may be reduced in humans, however, as the compliance correction incorporated into the PulseCO algorithm is human-specific.
Despite its limitations, PulseCO has demonstrated strong correlation to thermodilution CO5, 6, 18, 24 and direct CO measurement by aortic flow probe7 in patients after cardiac surgery, and maintains advantages over both. PulseCO offers clinicians the dual benefit of: a) continuous CO monitoring to facilitate treatment optimization while avoiding pulmonary artery catheterization necessary for thermodilution measurement and b) the ability to extend this into the postoperative setting, which is not feasible with aortic flow probes. CO is an important measure of myocardial performance in postoperative care of cardiac surgery patients. Maintenance of appropriate CO is critical in minimizing morbidity and mortality, and early detection of low CO with the subsequent initiation of therapy has been shown to improve patient outcomes.25, 26 BiVP is one treatment shown to improve CO in post-surgical patients, and its efficacy can be maximized by varying LV pacing site, AVD, and VVD using real-time CO measurement1–3. Unfortunately, currently accepted methods of measuring CO, such as thermodilution and flow probe, are generally invasive; thus, clinicians have developed increased interest in identifying less invasive means.5, 18, 27
The observed failure of PulseCO in consistently selecting the BiVP settings that optimized CO may partly reflect that response curves of CO versus AVD and VVD are generally curvilinear, without a sharp, distinct peak.28 Frequently there are two values in series that are almost equivalent, and in many cases the setting chosen by PulseCO and the flow probe differed by only one setting. However, CO was significantly lower with settings identified by PulseCO. Thus, other factors must have contributed to the discrepancy. It has been demonstrated that after a change in CO, PulseCO takes approximately 30 seconds to stabilize (likely reflecting the need for arterial pressure stabilization), while flow probes immediately reflect changes in CO.8 As testing interval was limited to 10 sec by the time constraints of intraoperative BiVP testing, PulseCO did not have the necessary time to fully reflect changes in CO. This is evident from the representative data shown in Figure 1 and may have affected the setting which appeared optimal. This also undoubtedly accounted in part for the observed underestimation of the change in CO by PulseCO; however others have shown a similar result with longer testing intervals.7, 29 The difference in selected settings may also partly be attributed to sudden changes in vascular tone that can occur immediately after CPB, thus affecting the arterial pressure waveform and PulseCO measurement, as discussed above.8 The BiPACS protocol has been designed to account for this by dividing testing into segments for each parameter. Thus far, over individual testing segments, we have shown only a 3.1±3.4% increase in systemic vascular resistance (SVR) unrelated to changes in BiVP setting (unpublished data). These small changes may be insufficient to cause PulseCO error, as it has been shown that PulseCO measurement is unaffected by large changes in SVR.30 Still, for PulseCO to be a useful tool for optimizing CO with BiVP, patient stability should be carefully controlled. The lithium thermodilution technique (part of the LidCO system that is coupled with PulseCO), while not a method of continuous CO measurement and thus limited in its capacities for real-time optimization immediately after CPB, is not affected by changes in vascular tone, and has been used in studies varying AVD and VVD with permanent devices,31 so may be useful in overcoming difficulties of patient instability.
An alternative to CO monitoring often utilized postoperatively is measurement of MAP. Changes in MAP are often assumed to reflect changes in CO before reflex changes in SVR occur. In swine, it has been shown that a linear relationship does indeed exist between MAP and CO before vasomotor response.32 However, in the current study, MAP was a poor surrogate for CO (as indicated by poor reliability). This may not be the case at later time points, and interestingly we have found that after chest closure MAP agrees well with PulseCO during optimization and may be effective for selecting optimal settings. In patients studied in the BiPACS trial (n=6), AVD selected by PulseCO and MAP were identical in 90% of cases and VVD were identical in 60% of cases, with correlations of 0.98 and 0.89 when comparing settings selected by the two methods (unpublished data).
As mentioned above, this study was limited by the short period available to complete the BiPACS protocol after CPB, which may have contributed to underestimation of changes in CO by PulseCO, as well as its error in selecting optimal settings. It should also be noted that the changes in CO seen during optimization were not due solely to effects of changes in BiVP settings, as some change can be attributed to patient instability seen immediately after CPB. In the future, comparison with later time points, such as the unanesthetized, post-extubation intensive care period, can be used for direct comparison and control. Finally, this study comprises only a small sample (n=7), and analysis at the conclusion of the BiPACS trial (5 years) may provide further insight.
Conclusion
Immediately following cardiac surgery, measurement by PulseCO reproducibly tracks changes in CO, but underestimates these changes when a short testing interval is used. This may not preclude the use of PulseCO for optimizing CO with BiVP, where the most important consideration is the selection of parameters that optimize CO. However, although it is considerably better than using MAP, in time-limited settings where short testing intervals are necessary, such as in the operating room after cardiac surgery, PulseCO may not precisely identify BiVP parameters that optimize CO. Its utility for optimization in this setting is therefore questionable. When the use of longer testing intervals is possible and under more controlled conditions, such as after chest closure, PulseCO may be more appropriate for optimization. Thus, further studies in the closed chest are indicated.
Acknowledgments
Grantors
This study was supported by a grant from the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01-HL080152 to Dr Spotnitz).
Footnotes
Presented at the 30th Annual Meeting & Workshops of the Society of Cardiovascular Anesthesiologists, June 20, 2008
References
- 1.Berberian G, Cabreriza SE, Quinn TA, Garofalo CA, Spotnitz HM. Left ventricular pacing site-timing optimization during biventricular pacing using a multi-electrode patch. Ann Thorac Surg. 2006;82:2292–2294. doi: 10.1016/j.athoracsur.2006.04.094. [DOI] [PubMed] [Google Scholar]
- 2.Berberian G, Kanter JP, Quinn TA, Spotnitz HM. Optimized perioperative biventricular pacing in setting of right heart failure. Europace. 2005;7:385–387. doi: 10.1016/j.eupc.2005.02.119. [DOI] [PubMed] [Google Scholar]
- 3.Berberian G, Quinn TA, Kanter JP, et al. Optimized biventricular pacing in atrioventricular block after cardiac surgery. Ann Thorac Surg. 2005;80:870–875. doi: 10.1016/j.athoracsur.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 4.Dean DA, Jia CX, Cabreriza SE, et al. Validation study of a new transit time ultrasonic flow probe for continuous great vessel measurements. Asaio J. 1996;42:M671–676. doi: 10.1097/00002480-199609000-00072. [DOI] [PubMed] [Google Scholar]
- 5.Missant C, Rex S, Wouters PF. Accuracy of cardiac output measurements with pulse contour analysis (PulseCO) and Doppler echocardiography during off-pump coronary artery bypass grafting. Eur J Anaesthesiol. 2008;25:243–248. doi: 10.1017/S0265021507002979. [DOI] [PubMed] [Google Scholar]
- 6.de Wilde RB, Schreuder JJ, van den Berg PC, Jansen JR. An evaluation of cardiac output by five arterial pulse contour techniques during cardiac surgery. Anaesthesia. 2007;62:760–768. doi: 10.1111/j.1365-2044.2007.05135.x. [DOI] [PubMed] [Google Scholar]
- 7.Marquez J, McCurry K, Severyn DA, Pinsky MR. Ability of pulse power, esophageal Doppler, and arterial pulse pressure to estimate rapid changes in stroke volume in humans. Crit Care Med. 2008;36:3001–3007. doi: 10.1097/CCM.0b013e31818b31f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berberian G, Quinn TA, Vigilance DW, et al. Validation study of PulseCO system for continuous cardiac output measurement. ASAIO J. 2005;51:37–40. doi: 10.1097/01.mat.0000150329.11072.c2. [DOI] [PubMed] [Google Scholar]
- 9.Stoelting RK, Hillier SC, editors. Pharmacology and Physiology in Anesthetic Practice. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 10.Eger EI., 2nd Age, minimum alveolar anesthetic concentration, and minimum alveolar anesthetic concentration-awake. Anesth Analg. 2001;93:947–953. doi: 10.1097/00000539-200110000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Roizen MF, Horrigan RW, Frazer BM. Anesthetic doses blocking adrenergic (stress) and cardiovascular responses to incision--MAC BAR. Anesthesiology. 1981;54:390–398. doi: 10.1097/00000542-198105000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Daniel M, Weiskopf RB, Noorani M, Eger EI., 2nd Fentanyl augments the blockade of the sympathetic response to incision (MAC-BAR) produced by desflurane and isoflurane: desflurane and isoflurane MAC-BAR without and with fentanyl. Anesthesiology. 1998;88:43–49. doi: 10.1097/00000542-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Quinn TA, Berberian G, Cabreriza SE, et al. Effects of sequential biventricular pacing during acute right ventricular pressure overload. Am J Physiol Heart Circ Physiol. 2006;291:H2380–2387. doi: 10.1152/ajpheart.00446.2006. [DOI] [PubMed] [Google Scholar]
- 14.Fleiss JL, editor. The design and analysis of clinical experiments. New York: John Wiley & Sons; 1986. [Google Scholar]
- 15.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 16.Kouchoukos NT, Sheppard LC, McDonald DA. Estimation of stroke volume in the dog by a pulse contour method. Circ Res. 1970;26:611–623. doi: 10.1161/01.res.26.5.611. [DOI] [PubMed] [Google Scholar]
- 17.Remington JW, Noback CR, et al. Volume elasticity characteristics of the human aorta and prediction of the stroke volume from the pressure pulse. Am J Physiol. 1948;153:298–308. doi: 10.1152/ajplegacy.1948.153.2.298. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton TT, Huber LM, Jessen ME. PulseCO: a less-invasive method to monitor cardiac output from arterial pressure after cardiac surgery. Ann Thorac Surg. 2002;74:S1408–1412. doi: 10.1016/s0003-4975(02)04059-6. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita K, Nishiyama T, Yokoyama T, Abe H, Manabe M. Cardiac output by PulseCO is not interchangeable with thermodilution in patients undergoing OPCAB. Can J Anaesth. 2005;52:530–534. doi: 10.1007/BF03016534. [DOI] [PubMed] [Google Scholar]
- 20.Fitchett DH, Simkus GJ, Beaudry JP, Marpole DG. Reflected pressure waves in the ascending aorta: effect of glyceryl trinitrate. Cardiovasc Res. 1988;22:494–500. doi: 10.1093/cvr/22.7.494. [DOI] [PubMed] [Google Scholar]
- 21.Latson TW, Hunter WC, Katoh N, Sagawa K. Effect of nitroglycerin on aortic impedance, diameter, and pulse-wave velocity. Circ Res. 1988;62:884–890. doi: 10.1161/01.res.62.5.884. [DOI] [PubMed] [Google Scholar]
- 22.Cooper ES, Muir WW. Continuous cardiac output monitoring via arterial pressure waveform analysis following severe hemorrhagic shock in dogs. Crit Care Med. 2007;35:1724–1729. doi: 10.1097/01.CCM.0000266590.25109.F2. [DOI] [PubMed] [Google Scholar]
- 23.Dyson DH, Sinclair MD. Impact of dopamine or dobutamine infusions on cardiovascular variables after rapid blood loss and volume replacement during isoflurane-induced anesthesia in dogs. Am J Vet Res. 2006;67:1121–1130. doi: 10.2460/ajvr.67.7.1121. [DOI] [PubMed] [Google Scholar]
- 24.Zollner C, Haller M, Weis M, et al. Beat-to-beat measurement of cardiac output by intravascular pulse contour analysis: a prospective criterion standard study in patients after cardiac surgery. J Cardiothorac Vasc Anesth. 2000;14:125–129. doi: 10.1016/s1053-0770(00)90003-x. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes A, Bennett ED. Early goal-directed therapy: an evidence-based review. Crit Care Med. 2004;32:S448–450. doi: 10.1097/01.ccm.0000145945.39002.8d. [DOI] [PubMed] [Google Scholar]
- 26.Pearse R, Dawson D, Fawcett J, et al. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial. Crit Care. 2005;9:R687–693. doi: 10.1186/cc3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman GM, Ghanayem NS, Tweddell JS. Noninvasive assessment of cardiac output. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2005:12–21. doi: 10.1053/j.pcsu.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Rabkin DG, Cabreriza SE, Curtis LJ, et al. Load dependence of cardiac output in biventricular pacing: right ventricular pressure overload in pigs. J Thorac Cardiovasc Surg. 2004;127:1713–1722. doi: 10.1016/s0022-5223(03)01319-9. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita K, Nishiyama T, Yokoyama T, Abe H, Manabe M. Effects of vasodilation on cardiac output measured by PulseCO. J Clin Monit Comput. 2007;21:335–339. doi: 10.1007/s10877-007-9093-9. [DOI] [PubMed] [Google Scholar]
- 30.Pittman J, Bar-Yosef S, SumPing J, Sherwood M, Mark J. Continuous cardiac output monitoring with pulse contour analysis: a comparison with lithium indicator dilution cardiac output measurement. Crit Care Med. 2005;33:2015–2021. doi: 10.1097/01.ccm.0000179021.36805.1f. [DOI] [PubMed] [Google Scholar]
- 31.Roberts PR, Allen S, Robinson S, et al. Use of lithium dilution assessment of cardiac output to optimise right/left ventricular activation in resynchronization therapy. Heart. 2002;87:146. [Google Scholar]
- 32.Prasso JE, Berberian G, Cabreriza SE, et al. Validation of mean arterial pressure as an indicator of acute changes in cardiac output. ASAIO J. 2005;51:22–25. doi: 10.1097/01.mat.0000150506.36603.1b. [DOI] [PubMed] [Google Scholar]