Abstract
Despite rapid advances in disease gene identification, the predictive power of the genotype remains limited, in part due to poorly understood effects of second-site modifiers. Here we demonstrate that a polymorphic coding variant of RPGRIP1L (retinitis pigmentosa GTPase regulator-interacting protein-1 like), a ciliary gene mutated in Meckel-Gruber (MKS) and Joubert (JBTS) syndromes, is associated with the development of retinal degeneration in patients with ciliopathies caused by mutations in other genes. As part of our resequencing efforts of the ciliary proteome, we identified several putative loss of function RPGRIP1L mutations, including one common variant, A229T. Multiple genetic lines of evidence showed this allele to be associated with photoreceptor loss in ciliopathies. Moreover, we show that RPGRIP1L interacts biochemically with RPGR, loss of which causes retinal degeneration, and that the 229T-encoded protein significantly compromises this interaction. Our data represent an example of modification of a discrete phenotype of syndromic disease and highlight the importance of a multifaceted approach for the discovery of modifier alleles of intermediate frequency and effect.
The ciliopathies are a collection of disorders with overlapping clinical manifestations that include renal cystic disease, polydactyly, retinal degeneration and defects of the central nervous system1, and provide a useful model to investigate both the effects of variation at a single locus and the potential epistatic interactions between alleles at functionally related loci. This is because several ciliopathy-causing genes can either cause a distinct recessive form of ciliary disease or contribute modulators of penetrance and expressivity. In some instances, multiple allelism at a single locus can partially explain phenotypic variability. For example, null mutations in NPHP3 cause MKS2, while hypomorphic NPHP3 alleles cause Nephronophthisis (NPHP)3. Similarly, hypomorphic CEP290/NPHP6 mutations are observed frequently in Leber congenital amaurosis (LCA) in both humans and in a mouse model4,5, whereas loss-of-function mutations lead to a constellation of ciliopathies that include MKS, NPHP, Bardet-Biedl Syndrome (BBS) and JBTS6-9 without a clear explanation for the phenotypic variation.
Consistent with the suggestion of second-site modifiers, several alleles are reported to modify ciliary phenotypes; a hypomorphic mutation in MGC1203 contributes to the overall penetrance and expressivity of BBS10, while heterozygous variants in CEP290 and AHI1 contribute to the phenotypic severity and pleiotropy of NPHP11. Finally, likely additive effects between alleles in bona fide BBS and MKS loci produce hybrid phenotypes of the two clinical entities7.
A paradigm emerging from the above studies is that genes mutated in one ciliopathy may contribute alleles across the entire clinical spectrum and that the assessment of total mutational load across the ciliary proteome12,13 will help dissect phenotypic causality and variability. Towards this end, we have initiated two complementary strategies – interactome studies on ciliary proteins and medical resequencing of known ciliopathy genes across a cohort of patients with diverse phenotypes (MKS and JBTS as severe, BBS as intermediate, NPHP and LCA as mild).
We first investigated RPGRIP1L, under the hypothesis that in addition to its association with MKS and JBTS14,15, it might also contribute alleles to other ciliary disorders. We screened 166 unrelated patients of northern European descent (17 MKS, 15 NPHP (with and without extra-renal phenotypes), 34 BBS, 100 LCA) and discovered 10 different novel nonsynonymous changes in 24 individuals (Table 1). We also detected two different common variants G1025S (rs2111119) and D1264N (rs3213758), both of which are present at similar allele frequencies when compared across our ciliopathy cohort, a cohort of 96 control individuals of northern European descent, and 60 CEPH controls from HapMap (data not shown).
Table 1.
Total mutational load in northern European ciliopathy patients with RPGRIP1L variants
| Clinical Diagnosis |
Patient ID |
RPGRIP1L Alleles |
Allele Frequency in Controls |
Other Alleles | |
|---|---|---|---|---|---|
| LCA | 3184 | S199G | 0/192 | ||
| LCA | 340 | A229T | 84/3016 | RPGR: I1148V het | |
| LCA | 341 | A229T | A229T | 84/3016 | LCA5: Q279X het |
| LCA | 1972 | L546F | 0/192 | ||
| LCA | 1321 | V647I | 0/192 | CRB1: C948Y hom | |
| LCA | 1378 | T677I | 0/260 | RPGRIP: R890X hom | |
| LCA | 1380 | T677I | 0/260 | ||
| LCA | 1617 | R937L | 0/192 | ||
| LCA | 3182 | A1183G | 4/192 | RPGR: I1148V het | |
| LCA | 3189 | A1183G | 4/192 | RPGR: I1148V het, N1081K het | |
| LCA | 3181 | D1264Y | 0/192 | RPGR: I1148V het | |
| LCA | 3187 | D1264Y | 0/192 | RPGR: I1148V het | |
| LCA | 3192 | D1264Y | 0/192 | ||
| SLS | F259-II1 | A229T | 84/3016 | NPHP5: R364X het | |
| SLS | F848-II1 | A229T | 84/3016 | NPHP5: T627M het, CEP290: R1978X het | |
| SLS | F99-II1 | A229T | 84/3016 | NPHP3: R397H het, S500P het | |
| JBTS | F256-II1 | A229T | 84/3016 | CEP290: Q1591X het | |
| BBS | AR74-05 | A229T | 84/3016 | ||
| BBS | AR400-03 | A229T | 84/3016 | ||
| BBS | AR672-03 | A229T | 84/3016 | ||
| BBS | AR775-03 | A229T | 84/3016 | MKS1: D286G het | |
| BBS | AR348-03 | L447S | 0/192 | ||
| BBS | AR623-03 | A1183G | 4/192 | ||
| MKS | MKS-060143 | R1236C | 0/192 | ||
None of the observed alleles are sufficient to explain disease manifestation under a Mendelian model. However, most alleles were either unique to our patient population, or were enriched compared to ethnically matched controls, and mapped within known functional domains of RPGRIP1L (Table 1; Suppl. Fig. 1).
However, the rarity of most alleles precluded us from delineating their impact on the clinical phenotypes. One notable exception, A229T, is sufficiently frequent to empower such a study. To assess allele neutrality without a preconceived model of inheritance, we performed Transmission Disequilibrium Testing (TDT); we screened 145 BBS families for which DNA was available from both parents. Focusing on families in which only one of the two parents was an A229T heterozygote, we identified 18 informative trios and detected significant over-transmission of the threonine-encoding allele in patients (15/18 transmissions; 83.3%, p<0.01).
Despite the modest number of trios, the TDT suggested that the A229T change is not neutral. To probe this possibility further, we re-evaluated our original resequencing data. We found a surprising enrichment of the 229T allele in northern European patients in whom retinal degeneration is mandatory for diagnosis, such as in BBS or Senior-Loken Syndrome (SLS) (5.9%; 4/68 chromosomes, and 15%; 3/20 chromosomes respectively; Table 1, compared to a 2.8% allele frequency in unaffected northern Europeans; Table 2). We therefore wondered whether this variant might contribute to the retinal defect, which is a frequent, but not ubiquitous, ciliopathy phenotype. If this is true, then comparisons between NPHP (no retinal degeneration) versus SLS cohorts (NPHP and retinal degeneration) should be informative. Even though each of these phenotypes is rare in the general population, we were able to study two northern European cohorts: 66 unrelated patients with isolated NPHP and 84 patients with SLS. Remarkably, 229T was absent from the NPHP group, but was detected at an allele frequency of 5.4% in SLS patients (Table 2). The small size of this cohort precluded meaningful statistical analysis. Nonetheless, these data suggested that the absence of 229T might have a protective effect from RP.
Table 2.
Frequency of RPGRIP1L A229T (c.685G>A; rs61747071) in ciliopathy patients of northern European descent
| Clinical Diagnosis |
n= | Genotype Counts |
Genotype Frequency |
Allele Counts |
Allele Frequency |
p-values |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases v. Controls1 |
RP v. Non-RP1 |
Cases v. Controls2 |
RP v. Non-RP2 |
||||||||||||
| GG | GA | AA | GG | GA | AA | G | A | G | A | ||||||
| Controls | 1508 | 1425 | 82 | 1 | 0.945 | 0.054 | 0.001 | 2932 | 84 | 0.972 | 0.028 | ||||
| NPHP | 66 | 66 | 0 | 0 | 1 | 0 | 0 | 132 | 0 | 1 | 0 | ||||
| MKS | 49 | 49 | 0 | 0 | 1 | 0 | 0 | 98 | 0 | 1 | 0 | ||||
| Ciliopathy Non-RP | 115 | 115 | 0 | 0 | 1 | 0 | 0 | 230 | 0 | 1 | 0 | 1.92E-03 | 1.54E-03 | ||
| LCA | 189 | 174 | 14 | 1 | 0.921 | 0.074 | 0.005 | 362 | 16 | 0.958 | 0.042 | ||||
| SLS | 84 | 75 | 9 | 0 | 0.893 | 0.107 | 0 | 159 | 9 | 0.946 | 0.054 | ||||
| JBTS | 30 | 25 | 5 | 0 | 0.833 | 0.167 | 0 | 55 | 5 | 0.917 | 0.083 | ||||
| BBS | 184 | 170 | 14 | 0 | 0.924 | 0.076 | 0 | 354 | 14 | 0.962 | 0.038 | ||||
| Ciliopathy RP | 487 | 444 | 42 | 1 | 0.912 | 0.086 | 0.002 | 930 | 44 | 0.955 | 0.045 | 6.45E-03 | 7.35E-05 | 0.021 | 1.66E-05 |
Fisher's exact test, 1-tailed; 2-tailed p-values remained significant (Ciliopathy Non-RP v. Controls, p=3.84E-03; Ciliopathy RP v. Controls, p=0.013; Ciliopathy Non-RP v. Ciliopathy RP, p=1.47E-04)
Permutation test
To investigate this possibility, we queried whether the 229T allele frequency is significantly different in a larger case-control cohort of northern European ciliopathy patients (Suppl. Table 1). We categorized our patients into two groups, with retinal phenotypes being the sole distinguishing factor. Both groups exhibited significantly different 229T allele frequencies compared to controls (n=3016 control alleles); the allele was absent from the non-retinal degeneration group (n=230 alleles; p=1.92E-03) but was enriched in the retinal degeneration group (combined: 4.5%, n=974 alleles, p=6.45E-03; Table 2). Furthermore, in agreement with our earlier data, we observed significantly different 229T allele frequencies between the non-retinal pathology and retinal degeneration ciliopathy groups (p=7.35E-05; Table 2). To evaluate the statistical significance of our findings further, we performed permutation analyses where the observed genotypes in the entire sample were assigned randomly without replacement in the three groups (ciliopathy +RP, ciliopathy −RP, controls). After 107 independent random assignments, we counted the number of times that the differences in minor allele frequency in each group matched or exceeded the actual differences. This study provided additional evidence that the association data did not occur by chance (Table 2). Importantly, we found A229T to be transmitted through at least three different haplotypes (three patients contained unique RPGRIP1L SNPs in cis with 229T), while the frequency of 229T was indistinguishable across cohorts collected at different sites (Suppl. Table 1), arguing against a stratification artifact.
Our genetic data suggest a role for 229T in the development of RP in ciliopathy patients. However, the low frequency of this allele in the population and its apparent modest effect in our cohorts limit the power of genetic studies. We therefore evaluated the potential consequences of A229T to protein function.
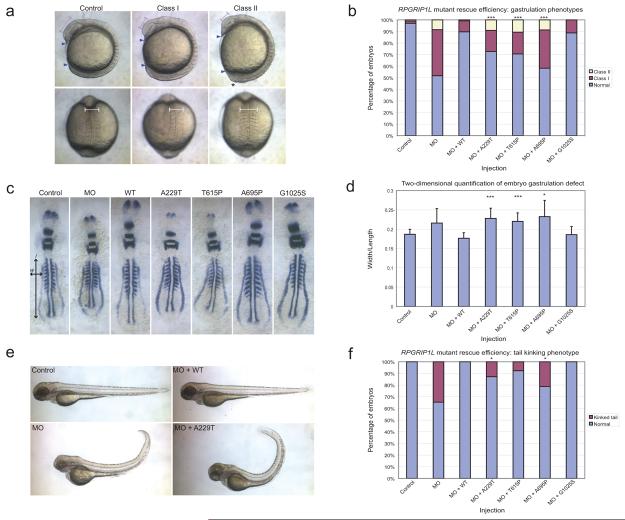
As a first test, we performed a genetic rescue experiment using an in vivo model. We have shown previously that suppression of ciliary and basal body proteins in zebrafish leads to measurable phenotypes in mid-somitic embryos, some of which are caused by defects in Wnt signaling16,17, a phenotype also observed in other experimental ciliopathy models18. The ability of human mRNA to rescue somitic phenotypes has proven useful for establishing the pathogenic potential of alleles by comparing the efficiency of rescue of wild type (wt) human messenger RNA (mRNA) compared to mRNA bearing the missense variant(s) under investigation7. We identified a single partial zebrafish transcript that, upon reciprocal BLAST analysis, showed the highest amino acid similarity between zebrafish and human (73% similarity, 55% identity), to which we designed a splice-blocking morpholino (MO). The MO was injected into wild-type (wt) embryos at the two-cell stage (50-100 embryos per injection) and scored at the eight to nine somite stage. Consistent with data from other ciliopathy genes7,10,16,17, we observed dose-dependent gastrulation defects that included a shortened body axis, thin somites with broad lateral extensions, minor kinking of the notochord, underdeveloped anterior structures and tail extension abnormalities, all of which enabled us to categorize embryos into two classes based on objective criteria (Class I or Class II; Fig. 1a; Suppl. Fig. 2a). These phenotypes resulted from loss of rpgrip1l, since RT-PCR and sequencing analysis of embryos showed 10 ng of MO to cause ∼50% reduction of rpgrip1l mRNA (Suppl. Fig. 2b). Importantly, the phenotypes could be rescued by co-injection of 50 pg of wt human mRNA (embryos scored blind to injection cocktails; Fig. 1b, Suppl. Table 2).
Figure 1. Functional assessment of the RPGRIP1L A229T variant in vivo.
a. Zebrafish embryos injected with rpgrip1l splice-blocking morpholino (MO) display gastrulation defects at the eight-nine somite stage; lateral and dorsal views of live embryos are shown. Embryos were categorized phenotypically based on the presence of a shortened body axis (anterior and posterior ends indicated with blue triangles) and small head/eyes only (magenta lines indicate eye size) (Class I; mild); or shortened body axis and small head/eyes in addition to at least one of the following: broad and thin somites (white line indicates somite span), notochord kinking, and tail extension defects (black asterisk) (Class II; severe).
b. Live scoring of embryos co-injected with MO and human RPGRIP1L mRNA indicates that A229T is pathogenic in our assay as indicated by the comparison of WT rescue efficiency to mutant rescue efficiency. MO, morpholino alone; WT, wild-type human RPGRIP1L mRNA. Known JBTS mutations T615P and A695P are positive controls; neutral variant G1025S (rs2111119) is a negative control. Statistical significance (mutant rescue vs. WT rescue) is depicted as (***), p<0.0001 (χ2); see Suppl Table 2 for all χ2 and p-values.
c. Representative examples of whole-mount embryos hybridized in situ with pax2, krox20, and myoD riboprobes.
d. Two-dimensional morphometric quantification of the efficiency of human RPGRIP1L mRNA to rescue rpgrip1l MO phenotypes. Whole embryos with eight-nine appreciable somites (n=8-20/injection) labeled with pax2, krox20, and myoD riboprobes were flat-mounted, photographed and measured in two dimensions. The width/length ratio was calculated as the width (w) spanning between the distal ends of the 5th anterior somites (horizontal arrow in panel c) vs. the length (l) of the notochord as indicated by the staining of adaxial cells (vertical arrow in panel c). Statistical significance (mutant vs. WT rescue) is depicted as (*), p<0.05 and (***), p<0.0001 (two-tailed student's t-test); see Suppl Table 3 for all p-values.
e. rpgrip1l morphant larvae display tail development defects at 5 days post-fertilization (dpf). Morphant larva (MO) with tail curvature and posterior structural disorganization can be rescued efficiently with WT human RPGRIP1L mRNA, however RPGRIP1L message harboring the 229T encoding allele does not completely rescue the tail phenotype.
f. Quantification of the efficiency of tail phenotype rescue in 5dpf larvae. Whereas WT RPGRIP1L message completely rescued the tail phenotypes, A229T as well as positive controls T615P and A695P result in the persistence of affected tail structures in injected larva. G1025S was used as a negative control. Statistical significance (mutant rescue vs. WT rescue) is depicted as (*), p<0.05 (χ2).
We therefore proceeded to compare the efficiency of rescue between human mRNA encoding each of 229A and 229T. In addition, we included two known pathogenic missense alleles, T615P and A695P, that cause JBTS14,15 as positive controls, as well as the neutral G1025S variant as a negative control. Embryos were co-injected with a cocktail of MO and each human mRNA (n=88-115 embryos per allele) and scored blind to the injection cocktail (Suppl. Table 2). We found that the 229T rescue gastrulation phenotype was significantly different from wt mRNA rescue (p<0.0001; Fig1b, Suppl Table 2). The assay was specific, since each of T615P and A695P were scored as pathogenic, but had no off-target effects; the rescue of the 1025S-encoding mRNA was indistinguishable from the 1025G message (Fig1b).
To substantiate these assessments, we measured embryonic structures labeled in situ with pax2, krox20, and myoD riboprobes. On whole embryo flat-mounts age-matched to eight to nine somites, we calculated the ratio of the width (w) spanning the distal ends of the 5th somites to the length (l) of the notochord as demarcated by adaxial cell labeling (n=8-20 embryos measured per variant). Consistent with our live embryo scoring, comparison of the mean w/l ratios between wt and mutant rescues indicated that both positive control alleles and A229T, but not G1025S, are pathogenic (Fig. 1c, d; Suppl. Table 3).
As a third line of in vivo evidence, we examined 5-day rpgrip1l morphant larvae (n=52-78 embryos/injection). Consistent with other ciliopathy morphants19, we observed tail extension abnormalities in 35% of rpgrip1l morphants, which was rescued by co-injection of wt human RPGRIP1L message (Fig. 1e, f). However, mRNA encoding 229T was unable to efficiently rescue the tail phenotype (p=0.031), consistent with both the T615P and A695P rescues (p=0.001 and 0.004 respectively). These observations, in addition to the absence of tail phenotypes in morphants rescued with G1025S mRNA, substantiated further the specificity of our assay (Fig. 1e, f).
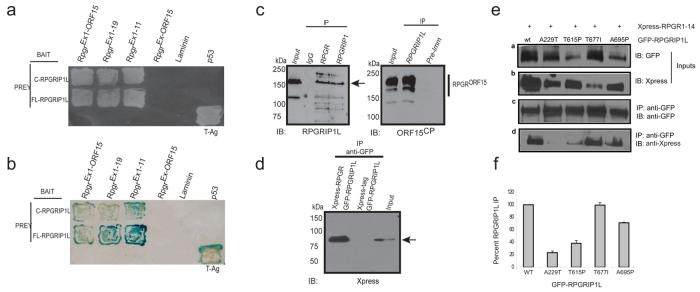
We next turned to the question of why A229T might have the observed effect on the retinal degeneration phenotype. We noted that mutations in one of the NPHP genes, NPHP5, are also associated with an increased incidence of SLS; all tested patients with NPHP5 null variants exhibit retinal degeneration compared to a 10% incidence of retinopathy in patients with mutations in other NPHP-associated genes20. NPHP5 interacts with RPGR20, a ciliary protein mutated in 70-80% of X-linked RP and 20-25% of simplex RP21-24. We therefore hypothesized that RPGRIP1L might also interact biochemically with RPGR and that the A229T allele might have a measurable effect on the affinity of this interaction. As part of our ongoing efforts to understand the pathobiology of RPGR, we had performed yeast two-hybrid (Y2H) screens of human retinal cDNA as prey using full-length RPGRORF15 as bait and identified the carboxyl-terminal domain of RPGRIP1L (RPGR-interacting domain; RID); this interaction was validated by known-bait and known-prey analysis using different domains of RPGR as bait (Fig. 2a, b). To corroborate the findings, we generated an anti-human RPGRIP1L antibody (Suppl. Fig. 3) and performed co-immunoprecipitations (IP) using bovine retinal extracts. In reciprocal IP experiments with RPGRIP1L or RPGR antibody, we detected the expected bands on immunoblots corresponding to RPGR or RPGRIP1L, respectively (Fig. 2c). Co-transfection of COS-1 cells followed by co-IP demonstrated that Xpress-tagged RPGR and GFP-RPGRIP1L proteins are part of the same complex (Fig. 2d). We then tested A229T and three reported JBTS variants14,15 that are shown to be pathogenic in our zebrafish assay. Triplicate experiments showed that the GFP-tagged A229T mutant protein showed considerably lower (∼80%) ability to immunoprecipitate RPGR and has a profound effect on RPGR binding compared to the JBTS mutations (Fig. 2e, f). We also found that RPGRIP1L localizes to the cilium and basal body of mouse IMCD3 cells and to the inner segment/connecting cilium of mouse photoreceptors (Suppl. Fig. 4), in the same spatiotemporal context as demonstrated previously for RPGR4,23,24. Hence, the abrogation of RPGRIP1L-RPGR interaction is likely to be relevant in photoreceptors.
Figure 2. RPGRIP1L interacts with RPGR and missense variant A229T abrogates this interaction.
a. Known-bait and known-prey analysis. Yeast two-hybrid analysis using indicated RPGR bait-encoding constructs and RPGRIP1L [carboxyl-terminal (C) or full-length (FL)] prey encoding constructs revealed interaction of RPGR exons 1-11 with carboxyl-terminal or full-length RPGRIP1L. Laminin was used as negative control, whereas p53/T-antigen (T-Ag) interaction served as a positive control.
b. RPGR-RPGRIP1L interaction was validated further by examining the activation of the LacZ gene in the β-galactosidase assay corresponding to (a).
c. Interaction of RPGR and RPGRIP1L in vivo. Bovine retinal extracts were subjected to immunoprecipitation (IP) using anti-RPGR, RPGRIP1, RPGRIP1L antibodies, normal immunoglobulin (IgG), or pre-immune serum (Pre-Imm). Precipitated proteins were analyzed by SDS-PAGE and immunoblotting (IB) using anti-RPGRIP1L (left) or RPGR-ORF15CP antibody (right). Arrow (left) indicates RPGRIP1L-imunoreactive band and the straight line in the right panel depicts the RPGR isoforms pulled down by RPGRIP1L. Asterisk (*) marks a non-specific immunoreactive band.
d. RPGR-RPGRIP1L interaction in transfected cells. COS-1 cells were transiently transfected with constructs encoding Xpress-tagged RPGR, GFP-RPGRIP1L or Xpress-tag alone. Proteins were extracted and subjected to immunoprecipitation (IP) using the anti-GFP antibody. Precipitated proteins were analyzed by SDS-PAGE and immunoblotting using anti-Xpress antibody. Input lane contains 20% of the protein extract used for IP. Arrow indicates the Xpress-immunoreactive band.
e. Interaction of RPGR with wild-type (WT) and mutant RPGRIP1L. COS-1 cells were transiently transfected with constructs encoding WT or mutant RPGRIP1L fused to GFP and Xpress-tagged RPGR. Panels ‘a’ and ‘b’ indicate the 20% of the GFP-RPGRIP1L and Xpress-RPGR input protein extract used for IP. Precipitation of GFP-RPGRIP1L using the GFP antibody was used as a positive control (panel ‘c’). Association of wt and mutant GFP-RPGRIP1L with Xpress-RPGR is represented in Panel ‘d’. T677I represents a bona fide pathogenic allele, but which likely does not contribute to retinal degeneration.
f. Quantitative analysis of the band intensities are represented as percent of mutant RPGRIP1L immunoprecipitated by RPGR. Association of wt GFP-RPGRIP1L with RPGR was considered as 100%. The results represent an average of three independent experiments.
Second-site modifications are emerging as prevalent phenomena in Mendelian disorders. For example, PLS3 is a protective modulator of SMN1 dysfunction associated with Spinal Muscular Atrophy (SMA)25; mutations in CNTF modify the age of onset in amyotrophic lateral sclerosis (ALS)26, while a mutation in MGC1203 can modify the global penetrance/expressivity in BBS10. However, the penetrance and expressivity of tissue-specific defects within the context of syndromic disease pose a considerable challenge, especially for alleles of modest frequency in the general population and modest effect on the phenotype, which cannot be captured by traditional population-based genetic tools. Our data suggest that, in patients of northern European descent, the presence of the 229T allele of RPGRIP1L has predictive value for retinal degeneration. It will be important to consider the nature and position of mutations at the primary locus, since A229T probably exerts its effect in the context of mutations in specific ciliopathy proteins that can complex with RPGR in the retina; this is consistent with the previously proposed poison model of genetic epistasis27.
It is possible that some of the other alleles reported herein might also exhibit a similar effect; however, their rarity will necessitate the evaluation of larger cohorts. Notably, in vivo evaluation of each of the rare alleles using the zebrafish rescue assay suggested that most are pathogenic (Table 3, Suppl. Table 2, Suppl. Table 3, Suppl. Fig 5) and that they can potentially interact with alleles at various sites of the ciliopathy genetic spectrum (Table 1). Also, destabilization of the RPGRIP1L-RPGR complex is unlikely to be the sole cause of retinal degeneration. RPGRIP1L also associates with RPGRIP1 (Fig. 2c), null mutations in which cause early-onset, severe retinal degeneration28. Three of the alleles found in our cohort, A1183G, R1236C and D1264Y map within the RPGRIP1-interacting domain of RPGRIP1L; hence, their contribution to disease might be mediated by loss of RPGRIP1 activity/function. Finally, RPGRIP1L and RPGR are members of a larger macromolecular complex whose composition and function will vary across tissue types and ciliary functions. Establishment of the content and the stoichiometry of such complex(es), coupled to the evaluation of ciliopathy alleles on the stability of the macromolecular complex in a tissue/function-dependent manner will enable the currently poor allelic stratification of variability in disease phenotypes.
Table 3.
Functional assessment of RPGRIP1L missense variants in an in vivo zebrafish model
| Conservation | In vivo modeling in zebrafish | |||
|---|---|---|---|---|
| Variant | Domains* | Species** | Live scoring | Quantitative |
| S199G | CCI | Ptr, Bta, Cfa, Mmu, Oan, Dre | pathogenic | pathogenic |
| A229T | CCI | Ptr, Mmul, Eca, Bta, Cfa, Rno, Mmu, Mdo, Dre | pathogenic | pathogenic |
| L447S | CCIV | Ptr, Mmul | benign | benign |
| L546F | CCV | Ptr, Mmul, Eca, Bta, Cfa, Rno, Mmu, Mdo, Oan, Gga, Dre | pathogenic | pathogenic |
| T615P | C2 | Ptr, Mmul, Eca, Bta, Cfa, Mmu, Mdo | pathogenic | pathogenic |
| V647I | C2 | Ptr, Mmul, Mdo, Oan, Gga, Dre, Spu | pathogenic | benign |
| T677I | C2 | Ptr, Mmul, Eca, Bta, Cfa, Rno, Mmu, Oan | pathogenic | pathogenic |
| A695P | C2 | Ptr, Mmul, Eca, Bta, Cfa, Rno, Mmu, Mdo, Oan, Dre, Spu | pathogenic | pathogenic |
| R937L | Ptr, Mmul, Cfa | pathogenic | pathogenic | |
| A1183G | RID | Ptr, Mmul, Eca, Bta, Cfa, Rno, Mmu, Mdo, Oan, Gga | pathogenic | pathogenic |
| R1236C | RID | Ptr, Mmul, Eca, Bta, Cfa, Rno, Mmu, Mdo, Oan, Spu | pathogenic | pathogenic |
| D1264Y | RID | Eca, Bta, Cfa, Rno, Mmu | pathogenic | pathogenic |
CC- Coiled coil domain; C2- protein kinase C (PKC) conserved region 2 motif; RID- domain with homology to the RPGR-interacting domain of RPGRIP1.
Ptr- Pan troglodytes; Mmul- M. mulatta; Eca- E. caballus; Bta- B. taurus; Cfa- C. familiaris; Rno- R. norvegicus; Mmu- M. musculus; Mdo- M. domestica; Oan- O. anatinus; Gga- G. gallus; Dre- D. rerio; Spu- S. purpuratus
Methods
Ciliopathy patients, controls, and mutational analysis
Control DNAs were obtained from healthy individuals with no clinical ciliopathy criteria (retinal degeneration, polydactyly, renal abnormalities, mental retardation or other central nervous system defects) from four collection points (Suppl. Table 1). Blood samples were obtained following informed consent. We amplified the exon sequence and intron/exon junctions of RPGRIP1L (NM_015272) from DNA extracted from lymphoblast cell lines or lymphocytes according to standard protocols. PCR products were sequenced with BigDye Terminator v3.1 chemistry on an ABI 3100 or ABI 3730 (Applied Biosystems), sequences were analyzed with PolyPhred, and variants were confirmed by resequencing and visual assessment of chromatograms. Primer sequences and PCR conditions are available upon request.
Statistical analyses
We used plink v1.04 (http://pngu.mgh.harvard.edu/purcell/plink/) for TDT analysis in BBS trios, and the PEPI404x package for Fisher's exact test. All other analyses were performed in Microsoft Excel.
DNA constructs
We generated full-length or specific domains of RPGR by PCR from retinal cDNA and subcloned them into the pcDNA 4 expression vector (Invitrogen). Full-length RPGRIP1L was amplified from human retinal cDNA, cloned into expression vector pEGFP-C1 (in vitro assays) or pCS2+ (in vivo assays) and confirmed by direct sequencing. Mutagenesis was performed using the QuickChange Site-Directed Mutagenesis kit (Stratagene), according to manufacturer's instructions.
Zebrafish embryo manipulation and morpholino injection
A splice-blocking MO (Gene Tools) was diluted to appropriate concentrations in deionized, sterile water (2, 4, 6, 8, and 10ng/nl for the dose response, and 10ng/nl for rescue experiments) and was injected into wt zebrafish embryos at the two-cell stage as described10. To rescue the MO phenotype, we transcribed mRNA from linearized pCS2+-RPGRIP1L vector with the SP6 mMessage machine kit (Ambion). We carried out in situ hybridization on whole embryos fixed with 4% paraformaldehyde with riboprobes against pax2, krox20, and myoD using standard protocols. Images were captured at 10X magnification and measurements were taken of the width spanning the 5th somites from the anterior end, and the length of the notochord as defined by myoD staining of adaxial cells. Embryos used for assessment of tail phenotypes at 5dpf were reared in 1-phenyl-2-thiourea (PTU)-treated embryo media, and anesthetized with Tricaine for imaging at 4X magnification. MO efficiency was determined by extracting total RNA from embryos with Trizol (Invitrogen) according to manufacturer's instructions. Oligo-dT-primed total RNA was reverse transcribed using SuperScriptIII reverse transcriptase (Invitrogen) and cDNA was subsequently PCR amplified and sequenced using standard procedures.
Yeast two-hybrid screen
We carried out the yeast two-hybrid (Y2H) analysis using the Matchmaker Gal4-two hybrid system (Clontech), according to manufacturer's instructions. We amplified the full-length cDNA of mouse RpgrORF15 or its different domains and cloned into the bait vector pGBKT7. The adult human retinal cDNA library was cloned into the ‘prey’ pGADT7 vector (Clontech) and used for the screen. Known-bait and known-prey analysis was carried out as described29.
Antibodies
Characterization of the ORF15CP antibody has been described24. Anti-RPGRIP1L antibody was generated by immunizing rabbits with the human peptide 1300HALQSVYKQYRDDLE1314 (Yenzym). RPGRIP1 antibody was a gift from Dr. Tiansen Li (Harvard Medical School); Antibodies against Xpress-tag and acetylated α-tubulin were procured from Invitrogen and Chemicon, respectively.
Mammalian cell culture, transient transfection, co-immunoprecipitation (coIP) and immunolocalization
We propagated COS-1 cells in DMEM plus 10%FBS, supplemented with Penicillin, Streptomycin and L-Glutamine. Co-IP experiments from retina and transfected cells have been described24. Immunolocalization studies in cells and in the retinal sections were carried out as reported previously4,30.
Supplementary Material
Acknowledgments
We thank the ciliopathy patients and their families for their continued support and encouragement. We also thank Heleen Arts for critical evaluation of the manuscript. This work was supported by grants R01EY007961 from the National Eye Institute (HK and AS), R01HD04260 from the National Institute of Child Health and Development (NK), R01DK072301, R01DK075972 (NK) R01DK068306, R01DK064614, R01DK069274 (FH), NRSA fellowship F32 DK079541 (EED) from the National Institute of Diabetes, Digestive and Kidney disorders, Intramural program of NEI (AS) the Macular Vision Research Foundation (NK), the Foundation for Fighting Blindness (HK, SSB, AS, and NK), the Foundation for Fighting Blindness Canada (RKK), Le Fonds de la recherche en sante du Québec (FRSQ) (RKK), Research to Prevent Blindness (AS), Harold Falls Collegiate Professorship (AS), the Midwest Eye Banks and Transplantation Center (HK), the Searle Scholars Program (MAB), the UK Medical Research Council (grant number G0700073; CAJ), NIHR Biomedical Research Centre for Ophthalmology (SSB) and EU-GENORET Grant LSHG-CT-2005-512036 (SSB). FH is an investigator of the Howard Hughes Medical Institute (HHMI) and a Doris Duke Distinguished Clinical Scientist (DDCF).
References
- 1.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–48. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann C, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008;82:959–70. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olbrich H, et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet. 2003;34:455–9. doi: 10.1038/ng1216. [DOI] [PubMed] [Google Scholar]
- 4.Chang B, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–57. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Hollander AI, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79:556–61. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baala L, et al. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet. 2007;81:170–9. doi: 10.1086/519494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leitch CC, et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet. 2008;40:443–8. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- 8.Sayer JA, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38:674–81. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 9.Valente EM, et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet. 2006;38:623–5. doi: 10.1038/ng1805. [DOI] [PubMed] [Google Scholar]
- 10.Badano JL, et al. Dissection of epistasis in oligogenic Bardet-Biedl syndrome. Nature. 2006;439:326–30. doi: 10.1038/nature04370. [DOI] [PubMed] [Google Scholar]
- 11.Tory K, et al. High NPHP1 and NPHP6 mutation rate in patients with Joubert syndrome and nephronophthisis: potential epistatic effect of NPHP6 and AHI1 mutations in patients with NPHP1 mutations. J Am Soc Nephrol. 2007;18:1566–75. doi: 10.1681/ASN.2006101164. [DOI] [PubMed] [Google Scholar]
- 12.Gherman A, Davis EE, Katsanis N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat Genet. 2006;38:961–2. doi: 10.1038/ng0906-961. [DOI] [PubMed] [Google Scholar]
- 13.Liu Q, et al. The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics. 2007;6:1299–317. doi: 10.1074/mcp.M700054-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arts HH, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007;39:882–8. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- 15.Delous M, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–81. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- 16.Gerdes JM, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–60. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- 17.Ross AJ, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–40. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 18.Corbit KC, et al. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–6. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- 19.Beales PL, et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–9. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- 20.Otto EA, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet. 2005;37:282–8. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- 21.Shu X, et al. RPGR mutation analysis and disease: an update. Hum Mutat. 2007;28:322–8. doi: 10.1002/humu.20461. [DOI] [PubMed] [Google Scholar]
- 22.Breuer DK, et al. A comprehensive mutation analysis of RP2 and RPGR in a North American cohort of families with X-linked retinitis pigmentosa. Am J Hum Genet. 2002;70:1545–54. doi: 10.1086/340848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong DH, et al. RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest Ophthalmol Vis Sci. 2003;44:2413–21. doi: 10.1167/iovs.02-1206. [DOI] [PubMed] [Google Scholar]
- 24.Khanna H, et al. RPGR-ORF15, which is mutated in retinitis pigmentosa, associates with SMC1, SMC3, and microtubule transport proteins. J Biol Chem. 2005;280:33580–7. doi: 10.1074/jbc.M505827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oprea GE, et al. Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science. 2008;320:524–7. doi: 10.1126/science.1155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giess R, et al. Early onset of severe familial amyotrophic lateral sclerosis with a SOD-1 mutation: potential impact of CNTF as a candidate modifier gene. Am J Hum Genet. 2002;70:1277–86. doi: 10.1086/340427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badano JL, Katsanis N. Beyond Mendel: an evolving view of human genetic disease transmission. Nat Rev Genet. 2002;3:779–89. doi: 10.1038/nrg910. [DOI] [PubMed] [Google Scholar]
- 28.Dryja TP, et al. Null RPGRIP1 alleles in patients with Leber congenital amaurosis. Am J Hum Genet. 2001;68:1295–8. doi: 10.1086/320113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng H, et al. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum Mol Genet. 2004;13:1563–75. doi: 10.1093/hmg/ddh173. [DOI] [PubMed] [Google Scholar]
- 30.He S, et al. Retinitis Pigmentosa GTPase Regulator (RPGR) protein isoforms in mammalian retina: insights into X-linked Retinitis Pigmentosa and associated ciliopathies. Vision Res. 2008;48:366–76. doi: 10.1016/j.visres.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.