Abstract
The present study was undertaken to determine whether germline encoded and polyreactive antibodies might be pathogenic and whether the breach of early tolerance checkpoints in Systemic Lupus Erythematosus (SLE) might lead to a population of B cells expressing germline encoded antibodies that become pathogenic merely by class-switching to IgG in a pro-inflammatory milieu.
We demonstrate here that IgM, DNA-reactive antibodies obtained from lupus patients that are unmutated and display polyreactivity can bind to isolated glomeruli and exhibit neurotoxic potential.
Thus, the IgM polyreactive repertoire in SLE includes antibodies that may acquire pathogenic function merely by undergoing class-switch recombination to become IgG antibodies.
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the production of a wide variety of autotantibodies, many of which react with nuclear antigens. In particular, antibodies against double-stranded (ds) DNA are regarded as a diagnostic marker for the disease and contribute to lupus nephritis. In both clinical studies and animal models, pathogenicity is associated with IgG isotype and with high-affinity binding to dsDNA. How the autoreactive B cells that produce these autoantibodies succeed in escaping normal tolerance mechanisms is not known. Patients with SLE have been demonstrated to have a defect in early B cell tolerance checkpoints, leading to the accumulation of high numbers of naive B cells that produce polyreactive antibodies that recognize both self and foreign antigen. A significant percentage of these B cells recognize dsDNA (1). Whether these antibodies have pathogenic potential is not known.
It is now appreciated that naïve B cells can undergo activation, class switch recombination, and ensuing secretion of IgG antibodies following exposure to elevated BAFF levels, endogenous ligands to toll-like receptors (TLRs), T cells overexpressing costimulatory molecules, or increased levels certain cytokines such as IL-21 (2–5). All these conditions that may lead to antigen-independent activation of B cells exist in lupus patients and may contribute to the increase in class-switched peripheral blood B cells that is present in a significant number of patients (6, 7). We, therefore, wished to know whether polyreactive antibodies might be pathogenic and whether the breach of early tolerance checkpoints in lupus patients might lead to a population of B cells making pathogenic IgG antibodies after activation by a pro-inflammatory milieu to class-switch to IgG.
Our laboratory has previously described a subset of anti-dsDNA antibodies that cross-reacts with a pentapeptide sequence, DWEYS, within the NR2A and NR2B subunits of the N-methyl D-aspartate receptor (NMDAR). These antibodies are present in murine lupus and in patients with SLE (8, 9). They deposit in glomeruli and cause proteinuria and have the potential to cause excitotoxic death of neurons if they penetrate the blood-brain barrier. Using a fluorochrome–labeled tetrameric DWEYS peptide (DWEYS-tetramer), which has higher avidity than monomer for peptide-reactive B cells, we have isolated IgM+ peptide-reactive B cells from the peripheral blood of patients with SLE. Antibodies derived from these B cells bind to peptide and are largely cross-reactive to dsDNA (10). This methodology enabled us to acquire self-reactive antibodies for further characterization.
We demonstrate here that many of these peptide, dsDNA cross-reactive antibodies obtained from lupus patients display the polyreactivity that characterizes the immature and naïve repertoire of both autoimmune and non-autoimmune individuals. Furthermore, these antibodies exhibit pathogenic potential in two major target organs in SLE, kidney and brain. These studies suggest that the polyreactive antibodies made by naïve B cell may become pathogenic, solely by class switch recombination.
Materials and Methods
Production of human anti-dsDNA monoclonal antibodies from peripheral blood of lupus patients
Human monoclonal antibodies were derived from peripheral blood B cells of 3 female SLE patients who met the revised ACR criteria for SLE (11). In brief, individual IgM expressing B cells, identified by reactivity with a fluorochome-tagged tetrameric form of the DWEYS peptide, were sorted into 96-well PCR plates and IgH (μonly) and IgL chain gene rearrangements were amplified in two rounds of PCR (50 cycles each) before being cloned into human Ig γ1 and κ expression vectors (gift of M.C. Nussenzweig, Rockefeller University, NYC). Human embryonic kidney fibroblast 293T cells were co-transfected with IgH and IgL encoding plasmid DNA by calcium phosphate precipitation as described previously (1, 12). Supernatants were collected after 5 days of culture. The sequences of these antibodies have been reported (10).
Purification of antibodies
Antibodies were purified form culture supernatants on a Protein G column (Amersham Biosciences, Uppsala, Sweden). The elution buffer was 0.1M glycine (pH 3.0). Eluted fractions were neutralized with 1M Tris-HCl (pH 9.0).
ELISAs
Antibody concentrations were determined using a standard curve constructed with human IgG1-kappa or lambda (Southern Biotechiology, Birmingham, AL). The capture antibody and detection antibody were unlabelled goat anti-human IgG and alkaline phosphatase conjugated goat anti-human kappa or lambda, respectively.
To test for polyreactivity, the following antigens, ssDNA, (100μg/ml), LPS (10μg/ml) (Sigma-Aldrich), and recombinant human insulin (5μg/ml) (Sigma-Aldrich), were coated to the plates. Clone #53 (gift of M.C. Nussenzweig) was used as a negative control in all ELISAs as it has previously been described not to bind to these antigens (1, 12) and twice its binding to antigen was used to determine the cut-off OD405.
Glomerular binding assay
Murine glomeruli were isolated and added to glass slides as described previously (13). Monoclonal antibodies were applied at 10–30 μg/ml for 1 hour at room temperature and visualized with FITC anti–human IgG (Inova Diagnostics, Inc, San Diego, CA). Monoclonal antibody B1 (30μg/ml), which does not recognize dsDNA or peptide by ELISA, was used as a negative control.
Neurotoxicity
Purified human monoclonal antibodies or saline alone (2μl) were injected stereotaxically into the hippocampus of C57Bl/6 mice as previously described (8, 9). Control antibody B1 was used at a concentration of 100μg/ml. G11, E2 and A8 were used at concentrations of 10–35μg/ml. Cresyl violet and fluorojade-B staining was performed 24 hours later (8, 9).
Results
Peptide and dsDNA cross-reactive antibodies are polyreactive
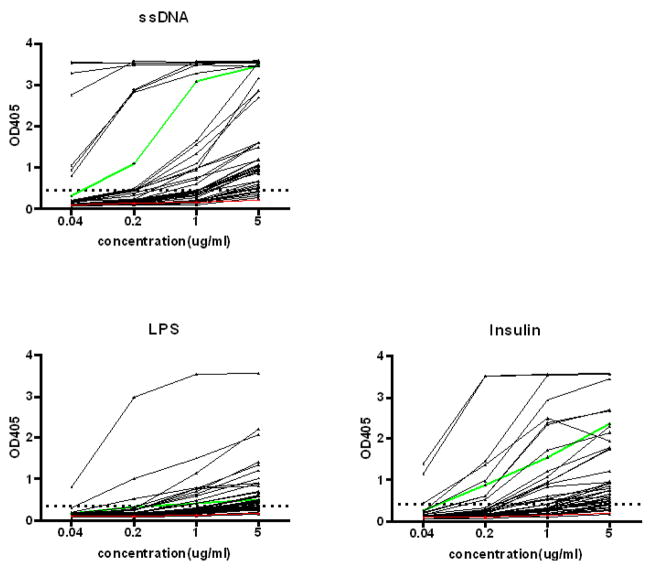
Previous studies have shown that antibodies in the immature, transitional and mature naïve B cell subsets are often polyreactive and many are autoreactive (1, 12), although only a subset of these binds dsDNA. This subset is expanded in the peripheral blood B cell repertoire of patients with SLE. We have isolated IgM+ B cells from the peripheral blood of patients with SLE that bind a peptide mimetope of dsDNA. Antibodies derived from these B cells were expressed as intact IgG1 and are largely cross-reactive to dsDNA (10). We were interested in asking whether those with reactivity to dsDNA were often polyreactive or whether the dsDNA-reactive B cell population is largely distinct from the polyreactive B cell population. Out of 47 dsDNA reactive antibodies nineteen were clearly derived from an antigen-naive population as they displayed no somatic mutation, while fourteen displayed limited mutation less than 2 base changes in either heavy chain, light chain or both, consistent with polymerase error. Fourteen displayed 3 or more mutations in either the heavy chain or both heavy and light chain, consistent with in vivo mutation. We analyzed these antibodies for reactivity with single stranded (ss) DNA, LPS and insulin. These antigens have been previously used to define the polyreactivity of the normal and autoimmune B cell repertoire and an increased number of B cells with reactivity to these antigens appear to be a marker of a dysregulated immune system (1). DNA binding by these antibodies was inhibitable by DWEYS peptide (data not shown), demonstrating that these two antigens share the same or a contiguous binding site. Almost all antibodies that bound dsDNA also bound ssDNA. Many also bound LPS, insulin or both, demonstrating that many of these DNA-reactive antibodies are polyreactive (Fig 1).
Figure 1. Polyreactivity ELISAs.
dsDNA-reactive antibodies were tested by ELISA for reactivity with ssDNA, LPS and insulin. A horizontal dotted line denotes the cut-off OD405 for positive reactivity. One dsDNA-binding antibody, #40, and one non dsDNA-binding antibody, #53, from M.C. Nussenzweig’s laboratory were used as positive and negative control antibodies as previously described (1, 12). The negative antibody, #53, is displayed in red; the positive antibody, #40, is shown in green.
Pathogenicity of peptide and DNA cross-reactive antibodies
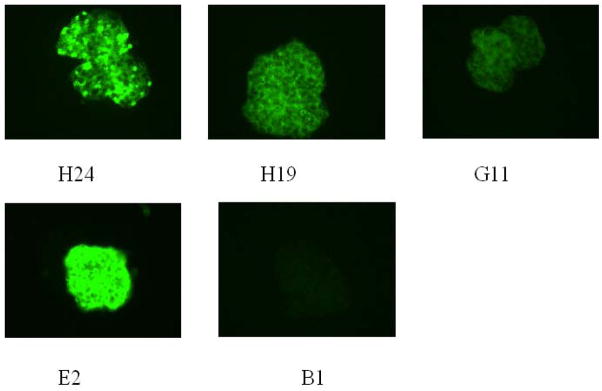
Since many of the peptide, dsDNA cross-reactive antibodies displayed a spectrum of polyreactivity that is thought to be non-pathogenic, we assayed a number of these polyreactive antibodies for glomerular binding. It has previously been shown that murine and human anti-DNA antibodies will bind glomerular structures in an ex vivo assay in which the glomerular antigens are thought to be largely in their native configuration. It has also been shown that mouse and human glomeruli share antigenic determinants and, in general, antibodies that bind glomeruli in vitro have renal pathogenic potential in vivo. Reciprocally, DNA-reactive sera that do not bind glomeruli in the glomerular binding assay are often derived from patients with no evidence of renal disease. Eleven antibodies were tested, 7 without mutation or with presumed PCR-introduced mutation and 4 exhibiting somatic mutation; all displayed glomerular binding while a control non-peptide, non-dsDNA binding antibody B1 did not (Figure 2). Furthermore, unmutated antibodies showed as strong binding to glomeruli as mutated antibodies. While we did not assay all 47 antibodies for glomerular binding, it is clear that many polyreactive antibodies displayed a characteristic that has been associated with renal pathogenicity in IgG antibodies. Furthermore, DNase treatment did not diminish glomerular binding (data not shown), demonstrating cross-reactivity to glomerular antigens.
Figure 2. Glomeruli binding assay.
Eleven polyreactive antibodies were tested for binding to isolated mouse glomeruli. Positive staining patterns are shown with four representative antibodies. Human monoclonal antibody B1 with no dsDNA binding was used as a negative control (17).
As human serum containing anti-DNA antibodies cross-reactive with DWEYS peptide and a murine monoclonal antibody with this cross-reactive antigenic specificity are capable of causing neuronal damage (9), we tested whether these polyreactive antibodies also displayed neurotoxicity. Three polyreactive antibodies, G11, A8 and E2 that bound peptide and DNA, all unmutated, were injected into the hippocampus of C57Bl/6 mice and the tissue was subsequently examined for evidence of neuronal stress, assessed by reactivity with fluorojade. Notably, they all caused neuronal stress in vivo at a concentrations of 10 to 35 μg/ml while injection of the control antibody, B1, did not lead to excitotoxicity even at a concentration of 100 μg/ml (Figure 3). Thus, three polyreactive antibodies all displayed neurotoxic potential.
Figure 3. Neurotoxicity of polyreactive antibodies.
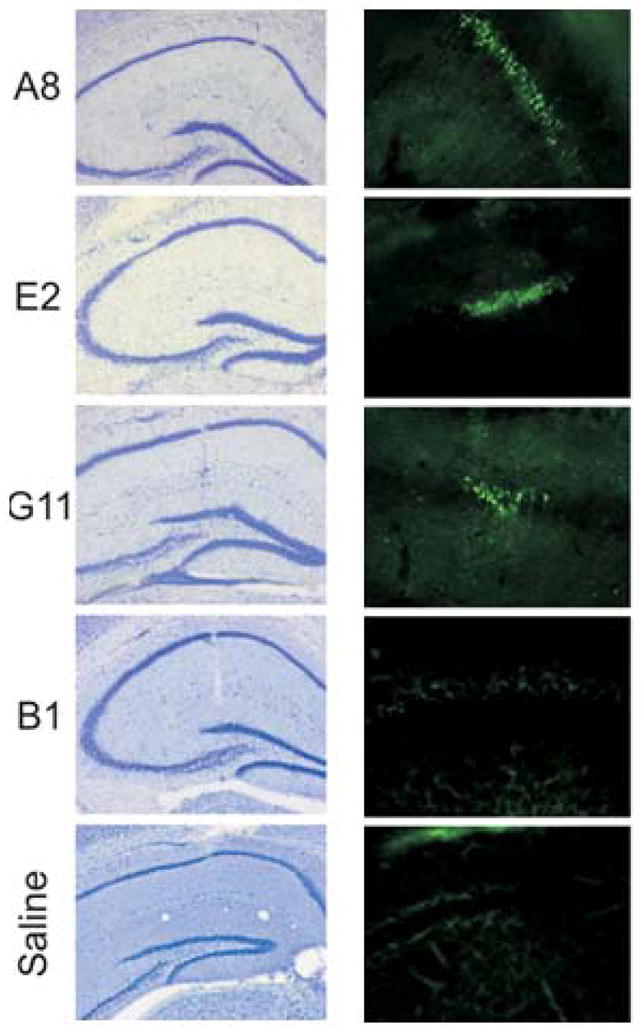
A focal region of neuron damage in mouse hippocampus is shown by cresyl violet staining (left panel) and fluorojade reactivity (right panel) 24 hours after intracerebral injection of polyreactive antibodies (10–35μg/ml) into the CA1 region compared with negative control antibody B1 (100μg/ml) (Mag x5)(17).
Discussion
The presence of autoantibodies directed against a wide variety of antigens is a hallmark of SLE. DNA-reactive IgG antibodies precede development of SLE and have been shown to play an important role in the pathogenesis of disease. The analysis of peripheral B cells in patients with SLE reveals a striking increase in autoreactive B cells among antigen-naïve B cells, suggesting a break in early B cell tolerance checkpoints. Most of the antibodies produced by these B cells display polyreactivity recognizing two or more of a panel of antigens that includes dsDNA, ssDNA, LPS, and insulin (1). It is important to note that the insulin reactivity that characterizes these antibodies is not the same as the insulin reactivity assay which is a characteristic serologic feature of individuals with type 1 diabetes and is detected in a fluid phase. The physiologic consequence of insulin reactivity detected by ELISA is not known. Similarly, whether anti-LPS antibodies detected by this ELISA are part of a protective repertoire is not known. Antibodies with the same polyreactive antigenic specificities can be cloned form B cells in the blood of non-autoimmune individuals. Whether they are the same as those cloned from lupus patients is not known.
We isolated IgM+ B cells reactive with a peptide mimetope of dsDNA to ask whether the naïve repertoire of SLE patients contained pathogenic antibodies or whether somatic mutation was necessary for pathogenicity. We isolated IgM antibodies that bound both a peptide mimetope of DNA and dsDNA. Many of these antibodies exhibited the polyreactivity that has been shown to characterize the early B cell repertoire and to be more common in dysregulated B cell repertoires. We asked whether these polyreactive antibodies had pathogenic potential. We wanted to explore the argument that has been put forth that pathogenic autoantibodies have high affinity for autoantigen and are less polyreactive than their germline counterparts. Eleven antibodies were tested for binding to isolated mouse glomeruli and all were positive. Although it is not known that whether all glomerular-binding antibodies can trigger an inflammatory process and lead to lupus nephritis, tissue-binding suggests the pathogenic potential of these antibodies. Furthermore, treatment of glomeruli with DNase did not diminish glomerular binding, demonstrating that the antibodies cross-reacted with glomerular antigens. This observation is consistent with previous studies showing that the renal elutes from both murine lupus models and lupus patients display reactivity to glomerular antigens.
Neuropsychiatric SLE (NPSLE) is an increasingly appreciated clinical manifestation of disease. While the mechanism of central nervous system injury in SLE is not clear yet, there are anti-neuronal antibodies in sera and brains of lupus patients. In recent studies, peptide and dsDNA cross-reactive antibodies have been shown to signal neuronal death through a NMDAR dependent pathway (8, 9). In this study, three polyreactive antibodies, which bind to dsDNA and NMDAR, were injected into the hippocampus of C57Bl/6 mice. They caused significant neuronal cell death while a control antibody did not, demonstrating the pathogenic potential of these polyreactive antibodies in brain.
The current study demonstrates that dsDNA-reactive IgM antibodies from lupus patients can be polyreactive and potentially pathogenic. As the antibodies we analyzed included both germline sequences and somatically mutated sequences, polyreactivity and pathogenicity may co-exist in both naïve and memory B cell subsets.
IgM memory B cells are diminished in lupus patients and an Ig-class switched subset lacking the memory cell marker CD27 has been described in lupus patients (6, 7). These observations could be related to increased Ig class switching of naïve B cells caused by elevated BAFF-levels, overexpression of costimulatory molecules and certain cytokines, such as IL-10, IL-21 (2–5). Antigen-independent class switching in SLE is, in fact, consistent with the reduced frequency of somatic mutation in IgG+ B cells of SLE patients compared to healthy controls (16). We now suggest that these antibodies may be involved in the end organ damage in SLE once they undergo class-switch recombination. Thus, in at least some patients with SLE, there may be a T cell independent pathway to disease activity.
Acknowledgments
Grant support: The study was supported by a grant from the National Institutes of Health (BD) and the Alliance for Lupus Research (BTV).
We would like to thank Sylvia Jones and Zhirong Huang for help in preparing the manuscript and Patricio Huerta for help with the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201(5):703–11. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briere F, Servet-Delprat C, Bridon JM, Saint-Remy JM, Banchereau J. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J Exp Med. 1994;179(2):757–62. doi: 10.1084/jem.179.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204(8):1959–71. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3(9):822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pene J, Gauchat JF, Lecart S, Drouet E, Guglielmi P, Boulay V, et al. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol. 2004;172(9):5154–7. doi: 10.4049/jimmunol.172.9.5154. [DOI] [PubMed] [Google Scholar]
- 6.Jacobi AM, Reiter K, Mackay M, Aranow C, Hiepe F, Radbruch A, et al. Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: delineation by expression of CD27, IgD, and CD95. Arthritis Rheum. 2008;58(6):1762–73. doi: 10.1002/art.23498. [DOI] [PubMed] [Google Scholar]
- 7.Wehr C, Eibel H, Masilamani M, Illges H, Schlesier M, Peter HH, et al. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol. 2004;113(2):161–71. doi: 10.1016/j.clim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 8.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7(11):1189–93. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 9.Kowal C, Degiorgio LA, Lee JY, Edgar MA, Huerta PT, Volpe BT, et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci U S A. 2006;103(52):19854–9. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Jacobi AM, Mackay M, Aranow C, Wang T, Chinnasamy P, et al. Identification of DNA-reactive B cells in patients with systemic lupus erythematosus. J Immunol Methods. 2008;338(1–2):79–84. doi: 10.1016/j.jim.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 12.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Z, Weinstein E, Tuzova M, Davidson A, Mundel P, Marambio P, et al. Cross-reactivity of human lupus anti-DNA antibodies with alpha-actinin and nephritogenic potential. Arthritis Rheum. 2005;52(2):522–30. doi: 10.1002/art.20862. [DOI] [PubMed] [Google Scholar]
- 14.Zhou ZH, Tzioufas AG, Notkins AL. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun. 2007;29(4):219–28. doi: 10.1016/j.jaut.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehrenstein MR, Cook HT, Neuberger MS. Deficiency in serum immunoglobulin (Ig)M predisposes to development of IgG autoantibodies. J Exp Med. 2000;191(7):1253–8. doi: 10.1084/jem.191.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci U S A. 2008;105(28):9727–32. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JY, Huerta PT, Zhang J, Kowal C, Bertini E, Volpe BT, et al. Maternal lupus and congenital cortical impairment. Nat Med. doi: 10.1038/nm.1892. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]