Abstract
The mechanisms underlying effects of aging on functions of pro-angiogenic endothelial progenitor cells (EPCs) are poorly understood. Previous studies demonstrated that human EPCs express high levels of antioxidant enzymes as compared to mature endothelial cells. Here, we hypothesized that aging impairs antioxidant capacity of EPCs. So called ‘early EPCs’ derived from cultured blood mononuclear cells were obtained from healthy young (average 24-year-old) and old (average 72-year-old) subjects. In EPCs of old subjects, the levels of glutathione peroxidase-1 (GPX1) protein and enzymatic activity were significantly reduced. The serum selenium levels in young and old subjects were not significantly different. Increasing selenium concentration in the cell culture also did not affect the protein levels of GPX1, suggesting the reduced GPX1 in old subject's EPCs is selenium-independent. Expressions of catalase, Mn-superoxide dismutase (MnSOD), and CuZnSOD were not affected by aging. EPCs of old subjects were more sensitive to oxidative stress induced by H2O2, as compared with EPCs of young subjects, suggesting that impairment of GPX1 during aging may contribute to low survival ability of EPCs in response to oxidative stress. The results indicate that GPX1 may represent a potential therapeutic target for enhancement of regenerative capacity of EPCs in old subjects.
Keywords: aging, endothelial progenitor cells, GPX1, oxidative stress, human subjects
Introduction
Accumulating evidence from both animal studies and pilot clinical trials has demonstrated that pro-angiogenic endothelial progenitor cells (EPCs) have therapeutic effects including stimulation of neovascularization and regeneration of injured endothelium (1). Aging is one of the major risk factors for the onset and development of cardiovascular diseases (2). Prior studies indicate that aging of human EPCs contributes to atherosclerosis (3), and endothelial dysfunction (4). Moreover, bone marrow-derived progenitor cells obtained from aged mice have reduced homing and engraftment (5), as well as impaired anti-atherosclerotic effect (6). However, the mechanisms underlying effects of aging on functions of EPCs are poorly understood.
Evidence continues to substantiate a strong relationship between oxidative stress and aging (7). Previous studies from our (8) and other groups (9,10) have demonstrated that EPCs express high levels of antioxidant enzymes and as a result are more resistant to oxidative stress as compared with mature endothelial cells. This phenotypic characteristic is critical for EPCs ability to perform regenerative function under conditions of oxidative stress induced by ischemia and vascular injury. Decreased or unbalanced levels of antioxidant enzymes have been found in various tissues in aged animals (11-13), as well as in aged human subjects (14,15). However, little is known about the effect of aging on levels of antioxidant enzymes in EPCs. In the present study, we hypothesized that antioxidant capacity of EPCs is impaired by aging.
Despite intensive search, the unique marker(s) that would enable reliable identification and isolation of EPCs from circulating blood has not been discovered (16). Functional and biochemical analysis of EPCs isolated by culturing peripheral blood mononuclear cells established two populations of cells with endothelial phenotype (8,16-22): early EPCs obtained 3- 7 days of culturing, and late EPCs (blood outgrowth EPCs, or endothelial colony-forming cells) present 2-3 weeks after isolation and culturing of circulating mononuclear cells in endothelial growth medium (EGM). Both populations stimulate angiogenesis and endothelial repair (1, 18-20). The early EPCs have been used in clinical trials designed to develop cell therapy for cardiovascular diseases (23,24). Therefore, experiments in the present study were designed to determine the effect of aging on the antioxidant capacity of early EPCs.
Materials and Methods
Subjects
The protocol for collection and use of human blood samples was approved by the Institutional Review Board at the Mayo Clinic. A total of 25 healthy male subjects were recruited and examined in the Mayo Clinic Center for Translational Science Activities: 13 young (aged 18-29 years) and 12 old (aged 65-83 years). To avoid the complexity of potential effect of reproductive hormones on EPCs, we only recruit male subjects. Subjects were nonsmokers, and free of histories of diagnosed cardiovascular diseases, diabetes, or malignancy. Subjects also had no conditions known to affect mobilization of progenitor cells such as inflammatory diseases, active infection, trauma, and invasive medical procedures for 6 months prior to the blood draw. Subjects did not take medications including ‘over the counter’ medications, vitamins, or supplements for 2 weeks prior to the blood draw. Subjects fasted overnight before blood (200 ml/subject) was drawn.
EPCs isolation, cell culture and phenotyping
EPCs were obtained as previously described (19,23). Briefly, human mononuclear cells were isolated from blood of healthy male volunteers by density gradient centrifugation with Ficoll-Paque Plus (Amersham Biosciences Corp.). After lysis of red blood cells, mononuclear cells were plated at a density of 2×107 cells/well on 6-well plates coated with human fibronectin (R & D Systems Inc.) in endothelial growth medium-2 (EGM-2, Cambrex Corp.), which is composed of endothelial cell basal medium-2 (EBM-2), 2% fetal bovine serum, fibroblast growth factor, vascular endothelial growth factor, insulin-like growth factor, epidermal growth factor, ascorbic acid, hydrocortisone, and heparin. Cells were cultured in a humidified incubator (37° C, 5% CO2). Culture medium was changed every other day. After 4 days of culturing, the attached cells [early EPCs, also referred to as circulating angiogenic cells (16)] were collected for experiments. Morphological appearance and fluorescent confocal microscopy were utilized to define endothelial cell phenotype of EPCs as previously described (8,19,22). In indirect immunofluorescent confocal microscopy analysis, cells were permeabilized with 0.1% Triton-X-100 for 2 min. Mouse anti-human Flk1 (Sigma-Aldrich Co.) and rabbit ant-GPX1 (Lab Frontier) were used at dilution of 1:50 and 1:100, respectively, Texas Red -X conjugated goat anti mouse IgG or anti rabbit IgG (Invitrogen) was used at 1:500.
Western Blot Analysis
Western blotting was performed as previously described (8,22). Equal amount of denatured proteins (20 μg/well) were loaded on the same gel. Rabbit polyclonal antibodies against manganese superoxide dismutase (MnSOD)(8), CuZnSOD (8), glutathione peroxidase -1 GPX1 (Lab Frontier) was used at dilutions of 1:1000, 1:500, and 1:250, respectively, and mouse monoclonal antibodies against catalase (Sigma-Aldrich Co.) at dilution of 1:300. Peroxidase-conjugated antibodies against rabbit or mouse IgG (Amersham Biosciences) was used at a dilutions of 1:5000 or 1:1000, respectively. Blots probed with actin (1:500, Santa Cruz Biotechnology, Inc.) were used as loading controls. The blots were then developed with ECL reagents (GE Healthcare) and exposed to Biomax MR films. The optical densities of the bands were measured by Scion Image (Scion Corp.). Protein expression was normalized to actin and expressed as relative densitometric units.
Zymograms of Antioxidant Enzymes
Total protein of each sample (100 μg/well) was separated in a native polyacrylamide gel. The enzymatic activity staining experiments for GPX was performed as described by Sun et al. (25). The optical densities of the bands were measured by Scion Image (Scion Corp.).
dUTP nick-end labeling (TUNEL) assay
Experiments were performed as described in our previous study (8). Briefly, day 3 EPCs were trypsinized and seeded on 2-well chamber slides (25,000 cells/well, in duplicate). After cells were treated with H2O2 for 24 h, cells on the slides were fixed with 2% paraformaldehyde and permeabilized with 0.1% Triton-X-100. DNA strand breaks were labeled with fluorescien-12-dUTP (Promega), and nuclei were co-stained with the fluorescent dye Hoescht 33528. TUNEL positive (green) cells were detected (in 12 random fields/well), using a fluorescent microscopy. Data are presented as % of total cell number.
Statistical analysis
Data are presented as mean ± SE; n = number of subjects. Differences between mean values of multiple groups were analyzed using ANOVA followed by Tukey test (SigmaStat 3.1 for Windows). Comparison between two groups was made using unpaired Student's t-test. P<0.05 was considered statistically significant.
Results
Characteristics of the subjects
Characteristics of the young and old subjects are shown in Table 1. There was no significant difference in lipid profile between young group and old group. The fasting plasma glucose levels of old subjects were higher than young subjects, but did not meet the criteria for the diagnosis of diabetes (26). The subjects were normotensive (as defined by systolic blood pressure [SBP] <140 mm Hg, and diastolic blood pressure [DBP] <90 mm Hg), though both SDP and DBP are higher in old group as compared with young group.
Table 1.
Subject characteristics
| Characteristics | Young Subjects | Old Subjects | P Value |
|---|---|---|---|
| Age (yrs) | 24 ± 1 | 72 ± 1 | <0.05 |
| Total cholesterol (mg/100 ml) | 156 ± 8 | 180 ± 9 | NS |
| HDL cholesterol (mg/100 ml) | 37 ± 2 | 42 ± 4 | NS |
| LDL cholesterol (mg/100 ml) | 118 ± 7 | 138 ± 7 | NS |
| Triglycerides (mg/100 ml) | 90 ± 16 | 119 ± 12 | NS |
| Fasting glucose (mg/100 ml) | 93 ± 2 | 102 ± 2 | <0.05 |
| Systolic blood pressure (mm Hg) | 119.2 ± 3 | 131.6 ± 4 | <0.05 |
| Diastolic blood pressure (mm Hg) | 65.3 ± 2 | 79.6 ± 2 | <0.05 |
Data are expressed as the mean ± SE. For blood pressure, n=11 in both young and old groups. For all other measurements, n=13 in young group, n=12 in old group.
Antioxidant enzyme profile
After culturing mononuclear cells in EGM-2 for 4 days, the attached cells (EPCs) were round or spindle shaped (Fig. 1A). EPCs were positive for up-take of AcLDL (Fig 1B), and expression of Flk1 (Fig 1C, D). The levels of GPX1 protein and enzymatic activity in EPCs of young subjects were significantly higher than those in EPCs of old subjects (Fig 2A, B). Immunoconfocal microscopy showed that the expression of GPX1 in EPCs of young subjects and old subjects were homogenously distributed, and was consistently stronger in EPCs of young subjects (a typical field is shown in Fig 2C). This indicates that higher levels of GPX1 in EPCs of young subjects are not caused by existence of subset of cell population expressing high levels of GPX1.
Fig 1.

Phenotype of EPCs. Mononuclear cells isolated from blood of healthy individuals were cultured on fibronectin-coated plates in EGM-2 for 4 days. The adherent cells (EPCs) were spindle and round shaped (A) (×10 magnification). B: Fluorescent confocal microscopy demonstrated that EPCs were positive for uptake of DiI AcLDL (red) (×60 magnification). C and D: Immunofluorescence confocal microscopy of EPCs (×100 magnification) labeled with Flk-1 (C), and normal mouse IgG (D, as a control). The secondary antibody was conjugated to Texas-Red. Cell nuclei were counterstained with Hoescht 33528.
Fig 2.
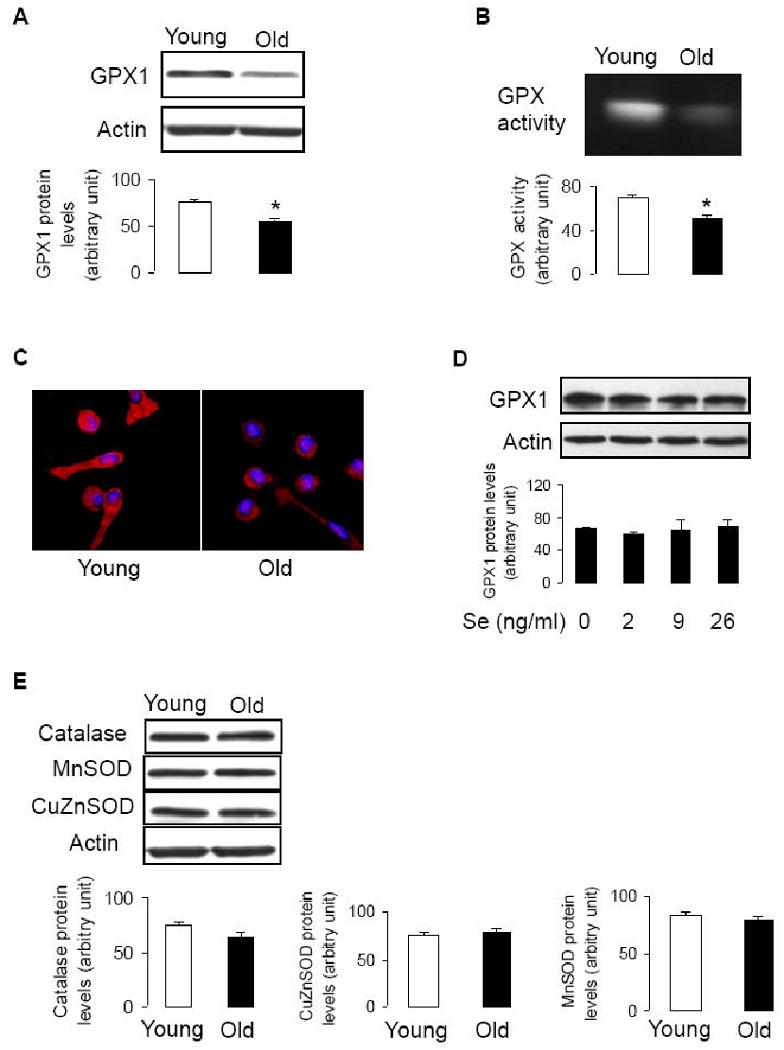
Profile of antioxidant enzymes in EPCs. A and B: Day 4 EPCs were collected for Western blotting (A, n=12-13, *P<0.05, compared to young subjects), and GPX enzymatic activity (B, n=7, *P<0.05, compared to young subjects). C: Immunofluorescence confocal microscopy of EPCs (×100 magnification) immunostained with GPX1. The secondary antibody was conjugated to Texas-Red. Cell nuclei were counterstained with Hoescht 33528. The representative fields are shown. D: Isolated mononuclear cells were cultured in EGM-2 in the presence or absence of sodium selenite for 4 days (medium and treatment was refreshed daily). n=3. E: Protein samples were collected from day 4 EPCs of young or old subjects. For catalase, n=12-13, for CuZnSOD, n=11-12, for MnSOD, n=10-11.
Since selenium is a critical factor affecting GPX1 protein (a selenoprotein) levels and activity (27, 28), we examined selenium levels in the blood of the subjects. The blood levels of selenium were not significantly different between young and old subjects (135.5±6.1 ng/ml, and 138.5±4.8 ng/ml, respectively; n = 6, P>0.05). Thus, decreased GPX1 levels in EPCs of old individuals appeared to be independent of blood levels of selenium. Since it has been reported that supplementation of selenium in cell culture increases GPX1 protein levels (28), we examined the effect of selenium in cell culture on GPX1 protein expression. Isolated mononuclear cells were cultured in EGM-2 supplied with sodium selenite (Se) for 4 days. The GPX1 protein levels in EPCs did not change in response to Se treatment (Fig 2D), though the similar concentrations of Se increased GPX1 expression in human endothelial cells (27,28). It is therefore unlikely that the low GPX1 levels in EPCs of old individuals are caused by selenium deficiency in the medium [EGM-2 contains minimum level of selenious acid (3.9 ng/ml, manufacturer information)].
We also examined protein expression of other antioxidant enzymes. The expression of catalase, MnSOD, and CuZnOSD proteins were not significantly different between EPCs of young and old subjects (Fig 2E). Thus, GPX1 was specifically reduced in EPCs derived from old subjects.
EPCs survival capacity in response to oxidative stress
Next we examined the survival ability of EPCs of young and old subjects in response to oxidative stress. EPCs number of old subjects significantly decreased in response to H2O2 and serum-free treatments, as compared with EPCs of young subjects (Fig 3A, B). TUNEL assay demonstrated that number of apoptotic cells significantly increased in EPCs of old subjects after treatment with H2O2, as compared with EPCs of young subjects (Fig 3C). These results were consistent with reduced GPX1 protein expression and enzymatic activity in EPCs of old subjects.
Fig 3.
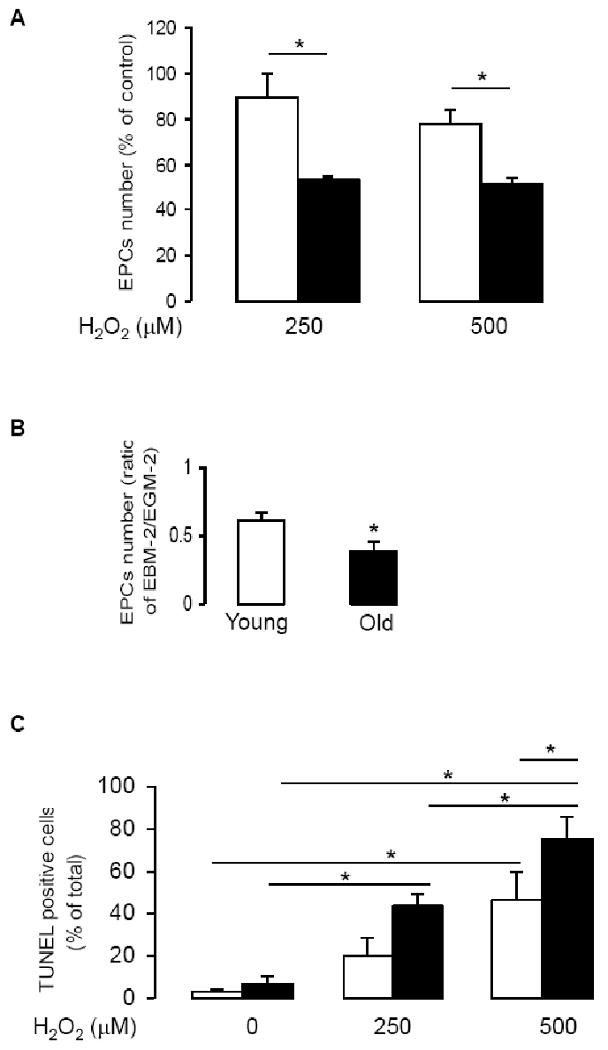
Decreased resistance to oxidative stress decreased in EPCs of old subject. A: Day 3 EPCs were seeded on 2-well chamber slides (25,000 cells/well, in duplicate), and incubated in EGM2 for 24 h. Cells were then treated with indicated concentrations of H2O2 for another 24 h, the attached cells were trypsinized and cell numbers were counted using a hemocytometer. Data are presented as % to control (EGM2 alone); n=6, *P<0.05. B: At day 4 of culturing, EPCs (in 6-well plates) were incubated with EGM2 (4 ml/well) or with EBM2 (a serum- and growth factor-free medium, 4 ml/well) for 24 h. EPCs were collected by trypsinization and counted (n=3, *P<0.05). C: Day 3 EPCs were trypsinized and seeded on 2-well chamber slides. After cells were treated with indicated concentrations of H2O2 for 24 h, TUNEL positive cells were counted (n=3, *P<0.05). Empty columns present young group, filled columns, old group.
Discussion
In the present study, we report two novel findings: 1) the levels of GPX1 protein and enzymatic activity are significantly reduced in EPCs derived from old subjects, and 2) aged EPCs are more sensitive to oxidative stress induced by H2O2 as compared to young EPCs.
To protect themselves from oxidative damage, cells have developed a sophisticated antioxidant enzyme defense system (29), which includes three major primary antioxidant enzymes. SODs (including mitochondrial MnSOD, cytosolic CuZnSOD, and extra-cellular SOD (EC-SOD) convert O2.- into H2O2. Catalase and GPX isoforms catalyze the reduction of H2O2. GPX also scavenges organic hydroperoxides (30). To date, five selenium-containing GPX isoforms have been identified in mammals (31). Classic GPX1 (cGPX) is located in the cytosol and mitochondria of most tissues (32). GPX1 activity is dependent on the incorporation of selenium into its catalytic site (31). Existing evidence suggests that GPX1, together with catalase, CuZnSOD, and MnSOD plays an important role in the aging process (33-36). Indeed, cells from GPX1-deficient mice displayed senescent-like features and were also susceptible to H2O2-mediated cell death (33). Although GPX1-deficient mice appear normal up to 15 months (37), the animals died earlier than control mice when stressed with paraquat and they were significantly more sensitive to cytotoxic effect of H2O2 (30). These studies suggest that GPX1 is a critical antioxidant enzyme, despite possible compensatory protective effects of catalase or peroxiredoxins under normal conditions or in situations of moderate oxidative stress. Consistent with our findings, EPCs isolated from GPX1 deficient mice also had reduced migration ability and increased sensitivity to H2O2 induced apoptosis (38). Additionally, in GPX1 deficient mice, the ability to increase EPCs levels in response to ischemic injury was reduced, and revascularization after hind limb ischemia was impaired (38).
To the best of knowledge, our study is the first human study demonstrating the impairment of GPX1 activity in EPCs of healthy old subjects. Since serum selenium levels were not different between young and old subjects, we ruled out the role of reduced selenium availability in decreased expression and function of GPX1. We also demonstrated that incubation of EPCs in various concentration of selenium for 4 days did not alter GPX1 protein levels. Thus, low levels of GPX1 in EPCs of old subjects were independent of selenium levels in blood and in cell culture medium. We did not measure intracellular levels of glutathione reductase and glutathione (GSH), and therefore we can not rule out the possibility that potential detrimental effect of aging on these two components of the antioxidant system may also contribute to increased cytotoxicity of H2O2 in EPCs of old subjects. We also wish to point out that we did not re-plate non-adherent mononuclear cells 24 h after the seeding, to exclude circulating mature endothelial cells from the EPCs culture. However, the circulating endothelial cells are very rare in the healthy subjects. Indeed, less than 3 cells/ml of blood have been detected in healthy older population (39,40). In our study, this translates into approximately 4 mature endothelial cells/106 isolated mononuclear cells in the EPCs culture. It is therefore very unlikely that rare circulating mature endothelial cells may significantly affect the differences in the level of GPX1 between EPCs of young and old subjects.
We also analyzed the resistance to oxidative stress of EPCs derived from young and old individuals. Consistent with low levels of GPX1, EPCs of old subjects had lower survival ability under the stress caused by H2O2 treatment. EPCs of old subjects were also more sensitive to serum-starvation. Since EPCs of young and old subjects had similar protein levels of catalase, the decreased resistance to H2O2 in aged EPCs is most likely caused by the low levels of GPX1.
In summary, our study provides novel information regarding the effect of aging on antioxidant capacity in human EPCs. In the EPCs of healthy old subjects, we observed selective loss of GPX1. These results suggest that enhancement of GPX1 activity may represent a potential therapeutic approach to improve regenerative function of EPCs during aging.
Acknowledgments
This work was supported in part by National Heart, Lung, and Blood Institute Grants: HL-53524 and HL-91867 (ZSK), HL-46493 and HL-83947(MJJ), National Institutes of Health (NIH) RR 25150 (MJJ), the American Heart Association (AHA) Scientist Development Grant 09SDG2190046 (TH), and the Mayo Foundation.
We would like to thank Karen P. Krucker, RN for helping with subject recruitment.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 3.Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 5.Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 7.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 8.He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 9.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 10.Cai H, Gehrig P, Scott TM, Zimmermann R, Schlapbach R, Zisch AH. MnSOD marks cord blood late outgrowth endothelial cells and accompanies robust resistance to oxidative stress. Biochem Biophys Res Commun. 2006;350:364–369. doi: 10.1016/j.bbrc.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 11.Ozturk O, Gumuslu S. Changes in glucose-6-phosphate dehydrogenase, copper, zinc-superoxide dismutase and catalase activities, glutathione and its metabolizing enzymes, and lipid peroxidation in rat erythrocytes with age. Exp Gerontol. 2004;39:211–216. doi: 10.1016/j.exger.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Cejkova J, Vejrazka M, Platenik J, Stipek S. Age-related changes in superoxide dismutase, glutathione peroxidase, catalase and xanthine oxidoreductase/xanthine oxidase activities in the rabbit cornea. Exp Gerontol. 2004;39:1537–1543. doi: 10.1016/j.exger.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J. 2005;19:419–421. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- 14.Matsubara LS, Machado PE. Age-related changes of glutathione content, glutathione reductase and glutathione peroxidase activity of human erythrocytes. Braz J of Med & Biol Res. 1991;24:449–454. [PubMed] [Google Scholar]
- 15.Sinha S. Anti-oxidant gene expression imbalance, aging and Down syndrome. Life Sci. 2005;76:1407–1426. doi: 10.1016/j.lfs.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 18.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 19.He T, Smith LA, Harrington S, Nath KA, Caplice NM, Katusic ZS. Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke. 2004;35:2378–2384. doi: 10.1161/01.STR.0000141893.33677.5d. [DOI] [PubMed] [Google Scholar]
- 20.Kong D, Melo LG, Mangi AA, Zhang L, Lopez-Ilasaca M, Perrella MA, Liew CC, Pratt RE, Dzau VJ. Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation. 2004;109:1769–1775. doi: 10.1161/01.CIR.0000121732.85572.6F. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He T, Lu T, d'Uscio LV, Lam CF, Lee HC, Katusic ZS. Angiogenic function of prostacyclin biosynthesis in human endothelial progenitor cells. Circ Res. 2008;103:80–8. doi: 10.1161/CIRCRESAHA.108.176057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N, Grünwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 24.Schächinger V, Aicher A, Döbert N, Röver R, Diener J, Fichtlscherer S, Assmus B, Seeger FH, Menzel C, Brenner W, Dimmeler S, Zeiher AM. Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation. 2008;118:1425–1432. doi: 10.1161/CIRCULATIONAHA.108.777102. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Elwell JH, Oberley LW. A simultaneous visualization of the antioxidant enzymes glutathione peroxidase and catalase on polyacrylamide gels. Free Rad Res Commun. 1988;5:67–75. doi: 10.3109/10715768809066913. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association. Standards of medical care in diabetes-2008. Diabetes Care. 2008;31(Suppl 1):S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 27.Miller S, Walker SW, Arthur JR, Nicol F, Pickard K, Lewin MH, Howie AF, Beckett GJ. Selenite protects human endothelial cells from oxidative damage and induces thioredoxin reductase. Clin Sci. 2001;100:543–550. [PubMed] [Google Scholar]
- 28.Handy DE, Hang G, Scolaro J, Metes N, Razaq N, Yang Y, Loscalzo J. Aminoglycosides decrease glutathione peroxidase-1 activity by interfering with selenocysteine incorporation. J Biol Chem. 2006;281:3382–3388. doi: 10.1074/jbc.M511295200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979;39:1141–1149. [PubMed] [Google Scholar]
- 30.de Haan JB, Bladier C, Griffiths P, Kelner M, O'Shea RD, Cheung NS, Bronson RT, Silvestro MJ, Wild S, Zheng SS, Beart PM, Hertzog PJ, Kola I. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. J Biol Chem. 1998;273:22528–2236. doi: 10.1074/jbc.273.35.22528. [DOI] [PubMed] [Google Scholar]
- 31.Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- 32.Esworthy RS, Ho YS, Chu FF. The Gpx1 gene encodes mitochondrial glutathione peroxidase in the mouse liver. Arch Biochem Biophys. 1997;340:59–63. doi: 10.1006/abbi.1997.9901. [DOI] [PubMed] [Google Scholar]
- 33.de Haan JB, Bladier C, Lotfi-Miri M, Taylor J, Hutchinson P, Crack PJ, Hertzog P, Kola I. Fibroblasts derived from Gpx1 knockout mice display senescent-like features and are susceptible to H2O2-mediated cell death. Free Rad Biol Med. 2004;36:53–64. doi: 10.1016/j.freeradbiomed.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 34.Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 35.Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat Gen. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 36.Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–672. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem. 1997;272:16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 38.Galasso G, Schiekofer S, Sato K, Shibata R, Handy DE, Ouchi N, Leopold JA, Loscalzo J, Walsh K. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res. 2006;98:254–261. doi: 10.1161/01.RES.0000200740.57764.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dignat-George F, Sampol J. Circulating endothelial cells in vascular disorders: new insights into an old concept. Eur J Haematol. 2000;65:215–220. doi: 10.1034/j.1600-0609.2000.065004215.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee KW, Lip GY, Tayebjee M, Foster W, Blann AD. Circulating endothelial cells, von Willebrand factor, interleukin-6, and prognosis in patients with acute coronary syndromes. Blood. 2005;105:526–532. doi: 10.1182/blood-2004-03-1106. [DOI] [PubMed] [Google Scholar]


