Abstract
The effect of estrogen on the number and size of cholinergic neurons in the basal forebrain was examined in surgically menopausal young and middle-aged cynomolgus monkeys. Young and middle-aged female monkeys were ovariectomized and treated with conjugated equine estrogens (Premarin) at doses that are equivalent to those currently prescribed to postmenopausal women. In the medial septum/diagonal band (MS/DB), no effect of treatment with Premarin was observed in the cholinergic neurons in either ovariectomized young or middle-aged monkeys. However, the number and size of cholinergic neurons in the MS/DB of middle-aged monkeys was greater than that in the young monkeys. In the nucleus basalis of Meynert (NBM) of middle-aged monkeys, the number of cholinergic neurons in the intermediate region (Ch4i) was greater in Premarin-treated monkeys as compared to controls and numbers of neurons in this region were greater at higher levels of estrogen. No effects of estrogen were observed in other NBM regions in the middle-aged monkeys and the size of cholinergic neurons was unaffected by Premarin. These findings suggest that treatment with Premarin has selective beneficial effects on cholinergic neurons in the basal forebrain but that these effects are both age and region specific.
Keywords: Premarin, nucleus basalis, medial septum, diagonal band, ovariectomy, stereology
1. Introduction
The basal forebrain cholinergic system (i.e., the medial septum (MS), the horizontal and vertical limbs of the diagonal band of Broca (DB), and the nucleus basalis of Meynert (NBM)) plays an important role in learning, memory and attention functions (e.g., Everett and Robbins, 1997; Olton et al., 1991; Parent and Baxter, 2004; Voytko et al., 1994) and projects to the hippocampal formation and to the neocortex (Dutar et al., 1995; Mesulam et al., 1983; Woolf, 1991). The cholinergic system may be a mechanism through which estrogen can affect cognition. For example in rodents, immunotoxic lesions of basal forebrain cholinergic neurons blocked the ability of estrogen to enhance spatial learning (Gibbs, 2002; Gibbs, 2007). Estrogen attenuated the ability of the muscarinic antagonist scopolamine to induce deficits in memory acquisition (Gibbs et al., 1998) and an estrogen-induced improvement in working memory was blocked by an M2 receptor antagonist (Daniel and Dohanich, 2001; Daniel et al., 2005.) In a non-human primate model, estrogen improved visual spatial attention in ovariectomized (OVX) monkeys, and this effect was modulated by scopolamine treatment (Voytko, 2002)
Basal forebrain cholinergic neurons and cholinergic fibers respond to levels of circulating estrogen in animals. Although the majority of this work has been conducted in rodents (Gibbs, 2000), the few studies performed in OVX monkeys suggests that estrogen can modulate aspects of primate cholinergic function, but that different cholinergic indices may respond differently. Treatment with estrogen for either one month or two years prevented decreases in cholinergic fiber density in layer II of the prefrontal cortex in young OVX monkeys (Kritzer and Kohama, 1999; Tinkler et al., 2004). However, treatment with estrogen for two years had no effect on numbers or size of cholinergic neurons in the NBM (Tinkler et al., 2004) or on choline acetyltransferase (ChAT) or acetylcholinesterase (AChE) activity in multiple cortical regions, including the MS/DB (Gibbs et al., 2002). In contrast, treatment with cyclical estrogen for only one month increased ChAT expression in the DB, but not NBM, of young OVX monkeys (Kompoliti et al., 2004). To date, only one study has investigated the effects of estrogen in the cholinergic system in older monkeys. Kompoliti et al. (2004) reported that ChAT expression was increased in the vertical limb of the DB, but not the NBM, in middle-aged OVX monkeys that received two injections of estrogen over one month, but that a loss of AChE-stained fibers was found in layer II of the entorhinal, insular, and cingulate cortices in these same animals. In this study, OVX control middle-aged monkeys had greater AChE fiber density in all regions sampled than OVX control young monkeys, the investigators concluded that the estrogen effect in the older monkeys may have been to reduce the AChE fiber density to that of the younger monkeys. However the number or size of the cholinergic neurons themselves was not evaluated.
While the majority of animal studies have used estradiol (E2), the most frequent form of estrogen therapy (ET) prescribed to postmenopausal women in the United States is conjugated equine estrogens, of which Premarin is the most frequently used (Ancelin and Ritchie, 2005). In contrast, various forms of E2 are prescribed more frequently to women outside of the United States (Ancelin and Ritchie, 2005; Rozenberg et al., 2000). In animal models, the central nervous system effects of Premarin are beginning to be evaluated in rodents (Celik et al. 2005, Jin et al. 2005, Rhodin et al., 2003) and in monkeys (Gibbs et al., 2002; Gibbs et al., 2006; Tinkler et al., 2004), however all these studies have involved young animals. Nothing is known about the effects of Premarin in the brain of older animals.
Given the limited information about estrogen effects in the cholinergic system of older monkeys, and specifically the limited information available about the effects of Premarin in the monkey brain, the purpose of the present study was to investigate the effects of Premarin on the cholinergic neurons in the basal forebrain of young and middle-aged OVX monkeys. We examined the effects of two years of treatment with Premarin on the number and size of cholinergic neurons in the MS/DB of young and middle-aged OVX monkeys, and on the number and size of cholinergic neurons in the NBM of middle-aged OVX monkeys; we previously reported the effects of two years of Premarin on cholinergic neurons in the NBM of young OVX monkeys (Tinkler et al., 2004). Our current study differed from the only other study conducted in the basal forebrain of older OVX monkeys (Kompoliti et al., 2004) in several major ways: 1) our monkeys received hormone therapy for two years, 2) Premarin was the formulation of estrogen in our therapy, 3) monkeys received continuous hormone therapy, and 4) our focus was on the number and size of the basal forebrain cholinergic neurons. Our hypotheses were that the number and size of cholinergic neurons in both the MS/DB and NBM of OVX monkeys treated with Premarin would be greater than in untreated OVX monkeys.
2. Results
2.1 Estrogen assays
Mean serum E2 levels were significantly greater in young Premarin-treated monkeys than young OVX monkeys (mean of 6, 12, and 24 month time points: OVX = 6.75 pg/ml ± 1.20 vs Premarin = 102.92 pg/ml ± 25.68; F (1, 9) = 28.90, p < 0.01) and in middle-aged Premarin-treated monkeys than middle-aged OVX monkeys (mean of 6, 12, and 24 month time points: OVX = 5 pg/ml ± 0.0 vs Premarin = 123.11 pg/ml ± 17.81; F (1,10) = 43.55, p< 0.01).
2.2 Medial septum/vertical limb diagonal band cholinergic neuron number and size in young and middle-aged Premarin-treated and OVX monkeys
Immunocytochemical processing for vesicular acetylcholine transporter (VAChT) revealed intensely stained scattered neurons in the MS and a greater number of densely packed neurons in the more ventrally located vertical limb of the DB (Fig. 1). Control sections that were processed identically except for the omission of the primary antibody did not display any staining.
Figure 1.
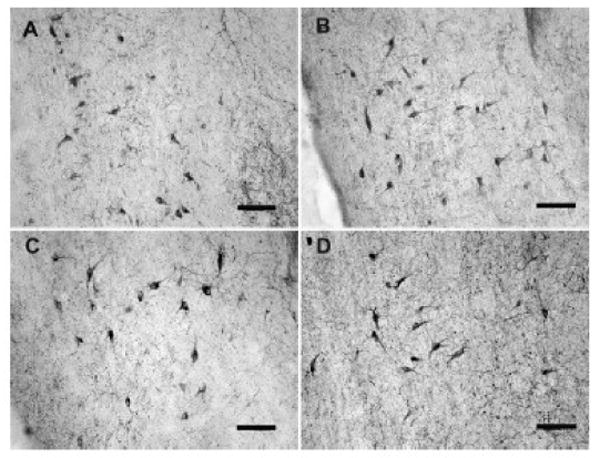
Photomicrographs of VAChT-stained neurons in the MS/DB in Premarin-treated and OVX young and middle-aged monkeys. A) OVX young animal. B) Premarin-treated young animal. C) OVX middle-aged animal. D) Premarin-treated middle-aged animal. Scale bars = 100 microns.
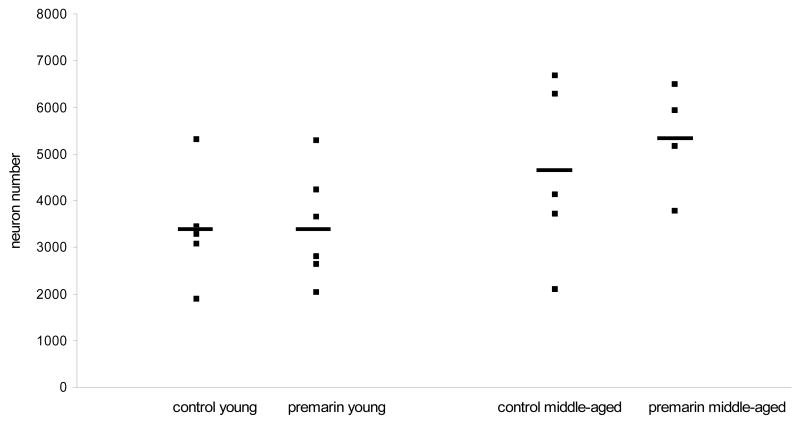
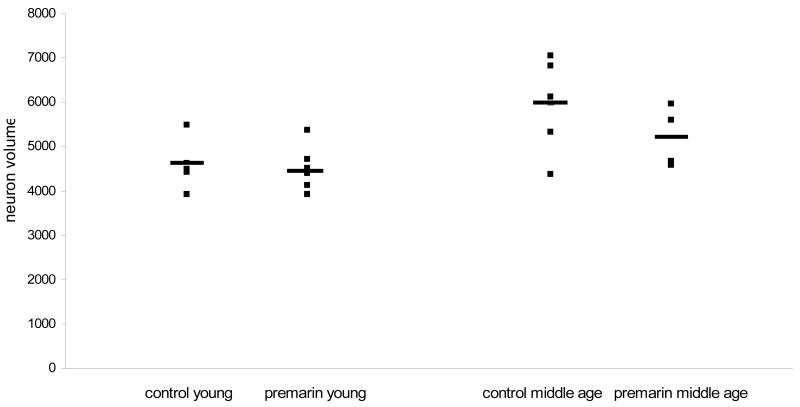
Stereological estimates of MS/DB cell number and size are presented in Table 1. No differences were found in the number of VAChT-positive MS/DB neurons between Premarin-treated and OVX young monkeys (F (1,9) = 0.001, p > 0.05) or between Premarin-treated or OVX middle-aged monkeys (F (1,7) = 0.192, p > 0.05) (Fig. 2). Similarly, there was no difference in the size of VAChT-positive neurons in the MS/DB between Premarin-treated and OVX young monkeys (F (1,9) = 0.046, p > 0.05) or between Premarin-treated and OVX middle-aged monkeys (F (1,7) = 1.643, p > 0.05) (Fig. 3). As there was no difference between the treatment groups of either age, the treatment groups within each age were collapsed and comparisons were made of numbers and size of VAChT-positive neurons in the MS/DB between the young and middle-aged monkeys. There was a significant increase in both the number (F (1,18) = 6.92, p = 0.02) and size (F (1,18) = 9.50, p < 0.01) of MS/DB VAChT-positive neurons in the middle-aged monkeys compared to the young monkeys. Collectively, the middle-aged monkeys had a 31% increase in numbers of VAChT-positive neurons in the MS/DB compared to the young monkeys and a 12% increase in size of these neurons compared to the young monkeys.
Table 1.
Estimates of cholinergic cell numbers and volume in basal forebrain.
| Number of VAChT-positive neurons | CE | Volume of VAChT-positive neurons (μm3) | CE | ||
|---|---|---|---|---|---|
| MS/DB | |||||
| Young | |||||
| Control | mean | 3390 | .08 | 4576.2 | .0024 |
| s.d. | 1229.2 | 574.0 | |||
| CV* | .362 | .125 | |||
| Premarin | mean | 3437.3 | .07 | 4506.3 | .0023 |
| s.d. | 1188.1 | 509.4 | |||
| CV | .344 | .113 | |||
| Middle-aged | |||||
| Control | mean | 4582.6 | .082 | 5934.0 | .0026 |
| s.d. | 1902.6 | 1104.2 | |||
| CV | .415 | .186 | |||
| Premarin | mean | 5332.8 | .08 | 5199.3 | .0025 |
| s.d. | 1177.9 | 670 | |||
| CV | .221 | .129 | |||
| NBM- middle-aged | |||||
| Ch4a | |||||
| Control | mean | 12883.5 | .065 | 7255 | .0015 |
| s.d. | 6771.7 | 220.7 | |||
| CV | .526 | .042 | |||
| Premarin | mean | 13123.3 | .078 | 7185 | .0013 |
| s.d. | 4583.7 | 661.4 | |||
| CV | .349 | .092 | |||
| Ch4i | |||||
| Control | mean | 5754 | .08 | 8249.6 | .0023 |
| s.d. | 1713.8 | 1353.6 | |||
| CV | .297 | .164 | |||
| Premarin | mean | 9480.3 | .058 | 7684 | .001 |
| s.d. | 2664.1 | 717 | |||
| CV | .27 | .091 | |||
| Chp | |||||
| Control | mean | 2209.3 | .1 | 8038.2 | .0047 |
| s.d. | 823.8 | 1768.2 | |||
| CV | .373 | .220 | |||
| Premarin | mean | 2211.0 | .085 | 6511.7 | .0047 |
| s.d. | 516.0 | 833.8 | |||
| CV | .233 | .128 |
s.d./mean
Figure 2.
Number of VAChT-stained cells in the MS/DB in Premarin-treated and OVX young and middle-aged monkeys. There were no significant differences in neuron number between the Premarin and OVX monkeys of either age group, however collectively the middle-aged monkeys had greater numbers of neurons than the young monkeys.
Figure 3.
Size of VAChT-positive neurons in the MS/DB in Premarin-treated and OVX young and middle-aged monkeys. There were no differences in neuronal size between the Premarin and OVX monkeys of either age group, however collectively the neurons in the middle-aged monkeys were larger than those of the young monkeys.
2.3 Nucleus basalis cholinergic neuron number and size in middle-aged Premarin-treated and OVX monkeys
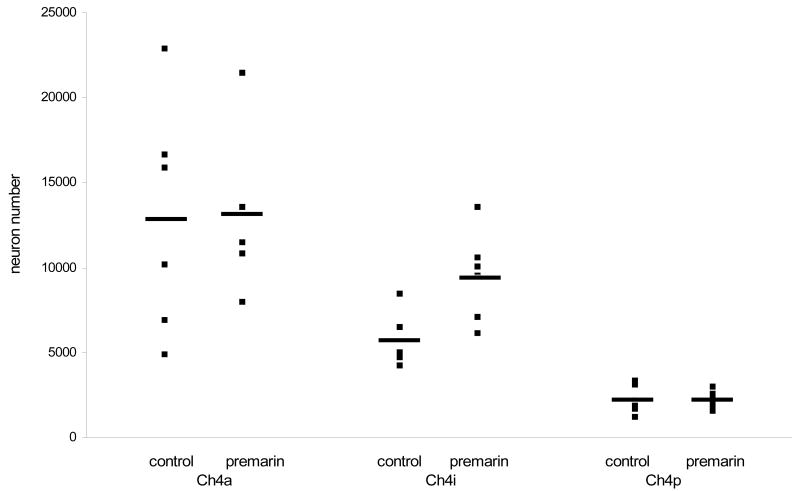
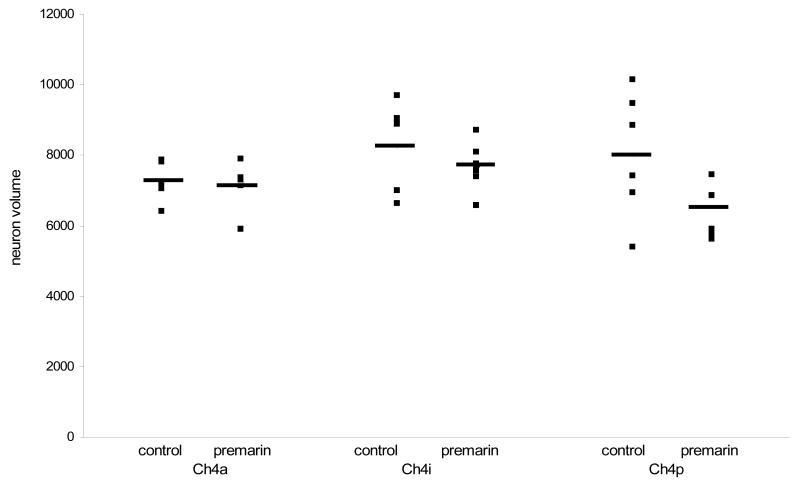
Immunoprocessing for VAChT revealed large, intensely stained cells in the NBM of the middle-aged monkeys (Fig. 4). Stereological estimates of NBM cell number and size are presented in Table 1. There was no difference between Premarin-treated and OVX middle-aged monkeys in the number of VAChT-positive neurons in Ch4a (F (1,10) = 0.005, p > 0.05) or in Ch4p (F (1,10) = 0.000, p > 0.05). However, Premarin-treated middle-aged monkeys had more VAChT-positive neurons in Ch4i than control monkeys (F (1,10) = 8.82, p < 0.02) (Fig. 5). There was no difference in size of the VAChT-positive neurons between Premarin-treated and OVX middle-aged monkeys in any region of the NBM (p >0.05 for all regions) (Fig. 6).
Figure 4.
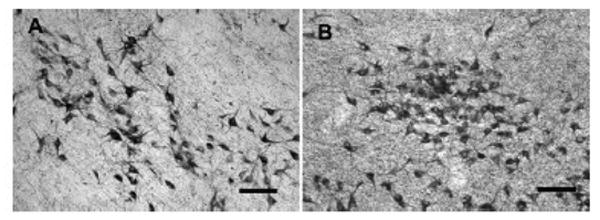
Photomicrographs of VAChT-stained neurons in the NBM in Premarin- treated and OVX middle-aged monkeys. A) OVX. B) Premarin. Scale bars = 100 microns.
Figure 5.
Number of VAChT-positive neurons in the anterior (Ch4a), intermediate (Ch4i), and posterior (Ch4p) NBM in Premarin-treated and OVX middle-aged monkeys. Numbers of neurons were greater in the Ch4i region of monkeys treated with Premarin than OVX monkeys. There were no differences between the groups for any other region. *, p < 0.02.
Figure 6.
Size of VAChT-positive neurons in the anterior (Ch4a), intermediate (Ch4i) and posterior (Ch4p) NBM in Premarin-treated and OVX middle-aged monkeys. There was no difference in the size of neurons between the groups in any region of the NBM.
Serum E2 was positively correlated with numbers of VAChT-positive neurons in the Ch4i region of the NBM in the middle-aged monkeys (r = 0.73, F (1,10) = 11.16, p < 0.01) (Fig. 7), but not with any other neuroanatomical assessments of neuron number or size for either the young or middle-aged monkeys.
Figure 7.
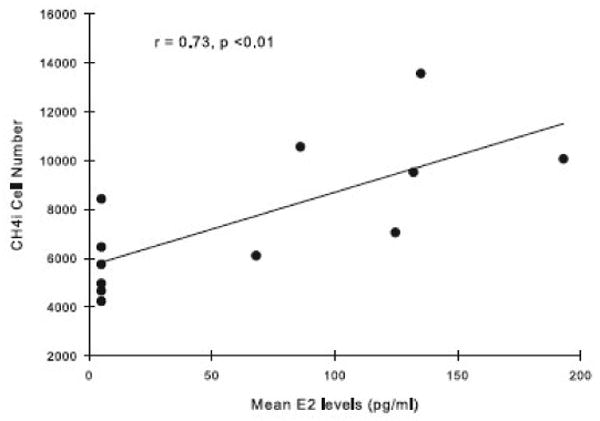
Relationship between numbers of VAChT-positive neurons in the Ch4i region of the NBM and serum E2 levels in Premarin-treated and OVX middle-aged monkeys. Numbers of Ch4i neurons significantly increased with rising levels of E2.
3. Discussion
This study is the first to examine the anatomical integrity of cholinergic neurons in the MS/DB of young OVX monkeys, and throughout the basal forebrain in middle-aged OVX monkeys, following long-term treatment with Premarin. Cholinergic neurons were affected by treatment with Premarin, but the responses were region specific.
3.1 The monkey model
Female macaque monkeys share many similarities in their reproductive and endocrine profiles to that of women. Female macaques have a 28-day menstrual cycle, with estrogen and progesterone patterns that closely mirror those of women (Jewett and Dukelow, 1972; Goodman et al., 1977) and they experience a similar menopause (Johnson and Kapsalis, 1995; Gilardi et al., 1997). In the present study, the treatment with Premarin produced levels of E2 in the monkeys that are seen during the follicular phase of the menstrual cycle in both female macaques and women (Speroff and Van de Wiele, 1971; Goodman et al., 1977).
3.2 Medial septum/vertical limb diagonal band in young and middle-aged Premarin-treated and OVX monkeys
Long-term treatment with Premarin, at doses that are equivalent to those prescribed to postmenopausal women, had no effect on morphometric indices of VAChT-positive neurons in the MS/DB of OVX young or middle-aged monkeys. Similar to our observations, neither ChAT or AChE activity in the MS were affected by similar durations of continuous treatment with the .04 mg/kg dose of Premarin in young OVX monkeys (Gibbs et al., 2002). Together, these observations suggest that Premarin has neither positive or adverse effects on these measures of the cholinergic system in this basal forebrain region.
Collectively, there was an increase in both the number and size of MS/DB VAChT-positive neurons in the middle-aged monkeys compared to the young animals. Although the young and middle-aged monkeys were subjects of different studies, these animals had identical experimental conditions (i.e, housed in the same facility and under the same conditions, fed the same diet, had the same surgeons, had similar blood sampling schedules, and experienced minimal other manipulations) and were immunoprocessed together. Therefore, it is unlikely that the results were influenced by experimental or processing conditions that differed between the two age groups. We do not know if the differences between the groups are related to true age-related differences in the normal indices of MS/DB neurons or to an age-related difference in response of these neurons to OVX. In the only study conducted to date, numbers of MS ChAT-positive neurons were decreased, but the size of these neurons was increased in old monkeys compared to young monkeys (Stroessner-Johnson et al., 1992). Although several methodological differences exist between studies, the increase in size of cholinergic identified neurons in older monkeys in our study and that of Stroessner-Johnson suggests that our findings in OVX monkeys are likely related to an effect of advancing age rather than to a difference in response to OVX. In addition, our findings suggest that age-related increases in the size of MS neurons may begin to occur at middle-age in monkeys. The increases in size of MS/DB cholinergic neurons of aged monkeys may reflect a compensatory reaction in response to alterations in the target fields of these neurons, e.g., hippocampus. Indeed age-related reductions in cholinergic innervation of the primate hippocampus have been reported (Calhoun et al., 2004; Conner et al., 2001) and may be present in the middle-aged monkeys of the present study.
3.3 Nucleus basalis in middle-aged Premarin-treated and OVX monkeys
We previously reported that two years of Premarin had no effect on either numbers or size of NBM VAChT-positive neurons in young OVX monkeys (Tinkler et al., 2004). In contrast to what we found in young monkeys, in the present study of middle-aged OVX monkeys we found a significant increase in the number of VAChT-positive neurons in the intermediate (Ch4i) region of the NBM following two years of Premarin treatment. Moreover, this observation occurred in the context of a dose of Premarin that was only half of that given to the young monkeys of our previous study (0.02 mg/kg vs 0.04 mg/kg; Tinkler et al., 2004). Additionally, we found that increasing levels of E2 in the middle-aged monkeys were associated with increasing numbers of VAChT-positive neurons in Ch4i. In studies of normal aging in monkeys, numbers of cholinergic neurons identified by p75 immunoprocessing in Ch4i, but not CH4a, were decreased in aged monkeys compared to young animals (Smith et al., 1999; Smith et al., 2004); however not all studies concur (Voytko et al., 1995). Collectively, these findings suggest that the cholinergic system may be compromised with normal aging in monkeys and thus may explain why Ch4i was more responsive to treatment with hormone therapy in our OVX middle-aged monkeys than in our OVX young monkeys.
Unlike neurons in the Ch4i region of the NBM, neurons in Ch4a or Ch4p did not respond to Premarin in the middle-aged monkeys of our study. Studies indicate that estrogen's effects on the cholinergic system in monkeys can be regionally specific and/or selective for particular indices of the cholinergic system. For example, in young OVX monkeys, cholinergic fiber density in layer II of the prefrontal cortex responded to Premarin but there was no response in the other prefrontal cortical layers or in the parietal cortex (Tinkler et al., 2004). In our current study, the mechanisms underlying Premarin's selectivity and specificity within the NBM are unknown.
Cholinergic neurons in Ch4i project to a number of cortical regions including orbital and periarcuate frontal cortex, insula, inferior parietal lobe, inferior temporal lobe, and parahippocampal cortex (Mesulam et al., 1983). The response to Premarin in the Ch4i of middle-aged monkeys in the present study may reflect changes that are occurring in those cortical regions in response to estrogen. For example, one month of cyclical ET reduced cholinergic innervation in the insular cortex of middle-aged OVX monkeys (Kompoliti et al., 2004). Perhaps similar alterations occurred in the cholinergic innervation of Ch4i target fields in our middle-aged monkeys and the increase in number of Ch4i VAChT-positive neurons signal these target region changes.
3.4 Implications for cognitive function and postmenopausal women
Many factors of the hormone therapy used in the present study in OVX monkeys are directly relevant to the primary hormone therapy that is prescribed to postmenopausal women in the United States. Specifically, 1) the hormone administration was a long-term continuous schedule, 2) a dose of Premarin was used that is equivalent to the 0.625mg dose most frequently prescribed to women prior to the recently reported adverse results of the Women's Health Initiative (WHI) (The Women's Health Initiative Steering Committee, 2004; Writing Group for the Women's Health Initiative Investigators, 2002) and its ancillary study, the Women's Health Initiative Memory Study (WHIMS) (Espeland et al., 2004; Rapp et al., 2003; Shumaker et al., 2003; Shumaker et al., 2004)], and 3) a dose of Premarin was used that is equivalent to the lower 0.312 mg dose of Premarin that currently is being prescribed to postmenopausal women following the WHI/WHIMS adverse findings. Moreover, because monkeys age approximately three times faster than humans, the two-year course of treatment of our monkeys is equivalent to six years of treatment of a postmenopausal woman. Thus, the results presented here are directly relevant to the current clinical practice being used to treat postmenopausal women and a length of time many postmenopausal women take hormone therapy.
While the dose of Premarin used in the middle-aged monkeys of our study was half of that used in the young monkeys, this dose has beneficial effects on physiological measures in postmenopausal women (Peeyananjarassri et al., 2005) and reduced the extent of coronary artery atherosclerosis in OVX monkeys (Appt et al., 2006). It is unknown if an equivalent dose of Premarin to that given to the young monkeys would have resulted in different outcomes in the middle-aged monkeys, but this is an important avenue for further exploration. Premarin is equivalent to E2 in exerting positive effects on neurons in cell culture (Brinton et al., 1997; Brinton et al., 2000) and in physiological systems of monkeys (Adams et al., 1990; Clarkson et al., 2001; Clarkson et al., 2002; Clarkson et al., 2004; Jayo et al., 1998; Jerome et al., 1994). Estradiol can improve cognitive function in both rodents (e.g., Gibbs, 1999; Gresak and Frick, 2006; Vaucher et al., 2002; Zurkovsky et al., 2007) and monkeys (Lacreuse, 2006; Rapp et al., 2003; Tinkler and Voytko, 2005; Voytko, 2002) and modulates cognitive processes through the cholinergic system (e.g., Daniel et al., 2001; Daniel et al., 2005; Dohanich et al., 1994; Fader et. al., 1998; Gibbs, 2002; Voytko, 2002). Although the effects of Premarin on cognitive processes has not been examined in animal models of menopause, the fact that the cholinergic system was modulated by either Premarin dose we have used in our investigations of surgically menopausal monkeys (prefrontal cholinergic fibers in Tinkler et al. [2004] and NMB cholinergic neurons in present study), suggests that, like E2, it may impact the cognitive processes in which these regions play a role, e.g. memory and attention function (Castner et al., 2004; Hopfinger et al., 2001; Voytko et al., 1994). Indeed, clinical studies of postmenopausal women taking Premarin have shown beneficial effects in these cognitive domains that are sensitive to manipulations of the cholinergic system in animals, although the outcomes of studies in women have been controversial (reviewed in LeBlanc et al., 2001; Maki and Hogervorst, 2003; Maki, 2005; Sherwin, 2006).
The female macaque monkey is an excellent model in which to examine the effects of ovarian hormones in the brain because their menstrual cycle and response to surgical menopause is similar to that of women. (Adams et al., 1990; Goodman et al., 1977; Jayo et al., 1998; Jerome et al., 1994; Jewett and Dukelow, 1972). Moreover, there are many parallels in the neural and cognitive aging of macaques with that of humans (reviewed in Voytko, 1997; Voytko and Tinkler, 2004). Thus, studies performed in OVX female macaques can be valuable to furthering our understanding of how menopause and estrogen affects the neural and cognitive profiles of women. Just as vital, these monkey models of menopause will be critical in sorting out the most appropriate formulation and regimen of ET that will have the greatest beneficial impact on the cognitive function and well-being of postmenopausal women.
4. Experimental Procedure
4.1 Animal subjects and tissue preparation
All procedures involving animals were conducted in compliance with state and federal laws, standards of the United States Department of Health and Human Services, and guidelines established by the Wake Forest University School of Medicine Institutional Animal Care and Use Committee.
The brains of 11 young female cynomolgus monkeys (Macaca fascicularis, ages 5-9 years) were available from a study that assessed the effects of OVX and ET on peripheral physiological parameters (M. Jayo, unpublished observations). These animals had been the subjects of our previous study examining the effects of Premarin on neurons in the NBM of young monkeys (Tinkler et al., 2004). The animals were socially housed in groups of 4-5 monkeys at Wake Forest University School of Medicine and fed a moderately atherogenic diet that was produced in the Wake Forest University School of Medicine Comparative Medicine Research Center diet lab. This diet was used previously in studies of the effects of hormone replacement on heart and bone in monkeys (Adams et al., 1990; Clarkson et al., 2001). Brains from 12 female middle-aged cynomolgus monkeys (Macaca fascicularis, ages 18-23 years) were available from a study that assessed the effects of Premarin on bone biomarkers (Lees et al., 2007). As with the young animals, these middle-aged monkeys were socially housed at Wake Forest University School of Medicine and fed a moderately atherogenic diet.
All animals received bilateral ovariectomies performed under ketamine (15 mg/kg, i.m.) and butorphanol (0.025 mg/kg, i.m.). Of the young monkeys, 6 were OVX and untreated for 24 months (OVX group), and 5 were OVX and given a daily oral dose of Premarin (0.04 mg/kg, equivalent to the standard dose of 0.625 mg prescribed to postmenopausal women; Wyeth-Ayherst, Radnor, PA) mixed with their food for 24 months. Of the middle-aged monkeys, 6 were OVX and untreated for 24 months (OVX group) and 6 were OVX and were given a daily oral dose of Premarin (0.02 mg/kg, equivalent to the dose of 0.312 mg prescribed to postmenopausal women; Wyeth-Ayherst, Radnor, PA) mixed with their food for 24 months.
For necropsy, the monkeys were restrained with ketamine (15 mg/kg, i.m.), anesthetized with sodium pentobarbital (35 mg/kg, i.v.) and perfused with lactated Ringer's solution. The brain was removed within ∼10 minutes of completion of perfusion, blocked coronally, and immediately immersion-fixed in 4% paraformaldehyde, pH 7.4, for two weeks. The size of the brain block containing the basal forebrain was approximately 3.0 -3.5 cm extending from the tip of the temporal pole caudally. The brains were cryoprotected in a graded series of phosphate buffered sucrose solutions and frozen at -80° C. The brains from young and middle-aged monkeys were equally frozen for ∼ five years before sectioning. Coronal sections were cut through the entire basal forebrain at a thickness of 50 μm on a freezing sliding microtome. Tissue sections were collected in cryoprotectant and stored at −20° C until immunoprocessing.
4.2 Immunocytochemistry
Series of sections were processed for the vesicular acetylcholine transporter (VAChT) that is found on the synaptic vesicles in cholinergic nerve terminals and is a specific marker for cholinergic neurons and fibers (Gilmor et al., 1996; Weihe et al., 1996). The same VAChT anti-sera used in the present study has been used previously to visualize both cholinergic neurons and fibers in nonhuman primate tissue (Schafer et al., 1995; Rico and Cavada, 1998; Shamy et al., 2007; Calhoun et al., 2004). Following procedures used previously (Tinkler et al., 2004), sections were rinsed in 0.1 M phosphate buffered saline (PBS, pH 7.4) to remove cryoprotectant, incubated in 0.3% H2O2 and 10% methanol in PBS for 15 minutes to eliminate endogenous peroxidase activity, and rinsed again in 2% normal goat serum (NGS) and PBS for 60 minutes. Sections were then incubated in primary antiserum for human VAChT (1:40,000, polyclonal, Phoenix Pharmaceuticals, Mountain View, CA) in PBS with 2% NGS and 0.3% Triton for 18 hours at room temperature. The sections were rinsed for 10 minutes in PBS, placed in biotinylated secondary antibody solution (1:200 goat anti-rabbit, Vector Labs, Burlingame, CA) for 30 minutes, rinsed in PBS for 5 minutes, and then incubated in avidin-biotin-complexed horseradish peroxidase (ABC Elite; Vector Labs) for 30 minutes. Sections were rinsed in acetate-imidazole for 10 minutes and reacted with the chromogen 3,3′-diaminobenzidine in 0.002% H2O2 to catalyze the reaction. Nickel sulfate was used to create a darker reaction product. Control sections were prepared in which the primary antibody was omitted. The large number of total sections that were immunocytochemically-processed precluded all sections from all animals being processed in a single batch. Immunocytochemical staining was performed in batches in which all processing parameters were kept constant between batches and each batch included sections from each group and each age to minimize differences in immunostaining between groups. Microscopic examination of sections, in regular steps through focus, revealed uniform staining throughout the sections.
4.3 Analyses: estimates of medial septum/diagonal band and nucleus basalis cholinergic neuron number and size
Unbiased stereological methods were used to quantify total cell number and size of VAChT-positive neurons in the MS/DB of both young and middle-aged monkeys, and in the NBM of only middle-aged monkeys; we previously reported the number and size of VAChT-positive cells in the NBM of young OVX and ET monkeys (Tinkler et al., 2004). Due to technical difficulties, the MS/DB in middle-aged monkeys was available for analyses in only four Premarin animals and five OVX animals. Cell counts and measurements of cell size were conducted on an Olympus BX51 microscope using StereoInvestigator software (MBF Bioscience, Williston, VT). The analyses were conducted blind with respect to animal treatment group. The boundaries of the MS/DB (cell groups Ch1 and Ch2; these areas were treated as one region in the analyses) and of the anterior (Ch4a), intermediate (Ch4i), and posterior (Ch4p) subregions of the NBM were identified according to the descriptions of Mesulam et al. (1983).
Cells were counted using the optical fractionator variation of the optical dissector method (West, 1993), that allows for systematic random sampling of a reference area. The reference area on each slide was outlined at low magnification, and counts and measurements were done with a 40× objective (numerical aperture = 0.9.) The first available section was taken as a random starting point, and cells were counted on every 8th section. Eight to ten sections were analyzed per brain. A uniformly spaced grid was placed over the area of interest, and a counting frame of defined size was randomly placed in this grid by the SteroInvestigator software. The size of the grid was determined by pilot studies, such that the coefficient of error was 0.10 or less (Gunderson, 1987). The grid size for analysis of the MS/ DB and Ch4p was 200 × 200 μM, and for Ch4a and Ch4i grid size was 250 × 250 μM. The area of the counting frame was 188 × 139 μM. The sections had a mean post-processing thickness of 22μm and a guard zone of 3μm at the top and bottom of each section, resulting in a counting frame or dissector height of 16 μM. Cells were counted if the nucleus was in focus and within/touching the inclusion boundary of the counting frame. The total number of cells was determined by the number of cells multiplied by the fraction of the tissue that was counted, as demonstrated by the equation N = Σ Q-(1/ssf)(1/asf)(tsf), where N is the total cell number, Q- is the number of counted cells on all sections, ssf is the sampling fraction (one out of every 8 slides), asf is the area sampled, and tsf is the thickness of the tissue divided by the height of the dissector.
Approximately 200 total cells were counted in the MS/DB and Ch4p of each brain. Approximately 300 cells were counted in the Ch4a and Ch4i region of each brain. Because of the small size of the total number of cells in the MS/DB, cells were counted twice; once by hand, and once with the optical fractionator, to ensure accuracy and reliability of the counter. The concordance between counts done by hand and by the optical fractionator was 98% for young animals and 89% for middle-aged animals. In those cases where the numbers between the two counts varied by more than several hundred cells, the cells were counted a second time with the optical fractionator. The data presented here represents the counts made with the optical fractionator.
Average cell size was determined by analyzing the cells counted using the planar rotator (Jensen and Gunderson, 1993) while using the optical fractionator to select the cells to be measured. Using the StereoInvestigator software, three parallel lines were superimposed over a vertical line passing through the nucleus of selected cells. Points of intersection between the lines and the cell boundary were marked, and neuron volume was estimated by StereoInvestigator using the equation V= π.tΣli2 where t equals one-third of the height of the line along the vertical axis, and li is the indicates the distance between the points of intersection of each parallel line and the vertical axis.
4.4 Serum estradiol
Concentrations of serum E2 were assayed at the Yerkes Primate Research Center's Assay Laboratory at Emory University in Atlanta, GA. Mean serum E2 concentrations were measured at 6, 12 and 24 months in the young and middle-aged monkeys. The final blood samples were taken one week before necropsy in young monkeys and at the time of necropsy in the middle-aged monkeys.
4.5 Statistical analyses
Separate one-way analyses of variance were used to determine the effects of treatment on the number and size of VAChT-positive neurons in the MS/DB of young monkeys and of the MS/DB and NBM of middle-aged monkeys. Regression analyses were conducted to determine if a relationship was present between serum E2 levels and anatomical measures in young and middle-aged monkeys.
Acknowledgments
The authors would like to thank Patricia Durant for her assistance in preparing some of the tissue for this project. The work presented in this paper was supported by a Cross Campus Collaboration grant from the Wake Forest University School of Medicine (C.B and M.L.V.) and by the NIA grant AG13204 (M.L.V.)
Abbreviations
- AChE
acetylcholinesterase
- ChAT
choline acetyltransferase
- DB
diagonal band of Broca
- ET
estrogen therapy
- E2
estradiol
- NBM
nucleus basalis of Meynert
- MS
medial septum
- OVX
ovariectomized/ovariectomy
- VAChT
vesicular acetylcholine transporter
- WHI
Women's Health Initiative
- WHIMS
Women's Health Initiative Memory Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MR, Kaplan JR, Manuck SB, Kroritnik DR, Parks JS, Wolfe MS, Clarkson TB. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys: Lack of an effect of added progesterone. Arteriosclerosis. 1990;10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- Ancelin ML, Ritchie K. Lifelong endocrine fluctuations and related cognitive disorders. Curr Pharm Des. 2005;11:4229–4252. doi: 10.2174/138161205774913228. [DOI] [PubMed] [Google Scholar]
- Appt SE, Clarkson TB, Lees CJ, Anthony MS. Low dose estrogens inhibit coronary artery atherosclerosis in postmenopausal monkeys. Maturitas. 2006;55:187–194. doi: 10.1016/j.maturitas.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Chen S, Montoya M, Hsieh D, Minaya J. The estrogen replacement therapy of the Women's Health Initiative promotes the cellular mechanisms of memory and neuronal survival in neurons vulnerable to Alzheimer's disease. Maturitas. 2000;34 2:S35–52. doi: 10.1016/s0378-5122(00)00107-9. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Tran J, Proffitt P, Montoya M. 17 beta-Estradiol enhances the outgrowth and survival of neocortical neurons in culture. Neurochem Res. 1997;22:1339–1351. doi: 10.1023/a:1022015005508. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Mao Y, Roberts JA, Rapp PR. Reduction in hippocampal cholinergic innervation is unrelated to recognition memory impairment in aged rhesus monkeys. J Comp Neurol. 2004;475:238–246. doi: 10.1002/cne.20181. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS, Williams GV. Animal models of working memory: insights for targeting cognitive dysfunction in schizophrenia. Psychopharmacology. 2004;174:111–125. doi: 10.1007/s00213-003-1710-9. [DOI] [PubMed] [Google Scholar]
- Celik O, Erdem G, Hascalik S, Karakas HM, Tamser M. Magnetic resonance spectroscopic comparison of the effects of resveratrol (3,4′,5-trihydroxy stilbene) to conjugated equine estrogen, tibolone and raloxifene on ovariectomized rat brains. Eur J Obstet Gynecol Reprod Biol. 2005;120:73–79. doi: 10.1016/j.ejogrb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Clarkson TB, Anthony MS, Cline JM, Lees CJ, Ederveen AG. Multisystem evaluations of the long-term effects of tibolone on postmenopausal monkeys. Maturitas. 2004;48 1:S24–9. doi: 10.1016/j.maturitas.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Clarkson TB, Anthony MS, Mikkola TS, Clair RW. Comparison of tibolone and conjugated equine estrogens effects on carotid artery atherosclerosis of postmenopausal monkeys atherosclerosis of postmenopausal monkeys. Stroke. 2002;33:2700–2703. doi: 10.1161/01.str.0000033130.82164.24. [DOI] [PubMed] [Google Scholar]
- Clarkson TB, Anthony MS, Wagner JD. A comparison of tibolone and conjugated equine estrogens effects on coronary artery atherosclerosis and bone density of postmenopausal monkeys. J Clin Endocrinol Metab. 2001;86:5396–5404. doi: 10.1210/jcem.86.11.8021. [DOI] [PubMed] [Google Scholar]
- Conner JM, Darracq MA, Roberts J, Tuszynski MH. Nontropic actions of neurotrophins : subcortical nerve growth factor gene delivery reverses age-related degeneration of primate cortical cholinergic innervation. Proc Natl Acad Sci. 2001;98:1941–1946. doi: 10.1073/pnas.98.4.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen–induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Lee CD. Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neurosci. 2005;132:57–64. doi: 10.1016/j.neuroscience.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Dohanich GP, Fader AJ, Javorsky DJ. Estrogen and estrogen-progesterone treatments counteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behav Neurosci. 1994;108:988–992. doi: 10.1037//0735-7044.108.5.988. [DOI] [PubMed] [Google Scholar]
- Dutar P, Bassant MH, Senut MC, Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiol Rev. 1995;75:393–427. doi: 10.1152/physrev.1995.75.2.393. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J, Women's Health Initiative Memory Study Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Everett BJ, Robbins TW. Central cholinergic systems and cognition. Ann Rev Psych. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Hendricson AW, Dohanich GP. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol Learn Mem. 1998;69:225–240. doi: 10.1006/nlme.1998.3820. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–223. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Oestrogen and the cholinergic hypothesis: implications for oestrogen replacement therapy in postmenopausal women. Novartis Found Symp. 2000;230:94–111. doi: 10.1002/0470870818.ch8. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42:245–275. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav. 2007;52:352–359. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Burke AM, Johnson DA. Estrogen replacement attenuates effects of scopolamine and lorazepam on memory acquisition and retention. Horm Behav. 1998;34:112–125. doi: 10.1006/hbeh.1998.1452. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Edwards D, Lazar N, Nelson D, Talameh J. Effects of long-term hormone treatment and of tibolone on monoamines and monoamine metabolites in the brains of ovariectomised, cynomologous monkeys. J Neuroendocrinol. 2006;18:643–654. doi: 10.1111/j.1365-2826.2006.01463.x. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Nelson D, Anthony MS, Clarkson TB. Effects of long-term hormone replacement and of tibolone on choline acetyltransferase and acetylcholinesterase activities in the brains of ovariectomized, cynomologus monkeys. Neurosci. 2002;113:907–914. doi: 10.1016/s0306-4522(02)00239-7. [DOI] [PubMed] [Google Scholar]
- Gilardi KVK, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57:335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Nash NR, Roghani A, Edwards RH, Yi H, Hersch SM, Levey AI. Expression of the putative vesicular acetylcholine transporter in rat brain and the localization in cholinergic synaptic vesicles. J Neurosci. 1996;16:2179–2190. doi: 10.1523/JNEUROSCI.16-07-02179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AL, Descalzi CD, Johnson DK, Hodgen GD. Composite pattern of circulating LH, FSH, estradiol and progesterone during the menstrual cycle in cynomolgus monkeys. Proc Soc Exp Biol Med. 1997;155:479–481. doi: 10.3181/00379727-155-39834. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gunderson HJG. The efficiency of systematic sampling in stereology and its prediction. J Micrsocopy. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Woldorff MG, Fletcher EM, Mangun GR. Dissociating top-down attentional control from selective perception and action. Neuropsychologia. 2001;39:1277–1291. doi: 10.1016/s0028-3932(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Jayo MJ, Register TC, Carlson CS. Effects on bone of oral hormone replacement therapy initiated two years after treatment in young adult monkeys. Bone. 1998;23:361–366. doi: 10.1016/s8756-3282(98)00106-9. [DOI] [PubMed] [Google Scholar]
- Jensen EBV, Gunderson HJG. The rotator. J Microsocopy. 1993;170:35–44. [Google Scholar]
- Jerome CP, Carlson CS, Register TC, Bain FT, Jayo MJ, Weaver DS, Adams MR. Bone functional changes in intact, ovariectomized hormone supplanted adult cynomolgus monkeys (Macaca fasicularis) evaluated by serum markers and dynamic histomorphometry. J Bone Miner Res. 1994;9:527–540. doi: 10.1002/jbmr.5650090413. [DOI] [PubMed] [Google Scholar]
- Jewett DA, Dukelow WR. Cyclicity and gestation length of Macaca fascicularis. Primates. 1972;13:327–330. [Google Scholar]
- Jin M, Jin F, Zhang L, Chen Z, Huang H. Two estrogen replacement therapies differentially regulate expression of estrogen receptors alpha and beta in the hippocampus and cortex of ovariectomized rat. Mol Brain Res. 2005;142:107–114. doi: 10.1016/j.molbrainres.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Kapsalis E. Ageing, infecundity, and reproductive senescence in free-ranging female rhesus monkeys. J Reprod Fertil. 1995;105:271–278. doi: 10.1530/jrf.0.1050271. [DOI] [PubMed] [Google Scholar]
- Kompoliti K, Chu Y, Polish A, Roberts J, McCay H, Mufson EJ, Leurgans S, Morrison JH, Kordower JH. Effects of estrogen replacement therapy on basal forebrain neurons and cortical cholinergic innervation in young and aged rhesus monkeys. J Comp Neurol. 2004;472:193–207. doi: 10.1002/cne.20050. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG. Ovarian hormones differentially influence immunoreactivity for dopamine β-hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1999;409:438–451. doi: 10.1002/(sici)1096-9861(19990705)409:3<438::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Lacreuse A. Effects of ovarian hormones on cognitive function in nonhuman primates. Neurosci. 2006;138:859–867. doi: 10.1016/j.neuroscience.2005.09.006. [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA. 2001;285:1489–1499. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- Lees C, Shen V, Brommage R. Effects of lasofozifene on bone in surgically postmenopausal cynomolgus monkeys. Menopause: J North Amer Menopause Soc. 2007;14:97–105. doi: 10.1097/01.gme.0000227858.50473.69. [DOI] [PubMed] [Google Scholar]
- Maki PM. A systematic review of clinical trials of hormone therapy on cognitive function: effects of age at initiation and progestin use. Ann NY Acad Science. 2005;1052:182–197. doi: 10.1196/annals.1347.012. [DOI] [PubMed] [Google Scholar]
- Maki P, Hogervorst E. The menopause and HRT. HRT and cognitive decline. Best Pract Res Clin Endocrinol Metab. 2003;17:105–22. doi: 10.1016/s1521-690x(02)00082-9. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:190–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Olton DS, Markowska AL, Voytko ML, Given B, Gorman L, Wenk GL. Basal forebrain cholinergic system: A functional analysis. Adv Exp Med Biol. 1991;295:353–372. doi: 10.1007/978-1-4757-0145-6_20. [DOI] [PubMed] [Google Scholar]
- Parent MB, Baxter MG. Septohippocampal acetylcholine: Involved in but not necessary for learning and memory? Learn Mem. 2004;11:9–20. doi: 10.1101/lm.69104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeyananjarassri K, Baber R. Effects of low-dose hormone therapy on menopausal symptoms, bone mineral density, endometrium, and the cardiovascular system: a review of randomized clinical trials. Climacteric. 2005;8:13–23. doi: 10.1080/13697130400012288. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–72. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Rhodin JA, Thomas TN, Clark L, Garces A, Bryant M. In vivo cerebrovascular actions of amyloid beta-peptides and the protective effect of conjugated estrogens. J Alzheimer's Dis. 2003;5:275–286. doi: 10.3233/jad-2003-5403. [DOI] [PubMed] [Google Scholar]
- Rico B, Cavada C. A population of cholinergic neurons is present in the macaque monkey thalamus. Eur J Neurosci. 1998;10:2346–2352. doi: 10.1046/j.1460-9568.1998.00246.x. [DOI] [PubMed] [Google Scholar]
- Rozenberg S, Fellemans C, Kroll M, Vandromme J. The Menopause in Europe. Int J Fertil Womens Med. 2000;45:182–189. [PubMed] [Google Scholar]
- Schafer MK, Weihe E, Erickson JD, Eiden LE. Human and monkey cholinergic neurons visualized in paraffin-embedded tissues by immunoreactivity for VAChT, the vesicular acetylcholine transporter. J Mol Neurosci. 1995;6:225–235. doi: 10.1007/BF02736782. [DOI] [PubMed] [Google Scholar]
- Shamy JL, Buckmaster CA, Amaral DG, Calhoun ME, Rapp PR. Reactive plasticity in the dentate gyrus following bilateral entorhinal cortex lesions in cynomolgus monkeys. J Comp Neurol. 2007;502:192–201. doi: 10.1002/cne.21313. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neurosci. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Smith DE, Rapp PR, McKay HM, Roberts JA, Tuscynski MH. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J Neurosci. 2004;24:4373–4381. doi: 10.1523/JNEUROSCI.4289-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Roberts J, Gage FH, Tuszynski MH. Age-associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proc Natl Acad Sci. 1999;96:10893–10898. doi: 10.1073/pnas.96.19.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speroff L, Van de Wiele RL. Regulation of the human menstrual cycle. Am J Obstet Gynecol. 1971;109:234–247. doi: 10.1016/0002-9378(71)90872-6. [DOI] [PubMed] [Google Scholar]
- Stroessner-Johnson HM, Rapp PR, Amaral DG. Cholinergic cell loss and hypertrophy in the medial septal nucleus of the behaviorally characterized aged Rhesus monkey. J Neurosci. 1992;12:1936–1944. doi: 10.1523/JNEUROSCI.12-05-01936.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Women's Health Initiative Steering Committee. Effects of Conjugated Equine Estrogen in Postmenopausal Women With Hysterectomy: The Women's Health Initiative Randomized Controlled Trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Tobin JR, Voytko ML. Effects of two years of estrogen loss or replacement on nucleus basalis cholinergic neurons and cholinergic fibers to the dorsolateral prefrontal and inferior parietal cortex of monkeys. J Comp Neurol. 2004;469:507–521. doi: 10.1002/cne.11028. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Voytko ML. Estrogen modulates cognitive and cholinergic processes in surgically menopausal monkeys. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:423–431. doi: 10.1016/j.pnpbp.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Vaucher E, Reymond I, Najaffe R, Kar S, Quirion R, Miller MM, Franklin KB. Estrogen effects on object memory and cholinergic receptors in young and old female mice. Neurobiol Aging. 2002;23:87–95. doi: 10.1016/s0197-4580(01)00250-0. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Functional and neurobiological similarities of aging in monkeys and humans. Age. 1997;20:29–44. doi: 10.1007/s11357-997-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML. Estrogen and the cholinergic system modulate visuospatial attention in monkeys (Macaca fascicularis) Behav Neurosci. 2002;116:187–197. doi: 10.1037//0735-7044.116.2.187. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci. 1994;14:167–186. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, Sukhov RR, Walker LC, Breckler SJ, Price DL, Koliatsos VE. Neuronal number and size are preserved in the nucleus basalis of aged rhesus monkeys. Dementia. 1995;6:131–141. doi: 10.1159/000106936. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Tinkler GP. Cognitive function and its neural mechanisms in nonhuman primate models of aging, alzheimer's disease and menopause. In: Taffee MA, Weed MR, editors. Nonhuman Primate Models of Neuropsychopathology, Frontiers of Bioscience Special Edition. Vol. 9. 2004. pp. 1899–1914. Front Biosci. [DOI] [PubMed] [Google Scholar]
- Weihe E, Tao-Cheng JH, Schafer MKH, Erickson JD, Eiden LE. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc Natl Acad Sci. 1996;93:3547–3552. doi: 10.1073/pnas.93.8.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- Woolfe NJ. Cholinergic Systems in Mammalian Brain and Spinal Cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Writing Group for the Women's Health Initiative Investigators. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results From the Women's Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neurosci. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]