Abstract
Human chorionic gonadotropin (hCG), a hormone produced during pregnancy, can elicit life-long refractoriness to carcinogenesis by differentiation of the breast epithelium. Human breast epithelial cells MCF-10F form tubules in collagen, mimicking the normal ductules, We have shown that 17 β-estradiol (E2) alter the ductulogenic pattern of these cells. The effect of the recombinant hCG (rhCG) in vitro was evaluated on the transformation of MCF-10F induced by E2. MCF-10F cells were treated with 70nM E2 alone or in combination with 50 IU/ml rhCG during 2 weeks, while the controls were treated with DMSO (the solvent in which E2 was dissolved) or rhCG alone. At the end of treatment, the cells were plated in type I collagen matrix (3D-cultures) for detecting 2 main phenotypes of cell transformation, namely the loss of ductulogenic capacity and the formation of solid masses. Although E2 significantly increased solid mass formation, this effect was prevented when MCF-10F cells were treated with E2 in combination with rhCG. Furthermore, E2 increased the main duct width (p<0.001), and caused a disruption of the luminal architecture, whereas rhCG increased the length of the tubules (p<0.001) and produced tertiary branching. In conclusion, rhCG was able to abrogate the transforming abilities of estradiol, and had the differentiating property by increasing the branching of the tubules formed by breast epithelial cells in collagen. These results further support our hypothesis, known as the terminal differentiation hypothesis of breast cancer prevention, that predicts that hCG treatment results in protection from tumorigenic changes by the loss of susceptible stem cells 1 through a differentiation to refractory stem cells 2 and increase differentiation of the mammary gland.
Keywords: Mammary epithelium, hCG, Estrogen, Neoplastic transformation, Branching morphogenesis
1. Introduction
Clinical and epidemiological studies have shown that prolonged or cumulative estrogenic exposure like early menarche or late menopause are associated with increased breast cancer incidence (Dorgan et al., 1996; Toniolo et al., 1995; Greenlee et al., 2000). Moreover, these effects continue even after menopause due to local tissue estrogen production (Geisler, 2003). We have demonstrated that estrogen induced neoplastic transformation in vitro characterized by the loss of the ductulogenic pattern in collagen matrix with disruption of the normal breast epithelial architecture (Russo et al., 2002; 2006b). At the early stages in the transformation process, estrogen produced a downregulation in the expression of genes related to cell adhesion, such as ITGB6 (integrin β6), LAMA3 (laminin α 3), LAMC2 (laminin γ2) and FN1 (fibronectin 1), and downregulation of other genes by epigenetic modifications (Fernandez et al., 2006; Huang et al., 2007).
At difference of estrogen, parity at early age, especially when younger than 24 years of age, decreases the incidence of breast cancer (Lambe et al., 1996). Compared with nulliparous women, women with at least one full term pregnancy have a 25% reduction in breast cancer risk and increasing number of pregnancies confers further protection (Hinkula et al., 2001; Key et al., 2001). This could be explained by comparison of the morphology of the breast of nulliparous and parous women. The breast of normally cycling women contains 3 types of lobules described as type 1 (Lob 1), type 2 (Lob 2) and type 3 (Lob 3) in ordrr of their increasing complexity, defined as the number of alveoli per lobule (Russo and Russo, 1994; Balogh et al., 2006; Russo et al., 2006a). The breast attains its maximum development during pregnancy when there is a progression of Lob 2 to Lob 3; this growth phase is followed by the secretory phase in fully differentiated lobules type 4 (Lob 4). With the progressive maturation of Lob 1 to Lob 2, Lob 3, and Lob 4 there is a progressive decrease in the percentage of proliferating cells and a reduction in the susceptibility of the cells transformation by carcinogens (Russo et al., 2006a). After post-lactational involution, Lob 4 regress to Lob 3, which remain present as the predominant structures in the breast until women reach the fourth decade of life, decreasing due to their involution to Lob 2 and Lob 1. In contrast to the difference of the breast in parous women, the nulliparous breast contains a great number of Lob 1, whose percentage remains almost constant throughout their lifespan. In nulliparous women, Lob 2 is present in moderate numbers and Lob 3 is almost totally absent. After menopause, the breast regresses in both nulliparous and parous women, which is manifested as an increase in the number of Lob 1, and a concomitant decline in the number of Lob 2 and Lob 3. At the end of the fifth decade of life, the breast of both nulliparous and parous postmenopausal women contains predominantly Lob 1, although the Lob 1 in parous women are refractory to carcinogenesis and have a “genomic signature” or gene expression profile different from the Lob 1 from nulliparous women (Russo et al. 2006a, 2008). We further demonstrated in a model of mammary carcinogenesis in rats that a term pregnancy results in substantial protection against DMBA-induced malignant transformation (Russo et al., 1991). Moreover, short-term treatment with recombinant human chorionic gonadotropin (rhCG), a placental hormone produced by the trophoblast, induces the same differentiation than pregnancy. Thus, the mammary epithelium is able to gain resistance to carcinogenesis by rhCG pretreatment (Russo et al., 2005). In one experiment in which virgin rats were treated with hCG for 21 days (the length of a pregnancy), followed by a 21-day rest period, and which were then administrered DMBA, a dramatic dose-dependent decline incidence was observed (Russo et al., 1990; 1991; Russo and Russo, 1994).
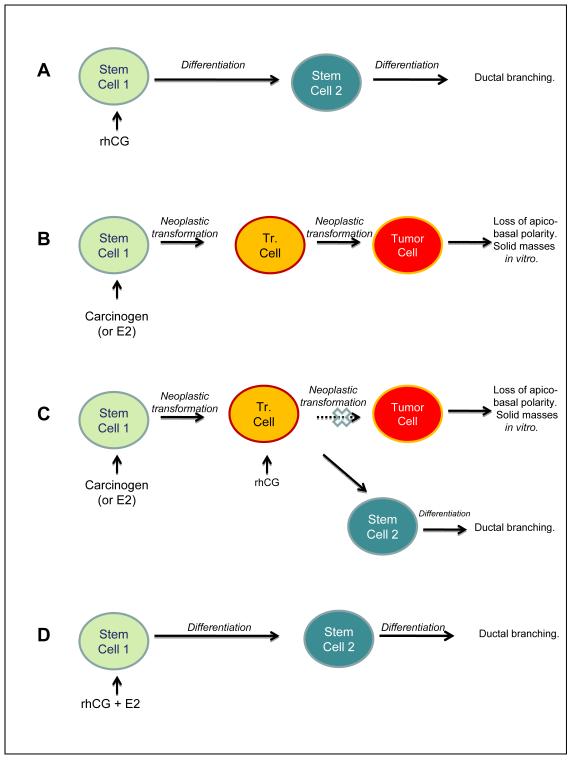
Based on these observations, it was proposed that in the breast, pregnancy or hCG is able to shift the stem cells 1 that are susceptible to be transformed by a carcinogen to stem cells 2 that are refractory (Russo et al., 1997; 2006a). Stem cells 2 - progenitor cells originating after post-lactation involution of the breast - can proliferate and differentiate under the stimulus of a new pregnancy (Russo and Russo, 1997; Russo et al., 2006a). This hypothesis, known as terminal differentiation hypothesis of breast cancer prevention, predicts that the loss of stem cells 1 through differentiation to stem cells 2 and a general increase in the mammary gland differentiation following pregnancy or rhCG treatment results in protection from tumorigenesis (Russo and Russo, 1987; 1997) (Fig. 1). Furthermore, our previous studies have shown than full term pregnancy induces a permanent genomic signature in the breast epithelial cells, associated with lower cell proliferation and efficient DNA repair capacity, creating a differentiated breast epithelium that are more resistant to carcinogenesis (Russo and Russo, 1997; Russo and Russo, 2007).
Fig. 1. The terminal differentiation hypothesis of breast cancer prevention.
Stem cells 1 are susceptible to be transformed by carcinogens (or E2) although stem cells 2 are refractory. This hypothesis predicts that: A) the loss of a population of susceptible stem cells 1 through differentiation to stem cells 2, and a general increase in the differentiation of the cells in the mammary gland following rhCG treatment results in protection from tumorigenic changes. B) Stem cells 1 exposed to carcinogens (or E2) give rise to early transformed cells (Tr. Cells) that progress to tumor cells. C) In the case of carcinogen (or E2) administration followed by rhCG treatment, transformed cells (Tr. cells) are able to avoid progression to tumorigenic stages. D) In the case of both carcinogen (or E2) and rhCG exposure, rhCG helps avoid the transformating action of E2 acting as an antagonist; differentiation of stem cells 1 to stem cells 2 occurs through the action of rhcG, and a general increase in differentiation of the mammary gland is seen.
The disruption of the tubular structures of the breast epithelial cells, including loss of apico-basal polarity and filling of the luminal space, is considered a hallmark in epithelial cancers (Debnath et al., 2003). The architectural features and branching morphogenesis during neoplastic transformation has critical importance for better understanding the mechanisms behind mammary epithelial carcinogenesis and its prevention. Presumably, early parity and/or hCG play critical roles in preventing the neoplastic process in the breast epithelium by preserving the normal 3-dimensional epithelial architecture. We report here that rhCG can prevent the transformation phenotypes induced by E2 and stimulate ductulogenesis by increasing the length of the ducts and producing tertiary branching of the breast epithelial cells in vitro.
2. Materials and methods
2.1. Human breast epithelial cell treatments with 17-β estradiol (E2) and recombinant human chorionic gonadotropin (rhCG)
MCF-10F, a human breast epithelial cell line spontaneously immortalized, estrogen receptor alpha (ERα)-negative and beta (ERβ)-positive and progesterone receptor (PR)-negative, was cultured in Dulbecco’s modified Eagle medium [DMEM/F-12, Gibco®; Formula 90-5212 EF: containing DMEM/F12 (1:1) with L-glutamine and phenol red, with D-glucose 315mg/L, with sodium pyruvate 55 mg/L] with 5% horse serum, 2.43 g/l sodium bicarbonate, 20 μg/l epidermal growth factor (EGF), 100 μg/l Vibrio cholera toxin, 10 mg/l insulin, 0.5 mg/l hydrocortisone, 1.05 mM calcium, antibiotics and antimicotic (100 units/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin). The cells were treated for 2 weeks with 70 nM E2 (E2 group) or 50 IU/ml rhCG (hCG group) or 70nM E2 in combination with 50 IU/ml rhCG (hCG+E2 group). E2 (Sigma Chemical Co., St. Louis, MO) was dissolved in dimethylsulphoxide (DMSO). Control MCF-10F cells were treated with 0.0467% DMSO (DMSO group). Another cell group was left untreated and maintained in the regular culture media (regular media group). In all groups, the culture medium was replaced daily. The E2 concentration used for the treatments was chosen based on previous results that gave optimal transformation with 70nM E2 (Russo et al., 2006b). A concentration of 50 IU/ml rhCG (Serono Inc, Rockland, MA) was chosen based in previous results showing that 10 IU/ml (0.5μg/ml) rhCG did not have any effect although 50 IU/ml (2.5 μg/ml) and 100 IU/ml (5μg/ml) had similar effects on the morphology of the cells grown in collagen matrix (data not showed).
2.2. Three-dimensional cultures in type I collagen
After 2 weeks of treatment, the ductulogenic capacity of the cells was evaluated. Cells were resuspended at 7.5×103 cells/ml in collagen matrix consisting of 2.68 mg/ml (89.3%) type I collagen (PureCol, Inamed Biomaterials CO., Fremont, CA), 8% 12.5X DMEM-F12 with antibiotics, 0.1 mg/ml insulin, 14mM NaHCO3 and 0.01N NaOH. A volume of 400μl of this mixture was plated into 24-well chambers pre-coated 400 μl collagen base at the same concentration. Eight wells were used for each group and 3,000 cells were plated per well. After solidification of the collagen, the cells were fed daily with DMEM medium as described above. Cells in the collagen matrix were examined under an inverted microscope (Nikon Eclipse, TS100, Japan). After 8 days, the total number of tubules and solid masses were counted. Spherical structures lacking any branching and >120 μm in diameter were consider as solid masses. Twenty ducts from each well were chosen at random and the length and diameter of the main tubule was measured using an ocular scale; also primary, secondary and tertiary branches arising from the main duct were counted. Analysis of variance (ANOVA) followed by Tukey’s test was used for comparing architectural parameters between the groups. Student t-test was used for comparing tertiary branching and p < 0.05 was accepted as statistically significant.
2.3. Immunohistochemical staining
At the end of the experiment, the collagen was removed from each well, fixed in 10% formalin, dehydrated, embedded in paraffin and cut into 3 μm slices. Paraffin embedded sections were mounted in slides and immunohistochemical stained with the antibodies: anti-Ki-67 (M7240, Dako North America Inc., CA); anti-low MW cytokeratin (AE1, Biogenex, MU075-UC, San Ramon, CA); anti-E-cadherin (E-Cad, 610182, BD Transduction Laboratories, San Jose, CA); anti-laminin γ-2 (sc-25341, Santa Cruz Biotechnology Inc., CA); anti estrogen receptor alpha (790-4324, Ventana Medical Systems Inc., Tucson, AR); and anti-vimentin (M0725, Dako North America Inc., CA). The slides were incubated at 37°C for 30 min with specific primary antibodies. The Ventana UltraView Universal DAB Detection Kit (Tucson, AZ) was used. The nucleus of the cells was staining with hematoxylin and a light microscope (Olympus, BX40, Japan) was used for evaluation. To calculate the proliferative index, 10 sections were used to evaluate the effect of each treatment; positive Ki67 positive cells were counted from a total of 100 cells per slide (Ki67 positive cells from a total of 1,000 cells per treatment), and only cells forming duct-like structures were considered for the calculation.
3. Results
3.1. Human chorionic gonadotropin (hCG) prevented the formation of solid masses induced by 17 β-estradiol (E2)
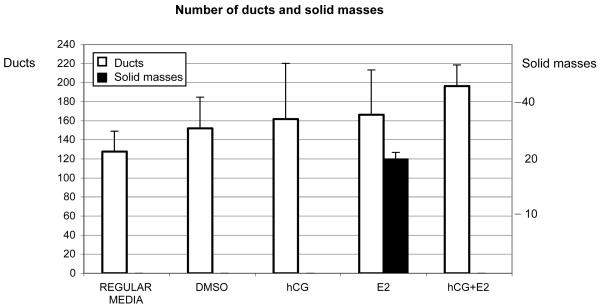
One of the advantages of using type I collagen matrix is that the human breast epithelial cells, like MCF-10F, forms tubules mimicking the normal ductules of the human breast. When MCF-10F cells were grown in regular media (regular media group) or in media with DMSO (DMSO group), with rhCG alone, or in combination with E2 (hCG and hCG+E2 groups) and plated in type I collagen matrix, tubules formed (Fig. 2). When the cells were treated with E2 alone (E2 group) and plated in collagen, some cells formed tubules and others solid masses (Fig. 2). E2 induced the formation of solid masses of 178 ± 33.16 μm in diameter; however, when the cells were treated with E2 in combination with rhCG the formation of solid masses was prevented (Fig. 2). The number of ducts was similar between the groups, except between the control in regular media and the hCG+E2 group in which the difference was significant (p=0.01 Tukey’s test) (Fig. 2).
Fig. 2. Number of tubules and solid masses on type I collagen matrix.
MCF-10F cells were treated during 2 weeks with 70 nM (E2 group), 50 IU/ml rhCG (hCG group), or 50 IU/ml rhCG in combination with 70 nM E2 (hCG+E2 group). Control cells were treated with DMSO (DMSO group) or maintained in the regular media (regular media group). After treatment, the number of ducts and solid masses were counted. Solid masses with a mean diameter of 178 μm appeared after treatment with E2, although when was used in combination with rhCG, solid masses did not appear in the collagen. The mean and the standard deviation (SD) are indicated.
3.2. The human chorionic gonadotropin (hCG) induced longer tubules with tertiary branching
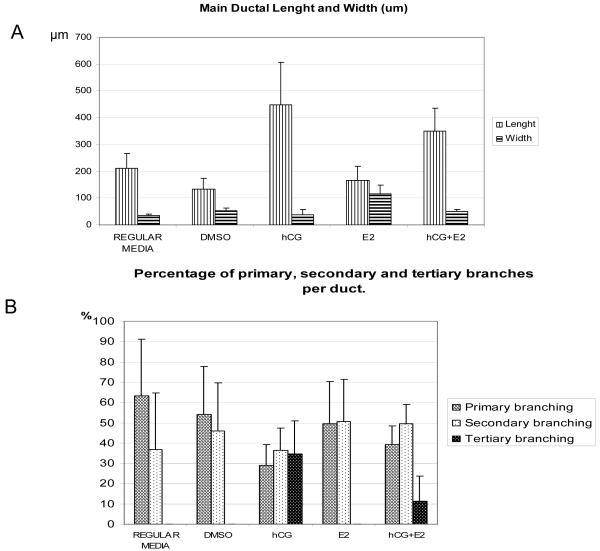
The tubules formed in collagen showed different morphology between the groups; the length and diameter of the main duct and the number of primary, secondary and tertiary branching arising from the main tubule was different according to the treatment received (Fig. 3). The ducts were significantly longer and were more branched when the cells were treated with rhCG (hCG alone or in combination with E2; Fig. 3). The length, width and branching of the tubules from each group are shown in Figure 4,. The tubules formed by the cells treated with rhCG (hCG group) were significantly longer compared to the other groups (p<0.001, Tukey’s test; Fig. 4A). Also the tubules formed by the cells treated with rhCG in combination with E2 (hCG+E2 group) were longer compared to the other groups (p<0.001; Fig. 4A). The diameters of the main tubules were significantly increased in width when the cells were treated with E2 alone (E2 group; p<0.001; ANOVA), but no significant differences were found among the other groups (Fig. 4A). With regard to primary, secondary and tertiary branching (Fig. 4B), all the tubules showed primary and secondary branches, with no significant differences between the treatments, except when the cells were treated with rhCG (alone or in combination with E2), which resulted in tertiary branching (Fig. 4B). All the ducts from the hCG group and 60% of the hCG+E2 group showed tertiary branching. The number of tertiary branches per duct was higher when the cells were treated with rhCG alone (hCG group) than when they were treated with rhCG in combination with E2 (hCG+E2 group) (Student’s t test, p<0.001).
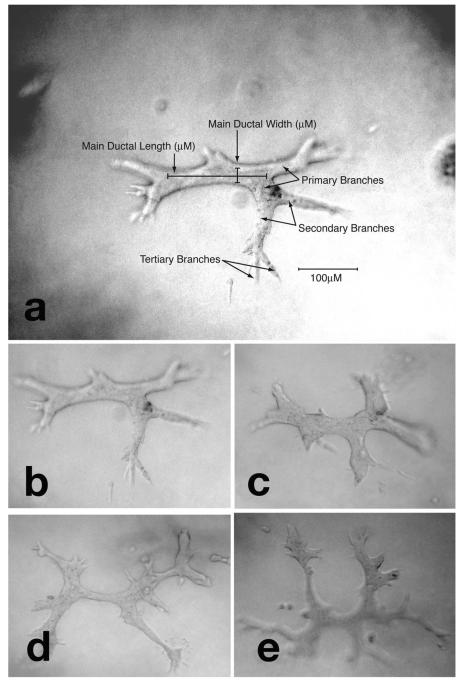
Fig. 3. Tubules on collagen matrix.
a) Parameters for the evaluation of 3-dimensional growth of human breast epithelial cells MCF-10F in collagen. The main ductal length and width, and primary, secondary and tertiary branches are indicated; b) tubule formed by MCF-10F cells growing in collagen after treatment with DMSO; c) tubule formed by MCF-10F cells after treatment with 70 nM 17 β-estradiol (E2): tubules are shorter and increased in width; d) tubule formed by MCF-10F after treatment with rhCG: the ducts are longer, thinner and present a significant higher number of secondary and tertiary branches; e) tubule formed by MCF-10F cells after treatment with rhCG in combination with 17 β-estradiol (hCG+E2 group): the presence of hCG prevented the alterations induced by E2.
Fig. 4. Tubule morphology on collagen matrix.
The main ductal length and width, and primary, secondary and tertiary branches are shown. A) Ductal length and width. The ducts were longer in the hCG groups (hCG and hCG+E2 groups). The tubules showed increased width in the E2 group. The ducts in hCG group are significantly longer than in DMSO, E2, control in regular media and hCG+E2 group (p<0.001; p<0.001; p<0.001 and p=0.008 respectively, Tukey’s test). Furthermore, the tubules are longer in the hCG+E2 group than in the DMSO (p<0.001 Tukey’s test), E2 (p<0.001 Tukey’s test) and the control in regular media (p<0.001 Tukey’s test) groups. The differences in the length of the tubules were not significant between control in regular media vs. DMSO, control in regular media vs. E2, and E2 vs. DMSO (p = 0.064, p = 0.052, and p = 0.79, respectively; Tukey’s test). The treatment with E2 (E2 group) increased the width of the ducts when compared to other groups (p<0.001 ANOVA followed by Tukey’s test). B) Percentage of primary, secondary and tertiary branching per duct. Tertiary branching was only observed in the rhCG groups (hCG alone or hCG+ E2).
3.3. The 17 β-estradiol (E2) treatment increased cell proliferation
Cell proliferation was evaluated using an antibody against Ki-67 antigen. (Ki67 antigen is a cell cycle related nuclear protein, expressed by proliferating cells in all phases of the active cell cycle (G1, S, G2 and M phase) but absent in resting (G0) cells.) Cross sections of the tubular structures showed a monolayer of epithelial cells around the lumen in all the groups except in the E2 group (Fig. 5). In the E2 treated group, the tubules showed an increase of both number of epithelial layers and Ki-67-positive cells (proliferative index = 54.9) indicating an increase in the cell proliferation rate; no difference in the proliferation rate was observed in the other groups (Table 1). Interestingly, there was no increase in the proliferation rate when the cells were treated with E2 in combination with rhCG (hCG +E2 group) (PI = 29.34) compared to the control in regular media (PI = 36.8) or DMSO (PI = 32) (Table 1). The epithelial cells layering the ducts were estrogen receptor alpha (ER α)-negative but positive for E- cadherin, laminin, vimentin and keratin (data not showed); no differences were found between the groups.
Fig. 5. Cell proliferation study by immunohistochemistry.
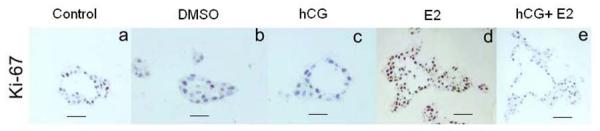
Cross sections of the tubules grown in collagen matrix after the different treatments are shown. Only some cells were positive for Ki67 antigen in the control in regular media (a), DMSO (b), hCG (c) and hCG+E2 (e). In cross section, MCF-10F cells are arranged as a monolayer around the lumen of the duct except in the cells treated with E2 (d). E2 impairs not only ductal morphology and lumen formation, but also causes loss of epithelial characteristics and proliferation as consequences of neoplastic transformation. An increased number of cells are positive for Ki67 antigen in the tubules formed by the cells treated with E2 (d) (Bar= 50 μm).
Table 1. Proliferative index.
In the ducts, the number of cells Ki67 positive was counted from approximately 100 cells that formed the ducts. The proliferative index with the standard deviation is indicated in each case.
| Group | Percentage of positive Ki67cells on ductal structures (Average ± SD) | |
|---|---|---|
| 1 | Control in regular media | 36.8 ± 6.7 |
| 2 | DMSO | 32 ± 3.45 |
| 3 | hCG | 31.63 ± 11.6 |
| 4 | hCG+E2 | 29.34 ± 7.35 |
| 5 | E2 | 54.9 ± 8.1 |
4. Discussion
Primary mammary epithelial cells grown in collagen matrix are able to form tree-like structures resembling in vivo ductulogenesis (Yang et al., 1980). We have previously demonstrated that treatment of these cells with E2 induces phenotypical changes indicative of neoplastic transformation (Russo et al., 2002; 2006b). In the present work, we show that the transformation of MCF-10F by E2 that is associated with impaired ductal morphogenesis can be abrogated by hCG. Treatment of MCF-10F cells with E2 induced formation of solid masses of 178 μm in diameter, although treatment with E2 in combination with hCG prevented their formation. Treatment with E2 in combination with rhCG result in cell differentiation and branch formation on collagen; rhCG prevented E2 acting as antagonist. Also, we have demonstrated that rhCG has a direct effect on branching of breast epithelium; significant ductal elongation was observed in hCG-treated groups and tertiary branches only appear in the presence of hCG (alone or in combination with E2). Furthermore, the increase in tubule width produced by E2 was inhibited by rhCG. In conclusion, the loss of differentiation and disorganized growth of transformed MCF-10F cells caused by estrogen was abrogated by hCG. Altogether our data clearly showed that estrogen induced neoplastic transformation, associated with altered ductal morphology and solid masses formation and hCG was able to abrogate them. The results presented here further support the terminal differentiation hypothesis of breast cancer protection. MCF-10F cells that are ERα-negative/CD44+ can form ductules in collagen, and can be transformed by carcinogens (Russo el al., 2006a; 2006b; Huang et al., 2007); it was proposed that these cells exhibit the characteristic of stem cells 1 (Russo et al., 2006a).
Ducts show a significant increase in width with incomplete lumen formation when the cells were treated with E2 (E2 group), an effect associated with increased cellular proliferation (more Ki-67 positive cells). The filling of the lumen would result from decreased central apoptosis, enhanced cellular proliferation, or their combination (Hebner et al., 2008). Luminal filling is the earliest morphologic alteration and is commonly reported in neoplastic processes (Hebner et al, 2008). It is also common in atypical ductal hyperplasia and ductal carcinoma in situ (DCIS).
The advantage of an in vitro 3D system is that it allows modeling of the epithelial architecture of the breast (Debnath et al., 2003; O’Brien et al., 2002; Shaw et al., 2004). Normal epithelial cells form duct-like structures, having apical-basal polarity and well-organized tubular structures with stable adherens junctions and cell-basement communications. Malignant transformation is associated with the loss of apical-basal polarity and monolayer morphology and significant deviations from normal epithelial behavior in 3D cultures (Shaw et al., 2004; Bissell and Radisky, 2001). In several studies, basement membrane (BM) like Matrigel was used to study the growth and differentiation of the breast epithelia instead of type I collagen matrix. Matrigel is a complex mixture of extracellular matrix proteins and growth factors extracted from Engelbreth-Holm-Swarm murine tumors; its components are laminin, type IV collagen, epidermal growth factor (EGF), transforming growth factor beta (TGF-beta) and insulin like growth factor (IGF) (Fata et al., 2004; Kleinman and Martin, 2005). The difference of type I collagen is that it provides structural support allowing the expression of the intrinsic properties of the breast epithelial cells; the use of BM permits one to study the role of different factors and extracellular matrix components that affect the branching (Lu et al., 2006; Fata et al., 2004). Some cells, e.g. MDCK kidney cells, form tubular structures in type I collagen in the presence of hepatocyte growth factor (HGF), but not in Matrigel (Santos et al., 1993). IMCD cells form multicellular cysts when suspended in Matrigel, whereas they form branching cords in type I collagen. However, when cultured in a mixture of collagen and Matrigel, these cells give rise to elongated branched tubules with visible lumen (Karihaloo et al., 2005). Studies of the matrix components demonstrated that collagen IV and vitronectin inhibit branching and tubule formation, whereas collagen I, laminin and fibronectin promote the process (Santos et al., 1993). Furthermore, EGF promotes proliferation and spheroid formation, and low TGF concentrations induce tubule formation (Shaw et al., 2004; Montesano et al., 2007). Therefore, the use of type I collagen gel matrix is more appropriate for our study of observing the paracrine effect of the hCG on the breast epithelial cells MCF-10F in the absence of these morphogenetic factors.
Although the cellular and molecular mechanisms of tubulogenesis are incompletely understood, a number of polypeptide growth factors stimulate the formation and branching of epithelial tubes (Hogan et al., 2002). We have now shown that rhCG produces longer tubules with tertiary branches even in the presence of estradiol. Popnikolov et al. (2001) have shown that. hCG treatment can result in branching and lobulo-alveolar development of the human breast epithelia although these effects were not observed in ovariectomized animals. Another factor known to affect branching morphogenesis is the hepatocyte growth factor/scatter factor (HGF/SF) (Berdichevsky et al., 1994; Montesano et al., 1991; Trusolino et al, 2002). Several members of the fibroblast growth factor family also have this affect (Bellusci et al., 1997), and heregulin and retinoids also stimulate branching (Offterdinger et al., 2003).
Both LH (luteinizing hormone) and hCG bind to LH/hCG receptor and stimulate adenylate cyclase in the internal membrane, converting adenosine triphosphate (ATP) into cyclic adenosine monophosphate (cAMP); cAMP stimulates the activation of a protein kinase, which among other actions, stimulates steroidogenesis in the mitochondria of the target cell by transforming cholesterol into pregnenolone Other actions include the induction of proteolytic enzymes, prostaglandin synthesis, inhibin production, induction of 17 beta-hydroxysteroid dehydrogenase and changes in gene metabolism (Guo et al., 2004). LH/hCG receptors are present in human breast tissue, rat breast tissue and different cell lines (Lojun et al., 1997; Meduri et al., 1997; Tao et al., 1997) although, it remains uncertain whether a separate hCG receptor exists (Gromoll et al., 2000). Gene expression profile studies of rats showed that the genomic signature induced by rhCG treatment was similar to that induced by pregnancy. The data indicated that hCG in pregnancy induces genomic changes that control a specific differentiation pathway that it is capable to change stem cells 1 to stem cells 2, although other compounds that induce branching were unable to induce that specific signature (Russo and Russo, 1997; Russo et al, 2008).
A population-based case-control study of breast cancer among women 40 years of age or younger, showed that those who received hCG as a part of a weight loss program (popular during the 1960s and 1970s) or as a component of infertility treatment were at lower risk of developing breast cancer (Bernstein et al., 1995). Another population-based cohort study of serum collected from first-trimester pregnant women showed that those with high levels of hCG had a lower risk of breast cancer than women with low hCG levels (Lukanova et al., 2008). The fact that parous women at early ages also develop breast cancer could be explained if hCG did not reach the level required to change stem 1 population to stem 2. It has been proposed by Russo (1997) that Lob 1 found in the breast of nulliparous women and parous women with breast cancer had never gone through the process of differentiation, retaining stem cells 1 that are targets for carcinogens and therefore susceptible to neoplastic transformation. Mutagenic insults or protective factors specifically operating before or during puberty are likely to have profound consequences for breast cancer later in life (Tokunaga et al., 1994; Aisenberg et al., 1997; Wu et al., 2002; Land et al., 2003; Horwich and Swerdlow, 2004). It has also been suggested that at puberty, during mammary gland development associated with stem cell 1 expansion, the gland is more sensitive to carcinogenic agents. Since stem cells 1 are target of tumorigenesis, reducing this population through a direct effect of hCG treatment would be beneficial.
In conclusion, rhCG abrogated the transforming abilities of E2 and had a differentiating action on human breast epithelial cells, MCF-10F, by increasing their branching, a phenotype indicative of cell differentiation. Our results suggested that rhCG has significant potential as a chemo-preventive agent, protecting normal breast cells from becoming malignant.
Acknowledgments
This work was supported by grants R21 ES015148, ES012771 from the National Institute Environmental Health Sciences and by R21 CA124522 from the National Cancer Institute.
Glossary
Abbreviations
- E2
17β-estradiol
- hCG
human chorionic gonadotropin
- rhCG
recombinant hCG
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisenberg AC, Finkelstein DM, Doppke KP, Koerner FC, Boivin JF, Willett CG. High risk of breast carcinoma after irradiation of young women with Hodgkin’s disease. Cancer. 1997;79(6):1203–10. doi: 10.1002/(sici)1097-0142(19970315)79:6<1203::aid-cncr20>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Balogh GA, Heulings R, Mailo DA, Russo PA, Sheriff F, Russo IH, et al. Genomic signature induced by pregnancy in the human breast. Int J Oncol. 2006;28(2):399–410. [PubMed] [Google Scholar]
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan B. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–78. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Berdichevsky F, Alford D, D’Souza B, Taylor-Papadimitriou J. Branching morphogenesis of human epithelial cells in collagen gels. J of Cell Sci. 1994;107:3557–3568. doi: 10.1242/jcs.107.12.3557. [DOI] [PubMed] [Google Scholar]
- Bernstein L, Hanisch R, Sullivan-Halley J, Ross RK. Treatment with human chorionic gonadotropin and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 1995;4(5):437–40. [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumors in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Dorgan JF, Longscope C, Stephenson HE, Falk RT, Miller R, Franz C, et al. Relation of prediagnostic serum estrogen and androgen levels to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:533–539. [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SV, Wu Y-Z, Russo IH, Plass C, Russo J. The role of DNA methylation in estrogen-induced transformation of human breast epithelial cells. Proc Am Assoc Cancer Res. 2006;47:375. [Google Scholar]
- Geisler J. Breast cancer tissue estrogens and their manipulation with aromatase inhibitors and inactivators. J Steroid Biochem Mol Biol. 2003;86:245–253. doi: 10.1016/s0960-0760(03)00364-9. [DOI] [PubMed] [Google Scholar]
- Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer Statistics. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Eiholzer U, Nieschlag E, Simoni M. Male hypogonadism caused by homozygous deletion of exon 10 of the luteinizing hormone (LH) receptor: differential action of human chorionic gonadotropin and LH. J Clin Endocr. Metabol. 2000;85(6):2281–86. doi: 10.1210/jcem.85.6.6636. [DOI] [PubMed] [Google Scholar]
- Guo S, Russo IH, Lareef MH, Russo J. Effect of human chorionic gonadotropin in the gene expression profile of MCF-7 cells. Int J Oncol. 2004;24(2):399–407. [PubMed] [Google Scholar]
- Hebner C, Weaver VM, Debnath J. Modeling morphogenesis and oncogenesis in three-dimensional breast epithelial cultures. Annu Rev Pathol Mech Dis. 2008;3:313–339. doi: 10.1146/annurev.pathmechdis.3.121806.151526. [DOI] [PubMed] [Google Scholar]
- Hinkula M, Pukkala E, Kyyronen P, Kauppila A. Grand multiparity and the risk of breast cancer: population-based study in Finland. Cancer Causes Control. 2001;12(6):491–500. doi: 10.1023/a:1011253527605. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Kolodziej PA. Organogenesis: molecular mechanisms of tubulogenesis. Nat Rev Genet. 2002;3:513–523. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- Horwich A, Swerdlow AJ. Second primary breast cancer after Hodgkin’s disease. Br J Cancer. 2004;90(2):294–8. doi: 10.1038/sj.bjc.6601499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Fernandez SV, Goodwin S, Russo PA, Russo IH, Sutter TR, et al. Epithelial to mesenchymal transition in human breast epithelial cells transformed by 17β-Estradiol. Cancer Res. 2007;67:11147–11157. doi: 10.1158/0008-5472.CAN-07-1371. [DOI] [PubMed] [Google Scholar]
- Karihaloo A, Nickel C, Cantley LG. Signals which build a tubule. Nepron Exp Nephrol. 2005;100:e40–45. doi: 10.1159/000084111. [DOI] [PubMed] [Google Scholar]
- Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2(3):133–40. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–86. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Lambe M, Hsieh CC, Chan HW, Ekbom A, Trichopoulos D, Adami HO. Parity, age at first and last birth, and risk of breast cancer: a population-based study in Sweden. Breast Cancer Res Treat. 1996;38:305–11. doi: 10.1007/BF01806150. [DOI] [PubMed] [Google Scholar]
- Land CE, Tokunaga M, Koyama K, Soda M, Preston DL, Nishimori I, Tokuoka S. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950-1990. Radiat Res. 2003;160(6):707–17. doi: 10.1667/rr3082. [DOI] [PubMed] [Google Scholar]
- Lojun S, Bao S, Lei ZM, Rao CV. Presence of functional luteinizing hormone/chorionic gonadotropin (hCG) receptors in human breast cell lines: implications supporting the premise that hCG protects women against breast cancer. Biol Reprod. 1997;57(5):1202–10. doi: 10.1095/biolreprod57.5.1202. [DOI] [PubMed] [Google Scholar]
- Lu P, Sternlicht MD, Werb Z. Comparative mechanisms of branching morphogenesis in diverse systems. J Mammary Gland Biol Neoplasia. 2006;11:213–28. doi: 10.1007/sl0911-006-9027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukanova A, Andersson R, Wulff M, Zeleniuch-Jacquotte A, Grankvist K, Dossus L, et al. Human chorionic gonadotropin and alpha-fetoprotein concentrations in pregnancy and maternal risk of breast cancer: a nested case-control study. Am J Epidemiol. 2008;168(11):1284–91. doi: 10.1093/aje/kwn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meduri G, Charnaux N, Loosfelt H, Jolivet A, Spyratos F, Brailly S, et al. Luteinizing hormone/human chorionic gonadotropin receptors in breast cancer. Cancer Res. 1997;57(5):857–64. [PubMed] [Google Scholar]
- Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Montesano R, Carrozzino F, Soulie P. Low concentration of transforming growth factor-beta-I induce tubulogenesis in cultured mammary epithelial cells. BCM Developmental Biol. 2007;7:1–16. doi: 10.1186/1471-213X-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LE, Zegers MM, Moskow KE. Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- Offterdinger M, Schneider SM, Grunt TW. Heregulin and retinoids synergistically induce branching morphogenesis of breast cancer cells cultivated in 3D collagen gels. J Cell Physiol. 2003;195:260–275. doi: 10.1002/jcp.10237. [DOI] [PubMed] [Google Scholar]
- Popnikolov N, Yang J, Liu A, Guzman R, Nandi S. Reconstituted normal human breast in nude mice: effect of host pregnancy environment and human chorionic gonadotropin on proliferation. J Endocrinol. 2001;168:487–96. doi: 10.1677/joe.0.1680487. [DOI] [PubMed] [Google Scholar]
- Russo J, Russo IH. Biological and molecular bases of mammary carcinogenesis. Lab Invest. 1987;57(2):112–37. [PubMed] [Google Scholar]
- Russo IH, Koszalka M, Russo J. Human chorionic gonadotropin and rat mammary cancer prevention. J Natl Cancer Inst. 1990;82(15):1286–9. doi: 10.1093/jnci/82.15.1286. [DOI] [PubMed] [Google Scholar]
- Russo IH, Koszalka M, Russo J. Comparative study of the influence of pregnancy and hormonal treatment on mammary carcinogenesis. Br J Cancer. 1991;64(3):481–4. doi: 10.1038/bjc.1991.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo J, Russo IH. Toward a physiological approach to breast cancer prevention. Cancer Epidemiol Biomarkers Prev. 1994;3(4):353–64. [PubMed] [Google Scholar]
- Russo J, Russo IH. Role of differentiation in the pathogenesis and prevention of breast cancer. Endocr Rel Cancer. 1997;4:7–12. [Google Scholar]
- Russo J, Lareef MH, Balogh GA, Guo S, Russo IH. 17 beta-estradiol is carcinogenic in human breast epithelial cells. J. Steroid Biochem. Mol. Biol. 2002;80:149–162. doi: 10.1016/s0960-0760(01)00183-2. [DOI] [PubMed] [Google Scholar]
- Russo J, Moral R, Balogh GA, Mailo D, Russo IH. The protective role of pregnancy in breast cancer. Breast Cancer Res. 2005;7(3):1–12. doi: 10.1186/bcr1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo J, Balogh GA, Chen J, Fernandez SV, Fernbaugh R, Heulings R, Mailo DA, et al. The concept of stem cell in the mammary gland and its implication in morphogenesis, cancer and prevention. Front Biosci. 2006a;11:151–72. doi: 10.2741/1788. [DOI] [PubMed] [Google Scholar]
- Russo J, Fernandez SV, Russo PA, Fernbaugh R, Sheriff FS, Lareef HM, et al. 17-Beta-estradiol induces transformation and tumorigenesis in human breast epithelial cells. FASEB J. 2006b;20:1622–1634. doi: 10.1096/fj.05-5399com. [DOI] [PubMed] [Google Scholar]
- Russo IH, Russo J. Primary prevention of breast cancer by hormone-induced differentiation. Recent Results Cancer Res. 2007;174:111–130. doi: 10.1007/978-3-540-37696-5_11. [DOI] [PubMed] [Google Scholar]
- Russo J, Balogh GA, Russo IH. Full-term pregnancy induces a specific genomic signature in the human breast. Cancer Epidemiol Biomarkers Prev. 2008;17(1):51–66. doi: 10.1158/1055-9965.EPI-07-0678. [DOI] [PubMed] [Google Scholar]
- Santos OFP, Nigan SK. HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-beta. Dev Biol. 1993;160:293–302. doi: 10.1006/dbio.1993.1308. [DOI] [PubMed] [Google Scholar]
- Shaw KR, Wrobel CN, Brugge JS. Use of three-dimensional basement membrane cultures to model oncogene-induced changes in mammary epithelial morphogenesis. J Mammary Gland Biol Neoplasia. 2004;9(4):297–310. doi: 10.1007/s10911-004-1402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX, Lei ZM, Rao CV. The presence of luteinizing hormone/human chorionic gonadotropin receptors in lactating rat mammary glands. Life Sci. 1997;60(15):1297–303. doi: 10.1016/s0024-3205(97)00073-8. [DOI] [PubMed] [Google Scholar]
- Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, Banerjee S, Koenig KL, Shore RE, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst. 1995;87:190–197. doi: 10.1093/jnci/87.3.190. [DOI] [PubMed] [Google Scholar]
- Tokunaga M, Land CE, Tokuoka S, Nishimori I, Soda M, Akiba S. Incidence of female breast cancer among atomic bomb survivors, 1950-1985. Radiat Res. 1994;138(2):209–23. [PubMed] [Google Scholar]
- Trusolino L, Comoglio PM. Scatter-factor and semaphoring receptors. Nat Rev Cancer. 2002;2:289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- Yang J, Guzman R, Richards J, Jentoft V, DeVault MR, Wellings SR, et al. Primary culture of human mammary epithelial cells embedded in collagen gels. J.Natl Cancer Inst. 1980;65:337–43. [PubMed] [Google Scholar]
- Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23(9):1491–6. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]