Abstract
Activation of the α2-adrenoceptor has been shown to produce antinociception. We have previously shown that the antinociceptive effect of clonidine, an α2-adrenoceptor agonist, is sex-specific and is abolished by exogenous estrogen in ovariectomized rats or high level of endogenous estrogen in proestrous females. Here, we investigated whether testosterone mediates the antinociceptive effect of clonidine in the trigeminal region of the male rat. Clonidine (7 µg/5 µl) was injected intracisternally through a PE-10 cannula implanted dorsal to the trigeminal region in orchidectomized (GDX) male Sprague-Dawley rats. In separate groups, testosterone propionate (250 µg/100 µl; GDX+T) or β-estradiol benzoate (100 µg/ 100 µl; GDX+E) were injected subcutaneously 24 and 48 hr. respectively prior to the N-methyl-D-aspartic acid (NMDA) - or heat-evoked nociceptive test. NMDA-induced number of scratches or duration of scratching behavior did not change significantly in control groups with or without hormonal replacement. Clonidine significantly reduced both measures only in the GDX+T group but not in GDX or GDX+E group. Clonidine also significantly increased head withdrawal latency (HWL) in the GDX+T group, but not in GDX or GDX+E group. The antinociceptive effect of clonidine was reversed by yohimbine, an α2-adrenoceptor antagonist, in GDX+T group. We conclude that testosterone is required for the expression of antinociception produced by selective activation of the α2-adrenoceptor in the trigeminal region of the male rat. These findings further our understanding of sex-related differences in the modulation of nociception and may provide insight into development and administration of analgesic agents in young vs. aging men.
Keywords: Sex-differences, clonidine, pain, head withdrawal test, NMDA
INTRODUCTION
Sex-related differences in the sensitivity to pain and response to analgesics have been reported with women exhibiting higher perceptual responses to experimentally induced pain and higher prevalence of many pain disorders, e.g. temporomandibular joint disorder (TMJ/TMD), migraine headaches, fibromyalgia, rheumatoid arthritis, irritable bowel syndrome etc. [3,16,17,31,43]. A number of G-protein-coupled receptors (GPCR) e.g. α2-adrenoceptors, µ-opioid, opioid receptor like-1 receptor (ORL1), etc. that regulate nociception, have also been shown to have sex-specific effects with a majority of studies reporting their diminished analgesic effects in females [4,6,7,12,20,28,34,35,39]. In support, we have previously shown that the antinociceptive effect of clonidine, an α2-adrenoceptor agonist, is sex-specific and is attenuated by high level of endogenous estrogen in normally cycling females as well as by exogenous estrogen in the ovariectomized females [35,41]. In contrast, testosterone has been shown to produce analgesia on a variety of nociceptive tests [5,21,22,25], including attenuation of temporomandibular joint pain/damage in male rats [18,19], and has also been shown by us to be essential for the expression of antinociceptive effects of clonidine and orphanin FQ (an ORL-1 receptor agonist) in the spinal cord on a heat-evoked tail flick assay [7,41]. However, the role of testosterone on α2-adrenoceptor-induced antinociception in the trigeminal region remains unknown. Hence, we examined whether testosterone mediates the antinociceptive effect of clonidine in the trigeminal region of the male rat. N-methyl-D-aspartic acid (NMDA) -induced nociceptive scratching behavior as well as heat-induced head withdrawal test was employed to determine the antinociceptive effect of clonidine in orchidectomized (GDX) and testosterone-treated GDX male rats. In addition, we also investigated if estrogen modifies the antinociceptive effect of clonidine in the trigeminal region of GDX rats.
MATERIALS AND METHODS
Subjects
Adult Sprague–Dawley orchidectomized (GDX) male rats (225–249 g) were obtained from Harlan Sprague-Dawley, Inc. (Indianapolis, IN) and were housed in the animal care facility at Meharry Medical College certified by the American Association for the Accreditation of Laboratory Animal Care (AAALAC) under a 12-h light/dark cycle (light 7 am–7 pm) in a temperature-controlled room (~22°C). Food and water were freely available. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Meharry Medical College and abided by the established guidelines of the National Research Council Guide for the Care and Use of Laboratory Animals and the International Association for the Study of Pain (IASP).
Implantation of cannulae
Two weeks after bilateral orchidectomy, a PE-10 cannula (Intramedic, Clay Adams, Parsippany, NJ; dead volume 10 µl) was implanted dorsal to the medullary dorsal horn region as described before [34,35]. Briefly, animals were surgically prepared under ketamine and xylazine anesthesia (72 and 4 mg/kg respectively; i.p.), their heads were shaved and secured in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). The skin above the head/neck region was incised, the atlantooccipital membrane was removed and the dura was exposed. A tiny opening was made in the dura using a 25 gauge sterile needle and the tip of a PE-10 cannula was inserted approximately 1.5 mm through the opening. The cannula was then secured with Mastisol (Mastisol-Ferndale Lab, Ferndale, MI) and dental cement to the base of the skull and the wound closed using autoclips. Animals were kept on a heating pad (36°C) till they regained consciousness and were returned to their home cages for recovery. Nociceptive testing was conducted 5–7 days after the surgery. As described before [35], using this intracisternal injection technique, the spread of drugs was limited to the trigeminal region. In addition, we have previously provided anatomical evidence that intracisternally administered clonidine produces antinociception by activating α2A-adrenoceptors in the medullary dorsal horn [44]; knockdown of α2A-adrenoceptors in the medullary dorsal horn abolished the antinociceptive effects of clonidine.
Testosterone / estrogen injections
A single subcutaneous (s.c.) injection of testosterone propionate (250 µg/100 µl sesame oil) was administered to GDX rats (GDX+T) 24 h prior to the behavior testing. This dose of testosterone has been shown to produce maximum copulatory behavior in GDX rats [9]. Estradiol benzoate (100 µg/100 µl sesame oil; s.c.) was injected in a separate group of GDX rats (GDX+E) 48 h prior to the nociceptive testing. We have previously shown that this dose of estrogen in ovariectomized rats, after 48 h, produces serum-estradiol levels similar to that of proestrous rats [7]. Control rats (GDX) were injected with the vehicle (100 µl sesame oil).
NMDA-induced nociceptive test
As described before [35], intracisternal injection of NMDA (2 nmol/10 µl) through the implanted PE-10 cannula produced intense scratching behavior which was confined to the orofacial region and lasted for <2 min. The number of scratches, the latency to and the duration of scratching behavior were recorded manually. Higher doses of NMDA (3 nmol and above) resulted in clearly aversive behavior (escape attempts and vocalization) in our pilot experiments and were excluded.
Heat-induced head withdrawal test
As described before [35], rats were partially anesthetized with sodium pentobarbital (25 mg/kg; i.p.) 30 min prior to the testing to achieve stable anesthesia during the testing. Pinch and blink responses remained intact at this level of anesthesia and stable baseline latencies were obtained up to two hours in GDX, as well as normal male rats (data not shown). Animals were kept in a Plexiglas tube with the head protruding through an opening and freely movable. Heat stimulus was applied to the shaved vibrissae pad and adjoining region using a modified tail flick analgesia meter (Model 33T, IITC Life Science, Woodland Hills, CA), and head withdrawal latencies were automatically recorded. Intensity of the heat stimulus was adjusted to achieve a baseline latency of 4–6 s. The cutoff time was set at 15 s to prevent any possibility of tissue damage. Three pre-drug (baseline) latencies were recorded followed by post-drug (test) latencies at 5 min intervals up to 30 min. and at 45, 60 and 90 min. Heat stimulus was applied alternatively to the right or left side of the head to prevent sensitization as a result of heating the same spot in succession.
Drugs
Clonidine (7 µg/5 µl; intracisternal) was injected in the medullary dorsal horn region 15 min. prior to the NMDA-induced nociceptive testing and at time 0 in the head withdrawal test. Yohimbine (30 µg/15 µl; intracisternal), an α2-adrenoceptor antagonist, was injected 5 min prior to the administration of clonidine in a separate group. These doses were selected from our detailed dose-response studies reported before [35,41]. There were no apparent sedative effects in animals given this dose of clonidine. All chemicals were obtained from Sigma-Aldrich, St. Louis, MO. Animals were sacrificed at the end of the testing by an overdose of sodium pentobarbital (150 mg/kg). Each animal was used for only one treatment condition. Since baseline head withdrawal latency or the number of NMDA-induced scratches did not significantly differ between GDX and normal males, reported by us before [35], the latter was excluded from the present study.
Data analysis
Data were analyzed using SPSS (SPSS Inc., Chicago, IL). All data were submitted to analysis of variance (ANOVA), corrected for repeated measures, where necessary, with appropriate within-(time) or between-group factors (group) and dependent variables (head withdrawal latency, number of scratches, latency to and duration of NMDA-induced scratching). Area under the curve (AUC) was calculated by trapezoid method using Prism (Graphpad Software, Inc., San Diego, CA) for the time course plot to obtain a single measure of the overall drug response and was analyzed by one-way ANOVA. Multiple comparisons were carried out using a post hoc t-test (Bonferroni) only where ANOVA yielded a significant main effect/interaction. Statistical significance was set at p <0.05. Data were plotted as mean ± SEM.
RESULTS
NMDA induces similar nociceptive behavior in GDX rats with or without sex-hormones
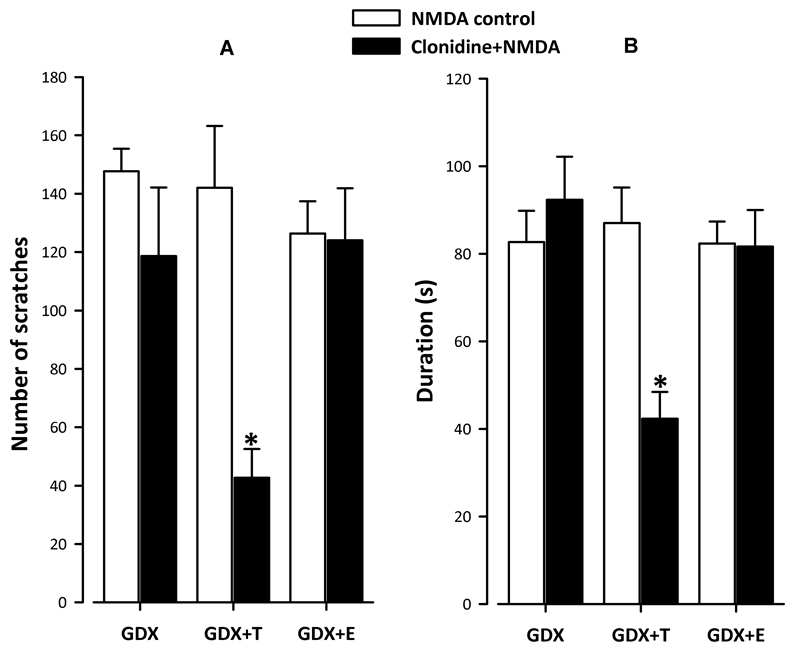
We first determined whether intracisternal injection of NMDA induces similar scratching behavior in the orofacial region of GDX rats with or without testosterone or estrogen treatment. As seen inFig. 1, the number of NMDA-induced scratches (Fig. 1A) or the duration of scratching behavior (Fig. 1B) was comparable in all experimental groups. These findings demonstrate that the perception of painper seon this assay was not modified by the presence of either testosterone or estrogen.
Figure 1. Testosterone is required for the expression of α2-adrenoceptor antinociception on the NMDA-evoked nociceptive test in the male rat.
Clonidine antinociception was observed only in testosterone treated orchidectomized rats (GDX+T) on the NMDA-induced nociceptive test. Significant main effect of group was obtained on the number of NMDA-induced scratches and the duration of scratching (F5,17=5.58 and 5.41 respectively; p<0.01). Post hoc comparisons revealed that while the number of scratches (Fig. 1A) or the duration (Fig. 1B) did not differ between control groups (NMDA injection only) with or without hormonal replacement, clonidine, given 15 min prior to NMDA, significantly reduced both measures only in the GDX+T group but not in GDX or GDX+E groups. Latency to scratching was not significantly affected between groups (F<1; data not shown). n = 3/group.
Testosterone is required for the α2-adrenoceptor-induced antinociception on NMDA-induced nociceptive test
To assess whether testosterone is required for the α2-adrenoceptor-induced antinociception on NMDA-induced nociceptive behavior, we tested the effect of a selective α2-adrenoceptor agonist, clonidine (given 15 min before NMDA), in GDX rats with or without testosterone. As seen inFig. 1, clonidine significantly reduced the number of scratches (Fig 1A) as well as the duration of the scratching behavior (Fig. 1B) only in testosterone-treated (GDX+T) GDX group. There was no significant effect of clonidine in the GDX group with its respective NMDA-only control. Further, as seen from unchanged number of scratches (Fig 1A) or duration (Fig 1B) of NMDA-induced scratching behavior, estrogen administration (GDX+E) did not alter the absence of clonidine-antinociception in GDX rats. The latency to scratching did not change significantly in any group regardless of the treatment (data not shown). These results demonstrate that testosterone is essential for the expression of clonidine-antinociception on NMDA-induced nociception in the trigeminal region of the male rat.
Testosterone is required for the α2-adrenoceptor-induced antinociception on a heat-induced head withdrawal test
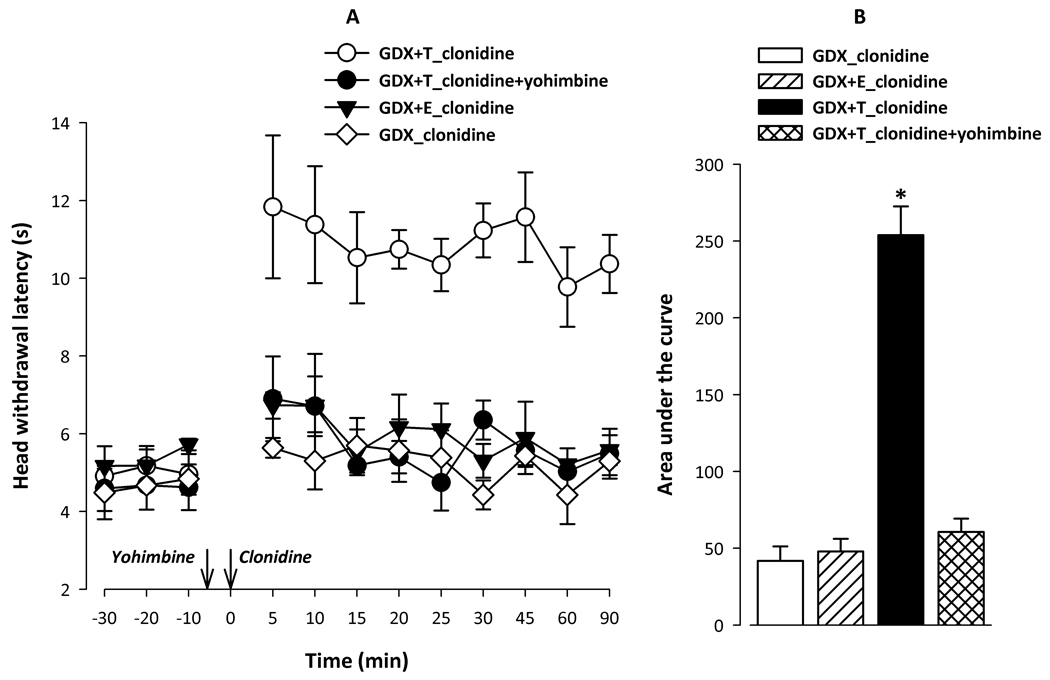
To determine whether testosterone is also required for the α2-adrenoceptor-induced antinociception on a natural stimulus evoked (i.e. heat vs. NMDA induced) nociception, we employed head withdrawal test. As seen inFig. 2A, baseline head withdrawal latencies were comparable in all GDX groups with or without hormone treatment reaffirming that basal pain perception did not change between different groups. However, α2-adrenoceptor activation by clonidine (given at time 0) produced a robust and significant increase in the head withdrawal latencies only in testosterone-treated GDX rats (GDX+T;Fig 2A). This increase was seen within 5 minutes of clonidine injection and persisted for the duration of the testing (90 min). However, clonidine failed to produce any effect in GDX group and the head withdrawal latencies remained at the baseline level following clonidine injection. In addition, as seen from unchanged head withdrawal latencies (Fig 2A), estrogen administration did not alter the absence of clonidine-antinociception in GDX rats (GDX+E).
Figure 2. Testosterone is required for the expression of α2-adrenoceptor antinociception on the heat-evoked head withdrawal test in the male rat.
Fig 2A: Clonidine antinociception was observed only in testosterone treated orchidectomized rats (GDX+T) on the heat-evoked head withdrawal test. ANOVA yielded significant main effects of Group (F3,10=58.67; p<0.001), Time (F11,110=7.04; p<0.001) and an interaction between Group × Time (F33,110=2.83; p<0.001). Post hoc comparisons revealed that the baseline head withdrawal latency did not significantly differ between different groups with or without hormonal replacement. Clonidine significantly increased head withdrawal latencies (i.e. produced antinociception) only in the GDX+T (n=4) group but not in GDX (n=3) or GDX+E (n=3) groups. The effect of clonidine was seen within 5 min of injection and persisted for the duration of the experiment (90 min) in the GDX+T group. This effect was completely reversed by yohimbine, a non imidazoline α2-adrenoceptor antagonist (n=4).
Fig 2B: The data was also subjected to area under the curve analysis to highlight the cumulative effect of clonidine. Clonidine significantly increased the AUC only in the GDX+T group (F3,13=64.86; p<0.001).
We then assessed whether the observed effect of clonidine was produced specifically by the activation of the α2-adrenoceptor and not by imidazoline receptors since clonidine has some affinity toward the latter. To address this we used yohimbine which is a nonimidazoline α2-adrenoceptor antagonist. As expected, yohimbine (given 5 min prior to clonidine) completely blocked the effect of clonidine in the GDX+T group and restored head withdrawal latencies to the control levels (Fig. 2A).
Further, area-under-the-curve analysis was conducted on time course data to obtain a single, readily comparable measure of the overall response, and as seen inFig. 2B, it further affirmed that clonidine was significantly effective only in GDX+T group as compared to all other groups. This demonstrates that testosterone is essential for the expression of clonidine-antinociception on the heat-induced head withdrawal test in the trigeminal region of the male rat.
DISCUSSION
We have demonstrated for the first time that testosterone is essential for the expression of antinociception induced by the α2-adrenoceptor in the trigeminal region of male rats. We have previously shown that intracisternal administration of clonidine in the trigeminal region of intact males produced a robust antinociception [35] which was attenuated by estrogen in females. The present study revealed that orchidectomy, and thus the absence of testosterone, resulted in a complete lack of an effect of clonidine treatment in males. Testosterone replacement in the GDX rats restored clonidine’s antinociceptive effect. The abolition of clonidine’s antinociceptive effect in the absence of testosterone was observed on both NMDA-induced nociceptive scratching behavior as well as on heat-evoked head withdrawal test suggesting that testosterone is essential for the expression of clonidine’s antinociceptive effects on different modalities of pain processing. These findings are consistent with those reported by us before [41], however, in the lumbar spinal region and only on a tail flick assay. Although the results of our previous study i.e. the requirement of testosterone for the expression of α2-adrenoceptor analgesia in the spinal cord, would predict a similar outcome in the trigeminal region, known region specific actions of gonadal hormones in the endocrinology field [11], as well as the possibility of site specific GPCR activation [42] provideda priorireasons to investigate hormonal effects in discrete regions (lumbar spinal cord vs. trigeminal region) independently. Further, trigeminal region is an important relay center for nociceptive signals originating in orofacial areas e.g. skin, tooth pulp, temporomandibular joint, cornea and dura [2,32,38] and despite sharing similarities, it is functionally more complex than the spinal dorsal horn [2].
The control NMDA-induced nociceptive behavior or the baseline head withdrawal latency did not significantly differ between GDX and testosterone replaced GDX rats. This finding is consistent with unchanged baseline nociceptive responses reported in a number of studies using heat evoked tail-flick or hot plate test [33], however, Borzan and Fuchs [1] reported that GDX rats had slightly but significantly lower foot withdrawal latency as compared to testosterone replaced GDX rats. The physiological significance of their finding may be questionable since the difference in the latency was only about 1 second. In another study, Romaro et al. [36] observed comparable baseline tail-flick latencies in sham operated and castrated males, however, cold water swim analgesia was reduced in castrated males and testosterone replacement significantly increased this analgesia. Kiefel and Bodnar [29] have also reported increased clonidine (intraperitoneal) analgesia by testosterone treatment in castrated rats. These results are in agreement with our present results and consistent with the interpretation that testosterone may play a crucial role in the expression of analgesia whether induced by cold water swim stress or by the selective activation of the α2-adrenoceptor.
The exact mechanism by which testosterone participates in the expression of clonidine antinociception is not yet known. However, it may act genomically and/or non-genomically. In support of a genomic action, Dygalo et al. [13] have shown that testosterone up-regulates the α2A-adrenoceptor mRNA in the cortical region of the castrated males, although testosterone had an opposite effect in the brain stem. Nevertheless, there is a possibility of testosterone-induced up regulation of the α2A-adrenoceptor mRNA in the trigeminal dorsal horn that may be responsible for the antinociceptive effects of clonidine via receptor over expression. Both α2-adrenoceptors and androgen receptors have been localized in the trigeminal region of the Sprague-Dawley rat [8,37]. Although a direct action of testosterone on the α2-adrenoceptor is not known, testosterone has been shown to directly modulate opioid receptor density or affinity and hence may affect other GPCRs including the α2-adrenoceptor similarly. It can also act antagonistically at NMDA receptors as reveled by increased [125I]MK801 binding sites in the hippocampus of castrated rats [30]. Finally, the metabolites of testosterone may also participate in its behavioral effects [14,15]. Gonadectomy has been shown to up regulate the levels of follicular stimulating (FSH) and luteinizing hormones (LH) in the pituitary [23,24] but the extent to which these hormones directly or indirectly affect the acute nociceptive tests employed in the present study is unknown and unlikely given that the baseline head withdrawal latencies as well as the number of NMDA-induced scratches did not differ between GDX rats and normal males reported by us before [35].
Estrogen, on the other hand, has been shown by us [7,34,35,41] and others [10,26,27,33,40] to attenuate antinociception induced by activation of a number of GPCRs, e.g. α2-adrenoceptor, µ-opioid receptors and ORL1 in ovariectomized rats and/or in intact females at proestrous stage (high circulating estrogen level). Although estrogen injection in orchidectomized rats did not exacerbate NMDA- or heat induced pain, it failed to change the absence of the antinociceptive effect of clonidine. This follows the general consensus that estrogen’s actions are not antinociceptive.
These results lead us to conclude that, i) antinociception produced by selective activation of the α2-adrenoceptor in the trigeminal region of the male rat requires the presence of testosterone, and ii) estrogen does not alter the lack of clonidine’s effect in GDX rats. These findings implicate α2-adrenoceptor as a promising target for analgesics in adult males as compared to young (before puberty), aging males, and adult females.
ACKNOWLEDGEMENTS
This work was supported by NIH grants 8SC1 NS063951, U54 NS041071 and RR03032.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Borzan J, Fuchs PN. Organizational and activational effects of testosterone on carrageenan-induced inflammatory pain and morphine analgesia. Neuroscience. 2006;143:885–893. doi: 10.1016/j.neuroscience.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 2.Bereiter DA, Hirata H, Hu JW. Trigeminal subnucleus caudalis: beyond homologies with the spinal dorsal horn. Pain. 2000;88:221–224. doi: 10.1016/S0304-3959(00)00434-6. [DOI] [PubMed] [Google Scholar]
- 3.Berkley KJ. Sex differences in pain. Behav. Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 4.Bartok RE, Craft RM. Sex differences in opioid antinociception. J. Pharmacol. Exp. Ther. 1997;282:769–778. [PubMed] [Google Scholar]
- 5.Ceccarelli I, Scaramuzzino A, Massafra C, Aloisi AM. The behavioral and neuronal effects induced by repetitive nociceptive stimulation are affected by gonadal hormones in male rats. Pain. 2003;104:35–47. doi: 10.1016/s0304-3959(02)00460-8. [DOI] [PubMed] [Google Scholar]
- 6.Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J. Pharmacol. Exp. Ther. 1996;279:767–773. [PubMed] [Google Scholar]
- 7.Claiborne J, Nag S, Mokha SS. Activation of opioid receptor like-1 receptor in the spinal cord produces sex-specific antinociception in the rat: estrogen attenuates antinociception in the female, whereas testosterone is required for the expression of antinociception in the male. J. Neurosci. 2006;26:13048–13053. doi: 10.1523/JNEUROSCI.4783-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clancy AN, Bonsall RW, Michael RP. Immunohistochemical labeling of androgen receptors in the brain of rat and monkey. Life Sci. 1992;50:409–417. doi: 10.1016/0024-3205(92)90375-y. [DOI] [PubMed] [Google Scholar]
- 9.Clark JT. Component analysis of male sexual behavior. Methods Neurosci. 1993;14:32–53. [Google Scholar]
- 10.Craft RM. Sex differences in opioid analgesia: "from mouse to man". Clin. J. Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Cyr M, Ghribi O, Di Paolo T. Regional and selective effects of oestradiol and progesterone on NMDA and AMPA receptors in the rat brain. J. Neuroendocrinol. 2000;12:445–452. doi: 10.1046/j.1365-2826.2000.00471.x. [DOI] [PubMed] [Google Scholar]
- 12.Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesth. Analg. 2008;107:83–95. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- 13.Dygalo NN, Kalinina TS, Sournina NY, Shishkina GT. Effects of testosterone on alpha2A-adrenergic receptor expression in the rat brain. Psychoneuroendocrinology. 2002;27:585–592. doi: 10.1016/s0306-4530(01)00094-4. [DOI] [PubMed] [Google Scholar]
- 14.Edinger KL, Frye CA. Testosterone's analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Behav. Neurosci. 2004;118:1352–1364. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- 15.Edinger KL, Frye CA. Testosterone's anti-anxiety and analgesic effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:418–430. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Fillingim RB, Maixner W. Gender differences in the responses to noxious stimuli. Pain Forum. 1995;4:209–221. [Google Scholar]
- 17.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: a review of recent clinical and experimental findings. J. Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer L, Clemente JT, Tambeli CH. The protective role of testosterone in the of temporomandibular joint pain. J. Pain. 2007;8:437–442. doi: 10.1016/j.jpain.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Flake NM, Hermanstyne TO, Gold MS. Testosterone and estrogen have opposing actions on inflammation-induced plasma extravasation in the rat temporomandibular joint. Am. J. Physiol Regul. Integr. Comp Physiol. 2006;291:R343–R348. doi: 10.1152/ajpregu.00835.2005. [DOI] [PubMed] [Google Scholar]
- 20.Flores CA, Wang XM, Zhang KM, Mokha SS. Orphanin FQ produces gender-specific modulation of trigeminal nociception: behavioral and electrophysiological observations. Neuroscience. 2001;105:489–498. doi: 10.1016/s0306-4522(01)00179-8. [DOI] [PubMed] [Google Scholar]
- 21.Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002;958:139–145. doi: 10.1016/s0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]
- 22.Gaumond I, Arsenault P, Marchand S. Specificity of female and male sex hormones on excitatory and inhibitory phases of formalin-induced nociceptive responses. Brain Res. 2005;1052:105–111. doi: 10.1016/j.brainres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Gharib SD, Bowers SM, Need LR, Chin WW. Regulation of rat luteinizing hormone subunit messenger ribonucleic acids by gonadal steroid hormones. J. Clin. Invest. 1986;77:582–589. doi: 10.1172/JCI112340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gharib SD, Wierman ME, Badger TM, Chin WW. Sex steroid hormone regulation of follicle-stimulating hormone subunit messenger ribonucleic acid (mRNA) levels in the rat. J. Clin. Invest. 1987;80:294–299. doi: 10.1172/JCI113072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hau M, Dominguez OA, Evrard HC. Testosterone reduces responsiveness to nociceptive stimuli in a wild bird. Horm. Behav. 2004;46:165–170. doi: 10.1016/j.yhbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Ji Y, Murphy AZ, Traub RJ. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. J. Neurosci. 2003;23:3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji Y, Murphy AZ, Traub RJ. Estrogen modulation of morphine analgesia of visceral pain in female rats is supraspinally and peripherally mediated. J. Pain. 2007;8:494–502. doi: 10.1016/j.jpain.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Kepler KL, Standifer KM, Paul D, Kest B, Pasternak GW, Bodnar RJ. Gender effects and central opioid analgesia. Pain. 1991;45:87–94. doi: 10.1016/0304-3959(91)90168-W. [DOI] [PubMed] [Google Scholar]
- 29.Kiefel JM, Bodnar RJ. Roles of gender and gonadectomy in pilocarpine and clonidine analgesia in rats. Pharmacol. Biochem. Behav. 1992;41:153–158. doi: 10.1016/0091-3057(92)90075-q. [DOI] [PubMed] [Google Scholar]
- 30.Kus L, Handa RJ, Hautman JM, Beitz AJ. Castration increases [125I]MK801 binding in the hippocampus of male rats. Brain Res. 1995;683:270–274. doi: 10.1016/0006-8993(95)00384-3. %19. [DOI] [PubMed] [Google Scholar]
- 31.LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev. Oral Biol. Med. 1997;8:291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- 32.Light AR. The initial processing of pain and its descending control: Spinal and trigeminal systems. Basel: Karger; 1992. [Google Scholar]
- 33.Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci. Biobehav. Rev. 2000;24:375–389. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 34.Nag S, Mokha SS. Estrogen attenuates antinociception produced by stimulation of Kolliker-Fuse nucleus in the rat. Eur. J. Neurosci. 2004;20:3203–3207. doi: 10.1111/j.1460-9568.2004.03775.x. [DOI] [PubMed] [Google Scholar]
- 35.Nag S, Mokha SS. Activation of alpha2-adrenoceptors in the trigeminal region produces sex-specific modulation of nociception in the rat. Neuroscience. 2006;142:1255–1262. doi: 10.1016/j.neuroscience.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Romero MT, Cooper ML, Komisaruk BR, Bodnar RJ. Gender-specific and gonadectomy-specific effects upon swim analgesia: role of steroid replacement therapy. Physiol Behav. 1988;44:257–265. doi: 10.1016/0031-9384(88)90147-3. [DOI] [PubMed] [Google Scholar]
- 37.Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT., Jr Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Res. Mol. Brain Res. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 38.Sessle BJ. Peripheral and central mechanisms of orofacial pain and their clinical correlates. Minerva Anestesiol. 2005;71:117–136. [PubMed] [Google Scholar]
- 39.Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tashiro A, Okamoto K, Bereiter DA. Morphine modulation of temporomandibular joint-responsive units in superficial laminae at the spinomedullary junction in female rats depends on estrogen status. Eur. J. Neurosci. 2008;28:2065–2074. doi: 10.1111/j.1460-9568.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 41.Thompson AD, Angelotti T, Nag S, Mokha SS. Sex-specific modulation of spinal nociception by alpha2-adrenoceptors: differential regulation by estrogen and testosterone. Neuroscience. 2008;153:1268–1277. doi: 10.1016/j.neuroscience.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tobin AB, Butcher AJ, Kong KC. Location, location, location...site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol. Sci. 2008;29:413–420. doi: 10.1016/j.tips.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 44.Wang XM, Zhang ZJ, Bains R, Mokha SS. Effect of antisense knock-down of alpha(2a)-and alpha(2c)-adrenoceptors on the antinociceptive action of clonidine on trigeminal nociception in the rat. Pain. 2002;98:27–35. doi: 10.1016/s0304-3959(01)00464-x. [DOI] [PubMed] [Google Scholar]