Abstract
Improved prevention and treatment of drug addiction will require deeper understanding of genetic factors contributing to susceptibility to excessive drug use. Intravenous operant self-administration methods have greatly advanced understanding of behavioral traits related to addiction. However, these methods are not suitable for large-scale genetic experiments in mice. Selective breeding of mice can aggregate ‘addiction alleles’ in a model that has the potential to identify coordinated effects of multiple genes. We produced mouse lines that orally self-administer high (MAHDR) or low (MALDR) amounts of methamphetamine, representing the first demonstration of selective breeding for self-administration of any psychostimulant drug. Conditioned place preference and taste aversion results indicate that MAHDR mice are relatively more sensitive to the rewarding effects and less sensitive to the aversive effects of methamphetamine, compared to MALDR mice. These results validate the oral route of self-administration for investigation of the motivational effects of methamphetamine, and provide a viable alternative to intravenous self-administration procedures. Gene expression results for a subset of genes relevant to addiction-related processes suggest differential regulation by methamphetamine of apoptosis and immune pathways in the nucleus accumbens of MAHDR and MALDR mice. In each line, methamphetamine reduced an allostatic state by bringing gene expression back toward ‘normal’ levels. Genes differentially expressed in the drug-naïve state, including Slc6a4 (serotonin transporter), Htr3a (serotonin receptor 3A), Rela (NFκB) and Fos (cFos), represent candidates whose expression levels may predict methamphetamine consumption and susceptibility to methamphetamine reward and aversion.
Keywords: selective breeding, conditioned place preference, conditioned taste aversion, preference drinking, gene expression, drug reward, addiction, eQTL
Introduction
Chronic methamphetamine (MA) use produces persistent neural changes that likely contribute to the high rate of relapse associated with MA addiction, but partially recover with protracted abstinence (Volkow et al., 2001). The neurochemical mechanisms that underlie the acute subjective effects of MA are well understood (Sulzer et al., 2005). However, less is known about neurochemical substrates responsible for transition from initial use to problem use and addiction, or about genetic factors that contribute to susceptibility to initiation and maintenance of MA use.
Twin and single nucleotide polymorphism (SNP) association studies confirm a genetic component in susceptibility to amphetamine dependence (Tsuang et al., 1999; Uhl et al., 2008), and genetic variants at a few loci have been suggested to influence vulnerability to MA dependence (Ikeda et al., 2007; Morita et al., 2008; Otani et al., 2008). However, as with any complex behavioral trait, contributions from multiple genetic loci that result in diversity in nervous system function likely contribute to MA addiction. Therefore, candidate gene approaches in animal models of MA addiction (e.g., knockout studies; Phillips et al., 2008) may not be adequate to model the more complex human condition. Large-scale genetic approaches using methods tractable for testing large numbers of animals are needed, because they have the potential to identify the coordinated effects of multiple genes.
Addiction is defined, in part, by the amount of drug used and the inability to refrain from use. Thus, animal models that measure voluntary drug self-administration have obvious translational value, assuming that self-administration can be attributed to the motivational effects of the drug. Most rodent studies that have examined psychostimulant self-administration have utilized operant intravenous methods in rats to ascertain motivation for the drug or for drug-associated cues (Sanchis-Segura & Spanagel, 2006). Intravenous methods offer the advantage of rapid drug delivery, and measurements of quantity, effort, and relapse-like behavior. These methods are more technically challenging in mice, the mammalian species most commonly used for genetic analyses, and this challenge prohibits large-scale genetic experiments like those required for SNP analysis and gene mapping. However, psychostimulants are also administered orally by humans, and this route can lead to addiction (www.nida.nih.gov). Consideration should be given to the possibility of using oral self-administration for large-scale testing to identify genetic mechanisms relevant to MA addiction.
Here we describe the successful bidirectional selective breeding of mice that orally self-administer high or low amounts of MA. Differences between the lines in the development of MA-induced conditioned place preference (CPP) and conditioned taste aversion (CTA), in the absence of differences in tastant consumption, validate the oral route of self-administration for investigation of the motivational effects of MA. Gene expression results, based on qPCR screening in nucleus accumbens (NAc) tissue of 384 genes putatively associated with addiction-related processes, provide candidate genes for further analysis, whose expression levels may predict MA consumption. Based on these data, we propose several hypotheses regarding genes and pathways that may influence an individual’s susceptibility to the rewarding or aversive properties of the drug.
Materials and Methods
Animals and Selective Breeding for MA Drinking
All experiments were performed in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Portland VA Medical Center’s Institutional Animal Care and Use Committee. Measures were taken to minimize discomfort. Mice were housed in shoebox cages (28.5 × 17.5 × 12 cm) with Bed-O-Cob ™ bedding, and maintained at 21 ± 1°C on a 12:12h light:dark schedule (lights on 0600h). All behavioral testing occurred during the light phase between 0800h and 1400h. Mice had free access to water and standard rodent diet (Purina 5001™, 4.5% fat content; Animal Specialties, Inc., Hubbard, OR), except as noted below. Breeding pairs and preweanling offspring were offered a higher fat diet (Purina 5020™, 9% fat content). Mice were weaned at age 20–22 d and thereafter group-housed with same-sex littermates in groups of 2–5 mice.
A reciprocal F1 cross between C57BL/6J (B6) and DBA/2J (D2) mice was performed in the Portland VA Medical Center’s animal facility, and these B6D2F1 animals were crossed to create a founding population of 120 male and female B6D2F2 mice for the MA drinking selective breeding project. This population was chosen because the current studies build on prior work that has utilized these strains as the founders of many populations used for gene mapping and expression profiling of addiction-related traits (Crabbe et al., 1999; Fehr et al., 2005; Kirstein et al., 2002; Palmer et al., 2005; Phillips et al., 1998). The F2 population was tested for voluntary MA consumption using the two-bottle choice procedure described below, beginning at age 56–64 d. Mass selection procedures were used in this short-term selection for high and low MA drinking, which is customary for selective breeding projects in which few generations of selection are completed (Belknap et al., 1997; Kamens et al., 2005; Phillips et al., 2005). Inbreeding was restricted by prohibiting brother-sister mating, avoiding mating of mice with common grandparents, and limiting the selection to 4 generations (S1–S4). This reduced the probability of the chance fixation of trait-irrelevant genes (Belknap et al., 1997; Phillips et al., 2005). The 13 male and 13 female mice showing the highest consumption of 40 mg/L MA were selected as breeders to establish the MA high drinking (MAHDR) line, and the 13 male and 13 female mice with the lowest consumption values were paired to establish the MA low drinking (MALDR) line. Male and female S1–S4 mice were age 51–73 d at the time of testing for MA consumption for selective breeding, and 112–159 offspring were tested in each generation from thirteen breeding pairs per line. Other MA traits were examined for genetic correlation with the selection trait using drug-naïve second (or later) litter offspring of the MAHDR and MALDR lines at the generations given below.
Drugs and other compounds
(+)Methamphetamine hydrochloride (MA), saccharin sodium salt, quinine hemisulfate salt, sodium chloride (NaCl) and potassium chloride (KCl) were obtained from Sigma (St. Louis, MO). For drinking solutions, all compounds were dissolved in tap water to the appropriate concentrations. For injections, MA was dissolved in sterile physiological saline (0.9% NaCl; Baxter Healthcare Corporation, Deerfield, IL). All injections were given intraperitoneally at a volume of 0.01 ml/g body weight.
Two-bottle choice MA drinking
Mice were tested for MA consumption using a standard two-bottle choice procedure in the home cage in which they were first isolate-housed and presented with two 25ml tubes containing tap water for 2 d to familiarize them with the drinking apparatus. Over the next 8 d, animals were given a choice between tap water and a MA solution. The 20 and 40 mg/L MA concentrations were chosen from prior work demonstrating increased MA consumption in a mouse line bred for reduced sensitivity to the acute stimulant effects of MA (Kamens et al., 2005). Each MA concentration was available for 4 consecutive days for 18 h/d and the solutions were offered in the order of ascending concentration. Prior work suggested that an 18-h access period avoids the possibility of drug-induced anorexia, compared to the significant weight loss observed when 24-h access was allowed. The relative position of the drug tube to the water tube was switched every other day to control for possible fluid tube position bias. Consumption was measured daily in ml (accuracy = 0.1 ml) and then converted to mg/kg based on body weight measured every 2 d. To measure possible leakage and evaporation, tubes containing the same solutions were placed on empty cages on the same animal racks, and the average volume lost from these tubes was subtracted each day from the individual drinking values. The averages of the second and fourth day consumption for each drug concentration were used as the individual consumption values. This method provides an adequate period for the animals to identify the location of the MA-containing solution and stabilize their intake. The average mg/kg/d consumption of the 40 mg/L concentration was used to select animals with high and low MA consumption for breeding at each generation. Preference ratios (ml MA solution consumed/total ml consumed from both drinking tubes) were also calculated.
Two-bottle choice tastant drinking
Because the question of tastant sensitivity is critical to the interpretation of differences in MA solution drinking, male and female MAHDR and MALDR mice (aged 70–80 d) of the second and fourth generations were tested for preference drinking of three tastant compounds with differing taste qualities (saccharin-sweet, quinine-bitter, and KCl-salty) versus tap water. Mice used in the tastant study had previously participated in a cocaine drinking study (manuscript in preparation) which ended 4 d prior to the start of tastant testing. Tastant solutions were each available 24 h/d for 4 consecutive days. Two concentrations were presented for each tastant, always with the lower concentration offered first (0.033% and 0.066% saccharin; 0.015mM and 0.03mM quinine; 100mM and 200mM KCl). The order of tastant presentation was counterbalanced across six groups to control for the possible effects of previous tastant exposure (n = 4–5 per line and order group). Consumption of the tastants was measured in ml (accuracy = 0.1 ml) on all days and then converted to mg/kg based on body weight measured every 2 d. These methods are consistent with our previous work (e.g., Kamens et al., 2005).
MA-induced conditioned place preference
Conditioning boxes (San Diego Instruments, San Diego, CA) were 30 × 15 × 15 cm, with walls and lids made of clear acrylic plastic. A black plastic wall, when inserted in the middle of the box, confined animals to the right or left half. The floors were removable half-panels constructed from one of three different materials: black plastic, “grid” (2.3mm stainless steel rods mounted 6.4mm apart), or “hole” (stainless steel with 6.4mm round holes on 9.5mm staggered centers). The grid and hole floor types are the same as those we and others have used successfully in previous drug conditioning studies (Bechtholt et al., 2004; Phillips et al., 2005). Each box was transected by 16 evenly spaced photocells and receptors located 2cm above the chamber floor. Beam breaks were recorded with Photobeam Activity System (PAS) software (San Diego Instruments, San Diego, CA) and used to determine activity levels and animal position. Each conditioning box was housed inside a sound-attenuation chamber, with a source of illumination and a ventilation fan, to minimize environmental disturbances during conditioning.
Place preference was assessed in third generation male and female MAHDR and MALDR mice (n = 9–12 per line, sex, and treatment group; aged 50–64 d). Conditioning sessions and testing were conducted on weekdays (Monday through Friday), during the light phase, consistent with previous studies using similar apparatus and procedures (Cunningham et al., 2006). Lights remained on inside the CPP test chambers during conditioning and testing. The mice were moved in their home cages to the testing room for a 1 h acclimation period prior to the start of testing. Each animal then received a single 5 min habituation session on the black plastic floor following saline injection, with full access to both sections of the conditioning chamber. This trial was intended to familiarize the mice with the apparatus and handling procedures. Beginning on the next day, twelve daily 15 min conditioning sessions were conducted, during which animals were restricted to one half of the chamber. Six conditioning sessions paired injection of 0.5 mg/kg MA with exposure to either the grid (G+) or hole (H+) floor, and six sessions on intervening days paired saline injection with the opposite floor type. Groups were counterbalanced for MA-paired floor (e.g., G+H− vs. G−H+) and order of conditioning sessions (e.g., G+H− vs. H−G+). CPP was tested following saline injection in a single 30 min session, during which the animals had access to the full chamber and both the grid and hole floor types. These methods and the dose of MA were chosen based on pilot studies that examined the duration of conditioning, number of trials, and dose in B6D2F2 mice. These pilot data indicated a restricted range of low MA doses that were efficacious in producing a place preference in the F2 mice (data not shown). Others have used this or a similar dose (Cunningham & Noble, 1992; Niwa et al., 2007).
Beginning 3 d after CPP testing, conditioning was extinguished by pairing saline with both floor types in six 15 min trials, two per treatment day. In order to confirm extinction of CPP expression, mice were retested for CPP after saline injection in a single 30 min session in which they had access to both floor types. One day later, reinstatement of CPP was tested by administering 0.5 mg/kg MA just prior to 30 min access to both floor types (Aguilar et al., 2008).
MA-induced conditioned taste aversion
MA-induced CTA was assessed in third generation MAHDR and MALDR mice (n = 5 per line, sex, and treatment group; aged 73–91 d). All mice were isolate-housed and presented with one 10ml tube containing tap water for 2 d (day -1 and 0) to familiarize them with the drinking apparatus. Beginning on the following day, animals were habituated to a fluid-restriction procedure for 4 d (day 1–4). Mice had access to water for 2 h (1000–1200h) each day, and were weighed daily to monitor for excessive weight loss (defined as loss of 20% or more of expected body weight). No excessive body weight loss was observed in this study. Beginning on day 5, animals were weighed and then offered a drinking tube containing 0.2M NaCl solution for 1 h (1000–1100h) in the home cage. This solution was chosen based on prior ethanol CTA studies (Lessov et al., 2001; Risinger & Cunningham, 1992; Sharpe et al, 2005). Three hours after removal of the salt solution, animals had 30 min access to tap water to prevent dehydration. On day 6, mice received 2 h of water access. This alternation of NaCl test and water access days continued until mice had the opportunity to consume NaCl on six occasions (thus, mice had access to the salt solution on days 5, 7, 9, 11, 13 and 15 and to water on intervening days 6, 8, 10, 12, and 14). Consumption of NaCl and water was measured in ml (accuracy = 0.1 ml) on all days. On the first NaCl access day (day 5), animals were familiarized with the novel drinking fluid, and no injections were administered. Consumption on this day was not included in the data analysis because it was not subject to the influence of conditioned drug effects. On all subsequent NaCl access days except the last (days 7, 9, 11, 13), mice were injected with saline, 1, or 2 mg/kg MA immediately following removal of the salt solution.
Statistical methods
Statistica (StatSoft, Tulsa, OK) was used for statistical analyses of the selection data and data from the behavioral experiments. Data were analyzed by factorial ANOVA with line, sex, generation, day, and treatment group as possible independent variables. Effects were considered significant at α ≤ 0.05. Animals of both sexes were used in all experiments, and follow-up analyses were performed with data from the sexes combined whenever sex did not interact with other factors. Significant interactions involving multiple factors were followed up by ANOVA including fewer factors to determine the sources of interaction. Two-way interactions were interpreted using simple main effect analysis and post-hoc mean comparisons (Newman-Keuls test) when appropriate. Heritability of the bidirectional selection trait was calculated using methods described by Falconer and Mackay (1996), based on the ratio of the response to selection, R, and the selection differential, S.
qPCR Gene Expression Analysis
The purpose of this expression study was to develop genetic hypotheses by identifying innate and MA-associated differences between the MADR lines in the expression of genes thought to influence addiction-related processes. Drug-naïve mice were fourth generation male MAHDR and MALDR mice (n = 6 per line and treatment group). Mice were injected with saline or 2 mg/kg MA, then maintained with free access to food and water for 4 h after injection until euthanized by cervical dislocation and immediately decapitated. This treatment method was chosen over oral MA consumption because it was necessary to control exposure dose among animals. By virtue of their selective breeding, MALDR mice consume practically no MA. Therefore, matching mice from the two lines for MA intake was not an option. Further, our behavioral data indicate important differences between the high and low lines after injected MA. The 2 mg/kg MA dose is a modest dose that would be expected to induce increased locomotor stimulation for about 2 h (Mori et al., 2004). The 4 h time point was chosen to allow time for MA-induced gene expression changes to occur (Cai et al., 2006). Brains were removed, and NAc samples were dissected using a razor blade and a 4°C dissection block. Tissue samples were rapidly frozen and stored at −80°C until use. Samples were pooled in groups of three for RNA preparation; thus, the data represent two independent measurements for each treatment group within each selected line.
Total RNA was prepared by the single-step acid guanidinium thiocyanate-phenol-chloroform extraction method using RNA STAT-60 (Tel-Test Inc., Friendswood, TX) and contaminating DNA was removed through spin column purification, as per manufacturer instruction (Zymo Research Corp, Orange, CA). RNA purity was confirmed by spectrophotometric analysis and the integrity of ribosomal RNA was confirmed on a 1% agarose gel stained with SYBR Gold Nucleic Acid Gel Stain (Molecular Probes, Invitrogen). The purified RNA samples underwent first-strand cDNA synthesis using qScript cDNA SuperMix according to manufacturer instructions (Quanta Biosciences, Gaithersberg, MD). Quantitative RT-PCR (qPCR) was performed by Bar Harbor Biotechnology (Trenton, ME) using the Mouse Mood Disorder 384-well StellARray qPCR array (Bar Harbor Biotechnology). The use of qPCR arrays affords the advantage of not having to confirm the expression differences, as is required for more comprehensive arrays, such as Affymetrix, although protein differences will ultimately require examination in both cases. Significantly changed genes in the data set were identified by Bar Harbor Biotechnology using their Global Pattern Recognition algorithm (Akilesh et al., 2003). Overrepresented functional groups were identified using the DAVID Bioinformatics Resources (david.niaid.nih.gov; Huang et al., 2007). The 384 genes on the Mouse Mood Array were used as the background gene list for pathway analysis. Finally, expression quantitative trait locus (eQTL) analysis was conducted using the nucleus accumbens B6 × D2 (BXD) recombinant inbred strain expression database at GeneNetwork (genenetwork.org; VCU BXD NA Sal M430 2.0) to identify potential genetic loci influencing the expression of genes that were differentially expressed basally between the MAHDR and MALDR lines. These strains carry only B6 and D2 alleles, which is also the case for the selected line mice. Each differentially regulated gene was queried against the nucleus accumbens dataset to identify probes targeting the gene of interest. For each probe associated with the differentially regulated gene, interval mapping was carried out using GeneNetwork across all chromosomes using permutation testing to identify significant and suggestive eQTL. Thus, we first identified whether an expression difference was present across the BXD strains and then mapped the genomic region that was correlated with expression. Secondly, we determined whether a B6 or D2 allele was driving this expression difference.
cFos immunohistochemistry
The difference in NAc Fos expression in drug-naïve MAHDR and MALDR mice was confirmed by immunohistochemistry (n = 3 per line). Brain tissue was prepared by modifications of previously described methods (Hill et al., 2007; Turek et al., 2008). Briefly, the brains were sliced into 40µm sections and stored in PBS with 0.1% sodium azide. Samples were washed with PBS, followed by 15 min incubation in 0.3% hydrogen peroxide in PBS. Blocking serum was added (450µL goat serum/10ml PBS/ 0.3% Triton), and sections were incubated for 4 h at room temperature. Sections were then incubated with primary antibody (rabbit anti-cFos, Santa Cruz Biotechnology, Santa Cruz, CA; diluted 1:2000 in PBS/Triton/0.1% BSA) for 16 h at room temperature. After washing with PBS, secondary antibody (biotinylated goat anti-rabbit, Vector Lab, Burlingame, CA; diluted 1:200 in PBS/Triton) was added. After a 1 h incubation at room temperature, sections were incubated with ABC solution (Vector Lab, Burlingame, CA), for 1 h at room temperature. DAB solution (Thermo Scientific Pierce Metal Enhanced DAB Substrate Kit Rockford Il) was added and sections were incubated for up to 5 min and mounted on slides. Cell counts were conducted by an individual who was blind to the mouse line origin of the tissue section. Slides were selected to match structure location based on the mouse brain atlas (Franklin & Paxinos, 2008) and were viewed with an Olympus microscope (BH2-RFL-T3) and a Canon XT digital camera. Three sections from each animal were used (corresponding to plates 18, 20, and 22 from Franklin & Paxinos, 2008). Each hemisphere was counted separately and included in the statistical analysis.
Results
Response to selection
Voluntary consumption of the 40 mg/L MA solution, the trait used for selection of breeders in each generation, is shown in Figure 1 panel a; preference for the 40 mg/L solution is shown in Figure 1 panel b. Values for the selected parents are shown above or below the offspring for each selection generation in panel a of Figure 1 to illustrate selection pressure. The response to selection was bidirectional (both lines diverged from the original population mean) and the lines differed in MA consumption in the first and all subsequent generations. Average MA consumption increased more than two-fold in the MAHDR line in the first generation. There was a lag in the response to selection in the MALDR line, but the average consumption dropped sharply in the second generation and subsequently remained near zero. ANOVA including data for the 4 selection generations (but excluding the F2) identified a significant interaction of line and generation (F[3,567]=20.9, p<0.0001), indicating increasing divergence across generations. A similar result was found for preference (F[3,567]=28.9, p<0.0001). There was a significant main effect of sex on MA consumption (F[1,567]=6.1, p<0.05), with females consuming more than males, but a sex difference did not emerge for preference. Sex did not interact with line or generation, indicating that differences across generations between the two lines were similar for the two sexes. Thus, data for the sexes were combined in further comparisons.
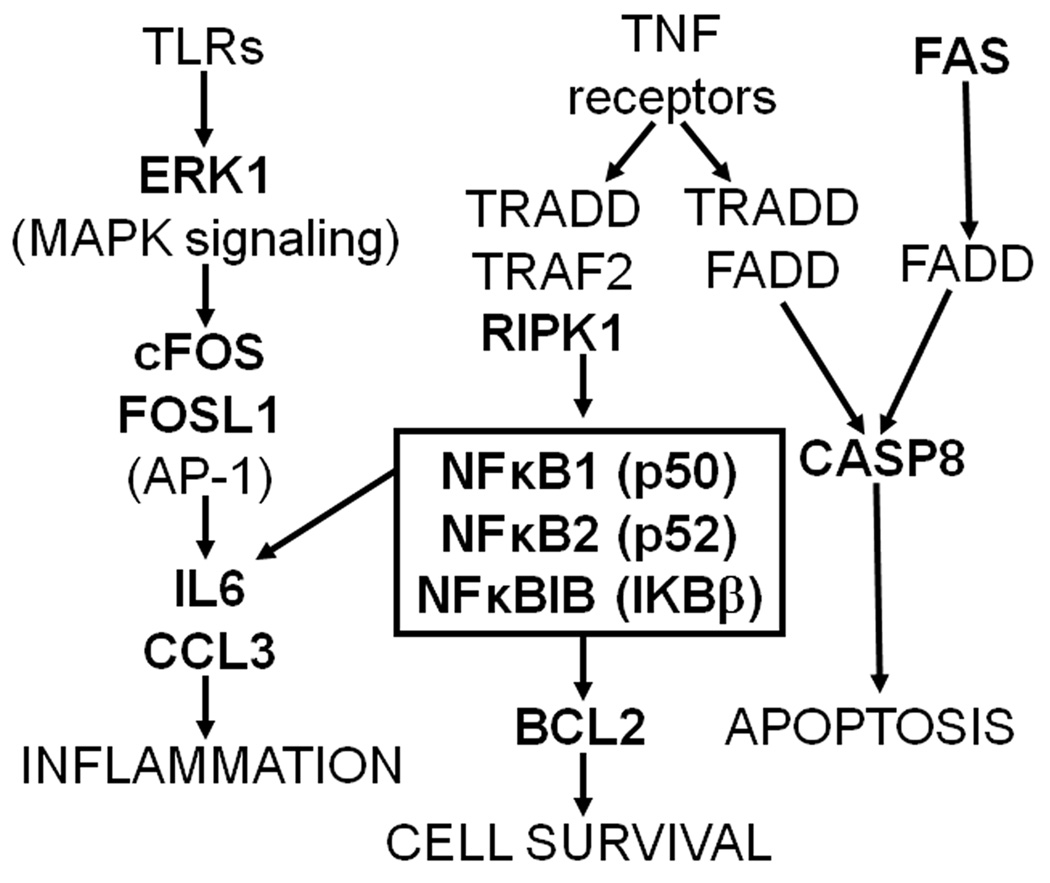
Figure 1. Bidirectional selective breeding produces significant differences in MA consumption.
(a) Means (± SEM; some error bars may be hidden by symbols) for the original B6D2F2 population and the parents and offspring of generations S1–S4 of short-term selection for high (MAHDR) and low (MALDR) MA drinking. Data shown are the average of consumption on the second and fourth days of access to the 40 mg/L MA solution. (b) Drug preference for the original B6D2F2 population and the offspring of generations S1–S4, calculated as the ratio of the volume of drug solution consumed over the total volume of fluid consumed. Data shown are the average preference on the second and fourth days of access to the 40 mg/L MA solution. (c) Offspring of the selected lines also differed in consumption of 20 mg/L MA. Data shown are means (± SEM) of average consumption on the second and fourth days of access. (n = 112–159 animals tested in each generation).
One-way ANOVAs were performed on data for each line, including the original F2 population, to determine significant changes in MA consumption from the originating population. A significant effect of generation was found for both the MAHDR (F[4,408]=44.6, p<0.001) and MALDR (F[4,403]=23.7, p<0.001) lines. Mean comparisons indicated that MA consumption in the MAHDR line was significantly increased above that of the starting F2 population in every generation of selection. In addition, MA consumption in the MAHDR line was significantly increased in generation S4 compared to generations S1 and S2. MA consumption in the MALDR line was significantly decreased compared to the F2 and S1 in generations S2 through S4. Calculation of the realized heritability for bidirectional selection of MA consumption indicates that 34% of the variability in this trait in this population can be attributed to genetic differences. This value is similar to the realized heritability for other short-term selective breeding experiments, such as for alcohol consumption (Belknap et al., 1997; Phillips et al., 2005) and MA-induced locomotor stimulation (Kamens et al., 2005).
Consumption of 20 mg/L MA (Figure 1 panel c) also differed between the lines, and exhibited a pattern similar to that seen for the selection trait. ANOVA including data for the four selection generations (but excluding the F2) indicated a significant interaction of line and generation (F[3,567]=12.8, p<0.0001), and also a significant three-way interaction with sex (F[3,567]=3.3, p<0.05). Separate analyses for each line including the original F2 population indicated a significant interaction of sex and generation in MAHDR animals (F[4,403]=3.1, p<0.02) but not in MALDR animals, that was due to greater consumption of MA in female, compared to male, MAHDR mice only in the fourth generation.
When total fluid consumption was examined for the four selected line generations (excluding F2 data), significant main effects of both line (F[1,567]=30.6, p<0.001) and generation (F[3,567] = 4.0, p<0.01), but no interaction, were found. Overall, the high line consumed about 0.5 ml more fluid than the low line (mean ± SEM = 6.4 ± 0.07 for the MAHDR and 5.9 ± 0.06 for the MALDR). The effect of generation was transient, with S3 mice consuming more fluid (about 0.3 ml more) than S1, S2 and S4 generation mice. The absence of a line by generation interaction indicates that differences in total fluid consumption cannot account for the large bidirectional changes in MA consumption in the selected lines.
Tastant drinking
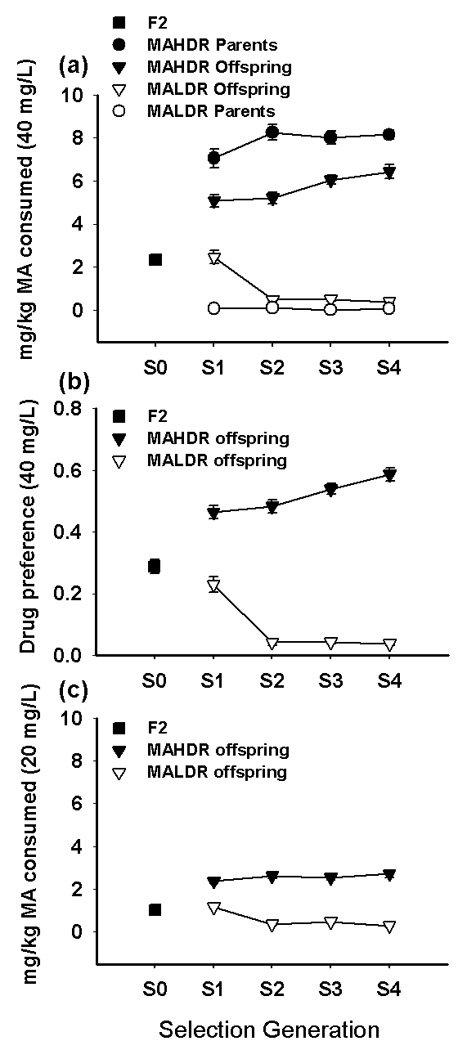
MA is known to have a bitter flavor (www.nida.nih.gov/Infofacts/methamphetamine.html). Because taste factors could be involved in MA consumption, we examined whether selective breeding for MA drinking produced lines that differ in their preference for various flavored solutions. No significant line differences were observed for consumption of saccharin or quinine solutions in either the S2 or S4 generations (Figure 2) and there were no significant line ×sex interaction effects. For KCl consumption, there was a significant 3-way interaction of concentration, line and sex (F[1,48]=4.9, p<0.05), associated with greater KCl consumption in MAHDR female compared to male mice (F[1,26]=8.5, p<0.01). This sex difference was not found in the MALDR line and there were no significant line differences in KCl consumption. This difference involving sex in the high line was transient, as no significant sex or line differences were observed for consumption of KCl in the S4 mice. We conclude from these data that alterations in taste sensitivity are unlikely to have played a role in the divergent MA consumption in these lines.
Figure 2. MAHDR and MALDR selected lines do not differ in consumption of sweet, bitter or salty flavored solutions.
Tastant preferences were measured in the S2 (panels a–c) and S4 (panels d–f) generations. Each solution was offered for four days versus tap water; data shown are the means (±SEM) of consumption on the second and fourth days of access to each solution. The lower concentration of each tastant was presented first, with the order of tastants counterbalanced across six groups (n = 4–5 per line and group). Several outliers (defined as values greater than 3 × the interquartile range from the mean) were removed from the data set: three from S2 quinine consumption and two from S2 KCl consumption. Extreme values suggest that the drinking tube may have been leaking.
MA-induced conditioned place preference
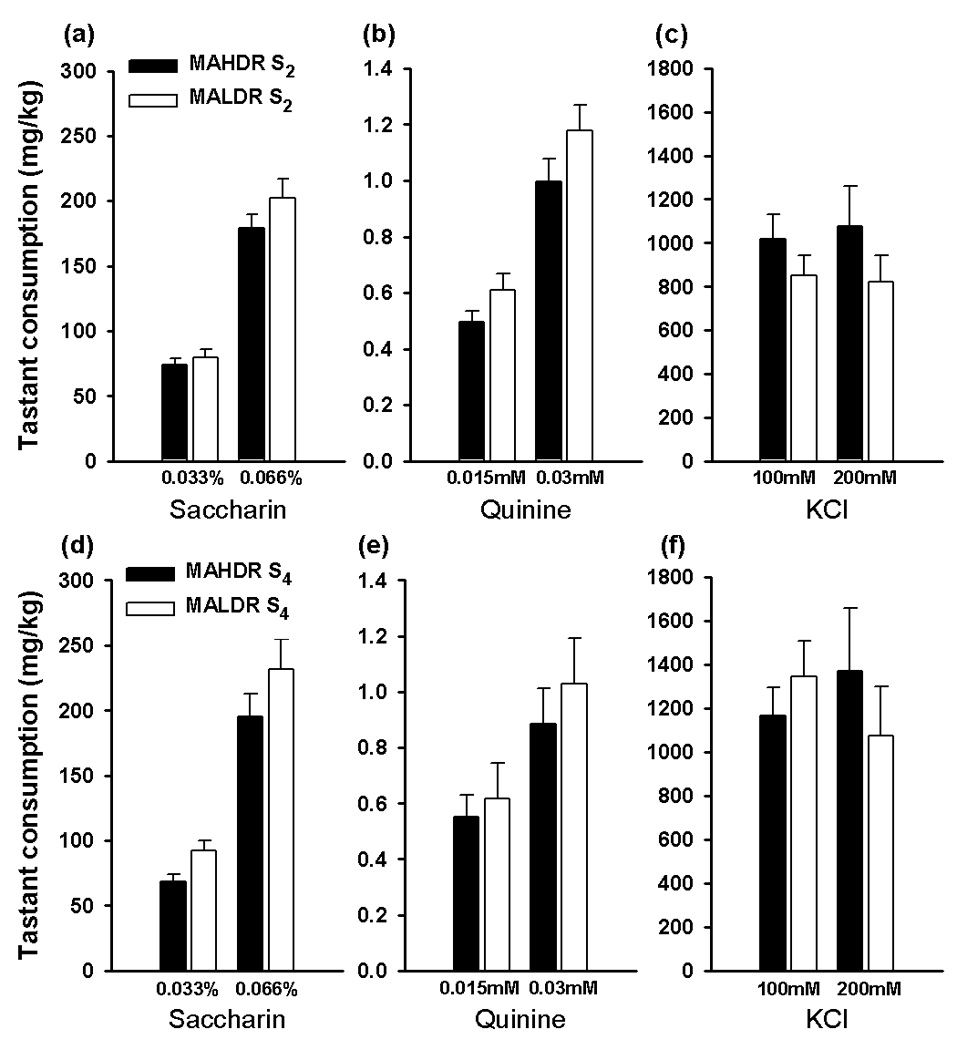
To provide evidence for differences in sensitivity to the rewarding effects of MA in the MAHDR and MALDR lines, we used a CPP procedure. The results for the preference test after conditioning are shown in Figure 3. Preference is indicated by more time spent on the grid floor by the grid+ compared to the grid− group (Figure 3 panel a). We observed a significant place preference in the MAHDR, but not the MALDR, line. This conclusion was supported by the statistical results, which indicated a significant interaction of drug-paired floor and line (F[1,77]=13.8, p<0.001) in the absence of sex effects, that when examined further revealed a significant effect of drug-paired floor in the MAHDR line (F[1,41]=55.0, p<0.001), but not MALDR line. Total beam breaks were similar in all groups during the preference test session (Figure 3 panel b); thus the difference in CPP between the lines cannot be attributed to different activity levels. Likewise, there were no differences in activity level between the lines during the conditioning sessions (data not shown), indicating that differential conditioning cannot be attributed to different levels of activity at the time of drug exposure.
Figure 3. MAHDR mice, but not MALDR mice, exhibit MA-induced conditioned place preference to a 0.5 mg/kg dose of MA.
(a) Sec/min spent on the grid floor of the CPP apparatus during the 30 minute conditioning test. There was a significant main effect of drug-paired floor in the MAHDR line (F[1,41]=55.0, p<0.0001) but not in the MALDR line. (b) Activity levels during the conditioning test, measured as total beam breaks during the session. There were no differences between the lines or treatment groups in activity levels. Subjects were S3 generation offspring of both sexes. Bars represent standard errors; n = 9–12 per line, sex, and treatment group.
Because significant MA-induced conditioning occurred only in the MAHDR line, extinction and reinstatement data are shown only for this line. Our methods fully extinguished CPP in the MAHDR animals; after the 6 extinction trials they no longer exhibited a preference for the MA-paired floor (data not shown). After administration of the 0.5 mg/kg dose of MA, reinstatement of CPP was seen in female, but not male, MAHDR mice (Figure 4 panel a). This characterization was statistically supported by a significant interaction of drug paired floor with sex (F[1,39]=4.8, p<0.05), followed by the identification of an effect of floor type in the MAHDR female mice (F[1,20]=12.4, p<0.01), but not in MAHDR male mice. Total beam breaks were about 50% higher compared to those observed during the conditioning (Figure 3 panel b) and extinction test sessions (data not shown) due to the administration of MA just before the reinstatement test. However, there were no significant differences in activity levels between the groups during this test session (Figure 4 panel b). These results indicate that selection for voluntary MA drinking has produced lines that are divergent in their sensitivity to the rewarding effects of MA. In addition, reinstatement of preference for MA-associated cues may occur more readily in high MA consuming female than male mice.
Figure 4. Female MAHDR mice exhibit reinstatement of MA-induced conditioned place preference in response to 0.5 mg/kg MA.
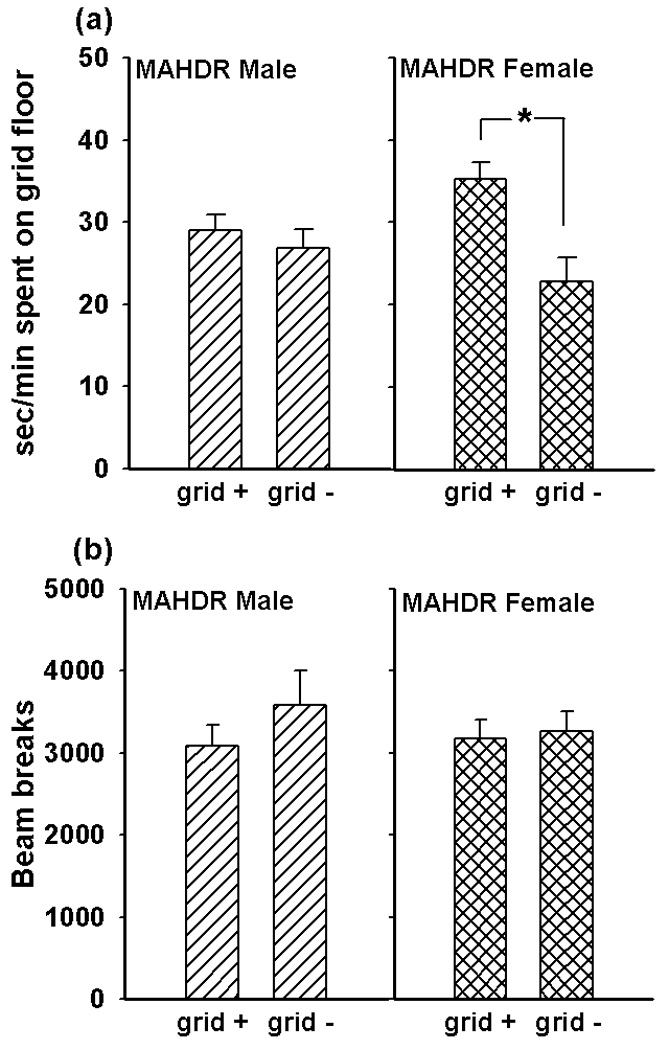
(a) Sec/min spent on the grid floor of the CPP apparatus during the 30 minute reinstatement test. There was a significant main effect of drug-paired floor in MAHDR females (F[1,20]=12.4, p=0.002) but not in males. (b) Activity levels during the reinstatement test, measured as total beam breaks during the session. There were no differences between the sexes or treatment groups in activity levels. Subjects were the same MAHDR animals tested for MA-induced conditioned place preference and represented in Figure 3. Bars represent standard errors; n = 9–12 per sex and treatment group.
MA-induced conditioned taste aversion
We used a CTA procedure to examine the possibility that selection for MA consumption was associated with differential sensitivity to the aversive effects of MA. The expectation is that consumption of a novel tastant solution will decrease from baseline consumption on days following MA treatment, due to the association of aversive drug effects with the novel taste, if the drug is perceived as aversive. As shown in Figure 5, we observed significant MA-induced CTA in the MALDR line but not in the MAHDR line. There were no significant differences in baseline consumption of 0.2M NaCl between the lines or treatment groups prior to conditioning (day 7). No significant effect of sex was detected, and sex did not interact with any other factor. When data from the sexes were combined, analysis showed a significant interaction of line, dose, and test day (F[8,212]=7.9, p<0.001). Separate ANOVAs for each line revealed a significant interaction of dose and test day in MALDR mice (F[8,104]=11.4, p<0.001), but not MAHDR mice. Simple main effect analyses indicated that there were significant differences in NaCl consumption across days in MALDR mice treated with 1 and 2 mg/kg MA, but not in those treated with saline. Mean comparisons indicated that NaCl consumption was significantly decreased compared to baseline consumption (day 7) in both MA dose groups on all four test days that followed MA administration. Analysis of average body weight in both of the lines did not identify any significant changes over the course of the conditioning trials, nor was body weight correlated with consumption of NaCl (data not shown). Thus, weight loss did not contribute to the decreased consumption in MALDR animals. These results suggest that selection for avoidance of consumption of MA has produced mice that find the subjective effects of MA relatively more aversive, compared to a line selected for high MA consumption.
Figure 5. Reduced MA consumption in MALDR mice is associated with relatively greater sensitivity to MA conditioned taste aversion in MALDR compared to MAHDR mice.
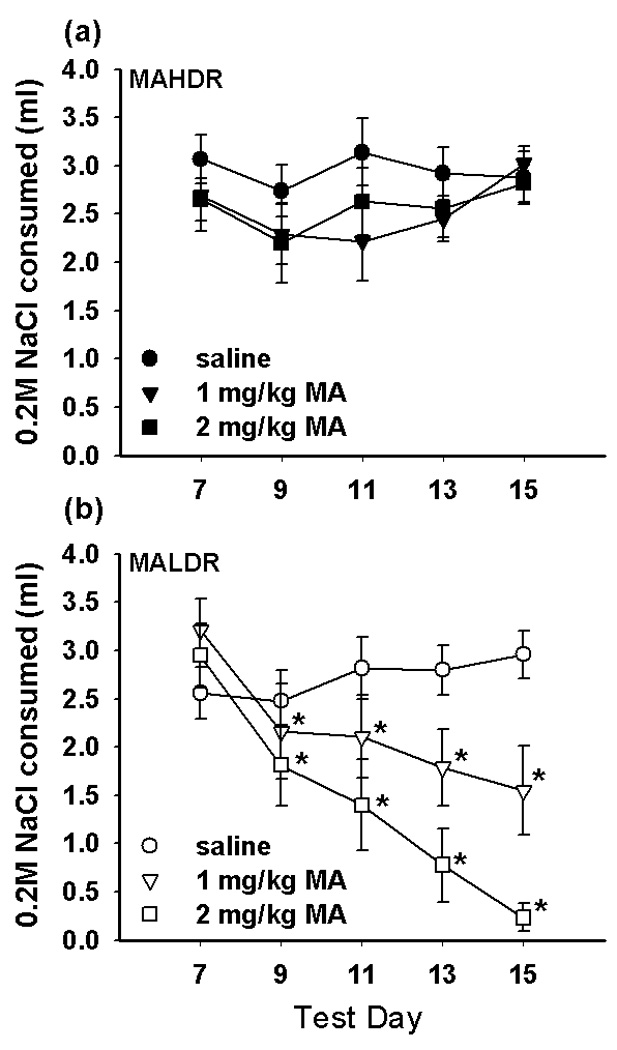
Animals (S3 generation; n = 5 per line, sex, and treatment group) received an injection of either saline, 1 mg/kg MA, or 2 mg/kg MA immediately following consumption of 0.2M NaCl on days 7, 9, 11, and 13. MAHDR animals (a) did not significantly decrease consumption of 0.2M NaCl relative to baseline (day 7) in any test session, regardless of MA dose. MALDR animals (b) in both MA dose groups showed significant aversion to 0.2M NaCl in all four test sessions following MA administration (*p < 0.01 versus day 7 consumption).
qPCR Gene Expression Array Analysis
We examined the expression levels of the 384 genes present on the Mouse Mood Disorder StellARray qPCR array using NAc tissue from MAHDR and MALDR mice that were either drug-naïve (saline-treated) or had received a single dose of 2 mg/kg MA 4 hours prior to obtaining brain tissue. Complete lists of all the genes that either differed between the lines, or were significantly altered following MA injection are available in Supplemental Table 1. The Mouse Mood Disorder array was chosen because many of the genes represented are relevant to findings from other studies of addiction-related processes. Thus, we have focused our initial efforts on a subset of genes most likely to be relevant to our selection phenotype, for the purposes of genetic hypothesis generation.
MAHDR and MALDR mice exhibited stark differences in MA regulation of gene expression. They shared only three genes in common that were regulated by MA. Two were heat shock proteins (Hsp40 and Hsce70), and their expression levels were increased in both lines after MA administration. Only one gene (transcription factor, Nfkb2) was downregulated in MALDR animals but upregulated in MAHDR animals. There were genes that showed significant expression differences between drug-naïve MAHDR and MALDR mice and also showed MA-induced expression changes. Some genes with higher basal expression in MAHDR mice were upregulated by MA in the MALDR line (Klk6, Adcyap1, Trpm2, Itsn1, Alg9), but another subset of these were downregulated by MA in the MAHDR line (Slc6a4, Slc6a2, Chrna1, Epb4.9, Fdft1, Grm4). Conversely, some genes with higher basal expression in MALDR mice were downregulated by MA in the MALDR line (Clcf1, Bcl2, Pla2g4a, Lef1, H2-Ea), while another subset of these was upregulated by MA in the MAHDR line (Fos). This type of regulation is consistent with an allostatic state, defined as “a state of chronic deviation of the regulatory system from its normal (homeostatic) operating level” (Koob & Le Moal, 2008), in which MA administration altered gene expression in both selected lines to bring them both back toward ‘normal’ levels.
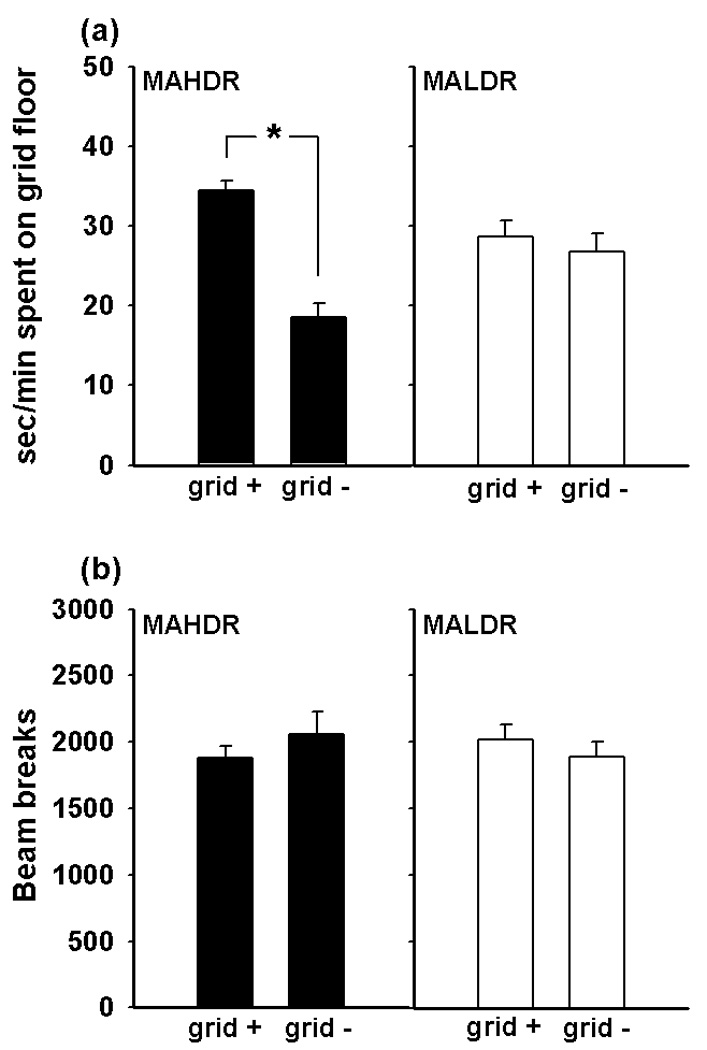
Altered regulation of common genes in opposite directions was rare, suggesting that selective breeding may have altered distinct neurochemical pathways in the two lines, rather than the same pathways in opposite directions. To test this hypothesis, we conducted a pathway analysis to identify Gene Ontology (GO) terms and signaling pathways that were overrepresented in our data set. The 384 genes on the Mouse Mood Array were used as the background gene list for pathway analysis, in order to account for any preexisting bias in functional classes among the genes in our data set. This analysis identified functionally significant clusters of genes, and also allowed for comparison of the selected lines on a higher functional level than that represented by individual gene products. Genes involved in apoptosis, immune response, and cytokine signaling were enriched among those significantly regulated by MA in the MALDR mice (Table 1). Overall, MA induced a coordinated downregulation of these gene classes in the low line. However, these functional classes were not overrepresented among genes significantly regulated by MA in MAHDR mice. Rather, genes participating in Toll-like receptor (TLR) signaling were enriched. This pathway shares some components (Nfkb2, Il6 [interleukin-6], Casp8 [caspase 8]) with those involved in apoptosis and immune responses. Additionally, two genes downregulated in MALDR animals (Ripk1 and Ccl3) also participate in TLR signaling. Interactions among some of these genes are described in Figure 6.
Table 1.
Overrepresented functional classes among genes significantly regulated by MA in MADR selected lines.
| Gene | Name | Apop | Immu | Cyto | Toll | Erbb | Oxid | Ster | MAHDR | MALDR | Saline |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clcf1 | Cardiotrophin-like cytokine factor 1 | • | • | ↓ | L | ||||||
| Ripk1 | Receptor (TNFRSF)-interacting serine-threonine kinase 1 | • | • | ↓ | |||||||
| Lta | Lymphotoxin A (aka TNF-beta) | • | • | • | ↓ | ||||||
| Pawr | PRKC, apoptosis, WT1, regulator | • | • | ↓ | |||||||
| Hspa5 | Heat shock 70kD protein 5 | • | ↑ | ↑ | |||||||
| Il6 | Interleukin 6 | • | • | • | • | • | ↓ | ||||
| Atf5 | Activating transcription factor 5 | • | ↓ | ||||||||
| Casp8 | Caspase 8 | • | • | ↓ | |||||||
| Il2rb | Interleukin 2 receptor, beta chain | • | • | ↓ | |||||||
| Gzmb | Granzyme B | • | ↓ | ||||||||
| Fas | Fas (TNF receptor superfamily member 6) | • | • | • | ↓ | ||||||
| Atm | Ataxia telangiectasia mutated homolog | • | ↓ | ||||||||
| Bcl2 | B-cell leukemia/lymphoma 2 | • | ↓ | L | |||||||
| H2-Ea | Histocompatibility 2, class II antigen E alpha | • | ↓ | L | |||||||
| Tgfb1 | Transforming growth factor, beta 1 | • | • | ↓ | |||||||
| Ccl2 | Chemokine (C-C motif) ligand 2 | • | • | ↓ | |||||||
| Ccl3 | Chemokine (C-C motif) ligand 3 (aka MIP-1alpha) | • | • | • | ↓ | ||||||
| Nfkb2 | Nuclear factor of kappa enhancer in B-cells (p52/p100) | • | • | ↑ | ↓ | ||||||
| Il1rn | Interleukin 1 receptor antagonist | • | ↓ | ||||||||
| Egfr | Epidermal growth factor receptor | • | • | ↑ | |||||||
| Mapk3 | Mitogen-activated protein kinase 3 (aka Erk-1) | • | • | ↓ | |||||||
| Fos | FBJ osteosarcoma oncogene (aka AP-1) | • | ↑ | ||||||||
| Esr1 | Estrogen receptor 1 (alpha) | • | • | ↓ | |||||||
| Erbb3 | V-erb-b2 erythroblastic leukemia viral oncogene homolog 3 | • | ↑ | ||||||||
| Ptgs2 | Prostaglandin-endoperoxide synthase 2 (aka Cox-2) | • | ↓ | ||||||||
| Atp5c1 | ATP synthase, H+ transporting, mitochondrial F1 complex | • | ↓ | ||||||||
| Ndufs8 | NADH dehydrogenase (ubiquinone) Fe-S protein 8 | • | ↓ | ||||||||
| Uqcrc2 | Ubiquinol cytochrome c reductase core protein 2 | • | ↓ | ||||||||
| Atp5g3 | ATP synthase, H+ transporting, mitochondrial F0 complex | • | ↓ | ||||||||
| Atp5j | ATP synthase, H+ transporting, mitochondrial F0 complex | • | L | ||||||||
| Esr2 | Estrogen receptor 2 (beta) | • | ↑ | ||||||||
| Thra | Thyroid hormone receptor alpha | • | ↓ | ||||||||
| Ar | Androgen receptor | • | ↑ | ||||||||
Arrows in the columns labeled ‘MAHDR’ and ‘MALDR’ indicate the direction of regulation of each gene in response to MA. The column labeled ‘Saline’ indicates which line, if any, showed higher expression of the gene in a drug-naïve state (H = MAHDR; L = MALDR). Overrepresented functional groups were identified using the DAVID Bioinformatics Resources (david.niaid.nih.gov; Huang et al., 2007). The 384 genes represented on the qPCR array were used as the background gene list for pathway analysis. Significance levels for each class: Apoptosis (Apop) p = 0.04; Immune response (Immu) p = 0.0013; Cytokine receptor interaction (Cyto) p = 0.0011; Toll-like receptor signaling pathway (Toll) p = 0.019; ERBB2 signaling pathway p = 0.061; Oxidative phosphorylation (Oxid) p = 0.0097; Steroid hormone receptors (Ster) p = 0.018.
Figure 6. Gene pathways including genes that were differentially expressed following acute MA exposure (2 mg/kg) in MAHDR and MALDR selected line mice.
Fas, which was downregulated in response to MA in MALDR animals, is upstream of Casp8, a pro-apoptotic factor that was downregulated in response to MA in MAHDR animals. Ripk1, which was downregulated in MALDR mice, encodes a kinase that functions upstream of the NFκB transcription factor, components of which were differentially expressed in both selected lines. NFκB activates transcription of Bcl2, an anti-apoptotic factor which was downregulated in MALDR animals, and also activates transcription of various inflammatory peptides. Mapk3, which encodes the ERK1 kinase and functions downstream of Toll-like receptors to activate the AP-1 transcription factor, was downregulated in MAHDR animals. Two components of AP-1, cFos (upregulated in MAHDR) and Fosl1 (downregulated in MALDR) were also identified in our analysis. AP-1, like NFκB, activates transcription of inflammatory peptides, such as those encoded by Il6 (downregulated in MAHDR) and Ccl3 (downregulated in MALDR). These results suggest that changes in the function of signaling pathways involved in apoptosis and immune responses are partially responsible for the divergence in MA consumption behaviors in these selected lines.
Three additional signaling pathways were identified that may be important for the response to MA in MAHDR mice: the ERBB2 (erythroblastic leukemia viral oncogene homolog 2) receptor pathway, steroid hormone signaling pathway, and oxidative phosphorylation pathway. Genes known to participate in ERBB2 signaling (Il6, Erbb3, Mapk3 [mitogen-activated protein kinase 3]) were up- or downregulated in response to MA in MAHDR animals. Estrogen receptors are a downstream target of this pathway, and three steroid hormone receptor genes were also significantly upregulated in the MAHDR data (Esr1 and Esr2 [estrogen receptors 1 and 2], Ar [androgen receptor]). Finally, several genes which are involved in oxidative phosphorylation were downregulated in MAHDR animals as compared to MALDR animals following MA exposure. These include a NADH dehydrogenase (Ndufs8), two mitochondrial ATP synthases (Atp5c1 and Atp5g3), and prostaglandin-endoperoxide synthase 2 (Ptgs2), formerly known as Cox2.
We also observed significant expression differences in many genes encoding neurotransmitter receptors and transporters. For instance, the data indicate differential regulation of three serotonin receptor subtypes (Htr1d, Htr4, and Htr3a), as well as the serotonin transporter (Slc6a4) in the selected lines. Htr1d is downregulated in response to MA in MALDR animals, whereas Htr4 is downregulated in response to MA in MAHDR animals. In the drug-naïve state, MAHDR animals express lower levels of Htr3a and higher levels of Slc6a4, compared to drug-naïve MALDR animals.
Many transcription factors are also represented in this data set, in addition to the previously described steroid hormone receptors, indicating that the gene expression alterations in MAHDR versus MALDR animals are likely much more extensive than those identified by this 384-gene array. In particular, we note that two components of the NFκB transcription factor (Nfkb1, Nfkb2), as well as the NFκB inhibitor (Nfkbib), are differentially expressed in response to MA. In addition, levels of Rela (which encodes NFκB p65) are significantly higher in drug-naïve MALDR animals. Of the genes listed here as differentially expressed, twenty have been previously identified as direct transcriptional targets of NFκB (www.nf-kb.org). Our results also indicate that MALDR animals exhibit higher levels of Fos expression prior to MA exposure, as compared to MAHDR animals. Expression levels of immediate early genes such as Fos are commonly used as a measure of neural activation following drug administration (Kovacs, 2008; Nestler, 2004), but these data suggest a pre-existing difference in the selected lines that may be related to MA consumption. We have confirmed by immunohistochemistry that this difference in gene expression is accompanied by a difference in the number of cFos expressing cells in the NAc of MALDR and MAHDR mice (mean ± SE = 152 ± 27 vs. 75 ± 4 cells for MALDR and MAHDR mice, respectively; p<0.03). Taken together, the gene expression results suggest that there are pre-existing differences in gene expression between the selected lines that could play a role in susceptibility to MA consumption, and also that the neurochemical response to MA is markedly different in MAHDR and MALDR animals.
The eQTL analysis identified seven significant eQTL on six different chromosomes associated with genes that were differentially expressed in drug-naïve MAHDR and MALDR mice. Five of the seven significant eQTL also overlapped with suggestive eQTL. These results are shown in Supplemental Table 2. In 3 cases (Bcl2, Alg9, H2-Ea), the differentially expressed gene was located in the same region as the eQTL, suggesting self-regulation of expression.
Discussion
Oral administration procedures have the benefit of being technically simple, thus allowing for large numbers of animals to be tested in large-scale genetic investigations. Our results demonstrate that mice will voluntarily self-administer MA orally, and that this trait has a strong genetic component. This is the first demonstration of selective breeding for self-administration of any psychostimulant drug. The current data show that MA consumption levels are genetically related to the motivational value of MA, but are not associated with differences in taste preference. We observed a rapid divergence of the high and low MA drinking lines, and heritability calculations indicate that 34% of the trait variance is associated with genetic variation in this population. Although there was a difference in total fluid consumption between the lines (MAHDR mice consumed about 8% more fluid than MALDR mice), this difference did not increase across selection generations, suggesting that it cannot account for the divergence in MA consumption between the lines.
Validation of the self-administration procedure using measures of reward and aversion is important for several reasons. First, some measures allow for comparison of the selected lines at controlled doses of MA. MALDR animals voluntarily consume virtually no MA. Moreover, the pattern of MA intake by MAHDR mice within each 24-h period is unknown. Thus, it was important to examine responses to pharmacologically relevant doses of MA at defined time periods after delivery. Second, drugs of abuse show reduced reinforcing effects when consumed orally, due to the latency between intake and pharmacological action (Meisch, 2001). Despite this limitation, our results suggest that selection for oral MA consumption has produced a difference between the selected lines in sensitivity to the positive effects of the drug. Third, the biphasic effects of many drugs, including methamphetamine (Cunningham & Noble, 1992), make the results of preference drinking experiments difficult to interpret in isolation. If one group of animals consumes less of the drug than another, one could conclude either of the following: 1) that the animals who consume less find the drug relatively more aversive and thus are avoiding it, or 2) that they are more sensitive to the rewarding effects of the drug and can consume less drug to get the same level of pleasurable effect.
To test the hypothesis that MAHDR and MALDR mice differ in sensitivity to the positive and negative motivational effects of MA, we tested the lines for development of MA-induced CPP and CTA. We observed reciprocal phenotypes at the doses tested, with the MAHDR line exhibiting CPP but not CTA, and the MALDR line exhibiting CTA but not CPP. Thus, selective breeding created a high consumption line that is more sensitive to the positive motivational effects of MA, and less sensitive to the aversive effects of MA, compared to a line bred for reduced voluntary MA consumption. These results may indicate a rightward shift in the MA dose-response curve in MAHDR animals and/or a leftward shift in the dose-response curve in MALDR animals; data from non-selected B6D2F2 mice are needed to make directional conclusions. Some data suggest high concordance between the ability of a drug to induce CPP and to stimulate IV self-administration in operant procedures (Bardo & Bevins, 2000). We are currently collaborating with another laboratory to measure intracranial self-administration of MA in the selected lines. This operant method will provide another assessment of the motivation to self-administer MA across a range of MA concentrations.
MA-induced CPP and CTA are genetically correlated with differences in MA consumption in the MAHDR and MALDR lines. This provides evidence that the genetic and neurochemical differences between these lines are relevant to not only MA consumption, but also to differences in sensitivity to the conditioned rewarding and aversive effects of MA. We used qPCR to screen for differences in the expression of a subset of genes in the NAc. Our results suggest that differences in pathways related to apoptosis and immune responses may contribute to the divergent responses to MA in our selected lines. Although these pathways are known to be involved in MA-induced neurotoxicity (Cadet et al., 2003; Jayanthi et al., 2005), our result is unexpected because a single modest dose of MA (2 mg/kg) was administered in our study. Previous work examining MA toxicity in mice has typically used either a single large dose (~25 mg/kg) or a multiple-dose regimen at short intervals (e.g., 2.5–10 mg/kg at 2h intervals; Cadet et al., 2003). It is possible that at lower non-toxic doses of MA, neuroprotective pathways are activated, although apoptosis is not triggered. Differences in the ability to mount a neuroprotective response following MA administration may have an impact on the rewarding and aversive effects of the drug. Administration of MA has been shown to decrease activity of the dopamine transporter VMAT2 (Eyerman & Yamamoto, 2007), and heterozygous VMAT2 knockout mice are more sensitive to the neurotoxic effects of MA (Fumagalli et al., 1999). Vmat2 was downregulated following MA administration in MALDR mice, suggesting that they may be more sensitive to the neurotoxic effects of MA.
Expression of Fos increases following administration of many drugs of abuse, and is commonly used as a marker of nerve cell activation (Kovacs, 2008; Nestler, 2004). Furthermore, upregulation of Fos following MA exposure is proposed to be important as a protective mechanism against neurotoxicity, since Fos knockout mice have increased sensitivity to MA-induced toxicity (Deng et al., 1999). Our results indicate that MALDR animals do not upregulate Fos in response to MA, and also have elevated levels of Fos expression in the absence of MA exposure, compared to MAHDR mice. Alterations in the regulation of Fos may contribute to the behavioral differences between the MAHDR and MALDR lines, and levels of Fos expression may partially predict MA consumption and susceptibility to MA reward and aversion.
Non-MA-treated MAHDR animals expressed lower levels of the NFκB subunit Rela compared to MALDR animals, and three other NFκB subunits showed differential expression in response to MA administration in these selected lines. The NFκB transcription factor is an important component of signaling pathways related to apoptosis and immune responses (Figure 6), and it is also implicated in psychostimulant-induced behavioral plasticity. Chronic cocaine administration upregulates NFκB in the NAc (Ang et al., 2001), and MA induces increased striatal NFκB activity (Asanuma & Cadet, 1998). Russo et al. (2008) have reported that inhibition of NFκB signaling in the NAc reduces sensitization to cocaine reward. Further investigation into the role of NFκB as a predisposing risk factor for the initiation and maintenance of MA self-administration is warranted.
We found decreased expression of Mapk3 (which encodes ERK-1 MAP kinase) in MAHDR animals after MA administration. ERK phosphorylation (but not that of other MAP kinases) is increased following MA-induced CPP, and reduction of ERK activity decreases expression of MA-induced CPP (Mizoguchi et al., 2004). Acute administration of amphetamine is also reported to increase ERK phosphorylation (Valjent et al., 2005). ERK-1 and ERK-2 have distinct functions in behavioral plasticity, and ERK-2 is the isoform most relevant to drug reward (Girault et al., 2007; Zhai et al., 2008). ERK-1 deficient mice exhibit increased ERK-2 signaling and enhanced cocaine-induced CPP (Ferguson et al., 2006). We found that in MAHDR animals, which display MA-induced CPP, Mapk3 expression was downregulated in response to acute MA administration. MALDR animals, which do not display MA-induced CPP in our protocol, did not exhibit MA-induced regulation of Mapk3 expression.
The current results are intriguing, and will be confirmed in a more comprehensive transcriptome analysis currently being conducted for the NAc and other brain regions, including prefrontal cortex and ventral midbrain, using Affymetrix microarray chips. Behavioral QTL (bQTL) are currently being mapped using DNA samples from the MAHDR and MALDR mice. These data will be compared with eQTL data to identify regions of comapping and indicate particular genes to pursue as possible quantitative trait genes (QTG) for MA drinking. In addition, to improve our confidence in the current findings and pursue genetic and behavioral mechanisms in greater detail, we are creating a second independent replicate set of MAHDR and MALDR lines. Data from the first replicate set of lines indicate that the oral MA self-administration procedure produced lines that also differ in sensitivity to the rewarding and aversive effects of MA. We are planning studies in which a recently developed method for examining intragastric self-infusion of ethanol (Fidler et al., 2006; 2008) will be used to examine self-administration of MA in the MADR lines via this route. Data showing higher levels of MA self-administration in MAHDR compared to MALDR mice would provide more convincing evidence that MA drinking is a useful method for large-scale genetic investigations of MA reward. We are also planning studies in the second replicate that will examine brain and blood levels of MA achieved, as well as clearance rate, as possible contributors to behavioral differences. Finally, we are currently working out the dose and timing parameters necessary for inducing conditioned place aversion with MA to examine the possibility that the lines will differ for this measure of aversion.
Supplementary Material
Acknowledgments
This research was supported by grants from the Department of Veterans Affairs and by NIH grants, P50DA018165 and T32DA07262. We appreciate the technical assistance of David Buck with collection of the cFos immunohistochemistry data.
References
- Aguilar MA, Rodriguez-Arias M, Minarro J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev. 2009;59:253–277. doi: 10.1016/j.brainresrev.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Akilesh S, Shaffer DJ, Roopenian D. Customized molecular phenotyping by quantitative gene expression and pattern recognition analysis. Genome Res. 2003;13:1719–1727. doi: 10.1101/gr.533003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang E, Chen J, Zagouras P, Magna H, Holland J, Schaeffer E, Nestler EJ. Induction of nuclear factor-κB in nucleus accumbens by chronic cocaine administration. J Neurochem. 2001;79:221–224. doi: 10.1046/j.1471-4159.2001.00563.x. [DOI] [PubMed] [Google Scholar]
- Asanuma M, Cadet JL. Methamphetamine-induced increase in striatal NF-κB DNA-binding activity is attenuated in superoxide dismutase transgenic mice. Mol Brain Res. 1998;60:305–309. doi: 10.1016/s0169-328x(98)00188-0. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Smith R, Raber J, Cunningham CL. Enhanced ethanol-, but not cocaine-induced, conditioned place preference in Apoe(−/−) mice. Pharmacol Biochem Behav. 2004;77:783–792. doi: 10.1016/j.pbb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Richards SP, O'Toole LA, Helms ML, Phillips TJ. Short-term selective breeding as a tool for QTL mapping: ethanol preference drinking in mice. Behav Genet. 1997;27:55–66. doi: 10.1023/a:1025615409383. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X. Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB. 2003;17:1775–1788. doi: 10.1096/fj.03-0073rev. [DOI] [PubMed] [Google Scholar]
- Cai NS, McCoy MT, Ladenheim B, Lyles J, Ali SF, Cadet JL. Serial analysis of gene expression in the rat striatum following methamphetamine administration. Ann NY Acad Sci. 2006;1074:13–30. doi: 10.1196/annals.1369.002. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Buck KJ, Cunningham CL, Belknap JK. Identifying genes for alcohol and drug sensitivity: recent progress and future directions. Trends Neurosci. 1999;22:173–179. doi: 10.1016/s0166-2236(99)01393-4. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Noble D. Methamphetamine-induced conditioned place preference or aversion depending on dose and presence of drug. Ann N Y Acad Sci. 1992;654:431–433. doi: 10.1111/j.1749-6632.1992.tb25989.x. [DOI] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Tsao LI, Cadet JL. Mull mutation of c-fos causes exacerbation of methamphetamine-induced neurotoxicity. J Neurosci. 1999;19:10107–10115. doi: 10.1523/JNEUROSCI.19-22-10107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J Neurochem. 2007;103:1219–1227. doi: 10.1111/j.1471-4159.2007.04837.x. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. 4th ed. Essex: Longman Group; 1996. [Google Scholar]
- Fehr C, Shirley RL, Crabbe JC, Belknap JK, Buck KJ, Phillips TJ. The syntaxin binding protein 1 gene (Stxbp1) is a candidate for an ethanol preference drinking locus on mouse chromosome 2. Alcohol Clin Exp Res. 2005;29:708–720. doi: 10.1097/01.alc.0000164366.18376.ef. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Clews TW, Cunningham CL. Reestablishing an intragastric ethanol self-infusion model in rats. Alcohol Clin Exp Res. 2006;30:414–428. doi: 10.1111/j.1530-0277.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- Filder TL, Powers MS, Ramirez JJ, Cunningham CL. Optimizing passive infusion parameters to produce ethanol self-infusion in C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 2008;32 Suppl:215A. [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Elsevier; 2008. [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci. 1999;19:2424–2431. doi: 10.1523/JNEUROSCI.19-07-02424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Hill KG, Ryabinin AE, Cunningham CL. FOS expression induced by an ethanol-paired conditioned stimulus. Pharmacol Biochem Behav. 2007;87:208–221. doi: 10.1016/j.pbb.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC, Lempicki RA. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35:W169–W175. doi: 10.1093/nar/gkm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Ozaki N, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y, Kishi T, Sekine Y, Iyo M, Harano M, Komiyama T, Yamada M, Sora I, Ujike H, Inada T, Iwata N. Possible association of beta-arrestin 2 gene with methamphetamine use disorder, but not schizophrenia. Genes Brain Behav. 2007;6:107–112. doi: 10.1111/j.1601-183X.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai N, Cadet JL. Calcineurin/NFAT-induced upregulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci USA. 2005;102:868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav. 2005;4:110–125. doi: 10.1111/j.1601-183X.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Kirstein SL, Davidson KL, Ehringer MA, Sikela JM, Erwin VG, Tabakoff B. Quantitative trait loci affecting initial sensitivity and acute functional tolerance to ethanol-induced ataxia and brain cAMP signaling in BXD recombinant inbred mice. J Pharmacol Exp Ther. 2002;302:1238–1245. doi: 10.1124/jpet.302.3.1238. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ. Measurement of immediate-early gene activation – c-fos and beyond. J Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Risinger FO, Phillips TJ. Attenuation of ethanol-induced conditioned taste aversion in mice sensitized to the locomotor stimulant effects of ethanol. Behav Neurosci. 2001;115:146–153. doi: 10.1037/0735-7044.115.1.146. [DOI] [PubMed] [Google Scholar]
- Meisch RA. Oral drug self-administration: an overview of laboratory animal studies. Alcohol. 2001;24:117–128. doi: 10.1016/s0741-8329(01)00149-5. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Mizuno M, Mizuno T, Nitta A, Noda Y, Nabeshima T. Regulations of methamphetamine reward by extracellular signal-regulated kinase 1/2/ets-like gene-1 signaling pathway via the activation of dopamine receptors. Mol Pharmacol. 2004;65:1293–1301. doi: 10.1124/mol.65.5.1293. [DOI] [PubMed] [Google Scholar]
- Mori T, Shinobu I, Narita M, Suzuki T, Sawaguchi T. Combined effects of psychostimulants and morphine on locomotor activity in mice. J Pharmacol Sci. 2004;96:450–458. doi: 10.1254/jphs.fpj04039x. [DOI] [PubMed] [Google Scholar]
- Morita Y, Ujike H, Tanaka Y, Kishimoto M, Okahisa Y, Kotaka T, Harano M, Inada T, Komiyama T, Hori T, Yamada M, Sekine Y, Iwata N, Iyo M, Sora I, Ozaki N, Kuroda S. The glycine transporter 1 gene (GLYT1) is associated with methamphetamine-use disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147:54–58. doi: 10.1002/ajmg.b.30565. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Mizoguchi H, Ito Y, Noda Y, Nagai T, Nabeshima T. A novel molecule "shati" is involved in methamphetamine-induced hyperlocomotion, sensitization, and conditioned place preference. J Neurosci. 2007;27:7604–7615. doi: 10.1523/JNEUROSCI.1575-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani K, Ujike H, Sakai A, Okahisa Y, Kotaka T, Inada T, Harano M, Komiyama T, Hori T, Yamada M, Sekine Y, Iwata N, Iyo M, Sora I, Ozaki N, Kuroda S. Reduced CYP2D6 activity is a negative risk factor for methamphetamine dependence. Neurosci Lett. 2008;434:88–92. doi: 10.1016/j.neulet.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Verbitsky M, Suresh R, Kamens HM, Reed CL, Li N, Burkhart-Kasch S, McKinnon CS, Belknap JK, Gilliam TC, Phillips TJ. Gene expression differences in mice divergently selected for methamphetamine sensitivity. Mamm Genome. 2005;16:291–305. doi: 10.1007/s00335-004-2451-8. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Huson MG, McKinnon CS. Localization of genes mediating acute and sensitized locomotor responses to cocaine in BXD/Ty recombinant inbred mice. J Neurosci. 1998;18:3023–3034. doi: 10.1523/JNEUROSCI.18-08-03023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Broadbent J, Burkhart-Kasch S, Henderson C, Wenger CD, McMullin C, McKinnon CS, Cunningham CL. Genetic correlational analyses of ethanol reward and aversion phenotypes in short-term selected mouse lines bred for ethanol drinking or ethanol-induced conditioned taste aversion. Behav Neurosci. 2005;119:892–910. doi: 10.1037/0735-7044.119.4.892. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Kamens HM, Wheeler JM. Behavioral genetic contributions to the study of addiction-related amphetamine effects. Neurosci Biobehav Rev. 2008;32:707–759. doi: 10.1016/j.neubiorev.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Genetic differences in ethanol-induced hyperglycemia and conditioned taste aversion. Life Sciences. 1992;50:PL113–PL118. doi: 10.1016/0024-3205(92)90463-y. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56 Suppl 1:73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Coste SC, Burkhart-Kasch S, Li N, Stenzel-Poore MP, Phillips TJ. Mice deficient in corticotropin-releasing factor receptor type 2 exhibit normal ethanol-associated behaviors. Alchohol Clin Exp Res. 2005;29:1601–1609. doi: 10.1097/01.alc.0000179371.46716.5e. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen MW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Harley RM, Xian H, Eisen S, Goldberg J, True WR, Faraone SV. Genetic and environmental influences on transitions in drug use. Behav Genet. 1999;29:473–479. doi: 10.1023/a:1021635223370. [DOI] [PubMed] [Google Scholar]
- Turek VF, Bennett B, Ryabinin AE. Differences in the urocortin 1 system between long-sleep and short-sleep mice. Genes Brain Behav. 2008;7:113–119. doi: 10.1111/j.1601-183X.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Liu QR, Johnson C, Walther D, Komiyama T, Harano M, Sekine Y, Inada T, Ozaki N, Iyo M, Iwata N, Yamada M, Sora I, Chen CK, Liu HC, Ujike H, Lin SK. Genome-wide association for methamphetamine dependence: convergent results from 2 samples. Arch Gen Psychiatry. 2008;65:345–355. doi: 10.1001/archpsyc.65.3.345. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H, Yanqin L, Wang X, Lu L. Drug-induced alteration in the extracellular signal-regulated kinase (ERK) signaling pathway: implications for reinforcement and reinstatement. Cell Mol Neurobiol. 2008;28:157–172. doi: 10.1007/s10571-007-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.