Abstract
Congenital deafness leads to atypical organization of the auditory nervous system. However, the extent to which auditory pathways reorganize during deafness is not well understood. We recorded cortical auditory evoked potentials in normal hearing children and in congenitally deaf children fitted with cochlear implants. High-density EEG and source modeling revealed principal activity from auditory cortex in normal hearing and early implanted children. However, children implanted after a critical period of seven years revealed activity from parietotemporal cortex in response to auditory stimulation, demonstrating reorganized cortical pathways. Reorganization of central auditory pathways is limited by the age at which implantation occurs, and may help explain the benefits and limitations of implantation in congenitally deaf children.
Keywords: Auditory evoked potential, critical period, deafness, development, neuroplasticity, source localization
1. Introduction
Development and organization of sensory pathways in the cortex is dependent on sensory experience. A lack of sensory input, such as in deafness, impedes the normal growth and early connectivity needed to form a functional sensory system – in some cases irretrievably (Wiesel and Hubel, 1965). Evidence from animal models of congenital deafness has revealed abnormal formation of auditory nerve fibers that terminate in the lower brainstem (Lee et al., 2003; Ryugo et al., 2005). Other animal models have shown reduced synaptic activity in the sensory deprived auditory cortex in response to acute electrical stimulation (Kral et al., 2002). In layer specific recordings from auditory cortex in congenitally deaf cats, supragranular layers showed less synaptic activity with decreased response amplitudes and increased response latencies compared to normal-hearing cats, while infragranular layers showed very little synaptic activity at all (Kral et al., 2000). Moreover, the synaptic currents had significantly reduced sink amplitudes, suggesting deficient corticothalamic and corticocortical projections. The reduced responsiveness of these physiological mechanisms may be due to a deterioration of the supporting anatomical structures during deprivation, to a reorganization of those structures, or to a combination thereof.
In humans, the cortical auditory evoked potential (CAEP) provides information about maturation of auditory pathways terminating in auditory cortex, and reflects recurrent cortical activity mediated by corticothalamic loops (Eggermont, 1992; Kral et al., 2002). These recurrent loops mediate subsequent corticocortical projections (Winguth and Winer, 1986) that may be disrupted after auditory deprivation. Restoring function to these modulatory projections may be possible with cochlear implantation, as long as the CAS remains maximally plastic and the effects of degeneration have not completely taken effect. Indeed, evidence from animal data reported by Klinke et al. (1999) have found that at least some deprivation effects can be reversed with chronic electrical stimulation to the auditory pathways. However, while early-implantation in a maximally plastic CAS restores maturation of the auditory pathways, providing auditory input after prolonged deprivation may not initiate stimulus driven reorganization with the same success.
The latency of the P1 CAEP has been used to examine CAS maturation in children with cochlear implants. Sharma et al. (2002a; 2002b; 2002c) and Ponton et al. (1996) examined P1 latencies in children with CIs and revealed prolonged latencies compared to normal-hearing children. Further analysis revealed that P1 latency appears to continue a developmental progression after implantation. Moreover, Sharma et al. (2002a; 2002b; 2002c) showed that age of implantation was a critical factor in P1 latency maturation. Children implanted after age 7 years did not show age appropriate P1 latencies, while children implanted prior to age 3.5 years showed P1 latencies within the normal developmental limits. Further examinations of P1 latencies in children with CIs compared to a group of age-matched normal-hearing peers, revealed no significant differences in P1 latency between the early-implanted and normal hearing children (Sharma et al., 2002a). Collectively, these data suggest a sensitive period of about 3.5 years for which auditory stimulation is necessary to promote normal maturation of the CAS as revealed by the P1 CAEP.
Children who are implanted after the age of seven years almost always show evidence of abnormal central auditory maturation when examining the latency of the P1 response (Sharma and Dorman, 2006; Sharma et al., 2002b; Sharma et al., 2005). However, if implantation occurs very early in childhood, then the P1 latency will typically follow a normal developmental trajectory (Sharma et al., 2002a; Sharma et al., 2002b). Furthermore, it is well established that the earlier in life a deaf child receives a cochlear implant, the better their development of natural language skills (Colletti et al., 2005; Connor et al., 2006; Francis and Niparko, 2003; Kang et al., 2004; Kirk et al., 2002). Taken together, these results support the conventional wisdom that early stimulation is necessary to support normal sensory development. Understanding the physiological mechanisms associated with both normal and abnormal sensory development may help us better understand and plan for successful rehabilitation in children with cochlear implants.
There is evidence from animal and human studies to suggest at least some level of cross-modal reorganization from one sensory modality when another modality is deprived of input (Armstrong et al., 2002; Doron and Wollberg, 1994; Lee et al., 2001; Yaka et al., 2000). Restoring auditory input to a reorganized system may mean that functional access to the cortex is limited to those pathways still available after a prolonged period of deprivation. It is likely that the time course of deterioration and reorganization of the deprived auditory pathways limits the success of restoring auditory input with a cochlear implant (CI). Therefore, restoring input very early to a highly plastic auditory system will likely lead to an improved chance of typical auditory function.
In research reported here, we used current source reconstruction and dipole source analyses derived from high density EEG recordings to estimate generators for the P1 response in three groups of children: Normal hearing children, congenitally deaf children who received a cochlear implant before the age of four years, and congenitally deaf children who received a cochlear implant after the age of seven years. At issue was whether cortical organization as reflected by the generators of the P1 response was the same for the three groups of listeners.
2. Results
2.1. P1 CAEP
Figure 1 shows the grand mean CAEPs from each of 64 scalp electrodes as butterfly plots in each of the three groups tested. CAEP responses in all subjects revealed morphologies consistent with those described previously by Sharma and others (2002a). The morphology of the grand average responses in the normal hearing (NH) children and in the early implanted children are nearly identical. However, the late implanted children revealed different morphologies, with generally lower amplitude responses.
Figure 1.
Stacked, butterfly plots of the CAEP from 64 scalp electrodes for A) normal hearing children, B) early implanted children, C) and late implanted children. The vertical black line in each plot represents time 0 ms; the onset of the stimulus. The time window for each plot includes a 100 ms pre-stimulus interval and a 600 ms post-stimulus interval. The vertical gray box over each plot represents the region of the P1 CAEP, on which the source analyses were performed.
P1 CAEP latencies and amplitudes were each treated as the response variable in a one-way ANOVA. Results of the ANOVA revealed a significant main effect for P1 latency [F(2,23)=35.52, p<0.0001]. A post-hoc comparison of means (Duncan’s test) reveled no significant difference between NH and early implanted children, but did find a significant difference between late implanted children and each of the other two groups (p>0/05). Results of the ANOVA for P1 amplitude revealed no significant effects between any of the three groups [F(2,23)=1.23, p=0.31].
2.2. Current source reconstructions
Current source reconstructions were performed using the sLORETA algorithm as presented in the Curry 6.0 software. Results of source reconstructions are shown in Figure 2 and Table 1. Figure 2A shows the mean source activity for the P1 CAEP in NH children as projected to a representation of the cortical surface. In NH children the sLORETA solutions place the primary source of activity around the right inferior temporal gyrus (ITG), and bilaterally in the superior temporal sulcus (STS). Current source reconstructions for the early implanted children are shown in Figure 2B. Similar to the NH children, early implanted children with an implant in the left ear show peak P1 CAEP activity over the right ITG and in the STS just at the junction of the middle temporal gyrus (MTG) contralateral to CI stimulation. Early implanted children with an implant in the right ear show activation in the contralateral STS and in the right ITG, but with less focal activity, likely caused by decreased power in the reconstructions due to less accurate noise estimations (i.e., smaller sample size). In addition to the activity in the right inferior temporal gyrus, the early implanted children reveal some source activity at the contralateral parietotemporal cortex, however this finding is purely descriptive in nature.
Figure 2.
Current density reconstructions of the P1 cortical auditory evoked potential projected to the cortical surface in A) normal hearing children, B) early implanted children, and C) late implanted children. Activity is represented as a normalized probability of cortical activity from the inverse solution; a distributed F-value. F-distribution values are labeled in the color bar for each group. Deep layer activity at the superior temporal sulcus in normal hearing and early implanted children is not visible in the surface projections, but can be seen in the cut-plane slices of temporal cortex in the lower panel of each figure section. Electrode positions for children with a right implant were mirrored on the scalp for visualization of contralateral sources.
Table 1.
Center of mass activity for sLORETA solutions
| MNI coordinates (mm) | ||||
|---|---|---|---|---|
| X | Y | Z | Cortical Region | |
| Normal Hearing | 46.2 | −20.4 | −28.2 | Right ITG |
| 66.8 | −24.5 | −0.8 | Right STS | |
| −55.1 | −8.7 | 4.1 | Left STS | |
| Early implanted | 63.6 | −8.7 | −0.8 | Contra MTG/STS |
| Late implanted | 56.3 | −26.5 | 24.2 | Contra PCG |
Note.
ITG=inferior temporal gyrus, STS=superior temporal sulcus, MTG=middle temporal gyrus, PCG=post-central gyrus Contra=contralateral to CI
Responses in late implanted children revealed source activity focused in parietotemporal cortex at the post-central gyrus contralateral to the CI stimulation as shown in Figure 2C. In the case of the children with implants in the right ear, the dispersion of activity was broader, indicating less specificity of the source localization, likely due to the less accurate noise estimations.
2.3. Dipole Source Analyses
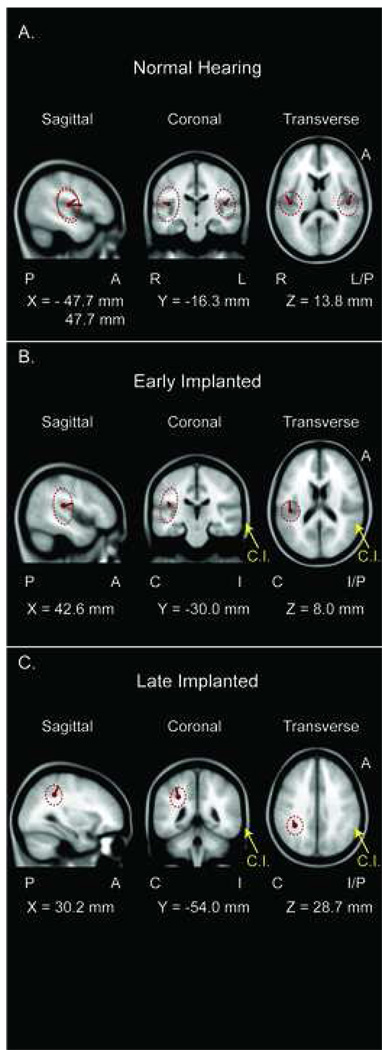
Fixed coherent dipoles were fitted to the peak P1 CAEP responses in each of the three groups based on the sLORETA reconstructions and a priori hypotheses of P1 source activity (Khosla et al., 2003; Scherg and Von Cramon, 1985; Scherg and Von Cramon, 1986). Dipole source modeling is an adequate supplementary analysis to current source reconstruction, as the analysis increases the accuracy of the temporal point source localization when spatio-temporal smearing may occur to overlapping peaks seen across the scalp recorded potential (Waberski et al., 2001). The best fit results for NH children revealed bilateral, symmetric dipoles at the transverse temporal gyri, medially (MNI coordinates: X=+/−47.7mm, Y=−16.3mm, Z=13.8mm). Figure 3A shows the dipole source reconstructions for NH children.
Figure 3.
Regional dipole source solutions for the P1 cortical auditory evoked potential in each of three groups. Dipoles are shown at the location of best fit in each of three views: sagittal, coronal, and transverse. Dotted lines that surround the dipoles represent the 95% confidence ellipsoids for the solution. A) Normal hearing children revealed bilateral, symmetric dipoles at the transverse temporal gyri, medially (MNI coordinates: X=−47.7mm/47.7mm, Y=−16.3mm, Z=13.8mm) with dipole strengths of q=27.9 µAmm and q=25.3 µAmm, respectively, and explaining 98.09% of the variance in the data. B) Early implanted children revealed a single dipole contralateral to the ear of stimulation (represented by the yellow arrow and C.I. label; “C”=contralateral, “I”=ipsilateral) at the transverse temporal gyrus, medially, and slightly posterior to that of the normal hearing group (MNI coordinates: X=42.6mm, Y=−30.0mm, Z=8.0mm) with a dipole strength of q=9.29 µAmm and explaining 83.67% of the variance in the data. C) Late implanted children revealed a single dipole contralateral to the ear of stimulation (represented by the yellow arrow and C.I. label) at the parietotemporal lobule, medially, just dorsal to the posterior cingulate cortex (MNI coordinates: X=30.2mm, Y=−54.0mm, Z=28.7mm) with a dipole strength of q=17.4 µAmm and explaining 73.3% of the variance in the data.
For early implanted children, the best dipole fit was revealed as a single dipole source originating from the right transverse temporal gyrus, medially (MNI coordinates: X=42.6mm, Y=−30.0mm, Z=8.0mm) explaining 86.59% of the variance (normalized). For the late implanted children, the best dipole fit was revealed as a single dipole source originating from the right parietotemporal cortex, just at the dorsal portion of the posterior cingulate cortex (MNI coordinates: X=30.2mm, Y=−54.0mm, Z=28.7mm) explaining 88.38% of the variance. Dipole source reconstructions for early and late implanted children are shown in Figures 3A and 3B, respectively.
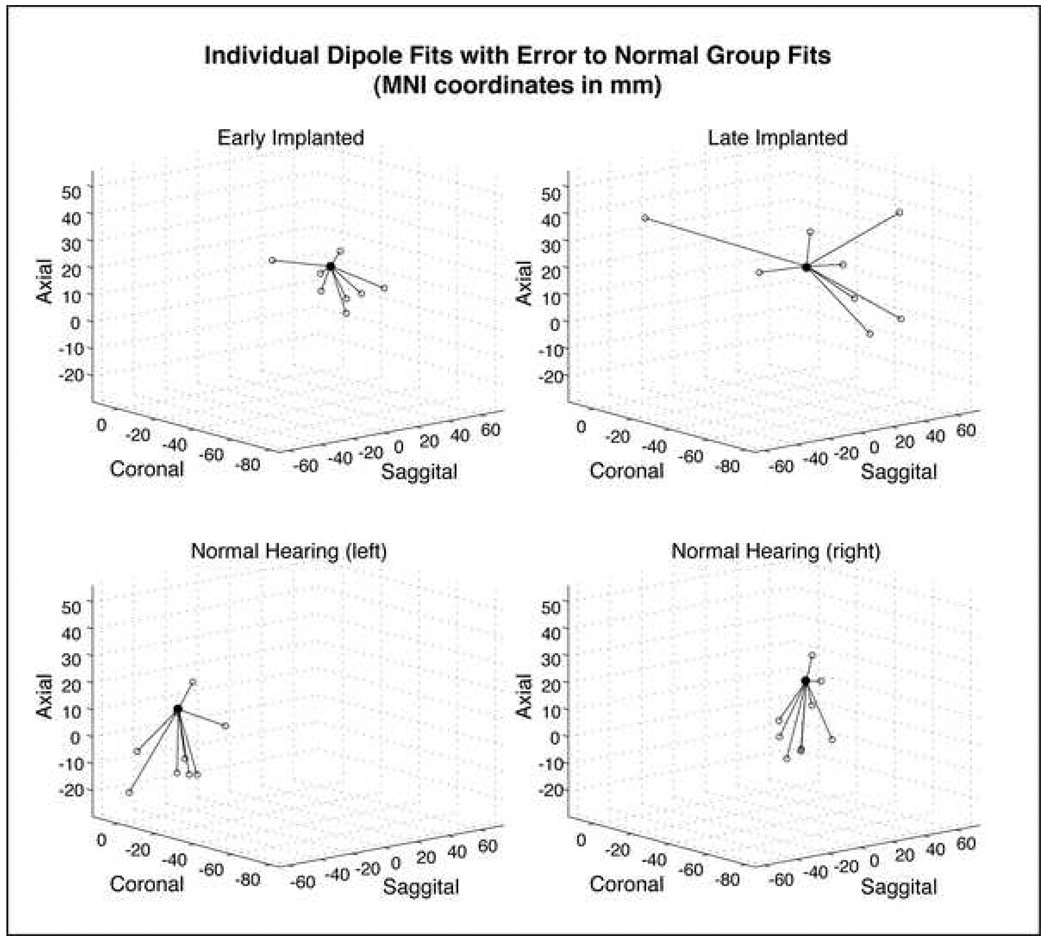
To verify solutions on grand average data, dipole solutions were computed for the P1 CAEP in each individual subject. Table 2 lists the MNI coordinates for each subject’s dipole fit. The solution error for each subject was considered as the Euclidian distance from the individual’s dipole to a reference dipole in auditory cortex, in this case the group dipole solution from normal hearing children. Figure 4 shows a representation of the solution error for each subject group. Error distances were treated as the response variable in a one-way ANOVA, with hearing group as the between subject variable. Results of that analysis revealed a significant main effect of distance error [F(3,30)=5.09, p=0.0057]. A post-hoc comparison of group means (Duncan’s test) revealed a significant difference in error distance between the late implanted group and all other groups (p>0.05). No significant differences were found between the NH group and the early implanted group.
Table 2.
Coordinates for individual dipole fits
| MNI Coordinates (mm) | ||||
|---|---|---|---|---|
| Subj | Stimulation | X | Y | Z |
| N1 | Binaural | +/−41.7 | −17.0 | −10.9 |
| N2 | Binaural | +/−55.2 | −21.9 | −7.9 |
| N3 | Binaural | +/−39.0 | −18.9 | −10.9 |
| N4 | Binaural | +/−51.0 | 2.6 | −5.0 |
| N5 | Binaural | +/−36.1 | −10.1 | −7.0 |
| N6 | Binaural | +/−34.2 | −29.7 | 8.7 |
| N7 | Binaural | +/−44.1 | −20.9 | 24.4 |
| N8 | Binaural | +/−51.8 | −18.9 | 13.6 |
| N9 | Binaural | +/−55.9 | 2.6 | −19.7 |
| E1 | Left CI | 34.7 | −21.9 | 13.6 |
| E2 | Left CI | 65 | −18 | 2.1 |
| E3 | Left CI | 36.8 | −33.6 | 0.9 |
| E4 | Left CI | 45.5 | −46.3 | 11.6 |
| E5 | Left CI | 32.8 | −23.8 | 7.7 |
| E6 | Right CI | −27.8 | −41.4 | 8.7 |
| E7 | Right CI | −34.8 | 3.5 | 13.6 |
| E8 | Right CI | −43.6 | −24.8 | 21.5 |
| L1 | Left CI | 36.4 | −27.9 | 30 |
| L2 | Left CI | 23.1 | −70.4 | 1.8 |
| L3 | Left CI | 58.4 | −32.6 | 3.8 |
| L4 | Left CI | 36.7 | −0.8 | 9.7 |
| L5 | Left CI | 27.9 | −52.2 | 23.4 |
| L6 | Right CI | −36.6 | −74.6 | 46 |
| L7 | Right CI | −43.6 | −69.5 | 4.8 |
| L8 | Right CI | 50.5 | −14 | 42 |
Note.
Subj=subjects by group where "N" is normal hearing, "E" is early implanted, and "L" is late implanted
Figure 4.
MNI coordinates (in mm) and error distances for individual dipole fits. In each panel, the filled circle represents the reference dipole at the transverse temporal gyrus (from the NH group fits), and each open circle represents the dipole fit for an individual subject. For the early implanted group (upper left panel) and the late implanted group (upper right panel), individual dipoles are shown for contralateral activity (i.e., contralateral dipoles for right implanted subjects have been mirrored for comparison). Only one late implanted subject was shown to have ipsilateral activation. Even with the ipsilateral data point removed, ANOVA results revealed a significant main effect of error distances for the late implanted group. Individual fits for the NH group are shown in two separate plots, representing dipole fits for the left auditory cortex (lower left panel) and the right auditory cortex (lower right panel).
Two normal hearing subjects revealed solutions in post-central gyrus, just superior to the superior temporal sulcus. Individual solutions in the early implanted children revealed dipoles in temporal cortex contralateral to the implant in six of the eight subjects. One early implanted subject revealed a solution in the contralateral insular cortex, a deep structure separating temporal and parietal cortices, and one subject revealed a dipole in the contralateral post-central gyrus. As expected, individual dipole solutions for the late implanted group revealed a large degree of variability for P1 sources, all outside of auditory regions (see Table 2). Sources for all but one late implanted subject were localized to the contralateral cortex. One late implanted subject revealed a dipole fit to the ipsilateral, pre-central gyrus.
Dipole sources in both the early and late implanted children were fit only to the hemisphere contralateral to CI stimulation, and each explained less than 90% of the data. To test whether a second dipole source could help explain the variance, we forced the dipole model to fit 2 dipoles for each of the implanted groups to rule out any missed components. In both cases, forcing a second dipole did not improve the detection of brain sources, but, instead, decreased the amount of explained variance and moved the dipole sources to anatomical regions that were physiologically unlikely to produce the recorded activity. Based on this information, and the closeness of fit with the sLORETA solutions, we accepted the single dipole fits as the most likely and most accurate fit model.
3. Discussion
We have found differences in generator sites for the P1 CAEP in normal hearing children, children who received a cochlear implant early in childhood and in children who received an implant later in childhood. We suggest that these differences are engendered by different degrees of cortical reorganization following different durations of deafness. It is important to note, however, that current density reconstructions and group dipole fits were performed on group average data, which inherently includes some expected variability from individual subjects. We examined this variability by estimating dipole sources for each individual subject. Those results confirm the general pattern of contralateral activity dominating the P1 response in implanted children, which was observed in 15 of the 16 implanted subjects.
3.1. Current source reconstructions
The results of the current source reconstructions in NH children revealed dominant activity in the right ITG, and bilateral STS. The stronger activity in the right hemisphere is consistent with the results of previous ERP studies showing greater right than left temporal activity during early auditory processing (Hine and Debener, 2007). However, it is important to note that the stronger right hemisphere activity in the present data has only been observed at a descriptive level, as this result is reflected in the current source reconstructions of group average data.
3.2. Dipole source analyses
To gain a clearer understanding of the underlying activity observed by the sLORETA solutions, we performed a dipole source analysis of the P1 CAEP activity in each group, and in each subject. Results of that analysis for normal hearing children revealed bilateral symmetric activations along the superior temporal sulcus, consistent with auditory cortical activity and with traditional findings of dipole sources for P1 activity (Ponton et al., 2002; Scherg and Von Cramon, 1985). Early implanted children revealed only a single dipole in the auditory cortex contralateral to auditory stimulation. These results are consistent with converging anatomical evidence that classical auditory pathways terminate, primarily, in the contralateral cortex (Demanez and Demanez, 2003; Menendez-Colino et al., 2007). Late implanted children revealed a single dipole in the parietotemporal cortex, consistent with the activity found in the current source reconstructions, and reflecting the lack of auditory activation in individual subjects in this group.
Of particular interest in the current results is the finding of contralateral only activation in the implanted children. These findings seemingly contradict evidence from adult CI users with activity localized bilaterally to auditory cortex (Debener et al., 2008) as well as evidence from adults with unilateral hearing loss that also reveal bilateral activity in auditory cortex (Hine et al., 2008; Ponton et al., 2001). However, because subjects in those previous studies had lost their hearing in adulthood, it is possible that auditory pathways with both ipsilateral and contralateral projections to auditory cortex were sufficiently preserved, thus even a monaural signal may have input to auditory areas in both hemispheres. In the present case, we hypothesize that such bilateral pathways may not be sufficiently developed during congenital deafness. If this is the case, then the present results might be evidence of a cortical de-coupling of auditory pathways during deprivation (see section 3.4.1, below).
There are at least three other confounds that may have influenced source localization in the present data. First, while implanted children only received monaural stimulation, the NH subjects received binaural stimulation. While it is possible that monaural stimulation of the NH subjects could have revealed unilateral hemispheric activity, evidence inferred from previous studies revealing bilateral activity to monaural stimulation would suggest this is not the case (Hine et al., 2008; Ponton et al., 2001).
A second possible confound is the issue of the head model for source localization. In the present study, the BEM was constructed from an averaged adult MRI. Previous studies have found that very little change in head size occurs after the age of 10 years (Bartholomeusz et al., 2002). Additionally, the BEM used in the present study was adjusted for a smaller white matter volume typically found in this age group (Schmithorst et al., 2008; Wilke et al., 2007). Because the source constraints were limited only to the volume surfaces (i.e., scalp, skull, and cortex), it is unlikely that this head model resulted in additional localization error related to the age of the subjects.
A third possible confound in the present data relates to the signal-to-noise ratio (SNR) of the P1 response, which is a function of the noise covariance matrix computed across all 64 scalp electrodes. Figure 1 clearly reveals a smaller P1 response in the late implanted children compared to the other two groups. Although results of the ANOVA did not reveal any significant differences for P1 amplitude, such results do not preclude an overall, smaller SNR in the late implanted group. In terms of localization error, a smaller SNR often results in source estimations pulled toward the center of the volume conductor. This may help explain the overall movement of source activity in the late implanted group, and may help explain the failure of the symmetric dipole model in implanted children. However, the convergence of individual dipole fits appears to support the present findings.
3.3. Early Implanted Children
The early-implanted children, like the normal-hearing children, showed dominant activity in the right ITG and STS (contralateral to stimulation). In addition, there was evidence of activity in the parietotemporal cortex, although it was minor and likely reflects the effects of two early implanted subjects with dipoles localized to post-central gyrus and insular cortex, respectively. We have found no other reports of parietotemporal activity during basic auditory tasks. The parietotemporal activity may indicate reorganization following a relatively brief period of deprivation. If this is the case, then cortical organization is very sensitive to even brief periods of auditory deprivation. As a consequence, very early implantation may be necessary to allow, at least, relatively normal organization of auditory pathways in congenitally deaf children. Interestingly, the two extra-auditory activations described above were from two of the three right implanted subjects, which may be evidence for a lateralization preference, possibly related to such a preference described for normal hearing subjects (Hine and Debener, 2007). However, such a conclusion is speculative at this time, and further research will be necessary to examine this possibility.
3.4. Late implanted children
Dipole solutions for late implanted children revealed focused activity in the pariteotemporal cortex contralateral to the stimulation. As we noted for the early implanted children, there seems to be no precedent for this outcome (parietotemporal activation) in normal hearing listeners or from imaging data from prelingually deaf patients who receive an implant as an adult (Allen et al., 2004; Truy, 1999). We should note that, while group results were localized to parietotemporal cortex, not all individual subjects revealed this pattern. However, none of the late implanted subjects revealed sources localized to areas associated with auditory cortex. As such, the general finding of pareietotemporal activation may serve to reflect the extent of cortical reorganization that may occur during deprivation.
3.4.1. Cortical de-coupling
For the late implanted children, it is unlikely that reorganized auditory pathways bypass auditory cortex altogether. For example, recent PET imaging data revealed activity in the left of STS of poor CI users versus bilateral STS of good CI users and normal controls, suggesting at least some auditory activation in that group (Coez et al., 2007). Auditory input first reaches the primary auditory cortex as early as 20 ms after the onset of stimulation (Lee et al., 1984). Reciprocal activity mediating cortico-cortical connections from infragranular cortex and thalamocortical loops are likely candidates for processes that generate the P1 CAEP observed around 100 ms in children. Although these processes represent auditory cortex activity, they may represent second order processing at that level. Eggermont and Ponton (2002; 2003) discussed the potential implications of auditory deprivation on infragranular development in the auditory cortex. It is likely that development of laminar projections in the cortex rely on experience with stimuli. Lack of stimulation then leads to a sort of atrophied development of the axons through the cortical layers. Lack of infragranular activity also reflects decreased activity of corticothalamic and collicular feedback projections (Kral et al., 2005; Raizada and Grossberg, 2003). These feedback projections do not seem to be activated in the auditory cortex of the deaf cat (Kral et al., 2005). Such reorganization during development leads to a partial decoupling of auditory pathways. If human development follows the same developmental trajectory in the absence of auditory input, then activation of typical P1 generators would likely not be observed when hearing is restored later in life. The observed activity in the parietotemporal cortex is a reasonable expectation in the presence of this reorganization.
3.4.2. Activation of the dorsal visual stream after implantation
In contrast to NH children and early implanted children, the late implanted children revealed activity outside of expected auditory areas. Previous studies of visual activity in deaf subjects have found increased temporal cortex activity in response to visual stimuli (Armstrong et al., 2002; Bavelier et al., 2001; Bavelier et al., 2000; Fine et al., 2005; Finney et al., 2001; Proksch and Bavelier, 2002). In normal pathways, parietotemporal cortex is associated with activity from the dorsal visual stream, which is sensitive to motion processing. It is possible that when auditory input is deprived during development, pathways associated with the dorsal visual stream begin to remap to dormant auditory areas. If this is the case, then it would be feasible that introducing a “new” sensory input late in childhood would artificially stimulate pathways involved in the visual stream.
3.4.3. Functional consequences of re-organization
If we must consider that the P1 CAEP in late implanted children is functionally different than the P1 CAEP in early implanted and NH children, then we must also consider the clinical implications of late implantation for behavioral development in this group of children. In a recent review, Kral and Eggermont (2007) discussed the mechanisms by which top-down processing modulates the development of the auditory system. In that paper, the authors conclude that a lack of reciprocating modulation from bottom-up processes may result in a reorganized auditory system, and “the ability to learn is compromised in sensory deprivation, resulting in a sensitive period for recovery” (Kral and Eggermont, 2007).
If reorganization has already taken place amongst the sensory systems, and a new system (e.g., hearing) is later introduced, what are the functional consequences to those systems currently organized in the auditory areas? One possibility is that introducing new stimulation would impede the functioning of those reorganized sensory processes as well. That is, introducing a new sensory stimulus also introduces a new competition for resources in the cortex. Another possibility is that the pathways are immediately relayed to areas of multisensory convergence without the reciprocating pathways. It is possible that the parietotemporal activity observed in these late implanted children is representing activation of polymodal cortical areas. Such activity should then have implications for multisensory processing. A recent study by Rouger et al. (2007) found possible evidence for multisensory enhancement in a group of CI users. However, that study was performed on postlingually deafened adults allowing, again, for the possibility that auditory pathways in congenitally deaf children are organized in a fundamentally different manner. Future testing of multisensory processing in these groups of children may help shed light on the consequences of this reorganization.
4. Conclusions
There is considerable evidence for a developmental sensitive period, during which the auditory cortex is highly plastic. If sensory input is deprived to the auditory system during this sensitive period, then the central auditory system is susceptible to large scale reorganization. Restoring input to the auditory system at an early age can provide the stimulation necessary to preserve the auditory pathways, although some evidence of reorganization may already have occurred. However, if auditory input is not restored until after this developmental period, then the reorganized pathways may exhibit abnormal functional characteristics as observed in the scalp recorded CAEPs. This abnormal activity may reflect processes of cross-modal neuroplasticity as evidenced by the parietotemporal activation observed from a source analysis of the P1 CAEP in late implanted children. Further testing will be needed to test the functional relationships between the observed reorganization and multisensory (e.g., auditory-visual) processing in these children.
5. Methods
5.1. Participants
Participants were placed in to one of three categories based on amount of hearing experience. Nine children aged 7.4 to 12.8 years (mean=10.62, SD=2.06) with normal hearing, speech, language, visual (normal or corrected-to-normal), and neurological development were categorized as normal hearing children (NH). All participants were screened for normal speech, language, and neurological development through parent questionnaire prior to testing. Only participants with hearing thresholds ≤20 dB HL and normal speech, language, and neurological development were included in the study.
Eight pre-lingually deafened CI users (5 left ear, 3 right ear) aged 9.57 to 14.7 years (mean=11.31, SD=1.82), implanted at ages 1.73 to 3.9 years (mean=2.79, SD=0.78) were categorized as early implanted children. Eight pre-lingually deafened CI users (5 left ear, 3 right ear) aged 10.0 to 13.3 years (mean=11.33, SD=1.12) implanted at ages 5.0 to 9.84 years (mean=7.84, SD=2.46) were categorized as late implanted children. Participants were screened by parent questionnaire for additional neurological conditions (e.g., mental retardation, cerebral palsy, autism, etc.) that may affect the CAEP recording. All participants and/or parents or legal guardians of all participants provided informed, written consent prior to participation in the study. All study procedures were approved by the Institutional Review Board at The University of Texas at Dallas where the testing took place.
5.2. Stimulus and Stimulation Paradigm
A synthetic speech syllable [ba] was presented with a steady inter-stimulus interval of 610 ms. The speech syllable is 97 ms in duration with a 27 ms consonant to vowel transition, and 7 cycles of the periodic vowel component. The starting frequencies of formants F1 and F2 are 312 and 650 Hz, transitioning to the vowel component with formant frequencies F1–F4 at 780, 1280, 2875, and 3625 Hz.
Stimuli were presented using the commercially available software Stim2 (Compumedics/Neuroscan, El Paso, TX) on a desktop computer with a 24 bit/192 kHz, stereo sound card routed to a GSI-61 audiometer (Grason-Stadler, Inc., Madison, WI). Stimuli were delivered through a loudspeaker placed at a 45° angle two meters from the head on the side of the implant or to the right ear in NH children. Presentation level of the stimuli was approximately 70 dB SPL measured at the head. Stimuli were presented in 2 blocks of approximately 500 presentations per block.
5.3. Test Conditions
Participants were seated in a comfortable chair in a sound treated booth. During the recording procedure, participants were engaged in a reading task. Previous testing has found this to be an effective means of engaging the participants and diverting attention from the stimulus (Sharma et al., 2002a; Sharma et al., 2002b; Sharma et al., 2002c). Because the EEG activity reflected in the CAEP represents an obligatory, pre-attentive response, participants do not need to pay attention to the stimulus.
5.4. EEG Recording Procedures
An electrode cap (Neuroscan Quickcap) with 64 sintered Ag/AgCl electrodes was placed on the participant’s head. Electrode positions were based on the Extended International 10–20 system for electrode placement. Two additional bi-polar channels were included to monitor eye movement, and were placed at the right and left lateral-canthi and superior and inferior orbits of the left eye. EEG activity was recorded using a commercially available Synamps2 68-channel acquisition unit (Compumedics/Neuroscan, El Paso, TX) and digitally stored on a PC computer. Incoming EEG was filtered from DC to 200 Hz at 1000 Hz sampling rate. Stimulus onset times were digitally encoded in the EEG, which is achieved by sending a time-locked, low voltage TTL signal from the stimulus computer to the Synamps unit.
5.5. Evoked Potential Analysis
All analyses were conducted off-line after completion of the recording session. In order to remove the slow DC drift in the recordings the EEG was passed through a high pass filter (1 Hz cutoff, zero-phase shift, 12 dB/octave). Continuous EEG was examined for contaminating artifact, and all EEG blocks containing excessive noise were rejected from further analysis. Eyeblink contamination was removed by applying a spatial filter using a linear derivation from the average eyeblink artifact. The linear derivation is computed from the spatiotemporal singular value decomposition (SVD) of the average eyeblink across the 64 recording channels and the two eye channels. The corrected EEG was then epoched around the stimuli using a 100 ms pre-stimulus interval and 600 ms post-stimulus interval. Each epoch was baseline corrected to the average amplitude of the entire waveform.
For subjects with a cochlear implant, the EEG files were then imported in to the Matlab environment using the EEGLAB Toolbox (EEGLAB, San Diego, CA) under the public GNU license (Delorme and Makeig, 2004). ICA was performed on the concatenated EEG trials using the Infomax approach (Bell and Sejnowski, 1995). Independent component activations were projected to the scalp and assessed for artifactual activity generated from the CI device (Gilley et al., 2006). Artifactual components were identified as components with an onset at time zero and an offset at about 97 ms (the stimulus duration), and as components with a scalp distribution originating from the temporal scalp region on the side of the implanted device (see Gilley et al., 2006; Debener et al., 2008 for complete ICA methods). Components meeting the criteria for a CI artifact were then removed from the ICA mixing matrix. The remaining components were then re-calculated to produce a filtered EEG dataset. When performed on the concatenated EEG trials, ICA for removal of the CI artifact has been shown to be adequate for both CAEP identification and dipole source analysis (Debener et al., 2008; Gilley et al., 2006).
After processing the EEG for CI artifact, the epochs were averaged to produce a CAEP. After averaging, the CAEPs were filtered with a low-pass filter (30 Hz, zero-phase shift, 12 dB/octave), and re-referenced to the common average reference of all electrodes across the scalp. Finally, data from the CI users with a right ear implant were inverted such that the responses from the right hemisphere were represented on the left side of the scalp. This transform was performed to correct for expected differences in activation strength from the hemisphere contralateral to the implant device, and to normalize responses to the left ear for group comparisons. The first robust peak of the CAEP, the P1, was then identified for each group. Latencies and amplitudes of the P1 CAEP were recorded for further analyses.
5.6. Current Source Reconstruction
Inverse solutions for the combined, contributing sources of the P1 response were computed using standardized low resolution brain electromagnetic tomography (sLORETA) (Pascual-Marqui, 2002) as implemented in the Curry 6.0 software package (Compumedics Neuroscan, Charlotte, NC). Results of the sLORTEA yielded a map of estimated current changes, computed as an F-statistic over the surface of the cortex in response to the auditory stimulus as a function of time.
5.7. Dipole Source Analysis
Inverse solutions using seeded dipole models were constructed for grand average P1 CAEP responses in post hoc analyses. A regional dipole model was used for the reconstructions, fit to the onset-to-peak region of the P1 CAEP. For normal hearing children, the model was based on 2 mirrored dipoles as described by Scherg and von Cramen (1985). For children with CIs, an initial model based on 2 mirrored dipoles was used for raw comparison against the normal hearing groups. As results of the mirrored dipole solutions in implanted children revealed unrealistic results (e.g., from the ventricles or sub-cortical locations) a second reconstruction was also performed using a single dipole as the source. The latter model was chosen based on the prediction that unilateral activation from a CI would result in input mostly to the cortex contralateral to the implanted device.
Anatomical constraints for the dipole solutions were limited by the boundary element method (BEM) (Fuchs et al., 2002; Fuchs et al., 2001). The BEM model was based on the segmentation of the average adult MRI from the Montreal Neurological Institute, with segmentation of white matter volume adjusted to 700 ml (Curry parameters) for children in this age group (Schmithorst et al., 2008; Wilke et al., 2007). BEM constraints were limited to three surfaces: cortex, scalp, and skull. No adjustments for head size were necessary for this age group (Bartholomeusz et al., 2002).
Acknowledgments
We would like to thank the two anonymous reviewers for their suggestions and expert opinion on this manuscript. We wish to thank the children and their families for their enthusiastic participation in this study, and to Kathryn Martin and Laura Veazey for their assistance in obtaining data for this study. We also wish to acknowledge the input of Aage Møller, James Jerger, and Teresa Mitchell while preparing this manuscript. This research was supported by funding from the National Institutes of Health (NIH-NIDCD R01-DC004552 and NIH-NIDCD R01-DC006257) to author AS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen A, et al. Perfusion SPECT in cochlear implantation and promontory stimulation. Nucl Med Commun. 2004;25:521–525. doi: 10.1097/00006231-200405000-00015. [DOI] [PubMed] [Google Scholar]
- Armstrong BA, et al. Auditory deprivation affects processing of motion, but not color. Brain Res Cogn Brain Res. 2002;14:422–434. doi: 10.1016/s0926-6410(02)00211-2. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz HH, et al. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics. 2002;33:239–241. doi: 10.1055/s-2002-36735. [DOI] [PubMed] [Google Scholar]
- Bavelier D, et al. Impact of early deafness and early exposure to sign language on the cerebral organization for motion processing. J Neurosci. 2001;21:8931–8942. doi: 10.1523/JNEUROSCI.21-22-08931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, et al. Visual attention to the periphery is enhanced in congenitally deaf individuals. J Neurosci. 2000;20:RC93. doi: 10.1523/JNEUROSCI.20-17-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Coez A, et al. Cochlear Implant Benefits in Deafness Rehabilitation: PET Study of Temporal Voice Activations. J Nucl Med. 2007 doi: 10.2967/jnumed.107.044545. [DOI] [PubMed] [Google Scholar]
- Colletti V, et al. Cochlear implantation at under 12 months: report on 10 patients. Laryngoscope. 2005;115:445–449. doi: 10.1097/01.mlg.0000157838.61497.e7. [DOI] [PubMed] [Google Scholar]
- Connor CM, et al. The age at which young deaf children receive cochlear implants and their vocabulary and speech-production growth: is there an added value for early implantation? Ear Hear. 2006;27:628–644. doi: 10.1097/01.aud.0000240640.59205.42. [DOI] [PubMed] [Google Scholar]
- Debener S, et al. Source localization of auditory evoked potentials after cochlear implantation. Psychophysiology. 2008;45:20–24. doi: 10.1111/j.1469-8986.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Demanez JP, Demanez L. Anatomophysiology of the central auditory nervous system: basic concepts. Acta Otorhinolaryngol Belg. 2003;57:227–236. [PubMed] [Google Scholar]
- Doron N, Wollberg Z. Cross-modal neuroplasticity in the blind mole rat Spalax ehrenbergi: a WGA-HRP tracing study. Neuroreport. 1994;5:2697–2701. doi: 10.1097/00001756-199412000-00072. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Stimulus induced and spontaneous rhythmic firing of single units in cat primary auditory cortex. Hear Res. 1992;61:1–11. doi: 10.1016/0378-5955(92)90029-m. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Ponton CW. The neurophysiology of auditory perception: from single units to evoked potentials. Audiol Neurootol. 2002;7:71–99. doi: 10.1159/000057656. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Ponton CW. Auditory-evoked potential studies of cortical maturation in normal hearing and implanted children: correlations with changes in structure and speech perception. Acta Otolaryngol. 2003;123:249–252. doi: 10.1080/0036554021000028098. [DOI] [PubMed] [Google Scholar]
- Fine I, et al. Comparing the effects of auditory deprivation and sign language within the auditory and visual cortex. J Cogn Neurosci. 2005;17:1621–1637. doi: 10.1162/089892905774597173. [DOI] [PubMed] [Google Scholar]
- Finney EM, et al. Visual stimuli activate auditory cortex in the deaf. Nat Neurosci. 2001;4:1171–1173. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- Francis HW, Niparko JK. Cochlear implantation update. Pediatr Clin North Am. 2003;50:341–361. doi: 10.1016/s0031-3955(03)00034-8. viii. [DOI] [PubMed] [Google Scholar]
- Fuchs M, et al. A standardized boundary element method volume conductor model. Clin Neurophysiol. 2002;113:702–712. doi: 10.1016/s1388-2457(02)00030-5. [DOI] [PubMed] [Google Scholar]
- Fuchs M, et al. Boundary element method volume conductor models for EEG source reconstruction. Clin Neurophysiol. 2001;112:1400–1407. doi: 10.1016/s1388-2457(01)00589-2. [DOI] [PubMed] [Google Scholar]
- Gilley PM, et al. Minimization of cochlear implant stimulus artifact in cortical auditory evoked potentials. Clin Neurophysiol. 2006;117:1772–1782. doi: 10.1016/j.clinph.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Hine J, Debener S. Late auditory evoked potentials asymmetry revisited. Clin Neurophysiol. 2007;118:1274–1285. doi: 10.1016/j.clinph.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Hine J, et al. Does long-term unilateral deafness change auditory evoked potential asymmetries? Clin Neurophysiol. 2008;119:576–586. doi: 10.1016/j.clinph.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Kang E, et al. Neural changes associated with speech learning in deaf children following cochlear implantation. Neuroimage. 2004;22:1173–1181. doi: 10.1016/j.neuroimage.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Khosla D, et al. Differential ear effects of profound unilateral deafness on the adult human central auditory system. J Assoc Res Otolaryngol. 2003;4:235–249. doi: 10.1007/s10162-002-3014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk KI, et al. Effects of age at implantation in young children. Ann Otol Rhinol Laryngol Suppl. 2002;189:69–73. doi: 10.1177/00034894021110s515. [DOI] [PubMed] [Google Scholar]
- Klinke R, et al. Recruitment of the auditory cortex in congenitally deaf cats by long-term cochlear electrostimulation. Science. 1999;285:1729–1733. doi: 10.1126/science.285.5434.1729. [DOI] [PubMed] [Google Scholar]
- Kral A, Eggermont JJ. What's to lose and what's to learn: development under auditory deprivation, cochlear implants and limits of cortical plasticity. Brain Res Rev. 2007;56:259–269. doi: 10.1016/j.brainresrev.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Kral A, et al. Congenital auditory deprivation reduces synaptic activity within the auditory cortex in a layer-specific manner. Cereb Cortex. 2000;10:714–726. doi: 10.1093/cercor/10.7.714. [DOI] [PubMed] [Google Scholar]
- Kral A, et al. Hearing after congenital deafness: central auditory plasticity and sensory deprivation. Cereb Cortex. 2002;12:797–807. doi: 10.1093/cercor/12.8.797. [DOI] [PubMed] [Google Scholar]
- Kral A, et al. Postnatal cortical development in congenital auditory deprivation. Cereb Cortex. 2005;15:552–562. doi: 10.1093/cercor/bhh156. [DOI] [PubMed] [Google Scholar]
- Lee DJ, et al. Effects of congenital deafness in the cochlear nuclei of Shaker-2 mice: an ultrastructural analysis of synapse morphology in the endbulbs of Held. J Neurocytol. 2003;32:229–243. doi: 10.1023/B:NEUR.0000010082.99874.14. [DOI] [PubMed] [Google Scholar]
- Lee DS, et al. Cross-modal plasticity and cochlear implants. Nature. 2001;409:149–150. doi: 10.1038/35051653. [DOI] [PubMed] [Google Scholar]
- Lee YS, et al. Recording of auditory evoked potentials in man using chronic subdural electrodes. Brain. 1984;107(Pt 1):115–131. doi: 10.1093/brain/107.1.115. [DOI] [PubMed] [Google Scholar]
- Menendez-Colino LM, et al. Activation patterns of the primary auditory cortex in normal-hearing subjects: a functional magnetic resonance imaging study. Acta Otolaryngol. 2007;127:1283–1291. doi: 10.1080/00016480701258705. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;24 Suppl D:5–12. [PubMed] [Google Scholar]
- Ponton C, et al. Maturation of human central auditory system activity: separating auditory evoked potentials by dipole source modeling. Clin Neurophysiol. 2002;113:407–420. doi: 10.1016/s1388-2457(01)00733-7. [DOI] [PubMed] [Google Scholar]
- Ponton CW, et al. Auditory system plasticity in children after long periods of complete deafness. Neuroreport. 1996;8:61–65. doi: 10.1097/00001756-199612200-00013. [DOI] [PubMed] [Google Scholar]
- Ponton CW, et al. Plasticity in the adult human central auditory system: evidence from late-onset profound unilateral deafness. Hear Res. 2001;154:32–44. doi: 10.1016/s0378-5955(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Proksch J, Bavelier D. Changes in the spatial distribution of visual attention after early deafness. J Cogn Neurosci. 2002;14:687–701. doi: 10.1162/08989290260138591. [DOI] [PubMed] [Google Scholar]
- Raizada RD, Grossberg S. Towards a theory of the laminar architecture of cerebral cortex: computational clues from the visual system. Cereb Cortex. 2003;13:100–113. doi: 10.1093/cercor/13.1.100. [DOI] [PubMed] [Google Scholar]
- Rouger J, et al. Evidence that cochlear-implanted deaf patients are better multisensory integrators. Proc Natl Acad Sci U S A. 2007;104:7295–7300. doi: 10.1073/pnas.0609419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, et al. Restoration of auditory nerve synapses in cats by cochlear implants. Science. 2005;310:1490–1492. doi: 10.1126/science.1119419. [DOI] [PubMed] [Google Scholar]
- Scherg M, Von Cramon D. Two bilateral sources of the late AEP as identified by a spatio-temporal dipole model. Electroencephalogr Clin Neurophysiol. 1985;62:32–44. doi: 10.1016/0168-5597(85)90033-4. [DOI] [PubMed] [Google Scholar]
- Scherg M, Von Cramon D. Evoked dipole source potentials of the human auditory cortex. Electroencephalogr Clin Neurophysiol. 1986;65:344–360. doi: 10.1016/0168-5597(86)90014-6. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, et al. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2008;29:696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Dorman MF. Central auditory development in children with cochlear implants: clinical implications. Adv Otorhinolaryngol. 2006;64:66–88. doi: 10.1159/000094646. [DOI] [PubMed] [Google Scholar]
- Sharma A, et al. Early cochlear implantation in children allows normal development of central auditory pathways. Ann Otol Rhinol Laryngol Suppl. 2002a;189:38–41. doi: 10.1177/00034894021110s508. [DOI] [PubMed] [Google Scholar]
- Sharma A, et al. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002b;23:532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Sharma A, et al. Rapid development of cortical auditory evoked potentials after early cochlear implantation. Neuroreport. 2002c;13:1365–1368. doi: 10.1097/00001756-200207190-00030. [DOI] [PubMed] [Google Scholar]
- Sharma A, et al. P1 latency as a biomarker for central auditory development in children with hearing impairment. J Am Acad Audiol. 2005;16:564–573. doi: 10.3766/jaaa.16.8.5. [DOI] [PubMed] [Google Scholar]
- Truy E. Neuro-functional imaging and profound deafness. Int J Pediatr Otorhinolaryngol. 1999;47:131–136. doi: 10.1016/s0165-5876(98)00131-1. [DOI] [PubMed] [Google Scholar]
- Waberski TD, et al. Spatio-temporal source imaging reveals subcomponents of the human auditory mismatch negativity in the cingulum and right inferior temporal gyrus. Neurosci Lett. 2001;308:107–110. doi: 10.1016/s0304-3940(01)01988-7. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965;28:1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- Wilke M, et al. Global and local development of gray and white matter volume in normal children and adolescents. Exp Brain Res. 2007;178:296–307. doi: 10.1007/s00221-006-0732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winguth SD, Winer JA. Corticocortical connections of cat primary auditory cortex (AI): laminar organization and identification of supragranular neurons projecting to area AII. J Comp Neurol. 1986;248:36–56. doi: 10.1002/cne.902480104. [DOI] [PubMed] [Google Scholar]
- Yaka R, et al. Pathological and experimentally induced blindness induces auditory activity in the cat primary visual cortex. Exp Brain Res. 2000;131:144–148. doi: 10.1007/s002219900295. [DOI] [PubMed] [Google Scholar]