Abstract
A subpopulation of cells expresses MyoD mRNA and the cell surface G8 antigen in the epiblast prior to the onset of gastrulation. When an antibody to the G8 antigen was applied to epiblast, labeled cells were later found in the ocular primordial and muscle and non-muscle forming tissues of the eyes. In the lens, retina and periocular mesenchyme, G8-positive cells synthesized MyoD mRNA and the bone morphogenetic protein inhibitor Noggin. MyoD expressing cells were ablated in the epiblast by labeling them with the G8 MAb and lysing them with complement. Their ablation in the epiblast resulted in eye defects, including anopthalmia, micropthalmia, altered pigmentation and malformations of the lens and/or retina. The right eye was more severely affected than the left eye. The asymmetry of the eye defects in ablated embryos correlated with differences in the number of residual Noggin producing, MyoD-positive cells in ocular tissues. Exogenously supplied Noggin compensated for the ablated epiblast cells. This study demonstrates that MyoD expressing cells serve as a Noggin delivery system to regulate the morphogenesis of the lens and optic cup.
Keywords: epiblast, MyoD, Noggin, eye development
INTRODUCTION
The epiblast gives rise to the three germ layers of the embryo (Bellairs, 1986). Although cells from this primitive epithelium are generally considered to be pluripotent, a small subpopulation within this tissue of the chick embryo expresses mRNA for the skeletal muscle specific transcription factor MyoD and a cell surface antigen recognized by the G8 monoclonal antibody (MAb) (George-Weinstein et al., 1996; Gerhart et al., 2000; Gerhart et al., 2001; Gerhart et al., 2004a; Strony et al., 2005). MyoD mRNA positive (MyoD+) epiblast cells do not synthesize detectable levels of MyoD protein or other skeletal muscle genes, including Myf5, Myogenin and sarcomeric myosin (George-Weinstein et al., 1996; Gerhart et al., 2000; Gerhart et al., 2007).
The significance of MyoD mRNA expression in the epiblast has been explored in a series of in vitro and in vivo experiments. MyoD+ epiblast cells recruit multipotent epiblast cells to the skeletal muscle lineage in culture by releasing an inhibitor of the bone morphogenetic protein (BMP) signaling pathway (Gerhart et al., 2004a). Although MyoD+ epiblast cells will differentiate into skeletal muscle when cultured under permissive conditions, in vivo, most appear to remain undifferentiated even in the somites that give rise to the skeletal muscles of the trunk and limbs (Gerhart et al., 2006; Gerhart et al., 2004a; Strony et al., 2005). Their role in the somites is to promote the differentiation of myogenic progenitor cells by releasing the BMP inhibitor Noggin (Gerhart et al., 2006). When MyoD+ epiblast cells are ablated in the epiblast, organs become herniated through the ventral body wall of older embryos due to a severe reduction in skeletal muscle (Gerhart et al., 2006).
MyoD+ cells labeled with the G8 MAb also are integrated into embryonic organs lacking skeletal muscle, including the heart and brain (Gerhart et al., 2006; Gerhart et al., 2007). In these locations, they continue to express MyoD mRNA and the G8 antigen and are not induced to form cardiac muscle or neurons. The heart, brain and other organs of chick fetus also contain small numbers of MyoD mRNA+ cells (Gerhart et al., 2001). In the adult mouse, MyoD protein was found in myoid cells of the thymus and in myofibroblasts derived from the liver and kidney (Grounds et al., 1992; Mayer and Leinwand, 1997; Redfield et al., 1997). In addition, MyoD promoter and/or enhancer element activity was detected outside of skeletal muscle in transgenic mice (Asakura et al., 1995; Chen et al., 2005; Kablar, 2004; Kablar and Rudnicki, 2002; Kirillova et al., 2007). With the exception of myofibroblasts that contain contractile proteins downstream of MyoD (Walker et al., 2001), the significance of small numbers of MyoD+ cells in non-skeletal muscle tissues is unknown.
In a previous report that focused on the role of MyoD+ epiblast cells in the somites, we noted that ablation of these cells in the epiblast produces facial and eye malformations in addition to body wall defects (Gerhart et al., 2006). In the following study, we examined the role of MyoD+ epiblast cells during the formation of non-muscle tissues of the eye. Eye development is regulated by a complex series of reciprocal tissue interactions that result in the specification of cells within the ocular primordia, morphogenesis and cell differentiation (Gilbert, 2006). The optic vesicle evaginates from the anterior/lateral neural plate as the adjacent ectoderm thickens to form the lens placode. Soon thereafter, the optic vesicle invaginates to form the optic cup and its derivatives, the retina and retinal pigmented epithelium (RPE), while the lens vesicle develops from the invaginating lens placode. The anterior margin of the optic cup gives rise to the iris and ciliary body and contributes progenitor cells to the retina (Gould et al., 2004). Cells fated to differentiate into the extraocular muscles emerge within the prechordal mesoderm and unsegmented paraxial mesoderm before populating the periocular mesenchyme (Couly et al., 1992; Gage et al., 2005; Jacob et al., 1984; Johnston et al., 1979; Noden, 1983).
Several families of secreted molecules, including BMPs, are involved in the orchestration of eye development. BMPs-2, -4, -5, -6, and -7 and BMP receptors BMPR-IA, -IB and -II have been mapped to the embryonic eye (Beebe et al., 2004; Belecky-Adams and Adler, 2001; Wordinger and Clark, 2007). Defining the processes regulated by the BMP signaling pathway during eye development has been achieved through genetic knockout and knockdown strategies and by over-expressing Noggin. These approaches revealed that BMP signaling is required for the formation of the lens, retina, RPE, ciliary body and cornea by mediating communication between tissues and regulating cell proliferation, apoptosis and differentiation (Beebe, 1986; Belecky-Adams and Adler, 2001; Belecky-Adams et al., 2002; Chang et al., 2001; Donner et al., 2006; Dudley et al., 1995; Faber et al., 2001; Faber et al., 2002; Furuta and Hogan, 1998; Hung et al., 2002; Jena et al., 1997; Litsiou et al., 2005; Luo et al., 1995; Rinaudo and Zelenka, 1992; Saika et al., 2001; Sanford et al., 1997; Trousse et al., 2001; Wawersik et al., 1999; Wordinger and Clark, 2007; Zhao et al., 2002).
While loss of function mutations in BMP-4 and overproduction of Noggin cause varying degrees of dysgenesis of ocular tissues (Belecky-Adams et al., 2002; Trousse et al., 2001; Zhao et al., 2002), overexpression of BMPs and null mutations in Noggin and Chordin also result in eye defects (Bachiller et al., 2000; McMahon et al., 1998; Ogita et al., 2001). The BMP inhibitors Noggin, Follistatin and Chordin shift the fate of ectoderm cells from the epidermal lineage to neuroectoderm and direct the formation of anterior neural structures that give rise to the optic vesicle (Zilinski et al., 2004). These three BMP inhibitors, along with Ventroptin, Gremlin, and Dan have been mapped to the developing eye (Belecky-Adams and Adler, 2001; Ogita et al., 2001; Sakuta et al., 2001; Trousse et al., 2001; Zhao et al., 2002). Inhibition of the BMP signaling pathway also is required for the differentiation of the extraocular muscles (Tzahor et al., 2003; von Scheven et al., 2006). These studies illustrate the importance of modulating BMP signaling both temporally and spatially for eye development to proceed normally.
One source of BMP inhibitors in the developing eye is the neural crest (Tzahor et al., 2003). Neural crest cells release Noggin and Gremlin in the periocular mesenchyme; however, extraocular muscle differentiation occurs in their absence (Diehl et al., 2006; Evans and Gage, 2005; Olsson et al., 2001; Tzahor et al., 2003), indicating that an additional population of cells must serve as a source of BMP inhibitors in the periocular mesenchyme. Neural crest cells also populate the iris, ciliary body, cornea, trabecular meshwork and primary vitreous but they have not been detected in the lens or optic cup (Cvekl and Tamm, 2004; Gage et al., 2005; Ittner et al., 2005; Johnston et al., 1979; Le Lievre and Le Douarin, 1975).
The following experiments were designed to map the integration sites of MyoD+ cells during the formation of the embryonic eye, determine whether they express Noggin in different ocular tissues and characterize the eye defects that arise as a result of their ablation in the epiblast. This study demonstrates that MyoD+ cells are the major source of Noggin in non-muscle forming tissues of the eye and are required for the normal morphogenesis of the lens and optic cup.
MATERIALS AND METHODS
Tracking MyoD+ Epiblast Cells in the Embryo
White Leghorn chick embryos (BE Eggs) were removed from the shell and staged according to the method of Hamburger and Hamilton (Hamburger and Hamilton, 1951). Analyses of the positions of MyoD+ epiblast cells for the first 48 hours after laying were accomplished by removing stage 2 embryos from the yolk, rinsing in Dulbecco’s phosphate buffered saline (PBS) (Invitrogen), positioning the embryo on a raft in a well containing thin albumen (the less viscous portion of the egg white that is almost completely lacking in ovomucin), and labeling the cells with the G8 MAb and a goat anti-mouse IgM Mu chain secondary antibody conjugated with either fluorescein, Alexa 488 or rhodamine (Chemicon, Jackson Labs) (Gerhart et al., 2007). Following rinsing, labeled embryos were incubated at 37°C with 4.5% CO2 in air. Since embryos survive for only 48 hours in this culture system, the distribution of stage 2 MyoD+ epiblast cells during later stages of eye development was determined by placing the contents of the egg in a dish, labeling with the G8 MAb and a fluorescent secondary antibody while the embryo resided on the yolk, returning the embryo to the shell and incubating at 37°C in an egg incubator for three to five days (Gerhart et al., 2006; Gerhart et al., 2008). This ex-ovo/in-ovo whole embryo culture system supports development into the late fetal period (Gerhart et al., 2008).
Stage 2 embryos pre-labeled with G8 and secondary antibodies were incubated to reach stages 3–7 and fixed in 2% formaldehyde for 20–60 minutes. Nuclei were labeled with Hoechst dye (Sigma-Aldrich). Pre-labeled embryos grown to stages 17–25 were fixed overnight in 4% formaldehyde and embedded in paraffin. Ten µm transverse sections were counterstained with Hoechst dye and mounted in Elvanol (Dupont). Fluorescent cells were visualized with a Nikon Eclipse 800 epifluorescence microscope (Optical Apparatus) equipped with the following filters: excitation 530–560, barrier 573–648 for Cy3 and Rhodamine; excitation 465–495, barrier 515–555 for Alexa 488; excitation 330–380, barrier 435–485 for Hoechst dye. Photographs were taken under 4× NA 0.2, 40× oil NA and 60× oil NA 1.4 objectives. Images were produced with the Evolution QE Optronics video camera (Media Cybernetics) and the Image Pro Plus image analysis software program (Phase 3 Imaging Systems). Figures were annotated and adjusted for brightness and contrast using Adobe Photoshop 6.0.
Immunofluorescence Localization and In Situ Hybridization
Double labeling for the G8 antigen and MyoD or Noggin mRNA, or MyoD mRNA and Noggin protein was carried out as described previously (Gerhart et al., 2000; Gerhart et al., 2004b). The primary antibodies were applied first and tagged with either fluorescein conjugated goat anti-mouse IgM Mu chain or donkey anti-goat IgG (Chemicon). The goat polyclonal antibody to Noggin was obtained from R&D Systems. After staining with primary and fluorescent secondary antibodies, sections were incubated in Cy3 labeled 3DNA™ dendrimers (Genisphere, Inc.) conjugated with an antisense cDNA sequence for chicken MyoD mRNA (5′-TTCTCAAGAGCAAATACTCACCATTTGGTGATTCCGTGTAGTA-3′ (Dechesne et al., 1994) (L34006) or chicken Noggin (5′TCTCGTTAAGATCCTTCTCCTTGGGGTCAAA-3′) (Tonegawa and Takahashi, 1998) (NM_204123). Nuclei were counterstained with Hoechst dye. Sections were mounted in Gelmount (Electron Microscopy Sciences).
Ablating MyoD+ Epiblast Cells in the Epiblast
Determination of the effects of ablating MyoD+ epiblast cells on eye development was carried out by placing stage 2 embryos in a tissue culture dish, incubating the embryo with the G8 MAb and lysing labeled cells with baby rabbit complement (Cedar Lane, Inc.) while they resided on the yolk (Gerhart et al., 2006; Gerhart et al., 2008). Control embryos were incubated in Hanks buffer, the G8 MAb or complement (Comp) only, or the E12 MAb and complement. The E12 MAb binds to a separate subpopulation of epiblast cells that expresses the neurogenic transcription factor NeuroM (Strony et al., 2005). Cell lysis was visualized by incubating embryos in trypan blue (Gerhart et al., 2006). Embryos were returned to a host shell and incubated at 37°C for three to six days. The following numbers of control and treated embryos were examined for their external and histological morphology:
| External Morphology | Histology | |||
|---|---|---|---|---|
| St 14–18 | St 23–25 | St 14–18 | St 23–25 | |
| Buffer | 3 | 7 | 1 | 2 |
| G8 MAb | 1 | 6 | 1 | 1 |
| Complement | 2 | 4 | 0 | 1 |
| E12 MAb + Comp. | 0 | 5 | 0 | 2 |
| G8 MAb + Comp. | 3 | 11 | 3 | 11 |
Determination of whether additional cells expressed the G8 antigen in the epiblast after the ablation procedure was performed on stage 2 embryos was carried out by applying a secondary antibody conjugated with fluorescein immediately after washing out the complement, incubating embryos on rafts for three hours at 37°C, re-applying the G8 MAb and tagging it with a secondary antibody conjugated with rhodamine.
Supplementing Embryos with Noggin
Embryos were treated on the yolk with the G8 MAb and complement to lyse MyoD+ epiblast cells and then incubated in a host shell at 37°C until they reached stages 10–13. Affigel Blue agarose beads (BioRad) soaked in a solution of 100 ng of human recombinant Noggin (PeproTech) per ml of PBS were inserted on the right side of the embryo lateral to the most cranial somite, the third and fourth somites, and the most caudal three somites (Fig. 6A) (Gerhart et al., 2006). Control embryos received beads soaked in PBS alone. Embryos were placed in 60 ml capacity glass bowls (Dollar Tree), covered and cultured at 37°C for two to four days.
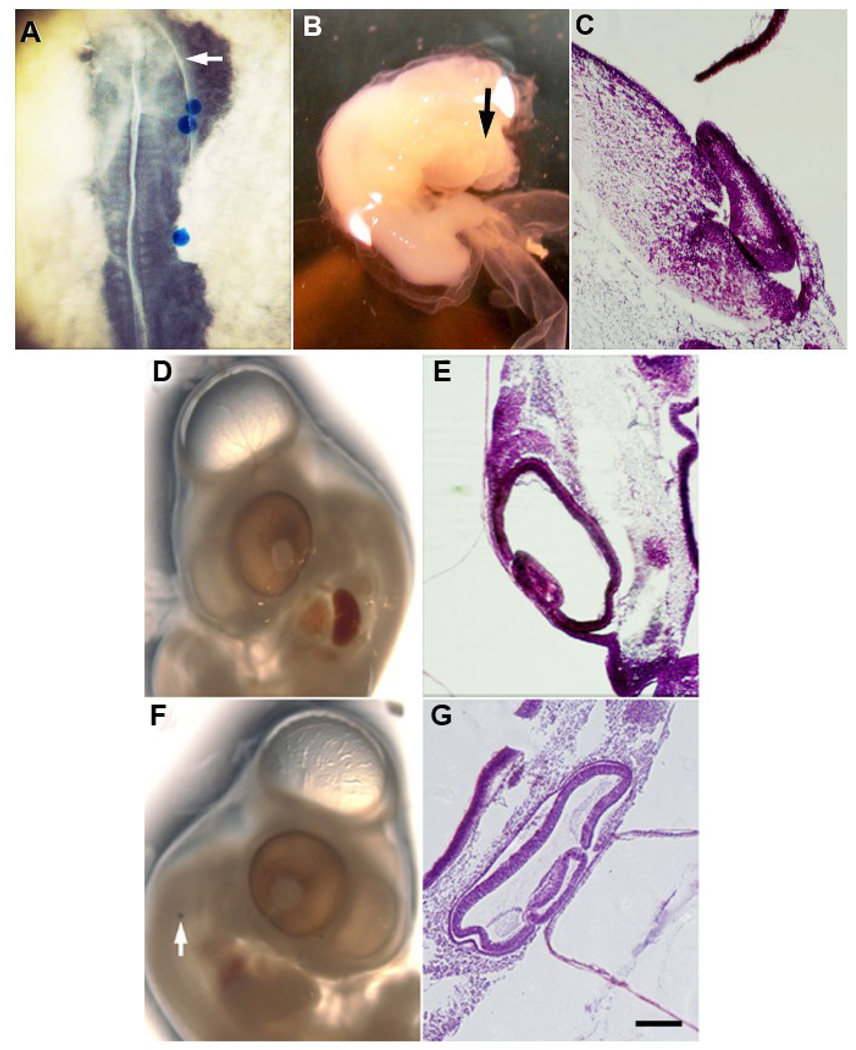
Figure 6. Effects of exogenous Noggin on eye development in embryos treated to ablate MyoD+ cells in the epiblast.
MyoD+ cells were ablated in the stage 2 epiblast and the embryos were incubated to stages 10–13. Affigel blue beads soaked in PBS or human recombinant Noggin were implanted on the right side of the embryo (A). The most proximal bead was adjacent to the first somite caudal to the developing eye. The arrow in A indicates the position of the eye. Stages 23–24 embryos were sectioned and stained with H & E. Ablated embryos implanted with PBS beads continued to exhibit eye defects (B and C). The left (D and E) and right eyes (F and G) of most ablated embryos implanted with Noggin soaked beads appeared normal. Scale bar: 56 µm in C, E and G.
RESULTS
Cells that Express MyoD mRNA in the Epiblast are Incorporated into the Developing Eye
MyoD+ epiblast cells were identified in the stage 2 epiblast based on their expression of the G8 antigen and MyoD mRNA (Fig. 1B) (Gerhart et al., 2006). Living stage 2 embryos were fluorescently labeled with the G8 MAb in order to track the sites of incorporation of MyoD+ cells during eye development. In a previous study we determined that cells labeled with the G8 MAb applied to the stage 2 epiblast were integrated into the developing somites, heart and central nervous system (Gerhart et al., 2006; Gerhart et al., 2007; Gerhart et al., 2008). Epiblast cells that expressed the G8 antigen within three hours after the embryo was incubated with the G8 MAb were not tagged by residual unbound antibodies or the transfer of antibodies from other cells (Gerhart et al., 2006).
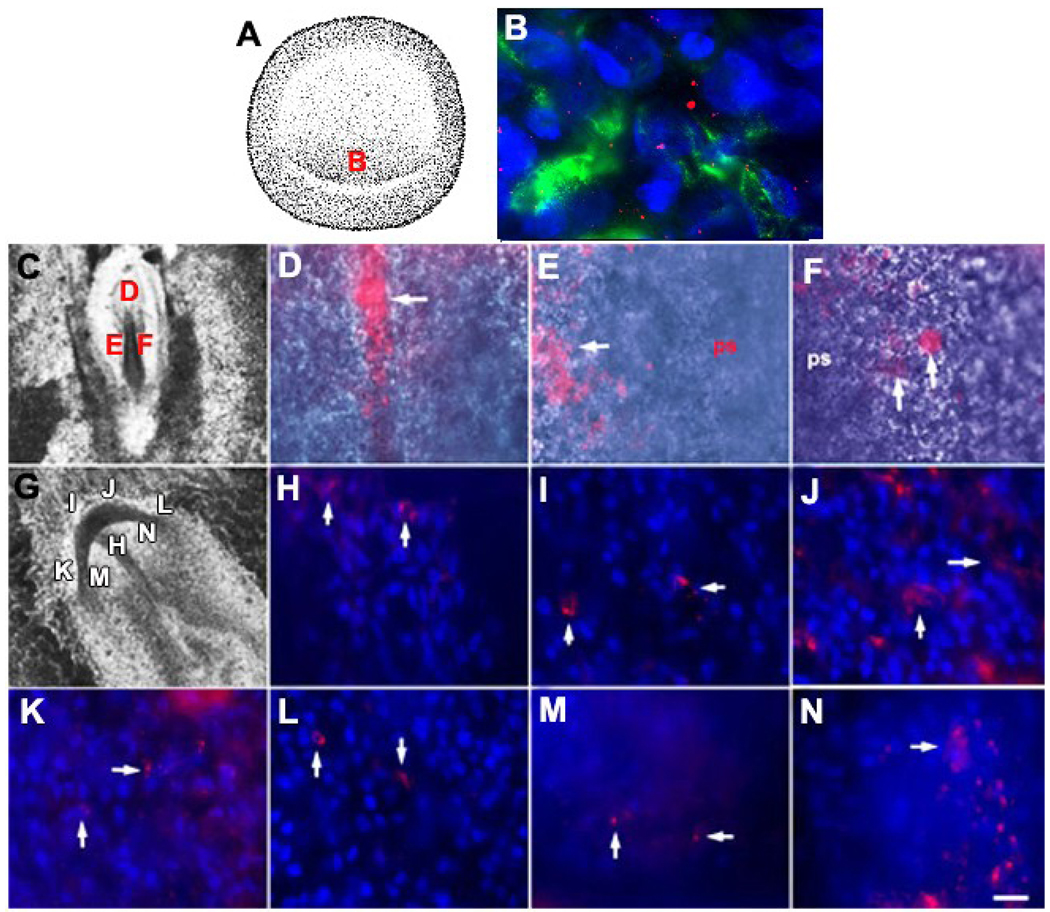
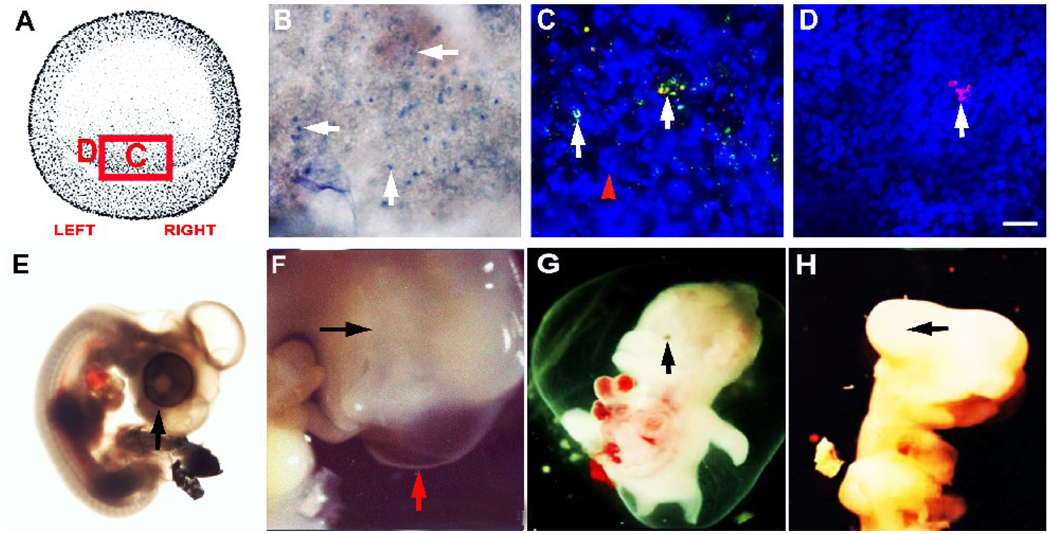
Figure 1. Identifying and tracking MyoD+/G8+ cells from the stage 2 epiblast into tissues of stages 5 and 7 embryos.
Whole stage 2 embryos were double labeled with the G8 MAb and fluorescein conjugated secondary antibodies (green) and Cy3 dendrimers to MyoD mRNA (red) (B). Nuclei were counterstained with Hoechst dye (blue). The area indicated in the drawing of the stage 2 embryo (A) is shown at high magnification in B. MyoD+ epiblast cells of the stage 2 embryo express the G8 antigen and MyoD mRNA (B). Whole stage 2 embryos were labeled with the G8 MAb and rhodamine conjugated secondary antibodies and incubated until they reached stages 5 (C–F) or 7 (G–N). Stage 7 embryos were counterstained with Hoechst dye. The areas designated with a letter in the DIC images of the embryos (C and G) are shown at high magnification in the remaining photomicrographs. Arrows indicate the locations of some of the fluorescent G8+ epiblast cells in whole embryos. By stage 5, G8+ epiblast cells had been incorporated into the head process (D). The epiblast on either side of the anterior portion of the primitive streak also contained G8+ epiblast cells (arrows in E and F). By stage 7, G8+ epiblast cells were found in the prechordal mesoderm (H), the anterior (I and J) and anterior/lateral preplacode regions of the ectoderm (K and L), and the anterior/lateral neural plate (M and N). Scale bar: 135 µm in C and G; 9 µm in D–F and H–N.
All MyoD+ epiblast cells were present in the posterior/marginal region of the stage 2 epiblast (Fig. 1A and B). Approximately 24 hours after applying the G8 MAb and fluorescent secondary antibodies (stage 5-5+), fluorescent cells were observed along the posterior margin of the epiblast, within the primitive streak (not shown), lateral to the streak and in the head process, a mesodermal structure that forms the notochord (Fig. 1D–F). By stage 7, fluorescent cells were observed in the unsegmented paraxial mesoderm (not shown) and prechordal mesoderm (Fig. 1H), the tissues of origin of the extraocular muscles (Couly et al., 1992; Gage et al., 2005; Jacob et al., 1984; Johnston et al., 1979; Noden, 1983). G8 labeled cells also were found in the region of the ectoderm containing the primordia of the sensory placodes (Litsiou et al., 2005), including the lens placode (Fig. 1I–L), and in the anterior/lateral neural plate from which the optic vesicle evaginates (Chow and Lang, 2001) (Fig. 1M and N). The movements of G8 labeled cells from the posterior epiblast suggest that some MyoD+ epiblast cells enter the mesoderm while others remain in the ectoderm.
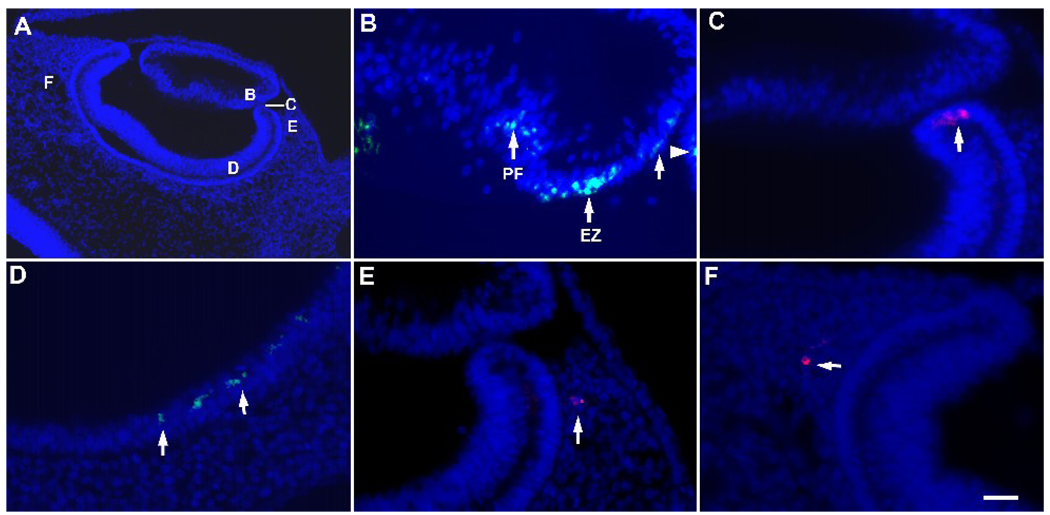
By stage 17, MyoD+ cells tagged with the G8 MAb were found as single cells or in foci of approximately two to eight cells within the eyes (Fig. 2). G8 labeled cells (G8+) constituted a minor subpopulation in the eye, and therefore, not all sections contained these cells in the same locations (Fig. 2 and Fig. 3). In the lens vesicle, G8+ cells were present in the equatorial zone where lens epithelial cells initiate differentiation, as well as in the anterior epithelium and posterior primary lens fiber region (Fig. 2B). The optic cup contained foci of G8+ cells in its anterior margin (Fig. 2C) and within the posterior region of the developing neural retina (Fig. 2D). The G8 expressing cells in the chick retina may be related to the rare population of retina cells that weakly express GFP under the regulation of enhancer and promoter elements of the MyoD gene (Kirillova et al., 2007). Cells labeled with the G8 MAb also were present within the periocular mesenchyme surrounding the lateral and posterior regions of the optic cup (Fig. 2E and F). The results of these experiments demonstrate that MyoD+/G8+ cells are incorporated into skeletal muscle (periocular mesenchyme) and non-skeletal muscle forming tissues (lens and optic cup) during eye development.
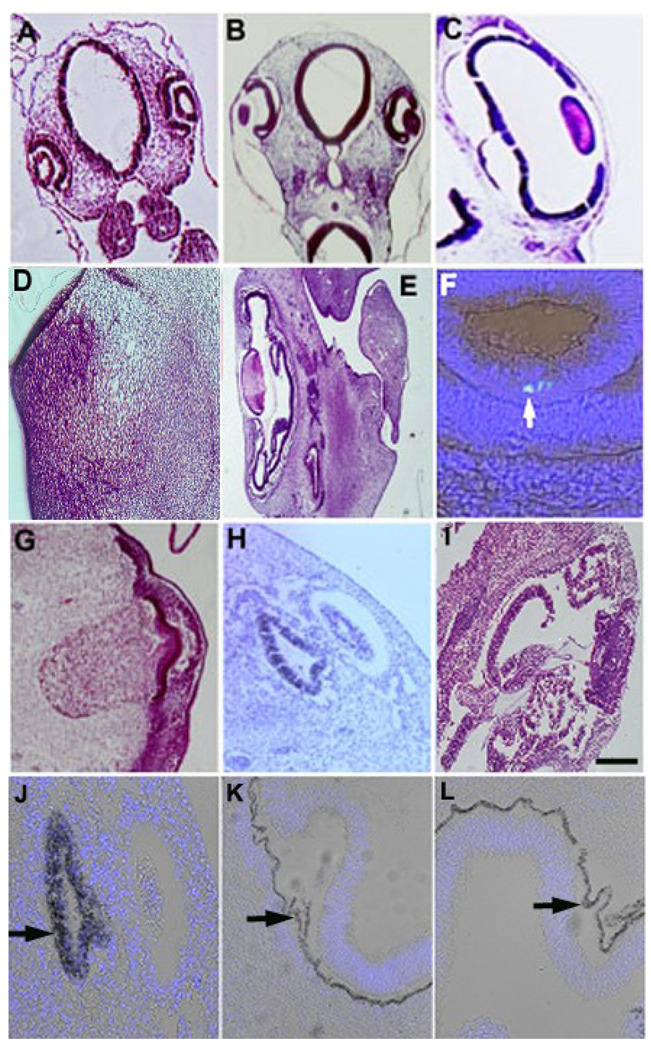
Figure 2. Distribution of G8+ epiblast cells in the eyes of stages 17 and 18 embryos.
Stage 2 embryos were labeled with the G8 MAb and a rhodamine (red) or fluorescein (green) conjugated secondary antibody, incubated to stages 17 or 18, fixed and sectioned. Nuclei were counterstained with Hoechst dye (blue). A representative section through the stage 17 eye is shown in panel A. The areas indicated by the letters in panel A are shown at high magnification in B–F. G8+ epiblast cells were present in the equatorial zone (EZ) and primary fiber region (PF) of the lens (arrows in B) and the anterior margin of the optic cup (arrowhead in B, arrow in C). G8+ epiblast cells also were present in the retinal layer of the posterior optic cup (D) and periocular mesenchyme (E and F). Scale bar: 27 µm in A; 13 µm in B–F.
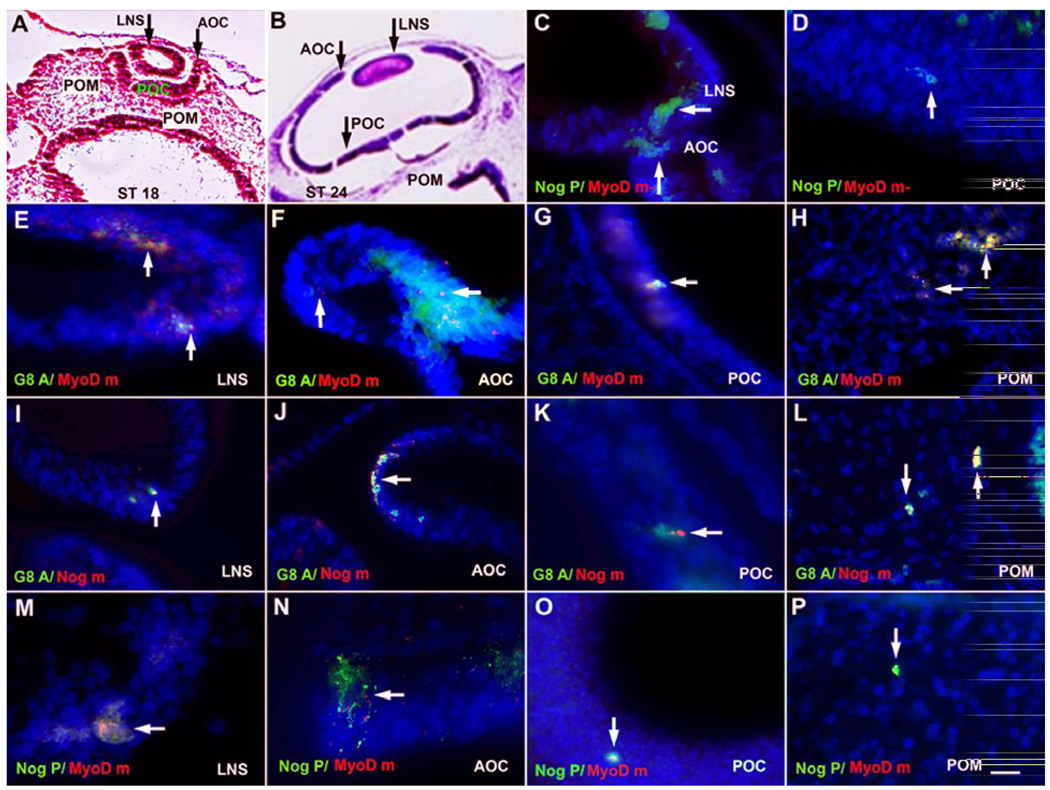
Figure 3. Co-expression of G8, MyoD mRNA and Noggin in the developing eye.
The eyes of stage 18 (A, C, E, G, H, M and O) and stage 24 (B, D, F, I, J, K, L, N and P) embryos were sectioned and stained with H & E (A and B) or double labeled with the G8 MAb (G8 A) (green) and dendrimers to MyoD mRNA (MyoD m) (red) or Noggin mRNA (Nog m) (red), or an antibody to Noggin protein (Nog P) (green) and dendrimers to MyoD mRNA. Nuclei were counterstained with Hoechst dye (blue). The overlap of red and green appears yellow-white in merged images. Cells co-expresssing G8 and MyoD mRNA were present in the lens (LNS) (E), anterior optic cup (AOC) (F), the retinal layer of the posterior optic cup (POC) (G), and periocular mesenchyme (POM) (H). Cells expressing the G8 antigen also expressed Noggin mRNA in the lens (I), anterior and posterior optic cup (J and K), and periocular mesenchyme (L). A few cells with Noggin mRNA were not stained with G8 in the lens (arrowhead in J). Noggin protein and MyoD mRNA were co-localized in some cells of the lens (M), anterior optic cup (arrow in N), posterior optic cup (O), and periocular mesenchyme (P). Other cells labeled for Noggin protein did not contain detectable levels of MyoD mRNA (MyoD m-) in the lens and optic cup (arrows in C and D). Scale bar: 56 µm in A and B; 13 µm in C–P.
G8+ Cells Express MyoD mRNA and Noggin in the Developing Eye
Cells labeled with the G8 MAb were examined for the expression of MyoD mRNA and Noggin mRNA and protein in representative sections through the eyes of three stages 17–18 and three stages 23–24 embryos (Table 1). All G8+ cells expressed MyoD mRNA. At stage 23–24, Noggin mRNA was found in all G8+ cells in the eyes and only 7% of the cells expressing Noggin lacked detectable levels of G8. Greater than 80% of the cells that expressed MyoD mRNA were stained with an antibody to Noggin protein. Two thirds of the cells with Noggin mRNA or protein contained G8 or MyoD mRNA.
Table 1.
Expression of the G8 antigen, MyoD mRNA and Noggin in the developing eye
| Stages 17–18 | Stages 23–24 | |||
|---|---|---|---|---|
| # of Cells | % | # of Cells | % | |
| G8+ cells with MyoD mRNA | 120/120 | 100 | 232/232 | 100 |
| MyoD mRNA+ cells without G8 | 1/121 | 1 | 0/232 | 0 |
| G8+ cells with Noggin mRNA | 39/40 | 98 | 28/28 | 100 |
| Noggin mRNA+ cells without G8 | 16/55 | 29 | 2/30 | 7 |
| MyoD mRNA+ cells with Noggin protein | 71/76 | 93 | 92/114 | 81 |
| Noggin protein+ cells without MyoD mRNA | 5/76 | 7 | 34/126 | 27 |
Tissue sections through three stages 17–18 and three stages 23–24 eyes were double labeled with the G8 MAb and either dendrimers to MyoD mRNA or Noggin mRNA, or MyoD dendrimers and antibodies to Noggin protein. # of Cells = the number of cells with or without both labels over the total number of cells labeled for the first marker. These numbers were derived by pooling the cell counts from representative sections from three embryos stained with the same probes. % = the number of cells with or without both labels ÷ total number of cells labeled for the first marker × 100. All G8+ cells expressed MyoD mRNA. Only one cell with G8 lacked detectable levels of Noggin mRNA. Some cells with Noggin mRNA or protein lacked detectable levels of G8 or MyoD mRNA, respectively.
The distribution of double and single labeled cells is shown in Figure 3. G8+/MyoD mRNA+ cells (Fig. 3E–H), G8+/Noggin mRNA+ cells (Fig. 3I–L), MyoD mRNA+/Noggin protein+ cells (Fig. 3M–P), and Noggin protein+/MyoD mRNA-cells (Fig. 3C and D) were found in the lens, anterior margin and posterior derivatives of the optic cup, and periocular mesenchyme of stages 17–18 and 23–24 embryos. A few Noggin mRNA+/G8− cells (Fig. 3J) and Noggin protein+/MyoD mRNA− cells were present in the lens and optic cup derivatives (Fig. 3C and D). These double labeling experiments demonstrate that G8+/MyoD+ cells are the major source of Noggin in ocular tissues.
Ablation of G8+/MyoD+ Cells in the Epiblast
MyoD+ cells were ablated in the epiblast by lysing G8 labeled cells with complement (Gerhart et al., 2006; Gerhart et al., 2008). Consistent with our previous experiments (Gerhart et al., 2006), the stage 2 epiblast contained 67 ± 3 G8+ cells (n = 6 embryos). All G8+ cells were located in the posterior/medial region of the epiblast. Cell lysis was demonstrated by incubation in trypan blue (Fig. 4B). Embryos treated with G8 and complement contained 66 ± 2 lysed cells (n = 4), whereas only one lysed cell was observed in two embryos treated with complement alone.
Figure 4. Lysis of MyoD+ cells in the epiblast and its effects on eye morphogenesis.
MyoD+ cells in the stage 2 epiblast were labeled with the G8 MAb and lysed with complement. The area in the box in the drawing of the stage 2 embryo in panel A is shown in panel B. The letters correspond to the areas shown at higher magnification in C and D. The uptake of trypan blue reveals the presence of lysed cells (B). A fluorescein conjugated secondary antibody was applied following treatment with complement (green). After incubating the embryos for three hours, the G8 MAb was reapplied and tagged with a secondary antibody conjugated with rhodamine (red). The overlap of red and green appears yellow-white in merged images. Most labeled cells were tagged with both fluorochromes (arrows in C). Nuclei appeared fragmented (arrowhead in C). Cells that expressed the G8 antigen within three hours after ablation were labeled with only rhodamine (D). These cells were concentrated on the left side of the epiblast a field away from the cells shown in panel C. The right (E) and left eyes were normal in control embryos treated with the E12 MAb and complement. One ablated embryo had an absence of visible eye tissue on the right (black arrow) and a normal eye on the left (red arrow) (F). Other embryos exhibited micropthalmia (G) or a lack of pigmentation (H). Scale bar: 56 µm in B; 9 µm in C–D.
To determine whether more cells emerged in the epiblast after ablation, a secondary antibody conjugated with fluorescein was applied to the embryo directly after washing out the complement to label cells that had bound the G8 MAb. Three hours later, the embryos were re-stained with another application of the G8 MAb and a secondary antibody conjugated with rhodamine. Cells tagged with fluorescein only or both fluorochromes corresponded to the original population of G8 labeled cells lysed with complement in the stage 2 epiblast, whereas cells stained with rhodamine only had expressed G8 after the initial labeling period (Fig. 4C). There were 11 ± 3 cells (n = 5 embryos) that were stained with rhodamine only, and therefore, had emerged in the epiblast after the ablation procedure (Fig. 4D). In four out of five embryos, 64–100% of these newly labeled cells were present on the left side of the embryo in a field adjacent to the lysed cells. This experiment demonstrates that additional cells express the G8 antigen after lysis of the original population, and in the majority of these embryos, the newly emerging G8 expressing cells were present on the left side of the posterior epiblast. These results suggest that subtle differences in the timing of ablation (early to late stage 2) could lead to phenotypes of varying severity.
Ablation of G8+/MyoD+ Cells in the Epiblast Adversely Affects Eye Development
On the third day after ablating MyoD+ cells in the epiblast, the eyes appeared normal externally. Examination of the complete set of serial tissue sections from two of these embryos revealed that the left eyes had a normal morphology while the lens vesicles of both of the right eyes were more densely stained than the control lenses and lacked a lumen (Fig. 5A and B). By the fifth day after ablation, all of the right eyes and 27% of the left eyes had externally visible eye defects, including clinical anopthalmia, micropthalmia, an abnormal shape and/or altered pigmentation (Table 2 and Table 3; Fig. 4F–H). The right eyes were more severely affected than the left eyes (Table 2 and Table 3). The asymmetry in the severity of the defects may reflect the emergence of additional G8+ cells on the left side of epiblast after the ablation procedure.
Figure 5. Histological appearance of the eye defects that arise after ablating MyoD+ cells in the epiblast.
Stage 2 embryos were treated with the G8 MAb and complement to ablate MyoD+ cells in the epiblast. Embryos were incubated to stage 17 (A and B) or stage 23 (C–L). Sections were stained with H & E (A–E and G–I), the G8 MAb tagged with fluorescein (green) and Hoechst nuclear dye (blue) (F), or Hoechst nuclear dye only (J–L). The eyes of control embryos treated with complement alone are shown in A and C. Unlike the control lens vesicle of a stage 17 embryo (A), the right lens did not contain a lumen (B). A vesicle was present in later sections through the left eye (not shown). One of the left eyes lacked morphologically distinct ocular tissues (D) (embryo #11, Table 3). Another left eye exhibited a normal lens with folds of retinal tissue (E) (embryo #8, Table 3). Residual MyoD+ epiblast cells stained with the G8 MAb were present in the lens of the left eye of an ablated embryo (F) (embryo # 2, Table 3 and Table 4). The right eye was either missing identifiable ocular tissues (G) (embryo # 10, Table 3) or contained a small lens and distorted optic cup (H) (embryo #8, Table 3) or folds of retinal tissue (I) (embryro #7, Table 3). Pigment was present throughout the optic cup of the eye shown in panel H (J). The RPE was present and pigmented in the right (K) and left (L) eyes containing retinal folds. Scale bar: 135 µm in A and B; 56 µm in C–E and G–I; 27 µm in J–L; 13 µm in F.
Table 2.
Incidence of eye defects in ablated embryos in the presence or absence of exogenous Noggin
| External Morphology | ||||
|---|---|---|---|---|
| # of Embryos | Both Eyes Normal | Left Eye Normal/ Right Eye Abnormal |
Both Eyes Abnormal | |
| External Morphology | ||||
| CT | 22 | 22 | 0 | 0 |
| Ablated | 11 | 0 | 8 | 3 |
| Ablated + Noggin | 5 | 5 | 0 | 0 |
| Internal Morphology | ||||
| CT | 6 | 6 | 0 | 0 |
| Ablated | 11 | 0 | 4 | 7 |
| Ablated + Noggin | 4 | 3 | 0 | 1 |
MyoD+ cells were ablated in the stage 2 epiblast by labeling with the G8 MAb and lysing with complement (Ablated). Control embryos (CT) were incubated in buffer, G8 or complement only, or the E12 MAb and complement. Beads soaked in Noggin were implanted into the stage 10 embryo of some ablated embryos (Ablated + Noggin). Embryos were incubated to stages 14–18 (days 2–3) or stages 23–26 (days 4–5). The morphology of the eyes was observed on the external surface of the embryo and in tissue sections (internal morphology). The frequency of eye defects increased after stage 18. Eye defects were more common in the right eye than the left eye. Exogenous Noggin compensated for the ablated MyoD+ epiblast cells.
Table 3.
Effects of ablating MyoD+ epiblast cells on ocular morphology
| External Morphology | Internal Morphology | |||
|---|---|---|---|---|
| Embryo | Left | Right | Left | Right |
| 1 | normal | misshapen | normal | misshapen retina |
| 2 | normal | misshapen | normal | retinal folds/no lens |
| 3 | normal | uneven pigment | normal | large lens |
| 4 | normal | anopthalmia | normal | anopthalmia |
| 5 | normal | misshapen | retinal folds/ misshapen lens |
misshapen retina/ misshapen lens |
| 6 | normal | no pigment | misshapen retina | small retina/no lens |
| 7 | normal | no pigment | slight retinal folds/ misshapen lens |
retinal folds/ misshapen lens |
| 8 | normal | anopthalmia | retinal folds | misshapen retina and lens |
| 9 | uneven pigment | decreased pigment | retinal folds/ uneven pigment |
retinal folds/ misshapen lens |
| 10 | micropthalmia | micropthalmia | retinal folds/no lens | pigmented tissue only |
| 11 | micropthalmia | micropthalmia | pigmented tissue only | pigmented tissue only |
MyoD+ cells were ablated in the stage 2 epiblast by labeling with the G8 MAb and lysing with complement. Embryos were incubated to stages 23–26. The morphology of the eyes was observed on the external surface of the embryo and in tissue sections (internal morphology). Eye defects were more severe in the right eye than the left eye. Misshapen morphology on the external surface indicates that the eye was not round. Other malformations include micropthalmia (small eye), altered pigmentation (uneven, decreased, small spot of pigmentation, or no pigment), misshapen retina (not cup shaped but there were no folds in the tissue), folds of neural retina tissue, misshapen lenses (small, densely stained and thickened in the anterior to posterior dimension), missing lenses, and anopthalmia (absence of morphologically identifiable ocular tissues).
None of the eyes in control embryos treated with buffer, the G8 MAb or complement alone, or the E12 MAb and complement had visible eye defects (Table 2 and Fig. 4E). The E12 MAb binds specifically to a subpopulation of epiblast cells that expresses the NeuroM transcription factor, but not MyoD or G8 (Gerhart et al., 2006; Strony et al., 2005). Ablation of the E12+ and G8+ subpopulations of epiblast cells results in different malformations, thereby demonstrating the specificity of the ablation procedure (Gerhart et al., 2006).
Histological analyses of the ablated embryos revealed eye defects of varying severity (Table 2 and Table 3, Fig. 5). All of the right eyes and 64% of the left eyes were abnormal. Half of the left eyes that appeared normal externally had internally visible defects. A frequently observed malformation was folding of the neural retina within the vitreous body (Table 3, Fig. 5E and I). In some embryos, retinal folding occurred in right and left orbits of normal size, suggesting that the retinal tissue had undergone abnormal expansion. Retinal folds were present in eyes with either a normal lens (Fig. 5E), or more commonly, eyes with lens defects (Table 3). The lens malformations included alterations in size (enlarged or reduced) or an absence of identifiable lens tissue (Table 3).
One of the ablated embryos contained a normal lens accompanied by retinal folds in the left eye (embryo #8, Table 3). The lens and optic cup in the right eye of this embryo were small and malformed and appeared to be arrested in development. Pigment was present throughout the optic cup (Fig. 5H and J, Table 3 embryo #8). Another embryo with retinal folds and no lens in the left eye lacked internally visible ocular tissue on the right (Fig. 5G, Table 3 embryo #10). The RPE had either a normal morphology or contained folds that were less pronounced than those of the neural retina (Fig. 5K and L). The embryo with the most severe phenotype had only a small, dense piece of pigmented tissue on both sides of the head (Fig. 5D, Table 3 embryo #11). The conclusion from these experiments is that ablation of MyoD+ cells in the stage 2 epiblast disrupts the morphogenesis of the lens and/or optic cup derivatives. While both eyes are affected by the loss of MyoD+ epiblast cells, the defects in the right eye are more severe than those in the left eye.
Noggin Producing MyoD+ Cells are Reduced in the Eyes of Ablated Embryos
Determination of whether the severity of eye defects correlated with the numbers of residual MyoD+ cells was carried out by comparing the number of G8+ and/or MyoD+ cells in every section through the eyes of stages 23–24 control (unablated) and ablated embryos (Table 4). All three control embryos contained fewer MyoD+ cells in the right lens than the left lens. Although the right lenses were smaller than the left lenses in two embryos (#1 and #3: number of sections containing lens tissue: right 30 and 28, left 55 and 36), both lenses were similar in size in the third embryo (#2: right 26, left 27). Two of the control embryos (#2 and #3) contained fewer MyoD+ cells in the right than the left neural retina and periocular mesenchyme. Differences in the numbers of MyoD+ cells in the right and left eyes did not correlate with the differences in the overall size of the eyes in control embryos, as one of the right eyes was larger than the left eye (embryo #2), another was smaller on the right (embryo #1), and the third pair of eyes was similar in size (embryo #2). Therefore, differences in the number of MyoD+ cells in the two eyes does not consistently correlate with the size of the tissue in which they were located.
Table 4.
Distribution of MyoD+/G8+ epiblast cells in control and ablated eyes
| Retina | Lens | POM | ||||
|---|---|---|---|---|---|---|
| left | right | left | right | left | right | |
| St. 23–24: Control | ||||||
| #1 | 48 | 52 | 45 | 6 | 20 | 20 |
| #2 | 55 | 28 | 30 | 10 | 27 | 11 |
| #3 | 87 | 49 | 54 | 11 | 39 | 33 |
| St. 23–24: Ablated | ||||||
| #2, Table 3 | 5 | 0 | 10 | 0 | 0 | 1 |
| #7, Table 3 | 5 | 0 | 0 | 0 | 1 | 0 |
| #9, Table 3 | 14 | 4 | 2 | 1 | 0 | 0 |
All sections through the eyes of three stages 23–24 control embryos and three embryos in which MyoD+ cells were ablated in the epiblast were labeled with the G8 MAb and/or dendrimers to MyoD mRNA. The number of G8 and/or MyoD mRNA positive cells was determined in the retina, lens and periocular mesenchyme (POM). In control embryos, the left lens consistently contained more MyoD+ epiblast cells than the right lens. Two out of three control embryos contained fewer cells in the right than left neural retina. The numbers assigned to the ablated embryos correspond to the numbers of the embryos described in Table 3. Ablation of MyoD+ cells in the epiblast reduced or eliminated MyoD+ cells in ocular tissues. Fewer MyoD+ epiblast cells were present in the right eyes of ablated embryos than the left eyes.
MyoD+ and G8+ cells were reduced or eliminated in ocular tissues of ablated embryos compared to control embryos (Table 4 and Table 5). One ablated embryo contained residual MyoD+/G8+ cells in the lens and retina (embryo #2, Table 4). Both of these tissues appeared to have a normal morphology (Table 3). MyoD+/G8+ cells were not found in the periocular mesenchyme of this eye, suggesting that sufficient numbers of these cells were present in the lens and retina to support their morphogenesis independently of the mesenchyme.
Table 5.
Distribution of Noggin+ cells in the eyes of ablated embryos
| Retina | Lens | POM | ||||
|---|---|---|---|---|---|---|
| Embryo | left | right | left | right | left | right |
| #7 | 3 Nog+/MyoD+ | 0 Nog+ | 0 Nog+ | 0 Nog+ | 0 Nog+ | |
| 3 Nog+/MyoD− | 1 Nog+/MyoD− | |||||
| #9 | 14 Nog+/G8+ | 4 Nog+/G8+ | 2 Nog+/G8+ | 1 Nog+/G8+ | 0 Nog+ | 0 Nog+ |
| 1 Nog+/G8− | ||||||
All sections through the stages 23–24 eyes of embryos ablated at stage 2 were double labeled for the G8 antigen or MyoD mRNA and Noggin mRNA or Noggin protein. The number of cells expressing Noggin (Nog) with or without MyoD or G8 was determined in the retina, lens and periocular mesenchyme (POM). Ablation of MyoD+ cells in the epiblast reduced or eliminated Noggin+ cells in ocular tissues. Fewer Noggin+ cells were present in the right eyes of ablated embryos than the left eyes.
Although the second embryo had the same number of residual G8+/MyoD+ cells in the retina of the left eye as the first ablated embryo, its retina had a folded appearance (embryo #7, Table 3 and Table 4). Only two G8+/MyoD+ cells remained in the lens of this eye. The left eye of the third embryo also contained retinal folds even though the folds contained more MyoD+/G8+ cells than the retina of the eye that lacked folds (embryos #9 and #2). The lens of this eye had only two MyoD+/G8+ cells but appeared to have a normal morphology. It is therefore possible that a critical number of residual MyoD+/G8+ cells in either the retina or the lens may compensate to some degree for their loss in the adjacent tissue.
The right eyes of ablated embryos contained fewer residual MyoD+/G8+ cells than the left eyes (Table 4). Whereas MyoD+/G8+ cells were present in the left eyes of all three embryos, these cells were not detected in the lens or retina of two of the right eyes of ablated (embryos #2 and #7, Table 4). One of these eyes lacked detectable lens tissue and the other had a misshapen lens (Table 3). A few MyoD+/G8+ cells were found in the right retina and lens of the third embryo (#9). This eye also had a misshapen lens. All of the right eyes exhibited retinal folding. These results demonstrate that the asymmetry in the severity of the eye defects correlates with a greater reduction in the number of residual MyoD+/G8+ cells in the right eye than the left eye.
The results displayed in Table 1 demonstrate that MyoD+/G8+ cells are the primary source of Noggin in stages 23–24 eyes. The eyes of ablated embryos were examined for Noggin expression to determine whether other cells compensated for the loss of Noggin producing, MyoD+/G8+ cells (Table 5). Six Noggin+ cells were observed in the left eye of embryo #7 and half of these cells expressed MyoD. Only one Noggin+ cell was present in the right eye of this embryo. This cell lacked detectable levels of MyoD mRNA. The second embryo contained 17 Noggin+ cells. Most of these cells were present in the retina and all but one Noggin+ cell was stained for G8. Five Noggin+/G8+ cells were detected in the right eye of this embryo. This analysis revealed that ablation of MyoD+ cells in the epiblast results in a reduction in Noggin producing cells in the eye. Comparison of the phenotype of the ablated eyes with the number of residual Noggin producing MyoD+ cells indicates that the severity of ocular malformations increases with decreasing numbers of a source for Noggin.
Exogenous Noggin Compensates for the Ablated MyoD+ Cells
Determination of whether exogenous Noggin could compensate for the missing MyoD+ cells was carried out by implanting beads soaked in Noggin into stages 10–13 ablated embryos after the lens placode and optic vesicle were specified within the ecotoderm and neuroectoderm, respectively. The most cranial implantation site was adjacent to the first somite on the right side of the embryo (Fig. 6A). This implantation site was expected to limit the amount of Noggin that diffused to the eye, thereby reducing the likelihood that eye defects would result from excessive blockage of BMP signaling, as occurs when Noggin is over-expressed in the eye (Huillard et al., 2005; Zhao et al., 2002). The beads eventually dislodged from the tissue but were secured in the vicinity of the implantation site by the embryonic membranes. As the embryo developed, the first bead was present in the cervical flexure adjacent to the eye (Fig. 6G).
Whereas the eyes of ablated embryos implanted with beads soaked in buffer continued to exhibit the same defects as ablated embryos lacking beads (Fig. 6B and C), the external morphology of both eyes from five stages 23–24 ablated embryos appeared normal after supplementation with exogenous Noggin (Table 2, Fig. 6D and F). Histological analyses revealed that the right and left eyes of three out of four ablated embryos that received Noggin soaked beads appeared normal (Table 2, Fig. 6E and G). The ability of exogenous Noggin to restore normal eye development after ablation in the majority of embryos, combined with the results of the previous experiment in which it was found that Noggin producing, MyoD+/G8+ cells were reduced or absent in ocular tissues of ablated embryos, suggests that MyoD expressing cells are a critical source of Noggin during eye development.
DISCUSSION
Cells that express MyoD mRNA and the G8 antigen emerge in the epiblast prior to the establishment of the three germ layers (Gerhart et al., 2000). We have used the G8 MAb to tag these cells in the epiblast and track their sites of integration as development progressed (Gerhart et al., 2006; Gerhart et al., 2007; present study). The strength of this approach is the specificity of the G8 MAb for cells that express MyoD (Gerhart et al., 2004a; Strony et al., 2005). However, there are limitations to using antibodies as lineage tracers, including the possibilities that unbound antibody may not be completely removed with rinsing and/or the antibody may be transferred between cells. The following evidence supports the conclusion that the majority of the G8 labeled cells that we detected in ocular tissues were derived from those cells that bound the G8 MAb in the epiblast. First, there was insufficient G8 MAb remaining in the embryo to stain epiblast cells that initiated the expression of the G8 antigen during the three-hour period following the completion of the labeling and ablation procedures (Gerhart et al., 2006 and present study). These newly emerging G8+ cells are only detected by reapplying the G8 MAb. Second, ablating cells that bound the G8 MAb in the epiblast severely reduced the number of G8+/MyoD+ cells in the eyes. Third, all cells that were labeled with the G8 MAb in the eyes of stages 23–24 embryos expressed MyoD and Noggin, suggesting that the G8 MAb was not transferred to paraxial myoblasts that do not appear to synthesize Noggin (Gerhart et al., 2006) or leukocytes that do not express MyoD. These results combined suggest that MyoD+/G8+/Noggin+ cells present in the eyes of chick embryos originate from cells that express MyoD mRNA and the G8 antigen in the epiblast. Confirmation of a lineage relationship between MyoD+ cells of the epiblast and the eyes awaits the development of a epiblast-specific MyoD reporter mouse.
Cells that express MyoD mRNA and the G8 antigen in the epiblast and in a variety of tissues in older embryos are not induced to differentiate into non-skeletal muscle cell types (Gerhart et al., 2001; Gerhart et al., 2007). Stable programming of MyoD+ cells in the epiblast may ensure that they are capable of releasing Noggin to titrate the activities of BMPs in diverse environments. MyoD mRNA is not translated into detectable levels of protein in epiblast cells (Gerhart et al., 2006; Gerhart et al., 2007), and unlike embryos in which MyoD+ cells are ablated in the epiblast, development proceeds normally in the MyoD knockout mouse (Rudnicki et al., 1992). Therefore, expression of MyoD mRNA appears to be a read out for, but not a regulator of MyoD+ epiblast cell function as a Noggin delivery system.
The importance of MyoD+ cells as a source of Noggin in the developing eye is exemplified by: (1) their expression of this BMP inhibitor in different ocular tissues, (2) the reduction in Noggin producing MyoD+/G8+ cells in the eyes following ablation in the epiblast, (3) the disruption of eye morphogenesis in ablated embryos, and (4) the ability of exogenous Noggin to fully or partially compensate for the missing MyoD+ cells. Noggin producing, MyoD+ cells appear to affect eye development after the initial specification of ocular tissues since the Noggin soaked beads were implanted into embryos containing lens placodes and optic vesicles. The Noggin bead experiment also raises the possibility that spatial restriction of Noggin’s release within the eye field is not critical for early stages of morphogenesis. However, the response of cells to Noggin may depend on where they lie within a gradient of BMPs and/or whether subpopulations of cells within the eye are rendered more sensitive to the effects of Noggin by expressing cell surface Noggin receptors (Ashe and Briscoe, 2006; Paine-Saunders et al., 2002; Simeoni and Gurdon, 2007).
The malformations that arise as a result of ablating MyoD+ epiblast cells provide clues as to the functions of these cells in different ocular tissues. Some ablated embryos exhibited small lenses. Since lens fiber cell differentiation is promoted by BMPs (Beebe et al., 2004; Belecky-Adams et al., 2002; de Longh et al., 2001; Faber et al., 2002), release of Noggin from MyoD+ cells may be important for modulating the initiation of differentiation in the equatorial zone. Noggin also may affect the maturation of lens fiber cells, as suggested by the appearance of MyoD+ cells in the posterior lens. The folding of the retina that occurs when MyoD+ cells are ablated in the epiblast suggests that the release of Noggin from these cells modulates the effects of BMPs on proliferation in the retina (Trousse et al., 2001). In the embryo containing a small and distorted optic cup, pigmentation was present throughout the tissue. This phenotype may reflect inappropriate BMP signaling since inhibition of BMPs is required for specifying the unpigmented neural retina (Muller et al., 2007).
BMP signaling also is required for the development of the ciliary body and iris but must be repressed for the differentiation of the extraocular muscles (Tzahor et al., 2003; von Scheven et al., 2006; Zhao et al., 2002). These tissues develop after day 5 when most embryos die as a result of eliminating MyoD+ cells in the epiblast. Therefore, examination of the roles of MyoD+ cells in the development of the ciliary body, iris and extraocular muscles will require ablation of MyoD+ cells within specific ocular tissues that is expected to extend the viability of the embryo. Targeted ablation of MyoD+ cells in specific ocular tissues also will enable us to distinguish their autonomous effects within the lens, optic cup and periocular mesenchyme from inductive interactions that occur between these tissues.
A common feature of the ablation experiments is that malformations are more pronounced on the right than the left side of the embryo, including the eyes (Gerhart et al., 2006; present study). One explanation for this phenomenon is that MyoD+ cells arising on the left side of the epiblast after the ablation procedure may be preferentially integrated into the left eye and release Noggin to afford partial protection from excessive BMP signaling. This hypothesis is supported by the fact that more residual MyoD+ epiblast cells are present in the left than the right eye after ablation at stage 2. However, the right eye may be more vulnerable to a reduction in MyoD+ cells than the left eye because there are fewer of these cells in the right lenses, and in some of the retinas, in normal, unablated embryos. Differences in the numbers of MyoD+ cells in the right and left eyes of normal embryos also may reflect asymmetric integration of MyoD+ cells that arise at different times within the epiblast or their different rates of proliferation.
The malformations that occur when MyoD+ cells are ablated in the epiblast (eye, facial and body wall defects) partially overlap with anomalies present in newborns with Axenfeld-Rieger (AR) syndrome (Gerhart et al., 2006; Hjalt and Semina, 2005; Lines et al., 2002; Lu et al., 1999; Shields et al., 1985). While mutations in the transcription factors PITX2, FOXC1, MAF and PAX6 are associated with AR and related syndromes in humans and mice, the causes of these disorders in many patients are unknown (Diehl et al., 2006; Gage et al., 1999; Gould et al., 2004; Graw, 2003; Hjalt and Semina, 2005; Kitamura et al., 1999; Lines et al., 2002). Abnormal behavior of neural crest cells results in the development of AR syndrome in mice (Evans and Gage, 2005; Gage et al., 2005; Kupfer and Kaiser-Kupfer, 1978). Both the neural crest and MyoD+ cells produce BMP inhibitors in the developing eye (Tzahor et al., 2003; present study), and the small number of Noggin+/MyoD− cells that we detect in ocular tissues may be derived from the neural crest. While neither MyoD+ cells nor neural crest cells can completely compensate for the loss of the other to prevent eye defects, the release of BMP inhibitors from the two cell types may overlap to regulate eye development and the expression of genes affected in ocular dysgenesis syndromes. In this regard, BMPs indirectly inhibit Pitx2 expression during the establishment of asymmetry in the body axis (Patel et al., 1999; Piedra and Ros, 2002; Schlange et al., 2001).
Our analyses of the developing embryo suggest that MyoD+ epiblast cells constitute a unique and stable lineage which functions to deliver Noggin to skeletal muscle and non-skeletal muscle forming tissues (Gerhart et al., 2006; Gerhart et al., 2007; present study). MyoD mRNA or protein has been detected in multiple fetal and adult organs lacking skeletal muscle (Chen et al., 2005; Gerhart et al., 2001; Grounds et al., 1992). In addition to playing crucial roles during skeletal myogenesis and eye development, cells that express MyoD mRNA and Noggin are likely to regulate the behavior of neighboring stem and progenitor cells in other tissues of the embryo and in the adult.
ACKNOWLEDGEMENTS
The authors thank Drs. Michelle Leonard and Janice Walker for critically reading the manuscript and Genisphere, Inc. for generously supplying dendrimers. This study was funded by the National Institutes of Health (grants HD43157 to MG-W, AR052326 to MG-W and KK) and the March of Dimes Foundation (MG-W and ASM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Asakura A, Lyons GE, Tapscott SJ. The regulation of MyoD gene expression: conserved elements mediate expression in embryonic axial muscle. Dev Biol. 1995;171:386–398. doi: 10.1006/dbio.1995.1290. [DOI] [PubMed] [Google Scholar]
- Ashe HL, Briscoe J. The interpretation of morphogen gradients. Development. 2006;133:385–394. doi: 10.1242/dev.02238. [DOI] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Beebe D, Garcia C, Wang X, Rajagopal R, Feldmeier M, Kim JY, Chytil A, Moses H, Ashery-Padan R, Rauchman M. Contributions by members of the TGFbeta superfamily to lens development. Int J Dev Biol. 2004;48:845–856. doi: 10.1387/ijdb.041869db. [DOI] [PubMed] [Google Scholar]
- Beebe DC. Development of the ciliary body: a brief review. Trans Ophthalmol Soc U K. 1986;105(Pt 2):123–130. [PubMed] [Google Scholar]
- Belecky-Adams T, Adler R. Developmental expression patterns of bone morphogenetic proteins, receptors, and binding proteins in the chick retina. J Comp Neurol. 2001;430:562–572. [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–3802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Bellairs R. The primitive streak. Anat Embryol (Berl) 1986;174:1–14. doi: 10.1007/BF00318331. [DOI] [PubMed] [Google Scholar]
- Chang B, Smith RS, Peters M, Savinova OV, Hawes NL, Zabaleta A, Nusinowitz S, Martin JE, Davisson ML, Cepko CL, Hogan BL, John SW. Haploinsufficient Bmp4 ocular phenotypes include anterior segment dysgenesis with elevated intraocular pressure. BMC Genet. 2001;2:18. doi: 10.1186/1471-2156-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Mortimer J, Marley J, Goldhamer DJ. MyoD-cre transgenic mice: a model for conditional mutagenesis and lineage tracing of skeletal muscle. Genesis. 2005;41:116–121. doi: 10.1002/gene.20104. [DOI] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development. 1992;114:1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26:374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Longh RU, Lovicu FJ, Overbeek PA, Schneider MD, Joya J, Hardeman ED, McAvoy JW. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development. 2001;128:3995–4010. doi: 10.1242/dev.128.20.3995. [DOI] [PubMed] [Google Scholar]
- Dechesne CA, Wei Q, Eldridge J, Gannoun-Zaki L, Millasseau P, Bougueleret L, Caterina D, Paterson BM. E-box- and MEF-2-independent muscle-specific expression, positive autoregulation, and cross-activation of the chicken MyoD (CMD1) promoter reveal an indirect regulatory pathway. Mol Cell Biol. 1994;14:5474–5486. doi: 10.1128/mcb.14.8.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl AG, Zareparsi S, Qian M, Khanna R, Angeles R, Gage PJ. Extraocular muscle morphogenesis and gene expression are regulated by Pitx2 gene dose. Invest Ophthalmol Vis Sci. 2006;47:1785–1793. doi: 10.1167/iovs.05-1424. [DOI] [PubMed] [Google Scholar]
- Donner AL, Lachke SA, Maas RL. Lens induction in vertebrates: variations on a conserved theme of signaling events. Semin Cell Dev Biol. 2006;17:676–685. doi: 10.1016/j.semcdb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Evans AL, Gage PJ. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet. 2005;14:3347–3359. doi: 10.1093/hmg/ddi365. [DOI] [PubMed] [Google Scholar]
- Faber SC, Dimanlig P, Makarenkova HP, Shirke S, Ko K, Lang RA. Fgf receptor signaling plays a role in lens induction. Development. 2001;128:4425–4438. doi: 10.1242/dev.128.22.4425. [DOI] [PubMed] [Google Scholar]
- Faber SC, Robinson ML, Makarenkova HP, Lang RA. Bmp signaling is required for development of primary lens fiber cells. Development. 2002;129:3727–3737. doi: 10.1242/dev.129.15.3727. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46:4200–4208. doi: 10.1167/iovs.05-0691. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- George-Weinstein M, Gerhart J, Reed R, Flynn J, Callihan B, Mattiacci M, Miehle C, Foti G, Lash JW, Weintraub H. Skeletal myogenesis: the preferred pathway of chick embryo epiblast cells in vitro. Dev Biol. 1996;173:279–291. doi: 10.1006/dbio.1996.0023. [DOI] [PubMed] [Google Scholar]
- Gerhart J, Bast B, Neely C, Iem S, Amegbe P, Niewenhuis R, Miklasz S, Cheng PF, George-Weinstein M. MyoD-positive myoblasts are present in mature fetal organs lacking skeletal muscle. J Cell Biol. 2001;155:381–392. doi: 10.1083/jcb.200105139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Baytion M, DeLuca S, Getts R, Lopez C, Niewenhuis R, Nilsen T, Olex S, Weintraub H, George-Weinstein M. DNA dendrimers localize MyoD mRNA in presomitic tissues of the chick embryo. J Cell Biol. 2000;149:825–834. doi: 10.1083/jcb.149.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Baytion M, Perlman J, Neely C, Hearon B, Nilsen T, Getts R, Kadushin J, George-Weinstein M. Visualizing the Needle in the Haystack: In Situ Hybridization With Fluorescent Dendrimers. Biol Proced Online. 2004b;6:149–156. doi: 10.1251/bpo84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Elder J, Neely C, Schure J, Kvist T, Knudsen K, George-Weinstein M. MyoD-positive epiblast cells regulate skeletal muscle differentiation in the embryo. J Cell Biol. 2006;175:283–292. doi: 10.1083/jcb.200605037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Neely C, Elder J, Pfautz J, Perlman J, Narciso L, Linask KK, Knudsen K, George-Weinstein M. Cells that express MyoD mRNA in the epiblast are stably committed to the skeletal muscle lineage. J Cell Biol. 2007;178:649–660. doi: 10.1083/jcb.200703060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Neely C, Pfautz J, George-Weinstein M. Tracking and ablating subpopulations of epiblast cells in the chick embryo. Biol. Proced. Online. 2008;10:74–82. doi: 10.1251/bpo145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Neely C, Stewart B, Perlman J, Beckmann D, Wallon M, Knudsen K, George-Weinstein M. Epiblast cells that express MyoD recruit pluripotent cells to the skeletal muscle lineage. J Cell Biol. 2004a;164:739–746. doi: 10.1083/jcb.200309152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S. Developmental Biology. Sunderland, M.A.: Sinauer Associates, Inc.; 2006. [Google Scholar]
- Gould DB, Smith RS, John SW. Anterior segment development relevant to glaucoma. Int J Dev Biol. 2004;48:1015–1029. doi: 10.1387/ijdb.041865dg. [DOI] [PubMed] [Google Scholar]
- Graw J. The genetic and molecular basis of congenital eye defects. Nat Rev Genet. 2003;4:876–888. doi: 10.1038/nrg1202. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Garrett KL, Beilharz MW. The transcription of MyoD1 and myogenin genes in thymic cells in vivo. Exp Cell Res. 1992;198:357–361. doi: 10.1016/0014-4827(92)90391-k. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hjalt TA, Semina EV. Current molecular understanding of Axenfeld-Rieger syndrome. Expert Rev Mol Med. 2005;7:1–17. doi: 10.1017/S1462399405010082. [DOI] [PubMed] [Google Scholar]
- Huillard E, Laugier D, Marx M. Defects in chicken neuroretina misexpressing the BMP antagonist Drm/Gremlin. Dev Biol. 2005;283:335–344. doi: 10.1016/j.ydbio.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Hung FC, Zhao S, Chen Q, Overbeek PA. Retinal ablation and altered lens differentiation induced by ocular overexpression of BMP7. Vision Res. 2002;42:427–438. doi: 10.1016/s0042-6989(01)00242-5. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Wurdak H, Schwerdtfeger K, Kunz T, Ille F, Leveen P, Hjalt TA, Suter U, Karlsson S, Hafezi F, Born W, Sommer L. Compound developmental eye disorders following inactivation of TGFbeta signaling in neural-crest stem cells. J Biol. 2005;4:11. doi: 10.1186/jbiol29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob M, Jacob HJ, Wachtler F, Christ B. Ontogeny of avian extrinsic ocular muscles. I. A light- and electron-microscopic study. Cell Tissue Res. 1984;237:549–557. doi: 10.1007/BF00228439. [DOI] [PubMed] [Google Scholar]
- Jena N, Martin-Seisdedos C, McCue P, Croce CM. BMP7 null mutation in mice: developmental defects in skeleton, kidney, and eye. Exp Cell Res. 1997;230:28–37. doi: 10.1006/excr.1996.3411. [DOI] [PubMed] [Google Scholar]
- Johnston MC, Noden DM, Hazelton RD, Coulombre JL, Coulombre AJ. Origins of avian ocular and periocular tissues. Exp Eye Res. 1979;29:27–43. doi: 10.1016/0014-4835(79)90164-7. [DOI] [PubMed] [Google Scholar]
- Kablar B. MyoD-lacZ transgenes are early markers in the neural retina,but MyoD function appears to be inhibited in the developing retinal cells. Int J Dev Neurosci. 2004;22:215–224. doi: 10.1016/j.ijdevneu.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Kablar B, Rudnicki MA. Information provided by the skeletal muscle and associated neurons is necessary for proper brain development. Int J Dev Neurosci. 2002;20:573–584. doi: 10.1016/s0736-5748(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Kirillova I, Gussoni E, Goldhamer DJ, Yablonka-Reuveni Z. Myogenic reprogramming of retina-derived cells following their spontaneous fusion with myotubes. Dev Biol. 2007;311:449–463. doi: 10.1016/j.ydbio.2007.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suehiro A, Motegi Y, Nakahara Y, Kondo S, Yokoyama M. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development. 1999;126:5749–5758. doi: 10.1242/dev.126.24.5749. [DOI] [PubMed] [Google Scholar]
- Kupfer C, Kaiser-Kupfer MI. New hypothesis of developmental anomalies of the anterior chamber associated with glaucoma. Trans Ophthalmol Soc U K. 1978;98:213–215. [PubMed] [Google Scholar]
- Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- Lines MA, Kozlowski K, Walter MA. Molecular genetics of Axenfeld-Rieger malformations. Hum Mol Genet. 2002;11:1177–1184. doi: 10.1093/hmg/11.10.1177. [DOI] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–4062. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- Mayer DC, Leinwand LA. Sarcomeric gene expression and contractility in myofibroblasts. J Cell Biol. 1997;139:1477–1484. doi: 10.1083/jcb.139.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F, Rohrer H, Vogel-Hopker A. Bone morphogenetic proteins specify the retinal pigment epithelium in the chick embryo. Development. 2007;134:3483–3493. doi: 10.1242/dev.02884. [DOI] [PubMed] [Google Scholar]
- Noden DM. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat. 1983;168:257–276. doi: 10.1002/aja.1001680302. [DOI] [PubMed] [Google Scholar]
- Ogita J, Isogai E, Sudo H, Sakiyama S, Nakagawara A, Koseki H. Expression of the Dan gene during chicken embryonic development. Mech Dev. 2001;109:363–365. doi: 10.1016/s0925-4773(01)00522-6. [DOI] [PubMed] [Google Scholar]
- Olsson L, Falck P, Lopez K, Cobb J, Hanken J. Cranial neural crest cells contribute to connective tissue in cranial muscles in the anuran amphibian, Bombina orientalis. Dev Biol. 2001;237:354–367. doi: 10.1006/dbio.2001.0377. [DOI] [PubMed] [Google Scholar]
- Paine-Saunders S, Viviano BL, Economides AN, Saunders S. Heparan sulfate proteoglycans retain Noggin at the cell surface: a potential mechanism for shaping bone morphogenetic protein gradients. J Biol Chem. 2002;277:2089–2096. doi: 10.1074/jbc.M109151200. [DOI] [PubMed] [Google Scholar]
- Patel K, Isaac A, Cooke J. Nodal signalling and the roles of the transcription factors SnR and Pitx2 in vertebrate left-right asymmetry. Curr Biol. 1999;9:609–612. doi: 10.1016/s0960-9822(99)80267-x. [DOI] [PubMed] [Google Scholar]
- Piedra ME, Ros MA. BMP signaling positively regulates Nodal expression during left right specification in the chick embryo. Development. 2002;129:3431–3440. doi: 10.1242/dev.129.14.3431. [DOI] [PubMed] [Google Scholar]
- Redfield A, Nieman MT, Knudsen KA. Cadherins promote skeletal muscle differentiation in three-dimensional cultures. J Cell Biol. 1997;138:1323–1331. doi: 10.1083/jcb.138.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaudo JA, Zelenka PS. Expression of c-fos and c-jun mRNA in the developing chicken lens: relationship to cell proliferation, quiescence, and differentiation. Exp Cell Res. 1992;199:147–153. doi: 10.1016/0014-4827(92)90472-k. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Braun T, Hinuma S, Jaenisch R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992;71:383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- Saika S, Liu CY, Azhar M, Sanford LP, Doetschman T, Gendron RL, Kao CW, Kao WW. TGFbeta2 in corneal morphogenesis during mouse embryonic development. Dev Biol. 2001;240:419–432. doi: 10.1006/dbio.2001.0480. [DOI] [PubMed] [Google Scholar]
- Sakuta H, Suzuki R, Takahashi H, Kato A, Shintani T, Iemura S, Yamamoto TS, Ueno N, Noda M. Ventroptin: a BMP-4 antagonist expressed in a double-gradient pattern in the retina. Science. 2001;293:111–115. doi: 10.1126/science.1058379. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlange T, Schnipkoweit I, Andree B, Ebert A, Zile MH, Arnold HH, Brand T. Chick CFC controls Lefty1 expression in the embryonic midline and nodal expression in the lateral plate. Dev Biol. 2001;234:376–389. doi: 10.1006/dbio.2001.0257. [DOI] [PubMed] [Google Scholar]
- Shields MB, Buckley E, Klintworth GK, Thresher R. Axenfeld-Rieger syndrome. A spectrum of developmental disorders. Surv Ophthalmol. 1985;29:387–409. doi: 10.1016/0039-6257(85)90205-x. [DOI] [PubMed] [Google Scholar]
- Simeoni I, Gurdon JB. Interpretation of BMP signaling in early Xenopus development. Dev Biol. 2007;308:82–92. doi: 10.1016/j.ydbio.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Strony R, Gerhart J, Tornambe D, Perlman J, Neely C, Dare J, Stewart B, George-Weinstein M. NeuroM and MyoD are expressed in separate subpopulations of cells in the pregastrulating epiblast. Gene Expr Patterns. 2005;5:387–395. doi: 10.1016/j.modgep.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Tonegawa A, Takahashi Y. Somitogenesis controlled by Noggin. Dev Biol. 1998;202:172–182. doi: 10.1006/dbio.1998.8895. [DOI] [PubMed] [Google Scholar]
- Trousse F, Esteve P, Bovolenta P. Bmp4 mediates apoptotic cell death in the developing chick eye. J Neurosci. 2001;21:1292–1301. doi: 10.1523/JNEUROSCI.21-04-01292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E, Kempf H, Mootoosamy RC, Poon AC, Abzhanov A, Tabin CJ, Dietrich S, Lassar AB. Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev. 2003;17:3087–3099. doi: 10.1101/gad.1154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Scheven G, Alvares LE, Mootoosamy RC, Dietrich S. Neural tube derived signals and Fgf8 act antagonistically to specify eye versus mandibular arch muscles. Development. 2006;133:2731–2745. doi: 10.1242/dev.02426. [DOI] [PubMed] [Google Scholar]
- Walker GA, Guerrero IA, Leinwand LA. Myofibroblasts: molecular crossdressers. Curr Top Dev Biol. 2001;51:91–107. doi: 10.1016/s0070-2153(01)51003-0. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Dev Biol. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Clark AF. Bone morphogenetic proteins and their receptors in the eye. Exp Biol Med (Maywood) 2007;232:979–992. doi: 10.3181/0510-MR-345. [DOI] [PubMed] [Google Scholar]
- Zhao S, Chen Q, Hung FC, Overbeek PA. BMP signaling is required for development of the ciliary body. Development. 2002;129:4435–4442. doi: 10.1242/dev.129.19.4435. [DOI] [PubMed] [Google Scholar]
- Zilinski C, Brownell I, Hashimoto R, Medina-Martinez O, Swindell EC, Jamrich M. Expression of FoxE4 and Rx visualizes the timing and dynamics of critical processes taking place during initial stages of vertebrate eye development. Dev Neurosci. 2004;26:294–307. doi: 10.1159/000082271. [DOI] [PubMed] [Google Scholar]