Abstract
The medial division of the central nucleus of the amygdala (CeAM) and the lateral division of the bed nucleus of the stria terminalis (BNSTL) are closely related. Both receive projections from the basolateral amygdala (BLA) and both project to brain areas that mediate fear-influenced behaviors. In contrast to CeAM however, initial attempts to implicate the BNST in conditioned fear responses were largely unsuccessful. More recent studies have shown that the BNST does participate in some types of anxiety and stress responses. Here, we review evidence suggesting that the CeAM and BNSTL are functionally complementary, with CeAM mediating short- but not long-duration threat responses (i.e., phasic fear) and BNSTL mediating long- but not short-duration responses (sustained fear or ‘anxiety’). We also review findings implicating the stress-related peptide corticotropin-releasing factor (CRF) in sustained but not phasic threat responses, and attempt to integrate these findings into a neural circuit model which accounts for these and related observations.
Keywords: anxiety, startle, amygdala, bed nucleus of the stria terminalis, corticotropin releasing factor
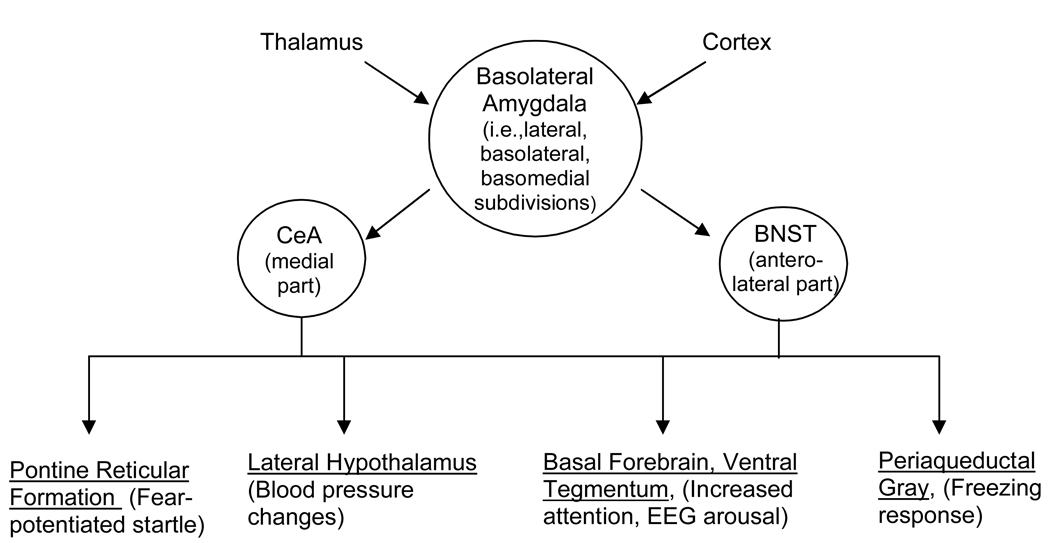
A now-familiar and widely-adopted neural circuit model of fear places the amygdala in center stage and assigns different functions to different amygdala subdivisions (Fig. 1). Thus, the basolateral group (BLA) screens incoming sensory information for threat cues and, if such cues are detected, passes this information on to the central nucleus of the amygdala’s medial subdivision (CeAM), which mediates threat responses by way of its many projections to areas that mediate specific fear-influenced behaviors (c.f., Davis, 1992; Fanselow and LeDoux, 1999; Maren, 2001; Pare et al., 2004).
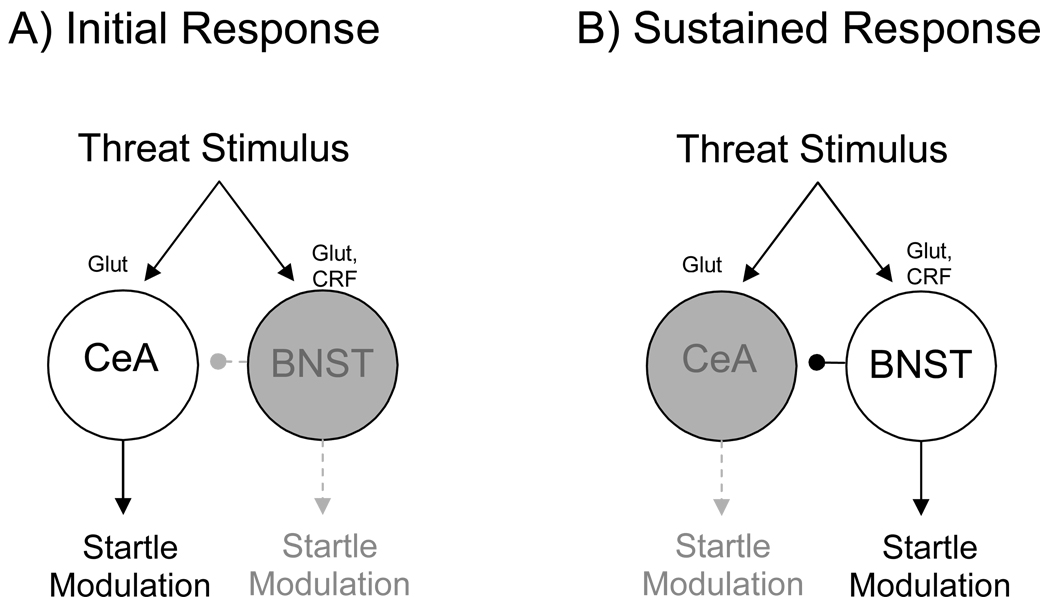
Figure 1.
The basolateral complex of the amygdala receives sensory input from afferent areas including various thalamic nuclei and cortical fields. If this information is processed as a threat signal (e.g., as a result of conditioning), it is passed on to the medial division of the central nucleus of the amygdala, and also to the anterolateral BNST – areas that project, in turn, to a common set of target areas (some shown here) that mediate fear-associated behaviors. Arrows indicate functional connections, and do not imply mono-synaptic projections.
In the following pages, we review evidence suggesting that this model is incomplete, and should be expanded to include the bed nucleus of the stria terminalis (specifically, it’s lateral subdivision – BNSTL). In fact, by many criteria, the CeA and BNST are closely related (Alheid et al., 1995; Alheid and Heimer, 1988; Johnston, 1923). Of particular interest, both receive projections from the BLA (c.f., Dong et al., 2001a; Pitkanen, 2000) and project in turn to many of the same brain areas that mediate fear-associated behaviors (e.g., Gray and Magnuson, 1987, 1992; Holstege et al., 1985; Hopkins and Holstege, 1978; Peyron et al., 1998; Rosen et al., 1991; Schmued, 1994; Schwaber et al., 1982). Although these similarities have been appreciated for some time, early lesion studies found no evidence for an involvement of the BNST in several fear-associated behaviors including freezing and blood pressure changes to 10- or 120-sec presentations of an auditory CS (LeDoux et al., 1988), or fear-potentiated startle to 3.7-sec presentations of a visual CS (Hitchcock and Davis, 1991), even though other studies have shown that these same responses are disrupted by CeA lesions or inactivation (e.g., Campeau and Davis, 1995; Goosens and Maren, 2001; Hitchcock and Davis, 1991; Iwata et al., 1986; Walker and Davis, 1997b). Thus, for many years then, CeAM but not BNSTL outputs were thought to mediate conditioned fear responses. We now believe that those earlier findings have been interpreted too broadly, and that the CeA and BNST serve similar but complementary functions. In the following pages, we present evidence that CeAM mediates short- but not long-duration threat responses (referred to elsewhere as phasic versus sustained responses), and the BNSTL long- but not short-duration threat responses. We suggest further that brain corticotropin releasing factor (CRF) plays a selective role in the latter (i.e., BNSTL-dependent response). Our evolution towards this position began with two sets of findings that are discussed in turn below.
CRF-Enhanced versus Fear-Potentiated Startle
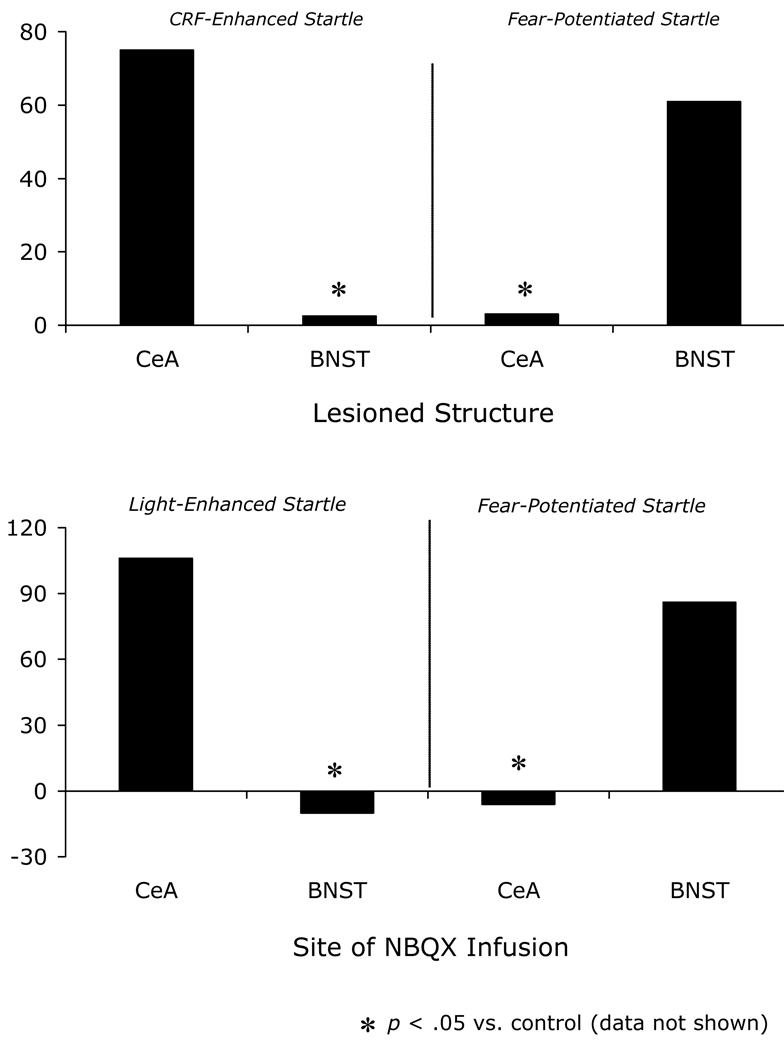
In 1986, Swerdlow et al. (1986) reported that CRF infusions into the lateral cerebral ventricle markedly increased startle amplitude in rats, and that these increases were prevented by pretreatment with the anxiolytic compound chlordiazepoxide. This “CRF-enhanced startle” effect was later replicated in our own laboratory by Liang et al. (1992b) who reported large dose-dependent startle increases that began approximately 30 minutes after the infusion had ended and which grew steadily over the course of a 2-hour test period. Lee and Davis (1997) subsequently identified the BNST as the likely location of the CRF receptors that mediated this effect. They showed, in particular, that excitotoxic lesions of the BNST but not CeA blocked CRF-enhanced startle (Fig. 2A), that infusion of the CRF receptor antagonist α-helical CRF9–41 (αhCRF) into the BNST, but not into the CeA, also blocked CRF-enhanced startle, and that intra-BNST CRF infusions mimicked the effect of intra-ventricular infusions. In fact, CRF receptor protein and mRNA are abundant in the BNST – much more so than in CeA (Ju et al., 1989; Potter et al., 1994; Van Pett et al., 2000; Wynn et al., 1984) – and consistent with this distribution, Liang et al. (1992a) had previously shown that intra-amygdala CRF infusions, in contrast to intra-BNST CRF infusions, do not increase startle. Lee and Davis (1997) also confirmed, in the same lesioned rats that were tested for CRF-enhanced startle, that CeA but not BNST lesions disrupted fear-potentiated startle. Overall then, these results demonstrated a double dissociation between the involvement of the BNST and CeA in CRF-enhanced versus fear-potentiated startle (Fig. 2A). These behavioral results, along with others that will be presented in the following pages, are summarized in Table 1.
Figure 2.
Panel A – Excitotoxic lesions of the BNST block CRF-enhanced startle but not fear-potentiated startle to a 3.7-sec shock-paired CS, whereas excitotoxic lesions of the CeA block fear-potentiated startle to the 3.7-sec CS, but do not affect CRF-enhanced startle (data from Lee and Davis, 1997). Panel B – Infusions of the AMPA receptor antagonist NBQX into the BNST block light-enhanced startle but not fear-potentiated startle to a 3.7-sec CS, whereas NBQX infusions into the CeA block fear-potentiated but not light-enhanced startle (data from Walker and Davis, 1997b). Potentiation scores are expressed as differences from control (i.e., either sham-lesioned or vehicle-infused).
Table 1.
This table summarizes the results of those studies that have directly compared (i.e., within the same study) the effect of (A) CRF receptor antagonists, (B) BNST inactivation, or (C) CeA inactivation on phasic versus sustained duration fear responses. Direct comparisons between the effects of different manipulations on the same response – either (D) phasic or (E) sustained are also shown. In some (shaded cells) but not all cases, these alternate comparisons are made using data which appear earlier in the table.
The comparisons consistently show that CRF receptor antagonists and BNST manipulations disrupt sustained but not phasic fear, whereas CeA manipulations often have the opposite effect. Interpretation of the effect of CeA manipulations is complicated by several factors. First, electrolytic CeA lesions would most likely transect BLA-to-BNST projections such that disruptive effects on sustained fear would be predicted based on the hypothesis outlined in this chapter. Second, sustained startle increases produced by CRF administration are thought to act directly on BNST neurons (with no involvement of the CeA) whereas sustained fear responses produced by environmental stimuli may involve release into the BNST of CRF from CeAL terminals. Thus, even though CRF- and sustained threat-induced startle increases are both of long duration, a differential involvement of the CeA is predicted due to the different origin of CRF in these paradigms. Third, studies showing that AMPA receptor antagonist infusions into the CeA do not disrupt sustained fear should be interpreted with caution insofar as sustained threat signals may activate relevant CeA neurons (i.e., those in the lateral subdivision) through non-AMPA (e.g., peptide) receptors. Even with these caveats however, the data are fairly consistent in suggesting a special role for the CRF and the BNST in sustained fear, and a negligible or at least different role for the CeA in phasic fear.
| A. Effect of CRF receptor antagonists on Short- vs. Long-Duration Fear Response | ||
|---|---|---|
| Study and Treatment | Effect on Phasic Fear | Effect on Sustained Fear |
| Walker et al. (2008) | FPS to 3.7-sec visual CS not disrupted | Light-Enhanced Startle (20 min exposure |
| to bright light) non-monotonically | ||
| Oral administration of the | blocked | |
| CRF-R1 antagonist | ||
| GSK876008 | Startle increases evoked by sustained | |
| exposure to shock-paired context non- | ||
| monotonically blocked | ||
| De Jongh et al. (2006) | FPS to 3.7-sec visual CS not disrupted | Light-Enhanced Startle (20 min exposure |
| to bright light) non-monotonically | ||
| i.c.v. infusion of the non | disrupted | |
| selective CRF antagonist | ||
| α-helical CRF | ||
| Walker, Miles, and Davis | FPS to 3.7-sec clicker CS not | FPS to 8-min clicker CS blocked |
| (unpublished) | disrupted | |
| FPS to 8-min filtered noise CS blocked | ||
| Oral administration of the | FPS to 3.7-sec filtered noise CS not | |
| CRF-R1 antagonist | disrupted | |
| GSK876008 | ||
| B. Effect of BNST Disruption on Short- vs. Long-Duration Fear Response | ||
|---|---|---|
| Study and Treatment | Effect on Phasic Fear | Effect on Sustained Fear |
| Gewirtz et al. (1998) | FPS to 3.7-sec visual CS not disrupted | Long-lasting sensitization of startle by |
| repeated footshocks across multiple days | ||
| Electrolytic lesion | blocked | |
| Lee and Davis (1997) | FPS to 3.7-sec visual CS not disrupted | CRF-enhanced startle blocked |
| Excitotoxic lesion | ||
| Waddell et al. (2006) | Conditioned Suppression to 60-sec CS | Conditioned Suppression to 10-min CS |
| not disrupted | blocked | |
| Excitotoxic lesion | ||
| Sullivan et al. (2004) | Freezing to 20-sec CS not blocked | Freezing to context CS blocked |
| Corticosterone response to 20-sec CS | Corticosterone response to context CS | |
| Electrolytic lesions | not disrupted | blocked |
| Walker and Davis (1997b) | Light-enhanced startle (20 min | Light-enhanced startle (20 min stimulus |
| stimulus duration) not disrupted | duration) disrupted (AMPAr antagonist | |
| AMPA receptor antagonist | (AMPAr antagonist infusion) | infusion) |
| infusion | ||
| C. Effect of CeA Disruption on Short- vs. Long-Duration Fear Response | ||
|---|---|---|
| Study and Treatment | Effect on Phasic Fear | Effect on Sustained Fear |
| Lee and Davis (1997) | FPS to 3.7-sec visual CS blocked | CRF-enhanced startle not disrupted |
| (excitotoxic lesion) | (excitotoxic lesion) | |
| Excitotoxic lesion | ||
| Sullivan et al. (2004) | Freezing to 20-sec CS blocked |
Freezing to context CS blocked |
| Electrolytic lesions | Corticosterone response to 20-sec CS | Corticosterone response to context CS |
| blocked | blocked | |
| Walker and Davis (1997b) | FPS to 3.7-sec visual CS blocked | Light-enhanced startle (20 min stimulus |
| duration) not disrupted | ||
| AMPA receptor antagonist | ||
| infusions | ||
| D. Effect on Phasic Fear Responses of CeA vs BNST Manipulations | ||
|---|---|---|
| Study and Treatment | Study and Treatment | Effect of BNST Manipulations |
| Hitchcock and Davis (1991) | FPS to 3.7-sec visual CS blocked | FPS to 3.7-sec visual CS not disrupted |
| Electrolytic (For CeA, | ||
| unilateral combined with | ||
| contralateral transection of | ||
| ventro-amygdalafugal | ||
| pathway; for BNST, | ||
| bilateral) | ||
| Lee and Davis (1997) | FPS to 3.7-sec visual CS blocked | FPS to 3.7-sec visual CS not disrupted |
| Excitotoxic lesion | ||
| Sullivan et al. (2004) | Freezing to 20-sec tone CS blocked | Freezing to 20-sec CS not disrupted |
| Electrolytic lesions | Corticosterone response to context CS | Corticosterone response to 20-sec CS |
| blocked | not disrupted | |
| Walker and Davis (1997b) | FPS to 3.7-sec visual CS blocked | FPS to 3.7-sec visual CS blocked |
| AMPA receptor antagonist | ||
| infusions | ||
| E. Effect on Sustained Fear Responses of CeA vs BNST Manipulations | ||
|---|---|---|
| Study and Treatment | Effect of CeA Manipulations | Effect of CeA Manipulations |
| Lee and Davis (1997) | CRF-enhanced startle not disrupted | CRF-enhanced startle disrupted |
| Excitotoxic lesion | ||
| Sullivan et al. (2004) | Freezing to context CS blocked | Freezing to context CS blocked |
| Electrolytic lesions | Corticosterone response to context CS | Corticosterone response to context CS |
| blocked | blocked | |
| Walker and Davis (1997b) | Light-enhanced startle (20 min | Light-enhanced startle (20 min stimulus |
| stimulus duration) not disrupted | duration) blocked | |
| AMPA receptor antagonist | ||
| infusions | ||
| Walker and Davis | FPS to 8-min filtered-noise CS not | FPS to 8-min filtered-noise enhanced |
| (unpublished observations) | disrupted | during 1st half of CS and blocked |
| during 2nd half minutes | ||
| AMPA receptor antagonist | ||
| infusions | ||
The involvement of BNST CRF receptors in anxiety-associated behaviors is not limited to startle increases, but appears instead to reflect a more general involvement in anxiety itself. For example, intra-BNST CRF infusions have also been found to elicit anxiety-associated behaviors in the elevated plus-maze and social interaction tests (Lee et al., 2008; Sahuque et al., 2006; and see Sajdyk et al., 2008 for similar effects produced by chronic inhibition of GABA synthesis in the BNST), to produce conditioned place aversions to places associated with CRF infusion (Sahuque et al., 2006), to elicit cardiovascular responses that are associated with fear and anxiety (Nijsen et al., 2001), and to have anorectic effects which, in the same study, were not similarly observed with intra-CeA CRF infusions (Ciccocioppo et al., 2003).
Light-Enhanced versus Fear-Potentiated Startle
During the same time that the BNST’s involvement in CRF-enhanced startle was being characterized, we were also evaluating the effect on startle of testing rats in illuminated versus darkened test cages. We found that rats tested in illuminated test cages show higher-amplitude startle responses than rats tested in the dark (Walker and Davis, 1997a) and that this effect was disrupted by several anxiolytic compounds (Walker and Davis, 1997a; Walker and Davis, 2002a; and see also de Jongh et al., 2002). In fact, illuminated environments are generally thought to be anxiogenic in this species, presumably because rats, and many other rodents, are at increased risk of predation (e.g., Crawley and Goodwin, 1980; DeFries et al., 1966; File and Hyde, 1978).
Although behaviorally very similar, fear-potentiated and light-enhanced startle are different in that fear-potentiated startle reflects anticipatory fear of a specific and imminent threat (i.e., shock), whereas light-enhanced startle is perhaps better thought of as a more diffuse sort of anxiety in response to a less certain threat – characteristics which have elsewhere been used to distinguish between ‘fear’ and ‘anxiety’, respectively (e.g., Blanchard et al., 2003; Blanchard et al., 1993). The temporal profile of startle increases in these two paradigms is also different, with fear-potentiated startle having a very rapid onset and offset (measured in msec and sec’s respectively – e.g., Davis et al., 1989), and light-enhanced (as well as CRF-enhanced) startle having a more gradual onset and offset (Walker and Davis, unpublished observations, de Jongh et al., 2003).
Subsequent findings indicated that the neural substrates of these two effects are also different. We found, specifically, that intra-CeA infusions of the AMPA receptor antagonist NBQX block fearpotentiated but not light-enhanced startle, whereas intra-BNST infusions block light-enhanced but not fear-potentiated startle (Fig. 2B) (Walker and Davis, 1997b). This double dissociation is very similar to that previously noted for CRF-enhanced versus fear-potentiated startle. NBQX infusions into the BLA, which as noted earlier projects to both structures (c.f., Dong et al., 2001a; Pitkanen, 2000; Sah et al., 2003), disrupted light-enhanced as well as fear-potentiated startle. For light-enhanced startle, the biggest effects occurred in rats with cannula placements in the more caudal part of the BLA, which is the primary site of origin of BLA-to-BNST projections (Dong et al., 2001a).
CRF Mediation of BNST-Dependent Light-Enhanced Startle but not CeA-Dependent Fear-Potentiated Startle
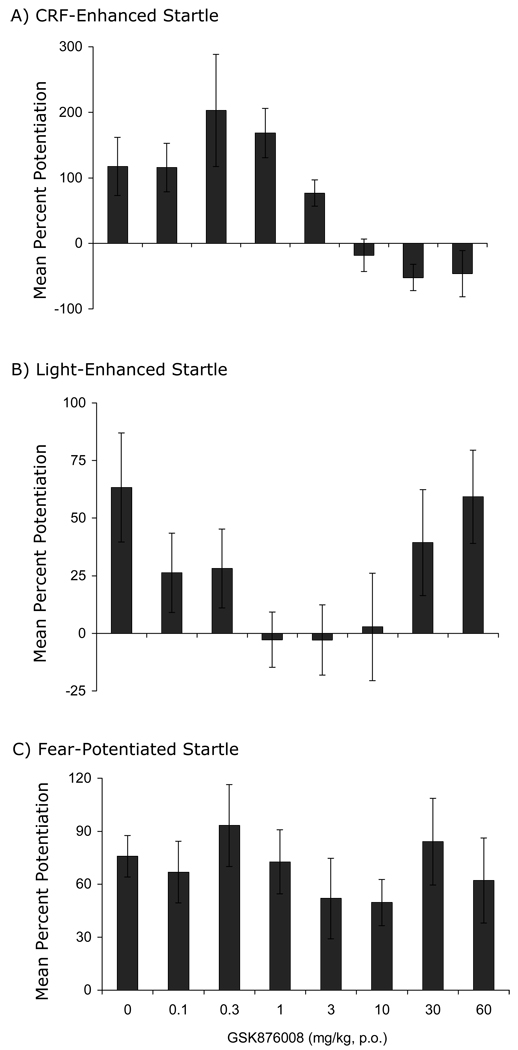
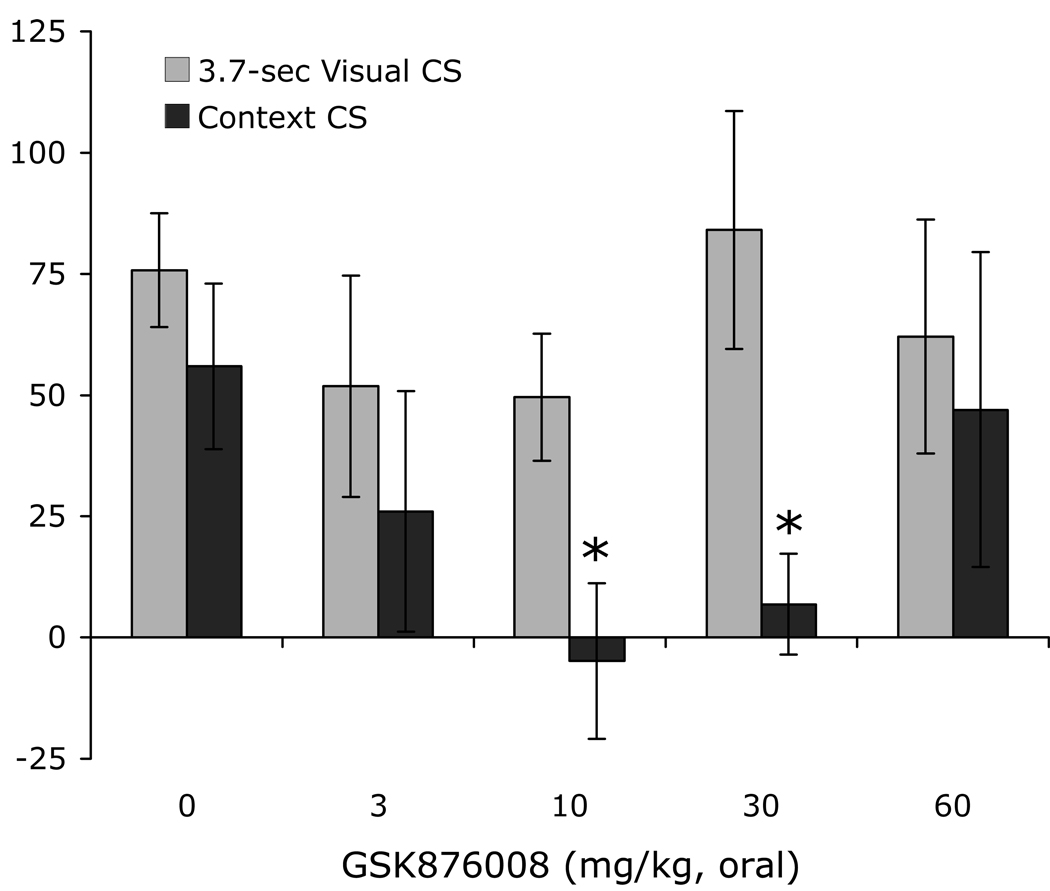
The involvement of the BNST in both CRF- and light-enhanced, but not fear-potentiated startle, suggest that light-enhanced startle, and perhaps BNST-dependent responses more generally, might be especially sensitive to CRF receptor blockade. Indeed, de Jongh et al. (2003) reported that i.c.v. infusions of the CRF-R1/2 antagonist αhCRF had no effect on fear-potentiated startle to a 3.7-sec light, but non-monotonically disrupted light-enhanced startle, significantly disrupting it at an intermediate (5 µg) dose but not at a higher dose (25 µg). Those results are consistent with a preferential involvement of CRF receptors in BNST- versus CeA-mediated effects, but might alternatively reflect preferential access of i.c.v.-infused αhCRF to the BNST neurons that lie immediately adjacent to the lateral ventricle, and more restricted access to CeA or other neurons that don’t. However, using oral administration of the new1 and selective CRF-R1 antagonist GSK876008 (Di Fabio et al., 2008,) at doses which dose-dependently (linear) disrupt CRF-enhanced startle, we too have observed a non-monotonic disruption of light-enhanced startle but no disruption in the same animals at the same doses of fear-potentiated startle (Fig. 3 - Walker et al., 2008). In fact, not only did we fail to see a significant disruption of fear-potentiated startle (i.e., to a 3.7-sec shock-associated light), but in a second experiment with a different group of rats (some trained with weaker-than-usual conditioning procedures to rule out differences in response strength as an explanation for differential vulnerability to CRF receptor blockade), we obtained a significant fit of the dose-response curve to a quadratic function with an apparent enhancement of fear-potentiated startle at an intermediate dose (Fig. 4). As we have observed similar effects in several other experiments, we believe that this enhancement of phasic fear by a CRF antagonist is both real and meaningful, and will present one possible account in a subsequent section.
Figure 3.
Rats were tested sequentially for CRF-enhanced startle, then light-enhanced startle, and then fear-potentiated startle. Prior to each test, the selective CRF-R1 antagonist GSK876008 was administered orally (for each test, each rat received the same dose that it received in the other two tests). GSK876008 dose-dependently disrupted CRF-enhanced startle (panel A; significant linear trend), non-monotonically disrupted light-enhanced startle (panel B; significant quadratic trend), and did not disrupt at all fear-potentiated startle (panel C). Data from Walker et al (2008)
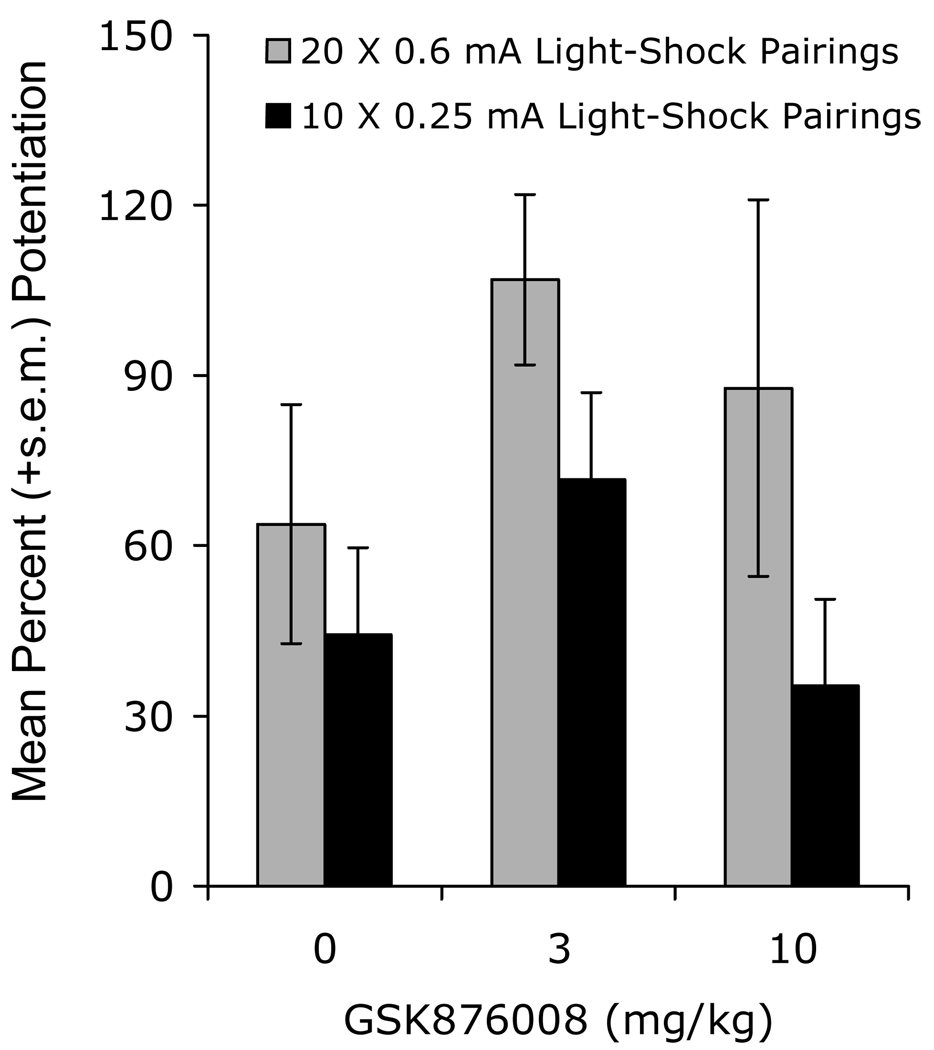
Figure 4.
The selective CRF-R1 antagonist did not disrupt, but modestly enhanced at the intermediate dose, fear-potentiated startle to a 3.7-sec CS (significant quadratic trend). Rats were trained with either normal (20 × 0.4 mA footshocks) or weak (10 × 0.25 mA footshocks) training procedures. Data from Walker et al. (2008).
Although our experiments with GSK876008 do not rule out a contribution of CRF-R2 receptors to fear-potentiated startle, a recent finding that CRF-R2 (as well as CRF-R1) knockout mice show normal fear-potentiated startle to a discrete CS (i.e., a 30-sec light/tone compound CS) suggest they may not (Risbrough et al., 2008). In that same study, CRF-R2 knockouts did show a significant attenuation of post-shock startle increases, which provided a positive behavioral control for the negative effect on startle increases to the discrete CS.
Two Hypotheses for the Differential Involvement of the BNST and CeA in Fear-Potentiated versus CRF- and Light-Enhanced Startle
Given the many similarities between fear-potentiated and light-enhanced startle – i.e., both use increased startle as a behavioral measure and light as a stimulus to produce this effect – their differential susceptibility to CeA versus BNST inactivation and to CRF-R1 blockade is perhaps surprising. These similarities are also quite useful however in that they greatly constrain the range of possible interpretations for the dissociations just noted. We previously suggested that there are two major possibilities – either that the CeA plays a special role in mediating conditioned fear responses and the BNST unconditioned responses or, alternatively, that the CeA plays a special role in mediating short-duration fear responses and the BNST longer-duration responses (Walker and Davis, 1997a). A similar argument can be made with respect to the involvement CRF receptors.
One approach to evaluating the relative merit of these alternatives would be to assess the effect of the aforementioned treatments on startle increases to a stimulus that is both conditioned and also of long duration. If, for example, BNST inactivation were to disrupt startle increases to such a stimulus, then the conditioned versus unconditioned account would no longer seem tenable, whereas the short-versus long-duration account would remain viable. We have now developed procedures to do this and have found, for both site-specific NBQX infusions and for CRF-R1 blockade, that the short-versus long-duration hypothesis is the better account.
The design that we are now using is depicted in Figure 5. Rats receive 8 presentations (ISI = 3 min) on each of 3 days of a variable duration (3 sec to 8 min) 60-Hz clicker stimulus (68 dB) together with co-terminating footshock (0.5 sec, 0.35 mA). The sequencing of the different duration CSs is different on each of the 3 conditioning days and thus, unpredictable. Twenty-four hours before the first conditioning session and 48 hours after the last, rats are presented with a series of startle-eliciting noise bursts (95 dB, ISI=30 sec) presented first in the absence (phase I) and then in the presence (phase II) of a fixed-duration 8-min CS. Training and testing occur in the same chamber, but various contextual elements are changed in order to minimize fear responses to the context itself. Specifically, during conditioning, the shockbars are exposed, the chamber lights are on, and an alcohol-wetted gauzepad (90% EtOH) is placed just outside the test cage as an olfactory stimulus. During testing, sandpaper inserts are placed over the floorbars, 2 beaded-wire chains are suspended from the top of the test chamber as a tactile stimulus, and the chamber lights are off. Because the duration of the CS during conditioning is variable, fear is maintained for the duration of the CS (8 min) during testing as the rats cannot predict in advance when the CS will end and the US occur (during testing, the US does not occur at all of course but the rat has no way of knowing this in advance). For each animal, and for each phase, a startle percent change score from the pre-conditioning to the post-conditioning test is calculated, which serves as the measure of sustained fear in this paradigm. Percent rather than absolute change scores are used because percent change scores remain stable, assuming stable fear levels, across markedly different startle baselines brought about by experimental manipulations, individual differences, or different noise-burst intensities (Walker and Davis, 2002b). As such, interpretation of treatment effects on fear-potentiated startle, using this measure, are not readily confounded by treatment effects on baseline startle that may also occur with some manipulations.
Figure 5.
Sustained Startle Test and Conditioning Procedure. For conditioning, rats receive 8 presentations of a variable duration (3 sec, 10 sec, 20 sec, 1 min, 2 min, 4 min, 6 min, and 8 min) 60-Hz 72-dB, clicker stimulus together with co-terminating footshock. Startle amplitude to 50-msec 95-dB noise bursts (ISI = 30 sec) is measured before and after conditioning, for 8 minutes in the absence and then for 8 minutes in the presence of the clicker. In normal rats, the clicker does not increase startle prior to conditioning, but does increase startle after conditioning. Black bars indicate periods when the clicker is present; arrows indicate footshock.
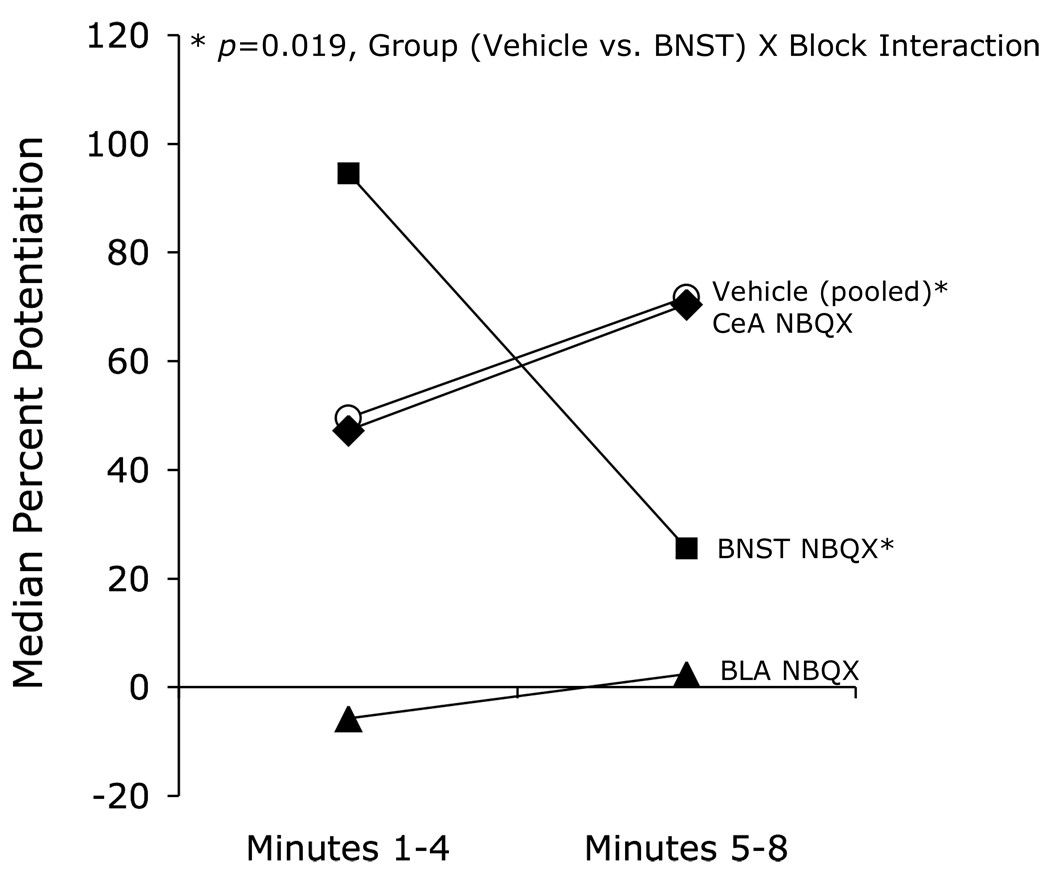
Using an earlier version of this design (8 presentations per day of a noise CS), we first evaluated the effect of pre-test NBQX infusions into the BLA, the CeA, and the BNST. As shown in Figure 6, BNST infusions decreased the late component of fear-potentiated startle (i.e., minutes 5–8 of the CS) but actually increased the early component (i.e., minutes 1–4). These results are consistent with the possibility that the BNST selectively mediates sustained fear responses, and may also be consistent with the enhancement of phasic (i.e., short-duration) fear-potentiated startle by the selective CRF-R1 antagonist GSK876008 that was noted in the preceding section (e.g., Fig. 4). Infusions into the BLA blocked both components and, in this study, infusions into the CeA blocked neither. The ineffectiveness of CeA infusions was a surprise given previous findings that electrolytic (Hitchcock and Davis, 1987) or chemical (Campeau and Davis, 1995) CeA lesions or intra-CeA NBQX infusions (Walker and Davis, 1997b) do block fear-potentiated startle to 3.7-sec CSs. It is possible that the cutoff for short-duration fear is very short and that we simply missed the CeA’s involvement (or more exactly, the involvement of CeA AMPA receptors). A finer scale analysis with more animals will be required to determine when short-duration fear becomes long-duration fear as defined by this measure.
Figure 6.
The effect on fear-potentiated startle to an 8-min 72-dB 2-kHz filtered white-noise CS was evaluated in rats after intra-cranial infusions of the AMPA receptor antagonist NBQX (3 µg/side in 0.5 µl phosphate-buffered saline). Intra-BNST infusions (N=11) decreased the sustained component of fear-potentiated startle, but augmented the early component, relative to vehicle infusions (N=25, pooled across structures). Due to an extreme outlier in the PBS group (606% potentiation during block 1) which distorted the normal distribution, these data were analyzed non-parametrically by Mann-Whitney on block 2 - block 1 difference scores (U=63, p=.019). Rats with amygdala infusions were divided, following histological verification of cannula placement, into BLA (N=6) or CeA (N=8) groups. Intra-BLA infusions appeared to disrupt both components, whereas intra-CeA infusions disrupted neither. These results are consistent with the view that the BNST participates selectively in sustained threat responses.
Again, this pattern does not appear to be idiosyncratic to studies in which startle is used as a response measure. Especially relevant are findings from Waddell et al. (2006), who reported that BNST lesions disrupt conditioned suppression (i.e., of bar pressing for food) to a 10-min but not 1-min clicker CS. Although these data are consistent with our hypothesis, the authors interpreted their findings somewhat differently. In particular, they suggested that the involvement of the BNST was a function of the CS-onset to US-onset interval (i.e., the BNST selectively mediates responses to a temporally distant CS), rather than the duration of the conditioned response. This is a viable interpretation because rats in their study were trained with either 1 or 10 min clicker presentations and, for both, the footshock did not occur until the very end of the CS. Because our study used US’s that were distributed throughout the CS, Waddell et al.’s (2006) interpretation would not seem to account for our data. A minute-by-minute analysis of their findings might indicate whether our interpretation would apply to theirs. That is, we would predict that a lesion-induced disruption of conditioned suppression would become increasingly apparent towards the end of the CS (i.e., as response duration grows), whereas their hypothesis would predict just the opposite (i.e., a lesion effect would be more apparent early on, when the US is still somewhat distant).
Preferential Involvement of CRF in Sustained versus Phasic Threat Responses
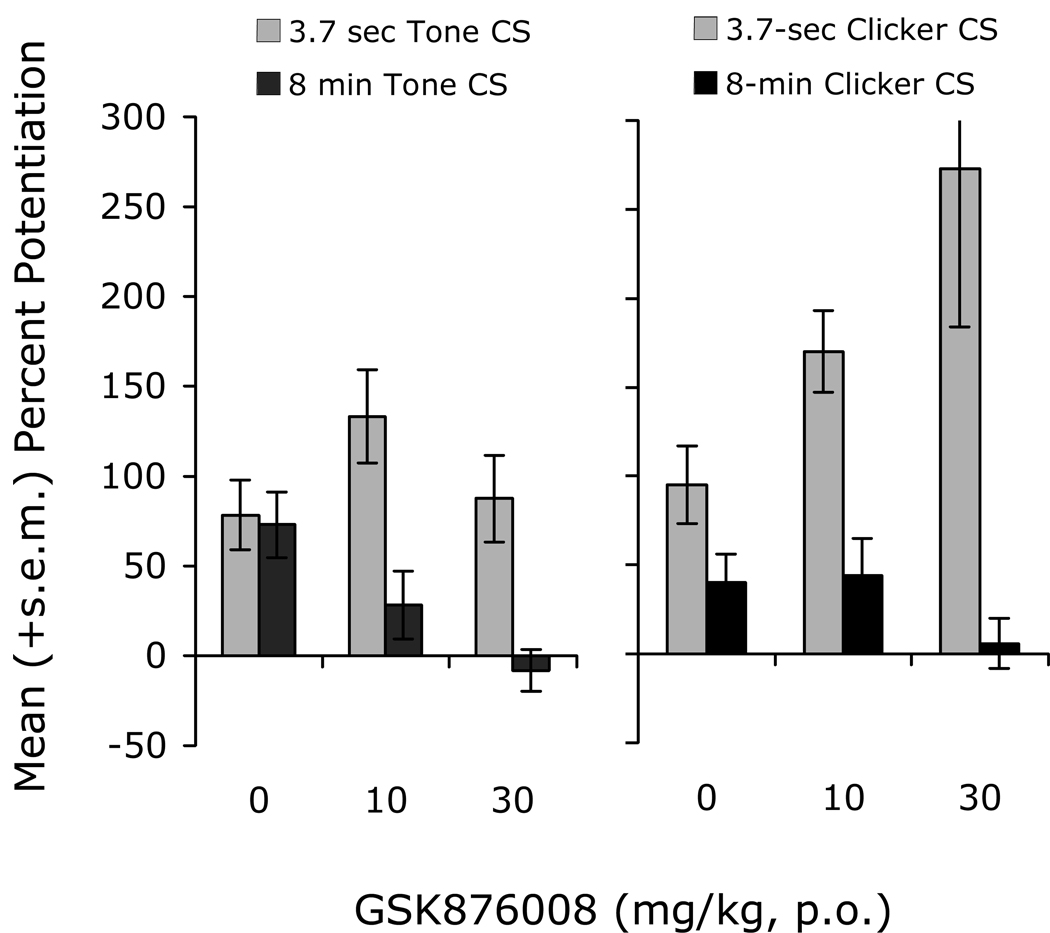
As noted above, we believe that CRF receptors participate preferentially in BNST- as opposed to CeA-dependent responses. As such, we have also compared the effect of CRF-R1 blockade (oral administration) on short- versus long-duration startle increases to conditioned fear stimuli. Data are shown for two experiments – one comparing the effect of the selective CRF-R1 antagonist GSK876008 on startle increases to a short (3.7-sec) versus long-duration (8-min) low-frequency-filtered 72-dB white noise CS (Fig. 7, left panel), and the other comparing the effect of GSK876008 on startle increases to a short (3.7-sec) versus long-duration (8-min) 60-Hz 72-dB clicker CS (Fig. 7, right panel). In both cases, oral administration of the CRF-R1 antagonist dose-dependently blocked fear-potentiated startle to 8-min CS presentations but did not disrupt, and again even enhanced in some animals, fear-potentiated startle to 3.7-sec CS presentations. GSK876008 also had no effect on phasic startle increases (i.e. to a 3.7-sec clicker CS presentation) in rats trained using the sustained fear conditioning paradigm (Fig. 8), confirming that it is the duration of the fear response during testing that confers sensitivity to CRF-R1 blockade, and not the nature of training.
Figure 7.
In two different experiments, the selective CRF-R1 antagonist GSK876008 disrupted potentiated startle to an 8-min CS but did not disrupt potentiated startle to a 3.7-sec presentation of the same stimulus.
Figure 8.
Rats trained with variable duration clicker presentations and co-terminating footshocks show an increase in startle not only to 8-min clicker presentations (as shown previously in Figure 7, right panel), but also to 3.7-sec presentation of the clicker as shown here. These increases are different however in that they are not disrupted by GSK876008. These results indicate that it is the duration of the response during testing rather than the duration of the CS during training that confers sensitivity to CRF-R1 blockade.
Does the Sustained Fear System Inhibit the Phasic Fear System? A Speculative Account of Unexpected Findings
One of the more curious aspects of these data is the non-monotonic disruption of light-enhanced following oral administration of GSK876008 (Walker et al., 2008; and see also de Jongh et al., 2003 for similar effects of the CRF antagonist α-helical CRF). We have also seen non-monotonic disruptions by GSK876008 of pre- to post-shock ‘baseline’ startle increases – which most likely reflect BNST-dependent context conditioning or possibly non-associative sensitization – and will discuss those findings in a later section. Curiously however, these non-monotonic effects are not consistent across behaviors in that linear dose-response curves for the same antagonist at similar concentrations were observed for CRF-enhanced startle in our study (Fig. 3), and for CRF-induced forepaw treading in gerbils, separation-induced vocalizations in rat pups, and defensive behavior by marmosets in the human threat test in Di Fabio et al. (2008). Although U-shaped dose-response functions for CRF receptor antagonists might, in some cases, be attributed to high antagonist levels displacing CRF from the CRF binding protein, which would then increase free CRF (Behan et al., 1996) and possibly mitigate the behavioral effects of lower doses, we do not believe that this explanation would account for our results insofar as GSK876008 is a non-peptide antagonist that would not be expected to interact with the CRF binding protein and in binding studies, demonstrably does not (up to 10 µM, D. Trist, personal communication).
We have also considered the possibility that interactions between the sustained and phasic threat response systems might account for these effects, and perhaps also for the augmentation of CeAM-dependent startle increases that have been observed in several other experiments. As indicated earlier, and depicted in Figure 9, we believe that threat-encoding neurons project to both CeAM and BNSTL, activating CeAM neurons rapidly and BNSTL neurons more slowly. Interestingly, CeAM also receives projections from BNSTL (see Fig. 10, and also Dong and Swanson, 2004; Dong et al., 2001b; Grove, 1988; Sun and Cassell, 1993) as well as from the lateral CeA (Huber et al., 2005; Petrovich and Swanson, 1997). As discussed in a later section, the lateral CeA (CeAL) is closely affiliated with BNSTL (Gray and Magnuson, 1987), and contains many CRF-positive neurons that project to BNSTL. In fact, these neurons are a significant source of the CRF which is found in the BNST (e.g., Sakanaka et al., 1986). Thus, we believe that CeAL may also be a component of the sustained fear system. Notably, projections from CeAL to CeAM are inhibitory (Collins and Pare, 1999; Huber et al., 2005). Those from BNSTL have not, to our knowledge, been functionally characterized, but evidence that BNST inactivation potently increases phasic fear-potentiated startle is consistent with this view (Meloni et al., 2006b). Thus, there is good evidence in support of the hypothesis that the sustained fear system exerts an inhibitory influence on the phasic fear system.
Figure 9.
Schematic drawing illustrating hypothetical involvement of the CeA and BNST in short- and long-duration startle increases. In this model, CeAM neurons respond immediately to neural activity which signals threat, whereas BNST neurons respond more slowly, perhaps requiring the sustained activation of CRF receptors. Once on-line however, the BNST takes over, mediating sustained startle increases and perhaps also inhibiting the CeAM neurons which mediated the initial response. Under ordinary circumstances, this would allow for a seamless transition from the early to the late components of a sustained threat response. Under some circumstances however – when, for example, there is a pre-existing level of activity in the BNST perhaps due to a low level of ongoing anxiety – inhibition of the BNST may actually promote CeAM-mediated threat responses as we have seen following intra-BNST NBQX infusions (and see also Meloni et al., 2006b) and on several occasions following systemic administration of a CRF-R1 antagonist. BNST-to-CeAM projections might also be involved in the non-monotonic effect of CRF receptor blockade observed by us (Walker et al., 2008) and also by (de Jongh et al., 2003). This would require that intermediate doses of the antagonist disrupt the facilitatory influence on startle of the BNST’s descending projections, but leave intact the inhibitory influence on startle of it’s projection to CeAM. If, at higher doses, both the behavioral effect of both projections were disrupted, then CeAM neurons would be disinhibited, allowing them once again to mediate startle increases, this time to a sustained threat stimulus. This model is of course purely conjectural, but would account for several otherwise surprising findings. Open circles and solid lines indicate active neurons and connections; gray circles and dashed lines indicate inactive or suppressed neurons and connections.
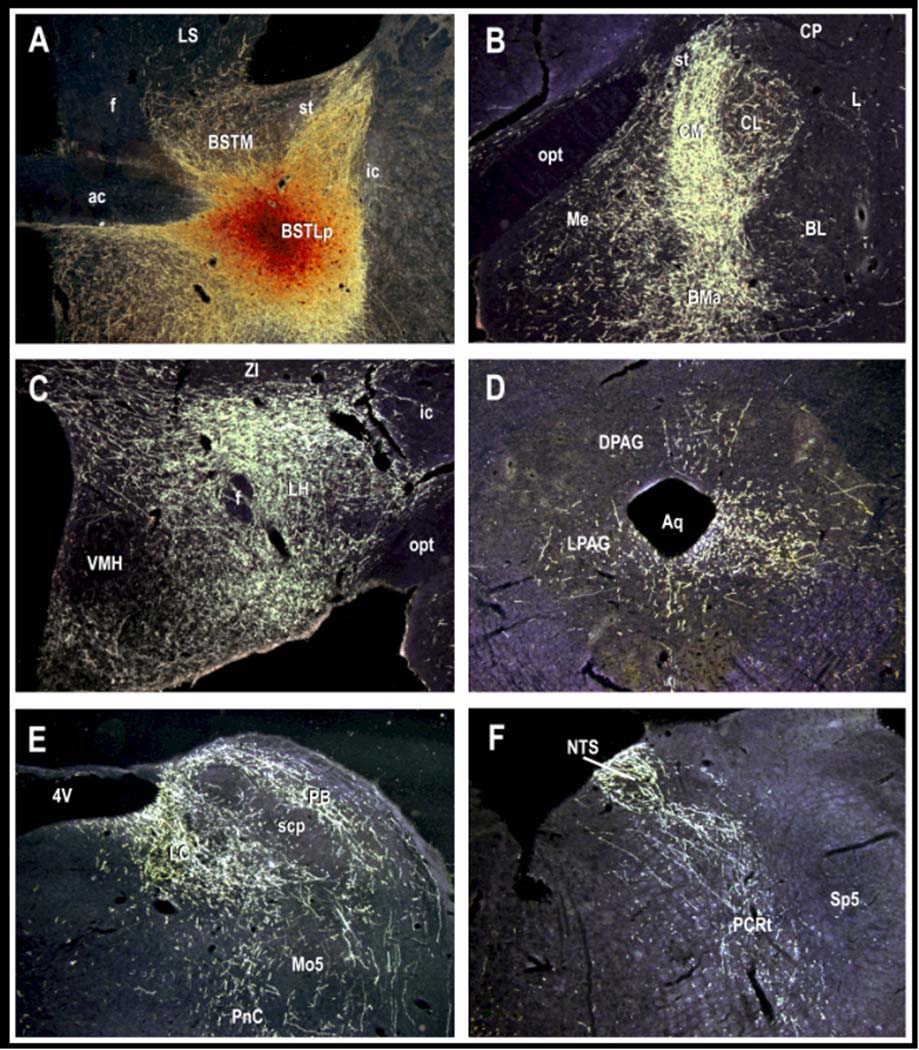
Figure 10.
Intra-BNST infusions of biotinylated dextran amine, an anterograde neuronal tracer (panel A), reveals projections to many of the same hypothalamic and brainstem areas (panels C–F) that the CeAM projects to (c.f., Davis, 2000; Davis and Whalen, 2001). These projections would allow the BNST to mediate many of the same types of threat responses that the CeAM is thought to mediate. The BNST also projects to CeAM (panel B), perhaps allowing the BNST to modulate CeAM output. The photomicrograph shown above was prepared by Dr. Chungjun Shi who has generously allowed us to reproduce it here.
What then might the behavioral implications of this circuitry be? In response to a sustained threat stimulus, the initial facilitatory effect on startle would be mediated by direct and indirect projections from CeAM to those brainstem neurons that mediate the basic startle reflex. During a sustained threat signal, as CRF is released into the BNSTL and its neurons gradually come on line, it too would influence startle by way of it’s own projections to these same areas. This increase in the BNSTL’s activity would be accompanied by a concomitant decrease in the CeAM’s contributions due to active inhibition by CeAL and perhaps BNSTL neurons (i.e., the sustained fear system). If however, the sustained fear system were inactivated (e.g., by high doses of a CRF receptor antagonist), then the inhibitory signal to CeAM would be lost, allowing CeAM neurons to once again participate in the behavioral response. If the effect on startle of the BNSTL’s inhibitory influence on CeAM neurons was less readily disrupted than its facilitatory effect on startle itself, this would generate a U-shaped dose-response function such as that observed with light-enhanced and perhaps also for context-potentiated startle. To state this more concretely, low doses of the CRF-R1 antagonist would block enough receptors in BNSTL to prevent sustained startle increases but not enough to disinhibit CeAM neurons. At higher doses, the CRF-R1 antagonist would prevent both effects, allowing CeAM neurons to once again mediate startle increases in response to BLA input.
This hypothesis also accounts for the differently-shaped, linear dose-response function observed for CRF receptor antagonist effects on CRF-enhanced startle and several other behaviors that might not involve the CeAM because, for these behaviors, disinhibition would be irrelevant as there would be nothing to disinhibit (e.g., CRF acts directly on CRF receptors in the BNST with no apparent involvement of the CeA). The model also predicts that in instances where there is already some degree of tonic inhibition (as appears generally to be the case for the CeA – e.g., Nose et al., 1991), CRF receptor blockade or BNSTL inactivation might actually augment responses to phasic threat signals as we have also observed in several (but not all) of our experiments with GSK876008, and what Meloni et al. (2006b) have reported following intra-BNST infusions of the GABA-A receptor agonist muscimol. The model is not wholly without challenges (mainly with respect to findings regarding “saturation” experiments which are discussed in a later section) and remains largely conjectural, but certainly amenable to experimental investigation. It would also predict, for example, that intra-CeA NBQX infusions, that do not normally disrupt light-enhanced startle, would disrupt light-enhanced startle in rats also treated with a high dose of the CRF receptor antagonist. The particular model presented here is one of probably many variations on the more general theme that high antagonist doses influence the output of different sets of neurons (e.g., due to differences in receptor reserve) with different behavioral effects, than lower antagonist doses.
CeA Influences on BNSTL Function: A Special Role for CeAL?
A possible role for CeAL in sustained fear was briefly alluded to in the preceding section and will be discussed in more detail here. In fact, the lateral subdivision of the CeA is distinct from the medial subdivision in a number of key respects, including cell morphology (McDonald, 1982), neurochemical content (Cassell et al., 1986; Sun and Cassell, 1993; Wray and Hoffman, 1983), and intrinsic (c.f., Jolkkonen and Pitkanen, 1998) as well as extrinsic connectivity (c.f., Pitkanen, 2000; Sah et al., 2003). Although both project to BNSTL (c.f., Dong et al., 2001a), projections from CeAL are unique in that they express a wide variety of peptides including, quite prominently, CRF (Cassell et al., 1986; Day et al., 1999; Gray and Magnuson, 1987; Moga and Gray, 1985; Otake and Nakamura, 1995; Shimada et al., 1989b; Veening et al., 1984; Wray and Hoffman, 1983). In fact, CeAL neurons are a major source of CRF within the BNSTL as judged by the marked (though certainly not complete) depletion of BNST CRF following CeA lesions (Sakanaka et al., 1986). Perhaps, sustained threat cues generate sustained high-frequency activity patterns in CeAL neurons which have been shown elsewhere to be especially favorable for peptide release (e.g., Bartfai et al., 1988; Bourque, 1991; Ip, 1994; Lundberg et al., 1986; Whim, 1989).
A handful of studies have explored the influence of CeA-to-BNST projections on stress- and/or threat-related behavior, although in most cases it has been difficult to specifically isolate the individual contributions of CeAL versus CeAM neurons. In one such study, Nakagawa et al. (2005) found that naloxone-precipitated morphine withdrawal increased Fos protein expression in the most lateral part of the CeA (i.e., the capsular subdivision) and also in the BNST (medial as well as lateral divisions). Excitotoxic lesions of either structure (i.e., CeA or BNST) significantly reduced place aversions to the context in which withdrawal occurred. Importantly however, whereas as excitotoxic CeA lesions significantly reduced CeA Fos expression, excitotoxic BNST lesions did not reduce CeA Fos expression. This pattern indicates an important contribution of both areas to the behavior, and suggests a serial flow of information from CeA to BNST.
Two other studies have evaluated the behavioral relevance of this pathway using variations on the crossed-lesion design. In the first, Erb et al. (2001) showed that neither unilateral intra-CeA TTX infusions nor unilateral infusions into the BNST of the CRF receptor antagonist D-Phe CRF12–41 disrupted shock-induced reinstatement of extinguished cocaine-seeking behavior, but that the combination of both treatments on opposite sides of the brain did. They concluded that a “CRF-containing pathway from CeA to BNST is involved in mediating the effects of CRF…on the reinstatement of cocaine seeking.” In the other, Jasnow et al. (2004) found that social defeat behavior in Syrian Hamsters was reduced by pre-defeat unilateral electrolytic CeA lesions and also by pre-test unilateral infusions into the BNST of the CRF receptor antagonist D-Phe CRF12–41. However, the combination of both treatments, again on opposite sides of the brain, produced an even greater effect, leading them to conclude that “stress activates CRF-containing neurons in the CeA, which then releases CRF within the BNST.”
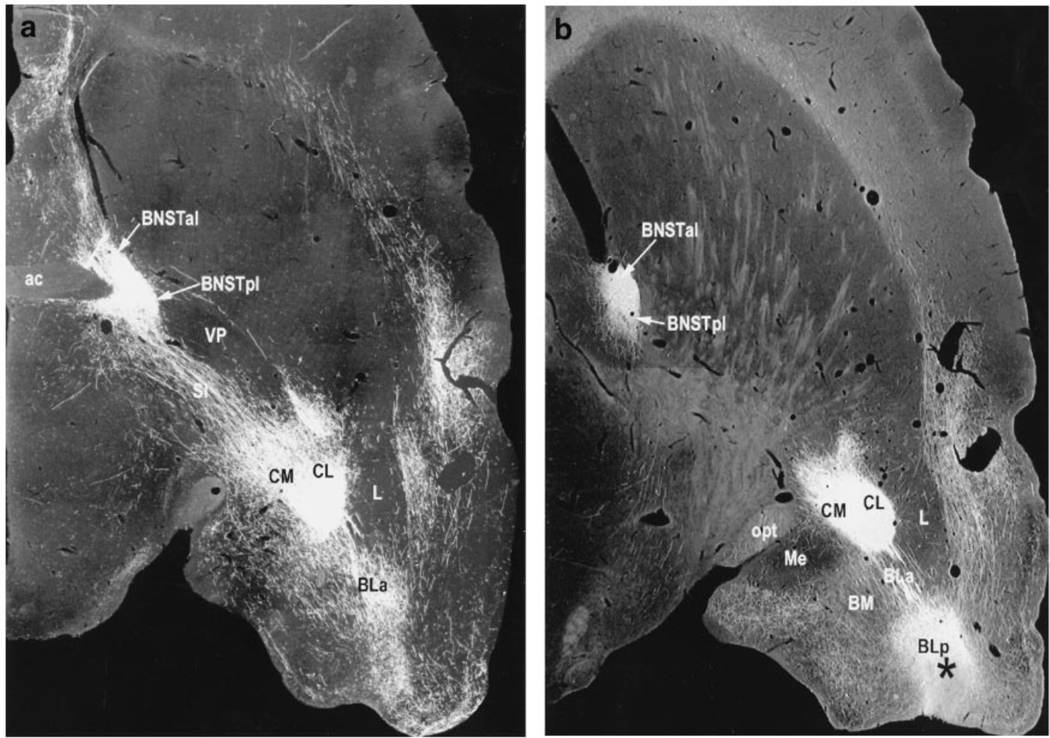
Although the results of both studies are consistent with the conclusions that were drawn, they are not definitive insofar as the crossed ‘lesion’ design was only partially implemented. The most telling outcome is these types of design is a demonstration that contralateral treatments are more effective than ipsilateral treatments, as this controls for the total amount of damage. Also, the use of TTX in Erb et al. (2001) and electrolytic lesions in Jasnow et al. (2004) would have interrupted projections from the BLA which pass through the CeA on their way to the BNST (c.f., Dong et al., 2001a; and see also Fig. 11 which is reproduced from Davis and Whalen, 2001). As discussed in a later section, these projections may play an important role in sustained fear. There are in fact, very few studies that have evaluated the CeA’s role in sustained fear responses using techniques in which effects on fibers-of-passage would not be a concern, and the results from these studies have been variable. Thus, excitotoxic CeA lesions were found to block conditioned corticosterone responses to a context CS in Van de Kar et al. (1991) and to either block (Goosens and Maren, 2001; Zimmerman et al., 2007) or not block (Koo et al., 2004) conditioned freezing responses to a context CS, in more recent studies.
Figure 11.
Photomicrographs prepared and generously provided by Dr. Chungjun Shi, of 30-µm horizontal sections through a rat brain, cut at a slight angle so as to include the amygdala and BNST in the same plane of section. Infusions of the anterograde tracer biotinylated dextran-amine (BDA) into the posterior BLA (BLp) reveal strong projections both to the medial and lateral CeA (labeled here as CM and CL) and also to the BNST. Because those that project to the BNST pass directly through the CeA, electrolytic CeA lesions or intra-CeA infusions of Na+-channel blockers such as TTX would interrupt this pathway.
As described earlier, we have found that intra-CeA infusions of the AMPA receptor antagonist NBQX do not block either light-enhanced startle (Fig. 2) or sustained startle increases to an 8-min noise CS (Fig. 6). Although these results are consistent with the view that the CeA is not involved in sustained fear, they too, as with negative results in general, are not definitive. It is possible, for example, that short-duration phasic fear responses are mediated by the activation of AMPA receptors on CeAM neurons, but that longer duration startle increases require the activation of other transmitter types acting on CeAL neurons. Van Nobelen and Kokkinidis (2006), for example, have found that startle increases which occur in the immediate aftermath of shock and which may reflect BNST-dependent (Gray et al., 1993; Sullivan et al., 2004) context conditioning (Kiernan et al., 1995; Richardson, 2000) are not blocked by intra-amygdala NBQX infusions but are blocked by intra-amygdala infusions of the GABA-A agonist muscimol, Thus, a definitive conclusion as to what role, if any, the CeA has in sustained fear will require further study using treatments that have more general effects on CeA function, or more selective effects on CeAL function. To this end, we are currently evaluating the role of CeAL and CeAM in sustained versus phasic fear using ligands for receptors which are differentially distributed in these areas.
Results from Behavioral Occlusion Studies Support the View that Phasic and Sustained Startle Increases are Mediated by Independent Systems
The findings reviewed above, obtained primarily from lesion and inactivation studies, strongly suggest that phasic and sustained startle increases, and perhaps phasic and sustained threat responses more generally, are mediated by different neural systems. Although the results from lesion and inactivation studies have contributed greatly to our current understanding of brain-behavior relationships, they can also lead to false conclusions when taken in isolation (e.g., due to distant or non-specific effects as argued in Anagnostaras et al., 2002). However, evidence for a dissociation between phasic and sustained threat response systems comes not only from loss-of-function studies in compromised animals, but also from behavioral occlusion experiments in healthy animals.
For these experiments, we reasoned that if a particular system that positively modulates startle amplitude were fully activated, then it would not be possible to increase startle further with a second treatment that acts through the same system. If, however, the second treatment acts through a different system, then further increases in startle amplitude would be possible. As an initial ‘proof-of-principle’ experiment, we first conditioned rats by pairing a 3.7-sec light with footshock and later tested them for fear-potentiated startle immediately after an injection of either strychnine or saline. Because strychnine increases startle by disinhibiting neurons in the brainstem and spinal cord (Kehne et al., 1981) and because this effect is independent of the amygdala (Hitchcock and Davis, 1986), we predicted that strychnine-enhanced startle would add to, rather than occlude amygdala-dependent fear-potentiated startle. As shown in Table 2 (Experiment 1), this is exactly what happened. Whether fear-potentiated startle was evaluated in saline-injected rats or in rats whose baseline startle amplitude had been elevated by strychnine (i.e., from a mean of 69 units to a mean of 376 units), the effect of the conditioned fear stimulus was the same – a 75% increase from non-CS to CS test trials (Walker and Davis, 2002b). Not all combinations of startle-potentiating stimuli show additivity however. As also indicated in Table 2, the effect on startle of an auditory CS occludes the effect on startle of a visual CS (Experiment 2), as does the transient startle-elevating effect of footshock itself (Experiment 3). As each of these phenomena is amygdala- but not BNST-dependent (e.g., Campeau and Davis, 1995; Gewirtz et al., 1998; Hitchcock and Davis, 1991; Sananes and Davis, 1992), occlusion was predicted and subsequently observed, thereby establishing the validity of this approach.
Table 2.
Additivity vs. Occlusion. Column 2 shows raw startle amplitude (bolded) in the absence of the visual CS startle under control conditions (top of cell) and after startle amplitude has been increased by one of several different treatments (bottom of cell). Column 3 shows startle amplitude in the presence of the visual CS for both conditions. The percent increase is indicated in column 4.
| (Startle Amplitude) | (Startle Amplitude) | % Increase by CS | |
|---|---|---|---|
| Experiment 1 (Amy + Brainstem) |
baseline (69) | + visual CS (126) | 75% |
| w/strychnine (376) | + visual CS (623) | 75% - no occlusion | |
| Experiment 2 (Amy + Amy) |
baseline (260) | + visual CS (455) | 88% |
| w/auditory CS (360) | + visual CS (408) | 18% - occlusion | |
| Experiment 3 (Amy + Amy) |
baseline (325) | + visual CS (617) | 89% |
| after footshock (612) | + visual CS (772) | 26% - occlusion | |
| Experiment 4 (Amy + BNST) |
baseline (115) | + visual CS (166) | 44% |
| w/CRF (702) | + visual CS (1040) | 48% - no occlusion | |
The telling experiment however was to see if CRF-enhanced startle would add to or occlude fear-potentiated startle (Experiment 4). As indicated in the Table 2, the conditioned fear stimulus increased startle just as effectively in vehicle-infused control rats (i.e., 44% increase from a mean baseline startle response of 115 units), as in rats whose baseline had already been increased by CRF (i.e., 48% increase from a CRF-elevated baseline of 702 units). These findings, then, provide complementary evidence in favor of the hypothesis that short-duration fear CSs increase startle through neural systems other than those that mediate CRF-enhanced startle. We predict, but have not yet tested, that CRF-evoked startle increases will occlude startle increases to a long-duration fear CS.
Findings from Context Conditioning Studies Also Implicate the BNST and CRF in Sustained but not Short-Duration Threat Responses
Because context is also a long-duration stimulus, which evokes long-duration responses, we would expect that those responses would be similarly vulnerable to BNST lesions/inactivation and to CRF receptor blockade. In fact, Gray et al. (1993) found many years ago that pre-training excitotoxic BNST lesions disrupt ACTH and corticosterone responses to a shock-paired context. This was later replicated by Sullivan et al. (2004) who reported that post-training electrolytic BNST lesions also disrupted freezing responses to a context CS but had no effect on either response when evoked by a 20-sec tone CS. In contrast, electrolytic CeA lesions disrupted responses to both CS types. Very recently, Resstel et al. (2008) reported that pre-test intra-BNST CoCl2 infusions, which block neurotransmitter release but not action potential propagation, also disrupts context-elicited freezing, as well as heart rate and arterial blood pressure increases. Time course analyses of context-evoked tachycardia suggested that the effect of BNST inactivation increased with time (10 min test), but that the effects on context-evoked blood pressure changes did not. Thus, evidence for a preferential involvement of the BNST in the early versus late component of these responses was mixed over these intervals, depending on the particular response being considered. Indirect evidence consistent with an involvement of the BNST in context-elicited fear can also be found in Waddell et al. (2006) who reported that the reinstatement of extinguished fear by footshock – a phenomenon thought to depend on conditioning to the shock-paired context (c.f., Bouton et al., 2006) – is also disrupted by BNST lesions. Overall, then, there is good evidence from several recent studies that an intact and functional BNST is required for context fear expression.
There is also evidence that CRF is involved. Several authors have reported that freezing to context CSs are disrupted by CRF receptor antagonists (Deak et al., 1999; Hikichi et al., 2000; Kalin and Takahashi, 1990) and a very recent report by Risbrough et al. (2008) indicates that fear-potentiated startle to a context CS, assessed immediately after footshock, is absent in CRF-R1 knockout mice and significantly reduced, compared to wild-type controls, in CRF-R2 knockouts. Notably, both knockouts showed normal fear-potentiated startle to a discrete CS.
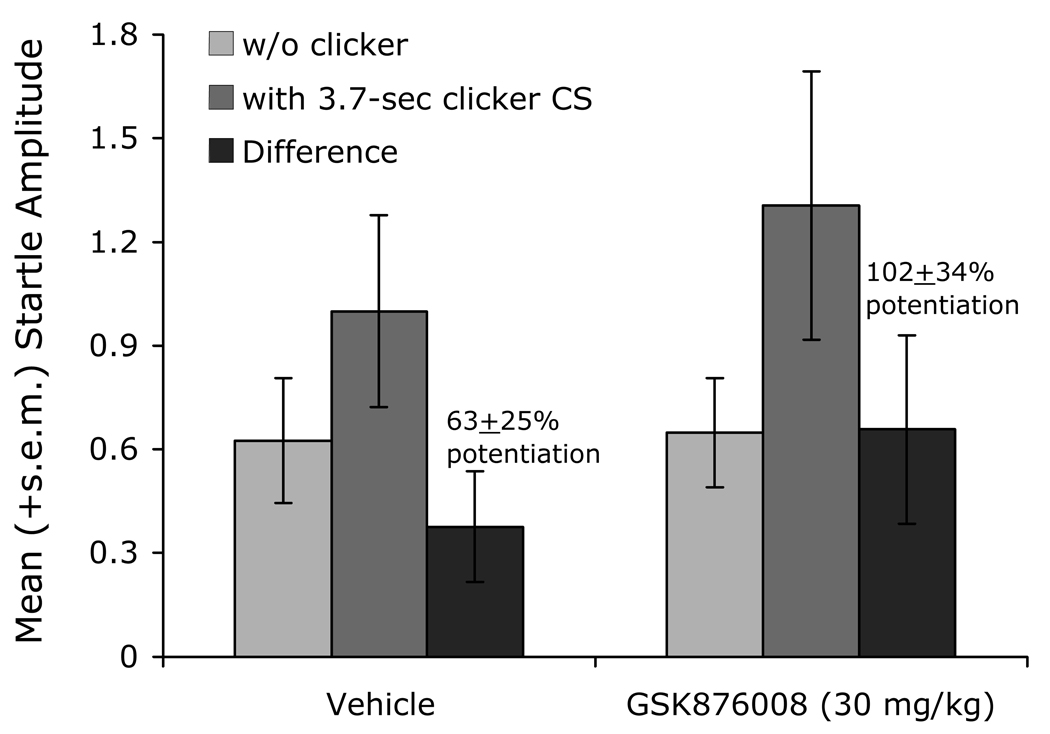
We too have observed what appears to be a selective disruption of context- as opposed to phasic cue-potentiated startle (i.e., Walker et al., 2008). Recall that we previously found that oral administration of the CRF-R1 antagonist GSK876008 did not disrupt fear-potentiated startle to a 3.7-sec light (Fig. 3, panel C). In that same study, the same rats were also tested for ‘baseline’ startle (i.e., in the absence of the explicit CS) prior to and then again after conditioning in the same context where conditioning took place – in both cases after receiving GSK876008. This allowed us to evaluate the effect of GSK876008 on pre- to post-conditioning startle increases. Control and low-dose rats did indeed show an increase in ‘baseline’ startle that was roughly comparable in magnitude to fear-potentiated startle to the explicit 3.7-sec visual CS. However, whereas fear-potentiated startle to the explicit CS was not disrupted by any dose of GSK86008, the pre- to post-conditioning startle increases were disrupted at doses of between 3 and 30 mg/kg but not, interestingly enough, at the higher dose of 60 mg/kg (Fig. 12) – a nonmonotonic dose-response curve similar to that previously observed with respect to light-enhanced startle. Because this study was not designed with the specific intent of evaluating context conditioning, we did not include context discrimination controls which would be necessary to rule out the possibility that these increases were due to non-associative sensitization. However, because long-lasting non-associative sensitization appears also to be a BNST-dependent phenomenon (e.g., Gewirtz et al., 1998, and more recent unpublished findings discussed in the following section), these findings, by either interpretation, would count as yet another example of BNST-dependent long-duration startle increases that are susceptible to CRF-R1 blockade. Note also that in control and low-dose rats, the effects on fear-potentiated startle to the 3.7-sec visual CS were not occluded by but instead summated with pre- to post-shock startle increases, as we would expect if phasic and sustained startle increases are mediated by different systems (see previous section on occlusion).
Figure 12.
This figure shows the effect of GSK876008 on pre- to post-shock changes in ‘baseline’ startle (i.e., on test trials without the 3.7-sec CS), which may be a conditioned response to the context CS. The shape of the dose-response curve was similar to that previously seen for light-enhanced startle in that intermediate doses of the CRF-R1 antagonist disrupted these increases.
It is tempting to speculate how broadly the above interpretation might apply. Context CSs are unique in that they are spatial, mulit-modal, and configural, and it is these characteristics to which the selective vulnerability of the responses they evoke – e.g., to hippocampal lesions – have most often been attributed. For BNST inactivation and CRF receptor blockade however, the more parsimonious interpretation is that their selective vulnerability is instead a function of response duration. In view of recent findings that long- but not short-duration responses to unimodal shock-associated CSs are also selectively vulnerable to hippocampal lesions (e.g., Otto and Poon, 2006; Parsons and Otto, 2008), we wonder if the involvement of the hippocampus in context-elicited fear might also depend on stimulus and/or response duration rather than the spatial, multi-modal, and configural nature of context CSs.
A possible strike against both hypotheses are results from Davis and colleagues who replicated the well-established effect of dorsal hippocampus lesions on context CS-elicited freezing but showed also, in the same animals, that context-dependent fear-potentiated startle (McNish et al., 1997) and contextual blocking (McNish et al., 2000) were intact. Other findings from those studies indicated that lesion-induced hyperactivity may have selectively interfered with the ability of the animals to freeze, without appreciably disrupting fear itself. These results suggest caution when interpreting the results of freezing studies. Importantly, however, other findings that have implicated the hippocampus in context fear are less readily explained by performance deficits (c.f., Anagnostaras et al., 2001), and it is those data to which a possible involvement in sustained fear would apply.
BNST Involvement in Shock-Dependent Non-Associative Startle Increases
To evaluate the possibility that a partial contribution of the BNST to phasic fear had been missed in earlier experiments due to ceiling effects, Gewirtz et al. (1998) trained and tested sham- and BNST-lesioned rats using a slow-acquisition repeated-test procedure which allowed for an evaluation of lesion effects over the entire course of conditioning (i.e., on weak fear responses which appear early in conditioning as well as stronger responses which occur later). On each of 20 days, rats received 10 startle-eliciting noise bursts to assess baseline startle amplitude, 2 pairings of a 3.7-sec light and coterminating footshock, and 3 test trials with and 3 test trials without the 3.7-sec CS. Fear-potentiated startle to the light was clearly evident by the fourth test day and continued to grow over the course of the study. Even during the first few days, however, when fear-potentiated startle was relatively weak, sham- and BNST-lesioned rats showed comparable levels of fear-potentiated startle. Unexpectedly however, BNST lesions did influence one aspect of performance. In shocked but not non-shocked control rats, baseline startle amplitude (i.e., to the 10 noise bursts delivered at the beginning of each session) grew steadily over the course of training. Indirect evidence suggested that the increase was due to non-associated shock-induced sensitization rather than context fear, although the latter could not be ruled out. Importantly, this increase was absent in BNST-lesioned rats.
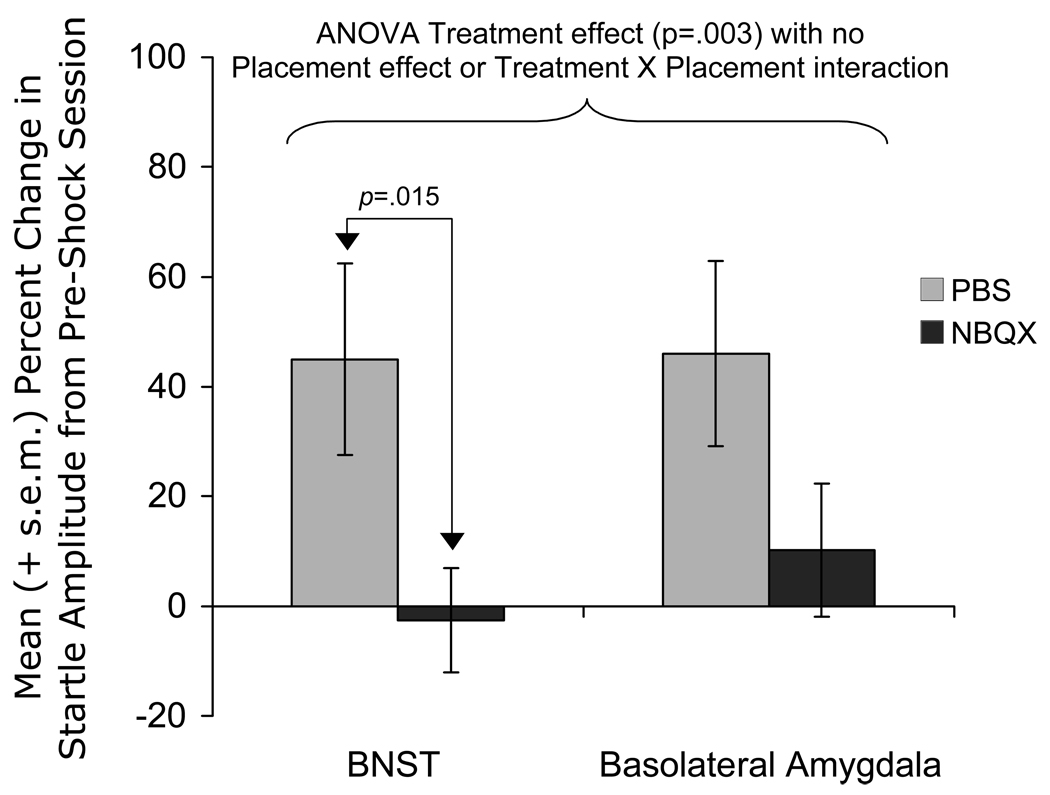
More recently, we evaluated the effect on shock-induced sensitization of pre-test intra-cranial NBQX infusions. While using an earlier version of the protocol shown in Figure 52, we again observed baseline increases in control rats but unreliable increases during the long-duration CS. As such, we limited our analyses to the baseline increases (and revised the procedure for obtaining sustained startle increases to the CS to that shown in Fig. 5). As shown in Figure 13, these baseline increases were prevented by pre-test NBQX infusions into the BNST and were significantly attenuated by pretest NBQX infusions into the BLA. NBQX did not reduce startle in rats that had not received shocks, indicating that NBQX was specifically disrupting the shock-related increase rather than startle in general.
Figure 13.
Pre-test infusions of NBQX infusions into BNST (3 µg/side) prevented pre- to post-shock session increases in ‘baseline’ startle amplitude. Although intra-BLA infusions did not significantly reduce these increases, the response was nominally comparable and an overall ANOVA found a significant Treatment (i.e., vehicle vs. NBQX) effect, without a Treatment X Placement interaction).
For several reasons, we believe that these increases reflect non-associative sensitization rather than context conditioning. First, the train and test contexts were made dissimilar by changing various elements of the context (as described earlier for the sustained fear training/testing procedure). Second, startle increases were shown in unimplanted animals from a control experiment to decay in approximately 2 weeks, either with or without repeated testing. That is, the decay occurred independently of, and was not accelerated by a nominal extinction procedure (i.e., repeated testing) as one would expect if the startle increases were due to conditioning. These data are shown in Figure 14. Moreover, once sensitization had decayed to pre-conditioning levels, re-introduction of the contextual elements that were present during shock again increased startle. This strongly suggests that the rats were able to discriminate the training from testing contexts, and that startle increases in the latter (test context) reflected sensitization rather than an over-generalized context response. Overall, the results of this series of experiments confirmed those of Gewirtz et al. (1998), extended them by showing a specific disruption of the expression of sensitization by BNST inactivation, and suggested a similar though perhaps weaker involvement of the BLA.
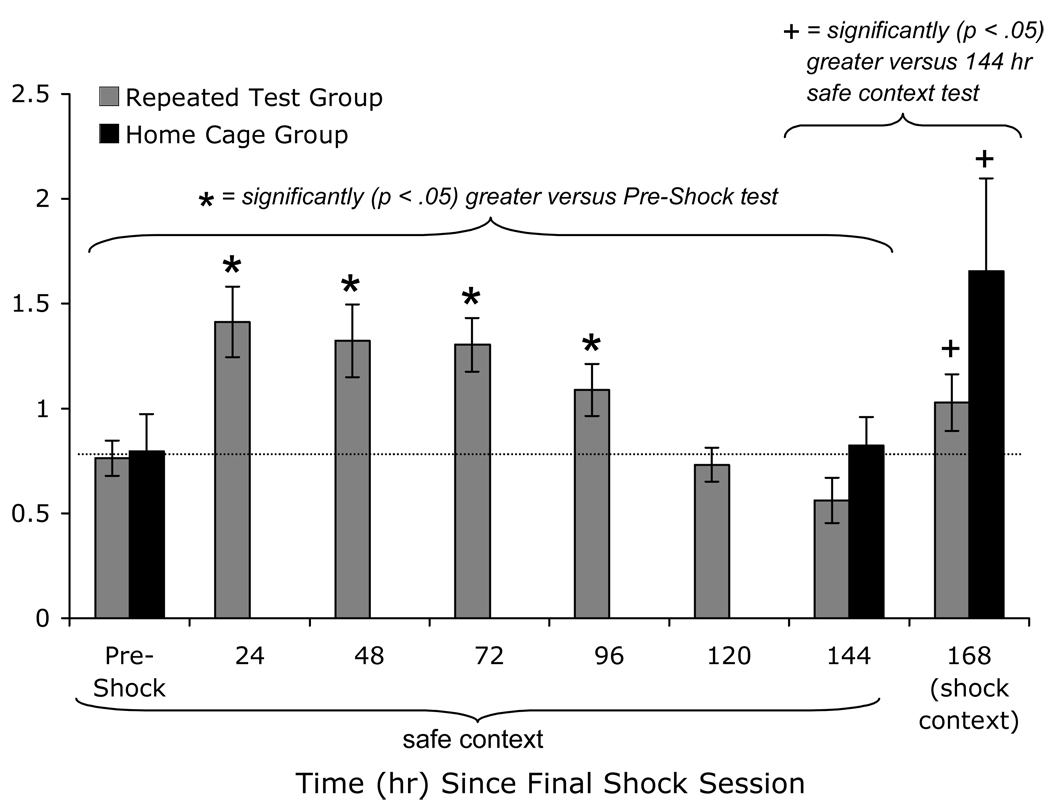
Figure 14.
Pre- to post-shock startle increases most likely reflect non-associative sensitization rather than context conditioning. First, rats were trained and tested in the presence of a different set of contextual cues. Second, when rats were repeatedly exposed to the test chamber in the absence of shock, startle amplitude decayed to pre-shock levels within 5 days of the final shock session and this effect did not require, nor was it accelerated by context exposure (i.e., a nominal extinction procedure) as would be expected if it were a conditioned effect. Third, when rats were re-tested in the presence of cues that were present during shock, rats in both groups showed an increase in startle, suggesting that they had formed an association between the context and shock, and that this association could be expressed under appropriate circumstances. Thus, rats show non-associative sensitization when tested within several days of shock in a neutral context, and conditioned fear when tested at longer intervals in the shock context.
A Specific Neural Circuit for Sustained Fear
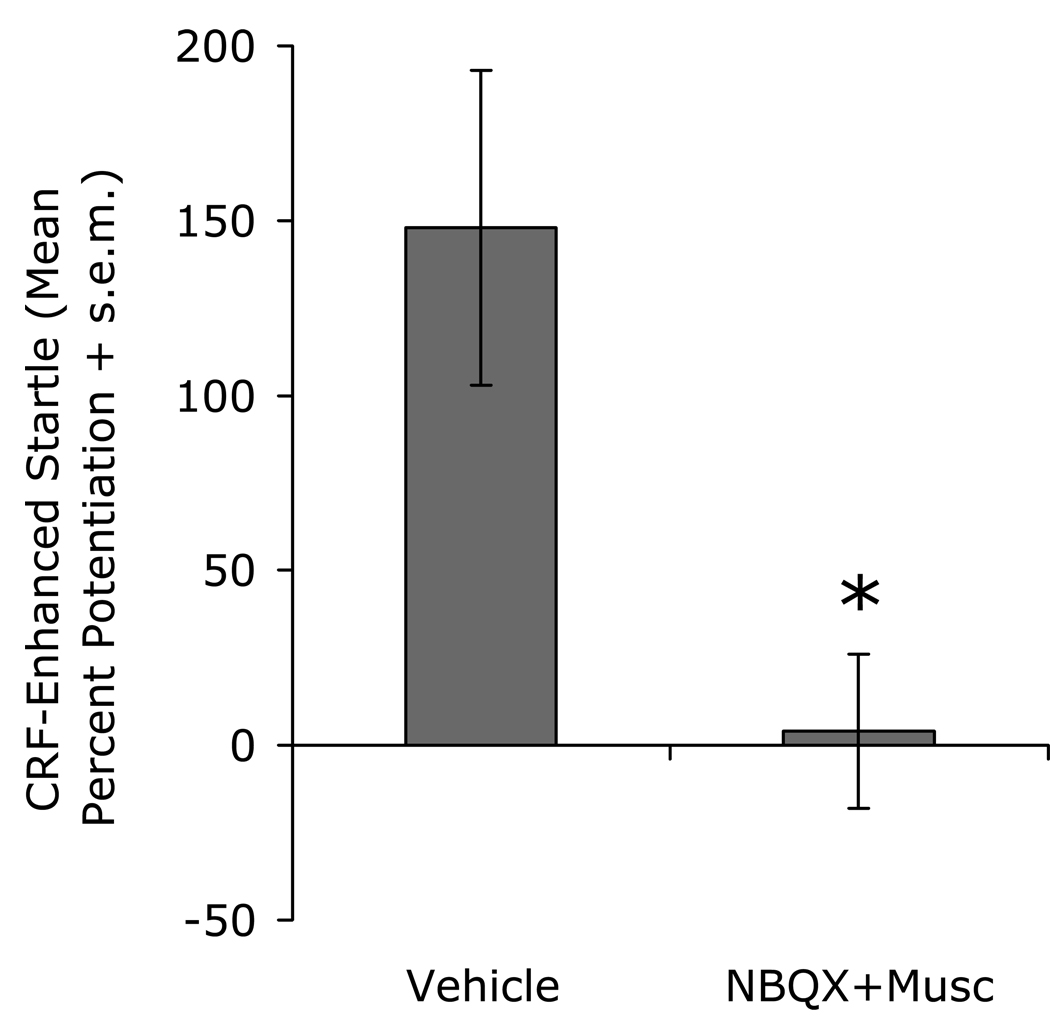
The results reviewed above suggest to us a particular relationship between the BLA and the BNSTL, and a particular role for CRF (see Fig. 15). Particularly influential to our thinking are results from an unpublished experiment in which we found that bilateral intra-BLA infusions of an NBQX / muscimol (3 µg / 0.1 µg) cocktail completely blocked CRF (1 µg, i.c.v.) - enhanced startle (Fig. 16). As noted earlier, CRF does not increase startle when infused into the BLA (Liang et al., 1992a), so we do not believe that i.c.v.-infused CRF is diffusing to and activating BLA CRF receptors. We suspect instead that CRF may increase startle by acting presynaptically on BLA terminals within the BNSTL where it promotes glutamate release and, therefore, the excitatory drive onto BNSTL neurons. In fact, very recent findings using CRF1-promotor-linked GFP, strongly suggest that many CRF1 receptors in the BNST are indeed located pre-synaptically (Justice et al., 2008), with very few post-synaptic receptors in the lateral BNST’s oval nucleus. The model illustrated in Figure 15 would also account for previously unexplained findings that electrolytic (Liang et al., 1992a) but not excitotoxic CeA lesions block CRF-enhanced startle (Lee and Davis, 1997) because, as indicated earlier (e.g., Fig. 11), electrolytic but not excitotoxic lesions would transect BLA to BNST projections.
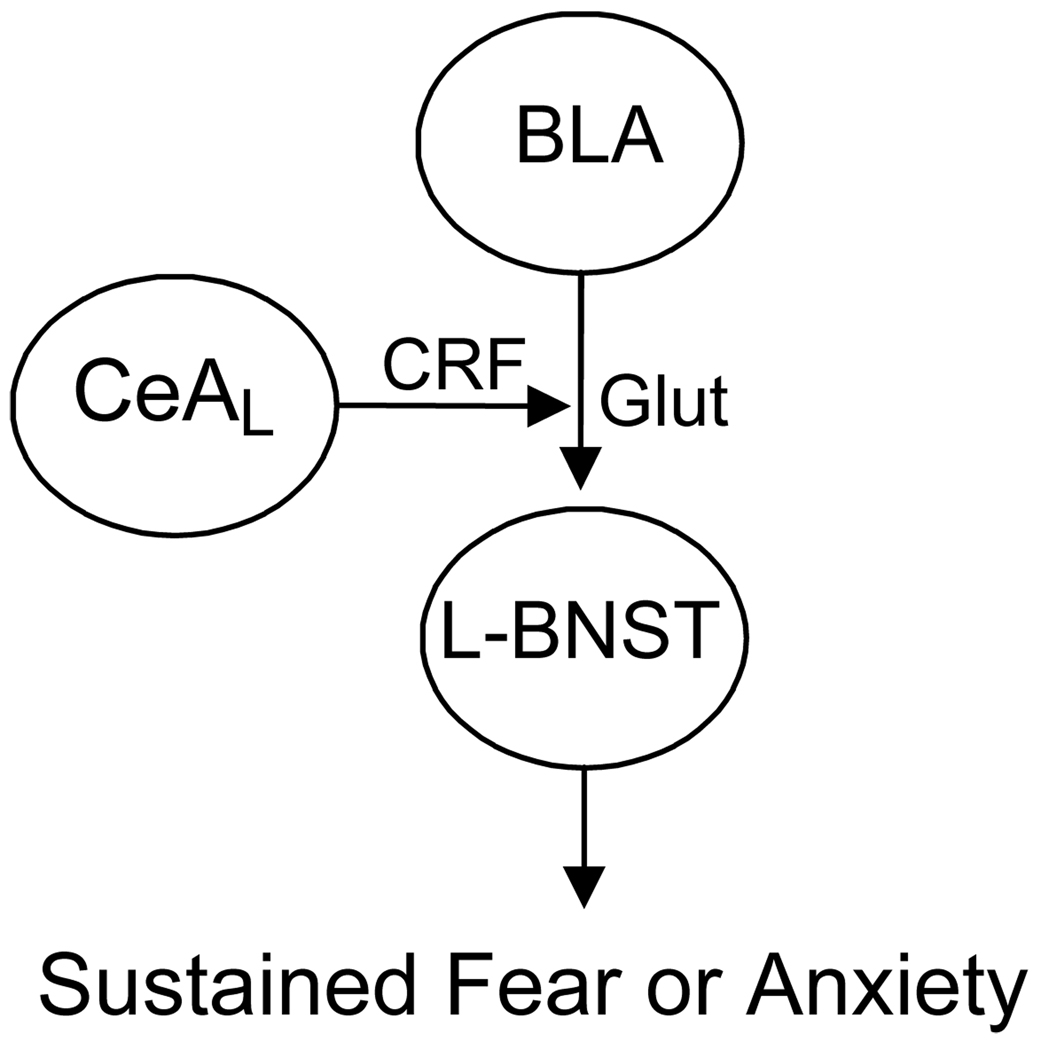
Figure 15.
Neural Circuit Model of Sustained Fear. In this model, threat-related information is relayed from the BLA to the BNST. The flow of information is gated by CRF, perhaps released from CeAL neurons, which binds to receptors on BLA terminals. Activation of these receptors increases glutamate release and, therefore, excitatory drive onto the BNST neurons that project to other areas, mostly in the brainstem, that mediate behaviors influenced by fear.
Figure 16.
The facilitatory effect on startle of i.c.v. CRF infusions was blocked by infusions into the basolateral amygdala of an NBQX (3 µg) / muscimol (0.1 µg) cocktail. As previous findings indicate that i.c.v.-infused CRF increases startle by acting on CRF receptors in the BNST and not the amygdala, these data suggest that CRF may increase startle by amplifying excitatory amygdala input onto BNST neurons.
Results from Dr. Maria Forray’s laboratory (Forray et al., 2005, and personal communication) are also relevant to this proposal. They have found that [K+]-evoked glutamate release is persistently elevated in rats receiving chronic immobilization stress (2 hr/day for 15 days), and that this increase is normalized by intra-BNST infusions of the CRF-R1 antagonist NBI 27914. Perhaps then, stress activates the BNSTL by increasing CRF levels which then potentiate the release of glutamate from BLA terminals. In fact, chronic or repeated stress of various sorts does increase total (Chappell et al., 1986; Santibanez et al., 2006; Stout et al., 2000) as well as extracellular (Olive et al., 2002) CRF in the BNST of rats, and stress (Albeck et al., 1997; Hatalski et al., 1998; Hsu et al., 1998; Kalin et al., 1994; Makino et al., 1999)] and the stress-related hormone corticosterone both increase CRF mRNA in CeA neurons (Makino et al., 1994; Shepard et al., 2000; Thompson et al., 2004; Watts and Sanchez-Watts, 1995).
A similar role has been posited for dopamine, which also enhances glutamatergic transmission in the BNST and appears to do so in a CRF-R1-dependent manner (Kash et al., 2008). In fact, Meloni et al. (2006a) reported that CRF-enhanced startle, in rats, is blocked by systemic injections of the D1 receptor antagonist SCH 23390. Vinkers et al. (2007) were unable to replicate this in mice however, and also reported normal CRF-enhanced startle in D1-receptor knockouts. They suggested that species or dosage differences (including non-selectivity at the doses used by Meloni and colleagues) may have accounted for the different outcomes of those two studies. Systemic administration of the D1 receptor agonist chloro-APB also increases startle (Meloni and Davis, 1999), but we have not been able to disrupt this effect with oral administration of the CRF-R1 antagonist GSK876008 (Miles and Davis, unpublished observations). Overall, these behavioral observations suggest in rats at least that dopamine may act downstream from BNST CRF receptors. Anatomical data however indicate that dopamine-positive terminals synapse onto CRF-positive BNSTL neurons (Meloni et al., 2006a; Phelix et al., 1994). In fact, these projections appear to play a trophic role as judged by the marked decrease in BNST CRF mRNA following 6-OHDA lesions to the median forebrain bundle (Day et al., 2002). In reality, of course, the circuitry is likely to be much more complex than a simple serial pathway in either direction. In any case, the existing anatomical, electrophysiological, and behavioral data suggest that further analyses of this potentially important influence on BNST function is well-warranted.
Does BNST Plasticity Mediate Stress-Induced Anxiety?
As discussed earlier, the standard model of fear conditioning suggests that conditioning-related plasticity occurs in the BLA, and that the CeA serves as a more-or-less passive relay to various other brain areas that mediate the specific signs and symptoms of fear. A revisionist view suggests that the CeA may play a more active role, perhaps being a site of plasticity itself (c.f., Pare et al., 2004). Consistent with this latter view is the finding that intra-CeA infusions of the protein synthesis inhibitor anisomycin disrupt fear memory consolidation (Wilensky et al., 2006). These sorts of studies must be interpreted with caution however as the BLA and CeA lie immediately adjacent to one another and as such, drugs infused into one may readily diffuse to the other. This is not a problem with the BNST however, which is well removed from both structures.
Using the same paradigm that we had previously used to show that intra-BNST NBQX infusions block the expression of non-associative stress-induced startle increases, we also evaluated the involvement of the BNST in the development of sensitization. For these experiments, NBQX was infused prior to shock and rats were retested 48 hours later (as before, in the presence of different contextual elements so as to minimize conditioned fear responses to the context). In contrast to vehicle-infused rats, those that received NBQX infusions showed virtually no evidence of sensitization (Fig. 17, left panel), despite vigorous reactions to the footshock itself (i.e., there was no evidence that the disruption of sensitization was secondary to an impairment of the animals ability to perceive the US). Many days later, after sensitization in controls had decayed to baseline, rats were tested once more – this time in the presence of the same contextual elements that were present during shock. As predicted, startle again increased in rats that had previously received vehicle infusions, but did not increase in rats that had received pre-shock NBQX infusions (Fig. 17, right panel). Thus, AMPA receptor activation in the BNST appears to be necessary not only for the expression of sensitization, as shown earlier, but also for the development of non-associative sensitization and also associative context fear.
Figure 17.
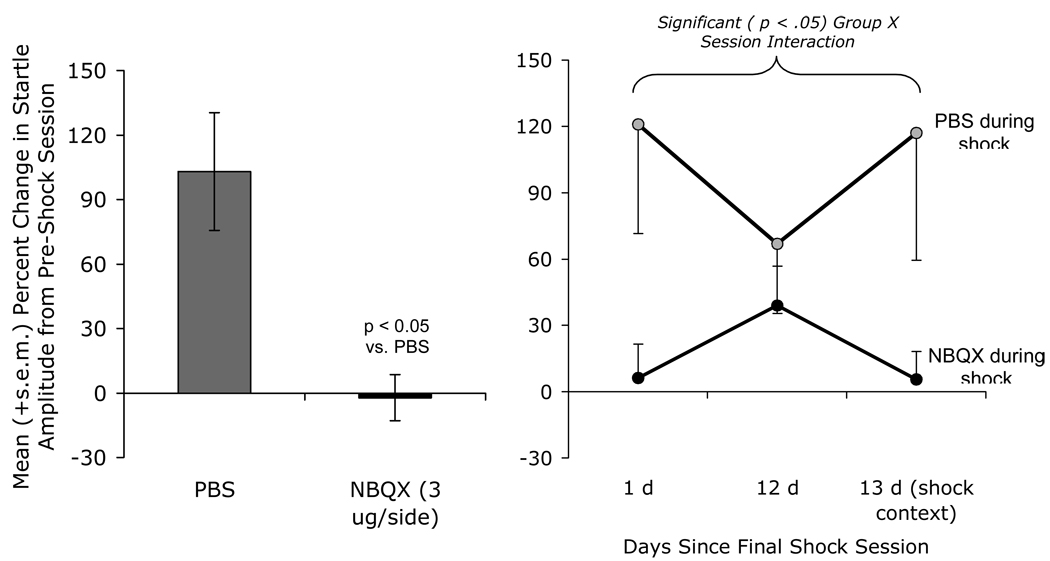
Pre-shock NBQX infusions (3 µg/side) prevented the development of non-associative shock-induced sensitization (left panel). A subset of these rats (N=19) received a 2nd test in the neutral context on day 12, and one more test in the shock context on day 13, after the sensitized response had decayed (in this experiment, by approximately 50%), the two groups performed similarly when tested in the neutral context. When tested in the shock context, the groups again diverged with rats that received pre-shock PBS infusions showing startle levels approximately 120% greater than their pre-shock values, while rats that had received pre-shock NBQX infusions showing startle levels nearly identical to pre-shock levels. Thus, intra-BNST NBQX infusions appeared to prevent the development not only of non-associative sensitization, but also of fear conditioning to the shock-associated context.
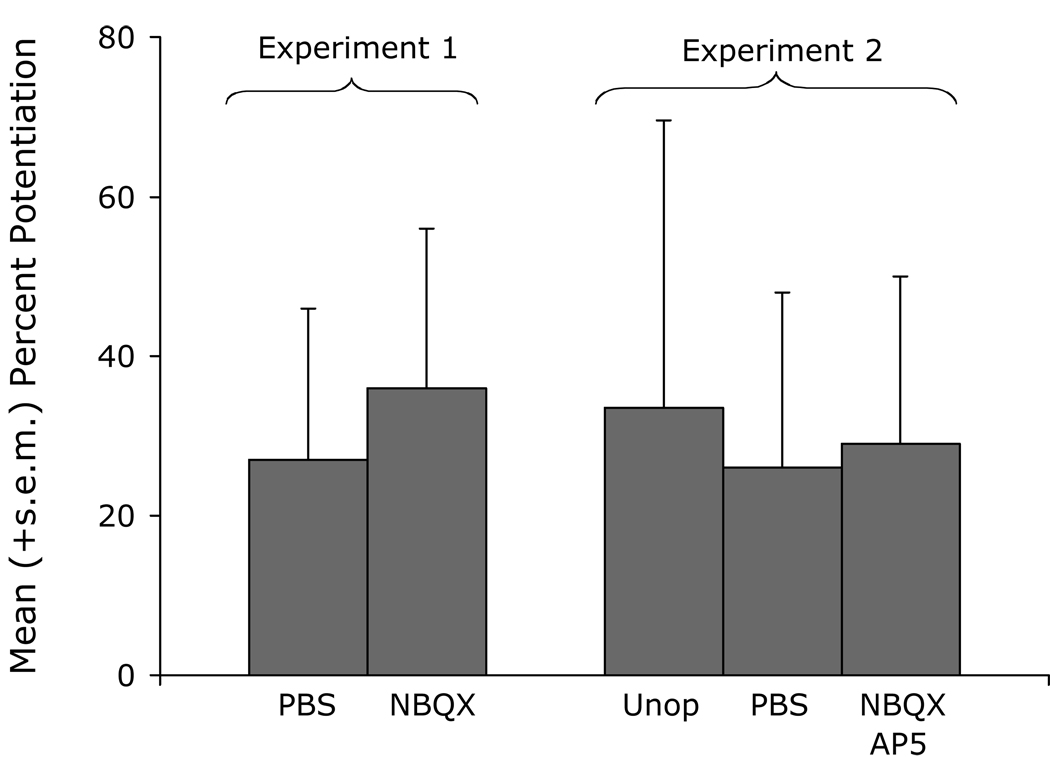
In the same series of experiments, we found no evidence that the BLA participates in the development of sensitization (Fig. 18). Specifically, neither NBQX nor an NBQX/AP5 cocktail disrupted sensitization when infused into the BLA prior to footshock, even though both of these treatments have been found in many previous studies to disrupt associative fear conditioning when infused into the BLA prior to phasic fear conditioning (c.f., Walker and Davis, 2002c). These data suggest that the BNST may be a more promising target than the amygdala with respect to the development of compounds with prophylactic value for stress-induced anxiety. The abundance of so many different peptides in the BNST, and in CeAL neurons which project to BNSTL (e.g., Batten et al., 2002; Cassell et al., 1986; Eiden et al., 1985; Gray and Magnuson, 1992; Haring et al., 1991; Harrigan et al., 1994; Honkaniemi, 1992; Honkaniemi et al., 1992; Huber et al., 2005; Ju et al., 1989; McDonald, 1989; Moga et al., 1989; Roberts et al., 1980; Roberts et al., 1982; Sakanaka et al., 1981; Shimada et al., 1989a; Shimada et al., 1989b; Woodhams et al., 1983; Wray and Hoffman, 1983) suggest many possibilities.
Figure 18.
Pre-shock infusions of NBQX (Experiment 1) or of an NBQX/AP5 cocktail (Experiment 2) into the BLA had no apparent effects on the development of shock-induced sensitization.
Our finding that BNST inactivation disrupts the development of sensitization without obvious effects on shock responses has parallels in several other studies from very different domains. Of particular interest are findings from Delfs et al. (2000) who found that intra-BNST infusions of a noradrenergic β1/β2 receptor antagonist cocktail (betaxolol + ICI 118,551), or of the non-selective β-receptor antagonist S-propranolol, or of the noradrenergic α2 agonist clonidine (which inhibits norepinephrine release via actions on presynaptic receptors (Forray et al., 1995; Forray et al., 1997) each block the development of withdrawal-induced place aversions with only modest (e.g., teeth chattering, eye twitching) or non-existent (e.g., wet dog shakes, jump escapes) effects on the acute somatic signs of withdrawal. Similar results have been reported by Cecchi et al. (2007). The noradrenergic system was targeted in these studies because of the extraordinarily high levels of norepinephrine found in the BNST (e.g., Brownstein et al., 1974; Kilts and Anderson, 1986) and released during stress (Pacak et al., 1995; Pardon et al., 2002).
Results from Deyama and colleagues, who have investigated the BNST’s role in pain and pain-motivated behavior, are again very similar. They found that conditioned place avoidance to a context associated with either somatic (intraplantar formalin injection) or visceral (intra-peritoneal acetic acid injection) pain is prevented by both pre-training excitotoxic BNST lesions (Deyama et al., 2007) and also by pre-training intra-BNST infusions of the noradrenergic β1-receptor antagonist timolol (Deyama et al., 2008a; Deyama et al., 2008b). These same manipulations do not however disrupt pain behavior itself (i.e., the immediate response to acetic acid or formalin injection).
Finally, Waddell et al. (2008) found that pre-shock intra-BNST infusions of the CRF receptor antagonist αhCRF disrupt the shock-induced reinstatement of extinguished fear-potentiated startle – an effect thought by many to be mediated by context fear (c.f., Bouton et al., 2006). It should also be noted however that a very recently published study found no effect of pre-defeat intra-BNST muscimol infusions on the development of conditioned defeat behavior in Syrian Hamsters (Markham et al., 2008). Thus, there is some behavioral and/or neurochemical specificity to these effects.
All in all then, results from several studies from very different domains indicate that normal BNST activity is necessary for the development of both associative and non-associative responses to a variety of aversive stimuli, in ways that cannot be accounted for by a simple mediation of aversion itself.
Direct Evidence of Stress-Related Plasticity in the BNST
The behavioral results reviewed above provide direct evidence for a critical involvement of the BNST in stress-induced plasticity, but only indirect evidence that such plasticity occurs in the BNST itself. Clearly however, the BNST is capable of such plasticity. In one of the first such demonstrations, Vyas et al. (2003) showed that chronic restraint stress (2 hr day/10 days) led to significant changes in dendritic morphology in the BNST, with increases being observed for the number of dendritic branch points and also for dendritic length – effects which were not observed in the amygdala. Similarly, Pego et al. (2008) has reported that a 4-week period of chronic unpredictable stress led to an overall increase of BNST volume and, at a finer level of analysis, of dendritic length for bipolar BNST neurons and total spine number. These effects were accompanied by and significantly correlated with an increase in anxiety-like behavior on the elevated plus maze (shown elsewhere to be modulated by BNST manipulations – e.g., Cecchi et al., 2002; Sahuque et al., 2006; and see also Waddell et al., 2006 for lesion effects on the conceptually similar zero-maze) whereas BNST-independent fear-potentiated startle was unaffected. Stress-related changes have also been found in the BNST, but again not in the amygdala, for the expression of the Kv4.2 potassium-channel subunit following a 4-day regimen of 1 hr/day restraint stress (Guo et al., 2007), and so too have changes in the expression levels of mRNA for different 5HT receptor subtypes (Hammack and Rainnie, this issue). In short, it seems increasingly clear that the BNST has a unique relationship with stress, being especially vulnerable to its effects and mediating many of its behavioral and autonomic consequences.
Summary, Conclusions, and Possible Clinical Relevance
A few short years ago, it was possible to author a review on the BNST’s involvement in stress and anxiety that was both comprehensive and concise. That this has become increasingly difficult is testimony to the ever-growing body of relevant findings and interest in this area. We have focused here on those findings most directly relevant to our hypothesis that the BNST plays a special role in longer-duration, sustained, anxiety-like responses and CeAM in shorter-duration fear responses. As the distinction between fear and anxiety remains uncertain, both at a definitional and biological level, it is premature to suggest that the BNST plays a special role in clinical anxiety (or other mood disorders such as depression which are increasingly thought to be related to anxiety and which also appear, based on results from animal models, to involve the BNST – e.g., Cooper and Huhman, 2005; Hammack et al., 2004; Jasnow et al., 2004). There is however a growing body of circumstantial evidence which is consistent with that view.
In healthy humans for example, context-elicited startle increases appear to be selectively vulnerable (i.e., compared to phasic, explicitly cued increases) to anxiolytics such as acute benzodiazepines (Grillon et al., 2006) and chronic SSRIs (Grillon et al., 2009). Moreover, in PTSD and panic disorder patients, it is these sustained rather short-duration startle increases that appear to be selectively exaggerated (c.f., Grillon, 2008).
In rats, Santibanez et al. (2006) have shown that chronic desipramine (a tricyclic antidepressant) prevents the lasting increase in CRF-like immunoreactivity in the BNSTL of animals exposed to repeated restraint stress. Also in rats, anxiolytic doses of the benzodiazepine chlordiazepoxide and the SSRI fluoxetine (chronic administration), assessed in the novelty-induced hypophagia paradigm, reduce novelty-induced fos activation in the BNSTL (Bechtholt et al., 2008; but see also Lino-de-Oliveira et al., 2001). Conversely, an analysis of Fos responses to several anxiogenic drugs indicates activation in several brain areas including, for each, BNSTL (Singewald et al., 2003). These effects of anxiolytic and anxiogenic treatments on BNST function act as a bridge between the animal and human literature but are at best circumstantial. Imaging of the BNST in anxious humans would perhaps be more validating, but is technically challenging due to the relatively small size of the BNST and its close proximity to the lateral ventricle. Nonetheless, a recent fMRI study (Straube et al., 2007) did find BNST activation in human subjects with spider phobias, but not control subjects, during anticipation of visual imagery associated with phobogenic stimuli (and see also Kalin, 2005 for what appears to be activation of the non-human primate BNST during a human intruder paradigm).
The results of these types of studies point increasingly to the BNST as a nodal point for at least some types of anxiety responses, and as a potentially important area-of-interest therefore for the development of new pharmacologic approaches to the treatment of anxiety and perhaps even depression. We have focused on the role of CRF, several other groups have explored the role of norepinephrine (Crestani et al., 2008; Delfs et al., 2000; Fendt et al., 2005; Liang et al., 2001; Morilak et al., 2003), and there is growing interest in the possible modulation of BNST-dependent anxiety by sex hormones (c.f., Toufexis, 2007) – an interest fueled by well-described differences in the incidence of anxiety disorders in men and women (c.f., Shekhar et al., 2001) and by findings that the BNST in rats as well as humans is sexually dimorphic (although these differences occur predominantly in the medial BNST which has not been implicated as consistently in anxiety anxiety - Allen and Gorski, 1990; Chung et al., 2002). Still, these initial forays are likely to be only scratches on the surface of a very deep vessel of possibilities. In fact the abundance of so many neuroactive peptides and peptide receptors within the BNST and its afferents, not only in rats as noted elsewhere, but also in humans (e.g., Lesur et al., 1989; Walter et al., 1991), indicates that there are many unexplored opportunities and avenues of investigation. The BNST is indeed target-rich environment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
GSK876008 is a novel non-peptide CRF-R1 antagonist synthesized by the Medicinal Chemistry Department of GlaxoSmithKline Medicines Research Centre (Verona, Italy). Following oral administration, GSK876008 shows a CNS bioavailability of 66% (58–73%, CL95) and a half life of 1.6 h (1.4–1.7 h CL95) at 10 mg/kg, with exposure increasing linearly up to doses of 300 mg/kg. In vitro, GSK876008 shows a functional potency (pA2) of 7.14 ± 0.12 on recombinant human CRF1 receptors expressed in CHO cells, but is inactive on human CRF2 receptors or the CRF binding protein, or on 83 other G-protein coupled receptors, channels, enzymes and transporters up to a concentration of 10 µM (di Fabio et al., 2008).
The procedure differed from that shown in Figure 5 in that rats received 14 (instead of 8) 0.4-mA (instead of 0.35 mA) footshocks that were randomly distributed across (instead of co-terminating with) 2 8-min fixed-duration CSs (instead of 8 variable duration CSs). The 1st CS began 5 minutes after the rats were placed into the test chamber. The 2nd 8-min CS began 8-min after termination of the 1st. Rats were removed from the test chamber 8 minutes after termination of the 2nd. The CS was a constant low-frequency-filtered noise rather than a clicker.
REFERENCES
- Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, et al. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci. 1997;17:4895–4903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid G, De Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system. New York: Academic Press; 1995. pp. 495–578. [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Allen LS, Gorski RA. Sex difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol. 1990;302:697–706. doi: 10.1002/cne.903020402. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. The hippocampus and Pavlovian fear conditioning: reply to Bast et al. Hippocampus. 2002;12:561–565. doi: 10.1002/hipo.10071. [DOI] [PubMed] [Google Scholar]
- Bartfai T, Iverfeldt K, Fisone G, Serfozo P. Regulation of the release of coexisting neurotransmitters. Annu Rev Pharmacol Toxicol. 1988;28:285–310. doi: 10.1146/annurev.pa.28.040188.001441. [DOI] [PubMed] [Google Scholar]
- Batten TF, Gamboa-Esteves FO, Saha S. Evidence for peptide co-transmission in retrograde- and anterograde-labelled central nucleus of amygdala neurones projecting to NTS. Auton Neurosci. 2002;98:28–32. doi: 10.1016/s1566-0702(02)00026-7. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Valentino RJ, Lucki I. Overlapping and distinct brain regions associated with the anxiolytic effects of chlordiazepoxide and chronic fluoxetine. Neuropsychopharmacology. 2008;33:2117–2130. doi: 10.1038/sj.npp.1301616. [DOI] [PubMed] [Google Scholar]
- Behan DP, Khongsaly O, Liu XJ, Ling N, Goland R, Nasman B, et al. Measurement of corticotropin-releasing factor (CRF), CRF-binding protein (CRF-BP), and CRF/CRF-BP complex in human plasma by two-site enzyme-linked immunoabsorbant assay. J Clin Endocrinol Metab. 1996;81:2579–2586. doi: 10.1210/jcem.81.7.8675581. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. The mouse defense test battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol. 2003;463:97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yudko EB, Rodgers RJ, Blanchard DC. Defense system Psychophamacol (Berl): an ethological approach to the pharmacology of fear and anxiety. Behav Brain Res. 1993;58:155–165. doi: 10.1016/0166-4328(93)90100-5. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Activity-dependent modulation of nerve terminal excitation in a mammalian peptidergic system. Trends Neurosci. 1991;14:28–30. doi: 10.1016/0166-2236(91)90180-3. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Brownstein M, Saavedra MM, Palkovits M. Norepinephrine and dopamine in the limbic system of the rat. Brain Res. 1974;79:431–436. doi: 10.1016/0006-8993(74)90440-5. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: cytological, hodological, and immunocytochemical study. J Comp Neurol. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Capriles N, Watson SJ, Akil H. β 1 adrenergic receptors in the bed nucleus of stria terminalis mediate differential responses to opiate withdrawal. Neuropsychopharmacology. 2007;32:589–599. doi: 10.1038/sj.npp.1301140. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, et al. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WC, DeVries GJ, Swaab DF. Sexual differentiation of the bed nucleus of the stria terminalis humans may extend into adulthood. J Neurosci. 2002;22:1027–1103. doi: 10.1523/JNEUROSCI.22-03-01027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 2003;23:9445–9451. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DR, Pare D. Reciprocal changes in the firing probability of lateral and central medial amygdala neurons. J Neurosci. 1999;19:836–844. doi: 10.1523/JNEUROSCI.19-02-00836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Corticotropin-releasing factor type II (CRF2) receptors in the bed nucleus the stria terminalis modulate conditioned defeat in syrian hamsters (mesocricetus auratus) Behav Neurosci. 2005;119:1042–1051. doi: 10.1037/0735-7044.119.4.1042. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazipines. Pharmacol Biochem Behav. 1980;13:167. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Crestani CC, Alves FH, Tavares RF, Correa FM. Role of the bed nucleus of the stria terminalis in the cardiovascular responses to acute restraint stress in rats. Stress. 2008:1. doi: 10.1080/10253890802331477. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The amygdala. volume 2. Oxford, United Kingdom: Oxford University Press; 2000. pp. 213–287. [Google Scholar]
- Davis M, Schlesinger LS, Sorenson CA. Temporal specificity of fear-conditioning: effects of different conditioned stimulus-unconditioned stimulus intervals on the fear-potentiated startle effect. J Exp Psychol Anim Behav Process. 1989;15:295–310. [PubMed] [Google Scholar]
- Davis M, Whalen P. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Day HE, Curran EJ, Watson SJ, Jr., Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1β. J Comp Neurol. 1999;413:113–128. [PubMed] [Google Scholar]
- Day HE, Vittoz NM, Oates MM, Badiani A, Watson SJ, Jr., Robinson TE, et al. A 6-hydroxydopamine lesion of the mesostriatal dopamine system decreases the expression of corticotropin releasing hormone and neurotensin mRNAs in the amygdala and bed nucleus of the stria terminalis. Brain Res. 2002;945:151–159. doi: 10.1016/s0006-8993(02)02747-6. [DOI] [PubMed] [Google Scholar]
- de Jongh R, Groenink L, van der Gugten J, Olivier B. The light-enhanced startle paradigm as a putative animal model for anxiety: Effects of chlordiazepoxide, flesinoxan and fluvoxamine. Psychophamacology (Berl) 2002;159:176–180. doi: 10.1007/s002130100914. [DOI] [PubMed] [Google Scholar]
- de Jongh R, Groenink L, van der Gugten J, Olivier B. Light-enhanced and fear-potentiated startle: Temporal characteristics and effects of α-helical corticotropin-releasing hormone. Biol Psychiatry. 2003;54:1041–1048. doi: 10.1016/s0006-3223(03)00468-2. [DOI] [PubMed] [Google Scholar]
- Deak T, Nguyen KT, Watkins LR, Spencer RL, Maier SF, et al. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- DeFries JC, Hegmann JP, Weir MW. Open-field behavior in mice: Evidence for a major gene effect mediated by the visual system. Science. 1966;154:1577–1579. doi: 10.1126/science.154.3756.1577. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Deyama S, Katayama T, Kondoh N, Nakagawa T, Kaneko S, Yamaguchi T, et al. Role of enhanced noradrenergic transmission within the ventral bed nucleus of the stria terminalis in visceral pain-induced aversion in rats. Behav Brain Res. 2008a doi: 10.1016/j.bbr.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Deyama S, Katayama T, Ohno A, Nakagawa T, Kaneko S, Yamaguchi T, et al. Activation of the β-adrenoceptor-protein kinase a signaling pathway within the ventral bed nucleus of the stria terminalis mediates the negative affective component of pain in rats. J Neurosci. 2008b;28:7728–7736. doi: 10.1523/JNEUROSCI.1480-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyama S, Nakagawa T, Kaneko S, Uehara T, Minami M. Involvement of the bed nucleus of the stria terminalis in the negative affective component of visceral and somatic pain in rats. Behav Brain Res. 2007;176:367–371. doi: 10.1016/j.bbr.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Di Fabio R, St-Denis Y, Sabbatini FM, Andreotti D, Arban R, Bernasconi G, et al. Synthesis and pharmacological characterization of novel druglike corticotropin-releasing factor 1 antagonists. J Med Chem. 2008;51:7370–7379. doi: 10.1021/jm800744m. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nucleus of the stria terminalis. J Comp Neurol. 2004;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res - Brain Res Rev. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001b;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Eiden LE, Hokfelt T, Brownstein MJ, Palkovits M. Vasoactive intestinal polypeptide afferents to the bed nucleus of the stria terminalis in the rat: An immunohistochemical and biochemical study. Neuroscience. 1985;15:999–1013. doi: 10.1016/0306-4522(85)90249-0. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychophamacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Fanselow M, LeDoux J. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. J Neurosci. 2005;25:5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Hyde JRG. Can social interaction be used to measure anxiety. Brit J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray MI, Andres ME, Bustos G, Gysling K. Regulation of endogenous noradrenaline release from the bed nucleus of stria terminalis. Biochem Pharmacol. 1995;49:687–692. doi: 10.1016/0006-2952(94)00498-b. [DOI] [PubMed] [Google Scholar]
- Forray MI, Bustos G, Gysling K. Regulation of norepinephrine release from the rat bed nucleus of the stria terminalis: In vivo microdialysis studies. J Neurosci Res. 1997;50:1040–1046. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1040::AID-JNR15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Forray MI, Gonzales M, Hadwed N, Gonzalez MP. Chronic immobilization stress increases glutamatergic transmission in the rat bed nucleus of the stria terminalis. In vivo microdialysis studies. Abstr Soc Neurosci. 2005 Program No. 187.3. [Google Scholar]
- Gewirtz JC, McNish KA, Davis M. Lesions of the bed nucleus of the stria terminalis block sensitization of the acoustic startle reflex produced by repeated stress, but not fear-potentiated startle. Prog Neuro-Psychophamacol Biol Psychiat. 1998;22:625–648. doi: 10.1016/s0278-5846(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. J Comp Neurol. 1987;262:365–374. doi: 10.1002/cne.902620304. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Gray TS, Piechowski RA, Yracheta JM, Rittenhouse PA, Bethea CL, Van de Kar LD. Ibotenic acid lesions in the bed nucleus of the stria terminalis attenuate conditioned stress-induced increases in prolactin, ACTH and corticosterone. Neuroendocrinology. 1993;57:517–524. doi: 10.1159/000126400. [DOI] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: Evidence from startle studies. Psychophamacology (Berl) 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Pine DS, Lissek S, Lawley M, Ellis V, et al. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C, Chavis C, Covington MF, Pine DS. Two-week treatment with the selective serotonin reuptake inhibitor citalopram reduces contextual anxiety but not cued fear in healthy volunteers: A fear-potentiated startle study. Neuropsychopharmacology. 2009;34:964–971. doi: 10.1038/npp.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove EA. Efferent connections of the substantia innominata in the rat. J Comp Neurol. 1988;277:347–364. doi: 10.1002/cne.902770303. [DOI] [PubMed] [Google Scholar]
- Guo J, Hazra R, Rainnie DG. Expression of potassium channel interacting proteins (KChIPs) and channel subunits in neurons of the anterolateral bed nucleus of stria terminalis (BNSTAL): modulation by stress? Abstr Soc Neurosci. 2007 Program Number 98.5. [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Haring C, Humpel C, Skofitsch G, Krobath J, Javorsky F, Saria A. Calcitonin gene-related peptide the amygdaloid complex of the rat: Immunohistochemical and quantitative distribution, drug effects on calcium dependent, potassium-evoked in vitro release. Synapse. 1991;8:261–269. doi: 10.1002/syn.890080404. [DOI] [PubMed] [Google Scholar]
- Harrigan EA, Magnuson DJ, Thunstedt GM, Gray TS. Corticotropin releasing factor neurons innervated by calcitonin gene-related peptide terminals in the rat central amygdaloid nucleus. Brain Res Bull. 1994;33:529–534. doi: 10.1016/0361-9230(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Hatalski CG, Guirguis C, Baram TZ. Corticotropin releasing factor mRNA expression in hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated repeated acute stress in the immature rat. J Neuroendocrinol. 1998;10:663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikichi T, Akiyoshi J, Yamamoto Y, Tsutsumi T, Isogawa K, Nagayama H. Suppression of conditioned fear by administration of CRF receptor antagonist CP-154,526. Pharmacopsychiatry. 2000;33:189–193. doi: 10.1055/s-2000-7587. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav Neurosci. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Fear-potentiated startle using an auditory conditioned stimulus: effect lesions of the amygdala. Physiol Behav. 1987;39:403–408. doi: 10.1016/0031-9384(87)90242-3. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. The efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behav Neurosci. 1991;105:826–842. doi: 10.1037//0735-7044.105.6.826. [DOI] [PubMed] [Google Scholar]
- Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res. 1985;58:379–391. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- Honkaniemi J. Colocalization of peptide- and tyrosine hydroxylase-like immunoreactivities with fos-immunoreactive neurons in rat central amygdaloid nucleus after immobilization stress. Brain Res. 1992;598:107–113. doi: 10.1016/0006-8993(92)90173-7. [DOI] [PubMed] [Google Scholar]
- Honkaniemi J, Pelto-Huikko M, Rechardt L, Isola J, Lammi A, Fuxe K, et al. Colocalization of peptide and glucocorticoid receptor immunoreactivities in rat central amygdaloid nucleus. Neuroendocrinology. 1992;55:451–459. doi: 10.1159/000126156. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Chen FL, Takahashi LK, Kalin NH. Rapid stress-induced elevations in corticotropin-releasing hormone mRNA in rat central amygdala nucleus and hypothalamic paraventricular nucleus: an in situ hybridization analysis. Brain Res. 1998;788:305–310. doi: 10.1016/s0006-8993(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Ip NY. Pattern of presynaptic nerve activity can determine the type of neurotransmitter regulating a postsynaptic event. Nature. 1994;311:472–474. doi: 10.1038/311472a0. [DOI] [PubMed] [Google Scholar]
- Iwata J, LeDoux JE, Meeley MP, Arneric S, Reis DJ. Intrinsic neurons in the amygdala field projected to by the medial geniculate body mediate emotional responses conditioned to acoustic stimuli. Brain Res. 1986;383:195–214. doi: 10.1016/0006-8993(86)90020-x. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav Neurosci. 2004;118:1052–1061. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Johnston JB. Further contributions to the study of the evolution of the forebrain. J Comp Neurol. 1923;35:337–481. [Google Scholar]
- Jolkkonen E, Pitkanen A. Intrinsic connections of the rat amygdaloid complex: Projections originating in the central nucleus. J Comp Neurol. 1998;395:53–72. doi: 10.1002/(sici)1096-9861(19980525)395:1<53::aid-cne5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW, Simerly RB. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. chemoarchitecture. J Comp Neurol. 1989;280:603–621. doi: 10.1002/cne.902800410. [DOI] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in bac transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol. 2008;511:479–496. doi: 10.1002/cne.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Takahashi LK. Fear-motivated behavior induced by prior shock experience is mediated by corticotropin-releasing hormone systems. Brain Res. 1990;509:80–84. doi: 10.1016/0006-8993(90)90311-x. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Takahashi LK, Chen FL. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 1994;656:182–186. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehne JH, Gallager DW, Davis M. Strychnine: Brainstem and spinal mediation of excitatory effects on acoustic startle. Eur J Pharmacol. 1981;76:177–186. doi: 10.1016/0014-2999(81)90499-4. [DOI] [PubMed] [Google Scholar]
- Kiernan MJ, Westbrook RF, Cranney J. Immediate shock, passive avoidance, and potentiated startle: Implications for the unconditioned response to shock. Animal Learn Behav. 1995;23:22–30. [Google Scholar]
- Kilts C, Anderson C. The simultaneous quantification of dopamine, norepinephrine and epinephrine in micropunched rat brain nuclei by on-line trace enrichment HPLC with electro-chemical detection distribution of catecholamines in the limbic system. Neurochem Int. 1986;9:437–455. doi: 10.1016/0197-0186(86)90086-0. [DOI] [PubMed] [Google Scholar]
- Koo JW, Han JS, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci. 2004;24:7654–7662. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, bed nucleus of the stria terminalis and amygdala in the excitatory effect of corticotropin releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Fitz S, Johnson PL, Shekhar A. Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacology. 2008;33:2586–2594. doi: 10.1038/sj.npp.1301674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesur A, Gaspar P, Alvarez C, Berger B. Chemoanatomic compartments in the human bed nucleus of the stria terminalis. Neuroscience. 1989;32:181–194. doi: 10.1016/0306-4522(89)90117-6. [DOI] [PubMed] [Google Scholar]
- Liang KC, Chen HC, Chen DY. Posttraining infusion of norepinephrine and corticotropin releasing factor into the bed nucleus of the stria terminalis enhanced retention in an inhibitory avoidance task. Chin J Physiol. 2001;44:33–43. [PubMed] [Google Scholar]
- Liang KC, Melia KR, Campeau S, Falls WA, Miserendino MJD, Davis M. Lesions of the central nucleus of the amygdala, but not of the paraventricular nucleus of the hypothalamus, block the excitatory effects of corticotropin releasing factor on the acoustic startle reflex. J Neurosci. 1992a;12:2313–2320. doi: 10.1523/JNEUROSCI.12-06-02313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Miserendino MJD, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J Neurosci. 1992b;12:2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lino-de-Oliveira C, Sales AJ, Del Bel EA, Silveira MC, Guimaraes FS. Effects of acute and chronic fluoxetine treatments on restraint stress-induced fos expression. Brain Res Bull. 2001;55:747–754. doi: 10.1016/s0361-9230(01)00566-4. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Rudehill A, Sollevi A. Frequency- and reserpine-dependent chemical coding of sympathetic transmission: Differential release of noradrenaline and neuropeptide y from pig spleen. Neurosci Lett. 1986;63:96–100. doi: 10.1016/0304-3940(86)90020-0. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- Makino S, Shibasaki T, Yamauchi N, Nishioka T, Mimoto T, Wakabayashi I, et al. Psychological stress increased corticotropin-releasing hormone mRNA and content in the central nucleus of the amygdala but not in the hypothalamic paraventricular nucleus in the rat. Brain Res. 1999;850:136–143. doi: 10.1016/s0006-8993(99)02114-9. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Markham CM, Norvelle A, Huhman KL. Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in syrian hamsters. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol. 1982;208:401–418. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Coexistence of somatostatin with neuropeptide Y, but not with cholecystokinin or vasoactive intestinal peptide, in neurons of the rat amygdala. Brain Res. 1989;500:37–45. doi: 10.1016/0006-8993(89)90297-7. [DOI] [PubMed] [Google Scholar]
- McNish KA, Gewirtz JC, Davis M. Evidence of contextual fear conditioning following lesions of the hippocampus: A disruption of freezing but not fear-potentiated startle. J Neurosci. 1997;17:9353–9360. doi: 10.1523/JNEUROSCI.17-23-09353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNish KA, Gewirtz JC, Davis M. Disruption of contextual freezing, but not contextual blocking of fear-potentiated startle, after lesions of the dorsal hippocampus. Behav Neurosci. 2000;114:64–76. doi: 10.1037//0735-7044.114.1.64. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Davis M. Enhancement of the acoustic startle response in rats by the dopamine D1 receptor agonist SKF82958. Psychophamacology (Berl) 1999;144:373–380. doi: 10.1007/s002130051020. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA., Jr. Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006a;26:3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Jackson A, Gerety LP, Cohen BM, Carlezon WA., Jr Role of the bed nucleus of the stria terminalis (BST) in the expression of conditioned fear. Ann N Y Acad Sci. 2006b;1071:538–541. doi: 10.1196/annals.1364.059. [DOI] [PubMed] [Google Scholar]
- Moga MM, Gray TS. Evidence for corticotropin-releasing factor, neurotensin, and somatostatin in the neural pathway from the central nucleus of the amygdala to the parabrachial nucleus. J Comp Neurol. 1985;241:275–284. doi: 10.1002/cne.902410304. [DOI] [PubMed] [Google Scholar]
- Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: Cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 1989;283:315–332. doi: 10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Cecchi M, Khoshbouei H. Interactions of norepinephrine and galanin in the central amygdala and lateral bed nucleus of the stria terminalis modulate the behavioral response acute stress. Life Sci. 2003;73:715–726. doi: 10.1016/s0024-3205(03)00392-8. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yamamoto R, Fujio M, Suzuki Y, Minami M, Satoh M, et al. Involvement of the bed nucleus of the stria terminalis activated by the central nucleus of the amygdala in the negative affective component of morphine withdrawal in rats. Neuroscience. 2005;134:9–19. doi: 10.1016/j.neuroscience.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Nijsen MJMA, Croiset G, Diamant M, De Wied D, Wiegant VM. CRH signalling in the bed nucleus the stria terminalis is involved in stress-induced cardiac vagal activation in conscious rats. Neuropsychopharmacology. 2001;24:1–10. doi: 10.1016/S0893-133X(00)00167-6. [DOI] [PubMed] [Google Scholar]
- Nose I, Higashi H, Inokuchi H, Nishi S. Synaptic responses of guinea pig and rat central amygdala neurons in vitro. J Neurophysiol. 1991;65:1227–1241. doi: 10.1152/jn.1991.65.5.1227. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Otake K, Nakamura Y. Sites of origin of corticotropin-releasing factor-like immunoreactive projection fibers to the paraventricular thalamic nucleus in the rat. Neurosci Lett. 1995;201:84–86. doi: 10.1016/0304-3940(95)12148-w. [DOI] [PubMed] [Google Scholar]
- Otto T, Poon P. Dorsal hippocampal contributions to unimodal contextual conditioning. J Neurosci. 2006;26:6603–6609. doi: 10.1523/JNEUROSCI.1056-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, McCarty R, Palkovits M, Kopin IJ, Goldstein DS. Effects of immobilization on in vivo release of norepinephrine in the bed nucleus of the stria terminalis in conscious rats. Brain Res. 1995;688:242–246. doi: 10.1016/0006-8993(95)00566-9. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, et al. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002;115:229–242. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. Journal Neurophysiology. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Parsons TC, Otto T. Temporary inactivation of dorsal hippocampus attenuates explicitly nonspatial, unimodal, contextual fear conditioning. Neurobiol Learn Mem. 2008;90:261–268. doi: 10.1016/j.nlm.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Pego JM, Morgado P, Pinto LG, Cerqueira JJ, Almeida OF, Sousa N. Dissociation of the morphological correlates of stress-induced anxiety and fear. Eur J Neurosci. 2008;27:1503–1516. doi: 10.1111/j.1460-9568.2008.06112.x. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 1997;763:247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Catecholamine-CRF synaptic interaction in a septal bed nucleus: afferents of neurons in the bed nucleus of the stria terminalis. Brain Res Bull. 1994;33:109–119. doi: 10.1016/0361-9230(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Pitkanen A. Connectivity of the rat amygdala complex. In: Aggleton JP, editor. Amygdala. New York: Oxford University Press; 2000. pp. 31–116. [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, et al. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci USA. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Alves FH, Reis DG, Crestani CC, Correa FM, Guimaraes FS. Anxiolytic-like effects induced by acute reversible inactivation of the bed nucleus of stria terminalis. Neuroscience. 2008;154:869–876. doi: 10.1016/j.neuroscience.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Richardson R. Shock sensitization of startle: learned or unlearned fear? Behav Brain Res. 2000;110:109–117. doi: 10.1016/s0166-4328(99)00189-8. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Geyer MA, Hauger RL, Coste S, Stenzel-Poore M, Wurst W, et al. CRF1 and CRF2 receptors are required for potentiated startle to contextual but not discrete cues. Neuropsychopharmacology. 2008;34:1494–1503. doi: 10.1038/npp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GW, Woodhams PL, Crow TJ, Polak JM. Loss of immunoreactive vip in the bed nucleus following lesions of the stria terminalis. Brain Res. 1980;195:471–475. doi: 10.1016/0006-8993(80)90082-7. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Woodhams PL, Polak JM, Crow TJ. Distribution of neuropeptides in the limbic system of the rat: the amygdaloid complex. Neuroscience. 1982;7:99–131. doi: 10.1016/0306-4522(82)90156-7. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Hitchcock JM, Sananes CB, Miserendino MJD, Davis M. A direct projection from the central nucleus of the amygdala to the acoustic startle pathway: anterograde and retrograde tracing studies. Behav Neurosci. 1991;105:817–825. doi: 10.1037/0735-7044.105.6.817. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, McGeehan AJ, Kinder JR, Hicks MP, Blanton MG, et al. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: Role of CRF receptor subtypes. Psychophamacology (Berl) 2006;186:122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk T, Johnson P, Fitz S, Shekhar A. Chronic inhibition of GABA synthesis in the bed nucleus of the stria terminalis elicits anxiety-like behavior. J Psychophamacol. 2008;22:633–641. doi: 10.1177/0269881107082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shiosaka S, Takatsuki K, Inagaki S, Takagi H, Senba E, et al. Experimental immunohistochemical studies on the amygdalofugal peptidergic (substance P and somatostatin) fibers in the stria terminalis of the rat. Brain Res. 1981;221:231–242. doi: 10.1016/0006-8993(81)90774-5. [DOI] [PubMed] [Google Scholar]
- Sananes CB, Davis M. N-methyl-D-aspartate lesions of the lateral and basolateral nuclei of the amygdala block fear-potentiated startle and shock sensitization of startle. Behav Neurosci. 1992;106:72–80. doi: 10.1037//0735-7044.106.1.72. [DOI] [PubMed] [Google Scholar]
- Santibanez M, Gysling K, Forray MI. Desipramine prevents the sustained increase in corticotropin-releasing hormone-like immunoreactivity induced by repeated immobilization stress in the rat central extended amygdala. J Neurosci Res. 2006;84:1270–1281. doi: 10.1002/jnr.21023. [DOI] [PubMed] [Google Scholar]
- Schmued LC. Diagonal ventral forebrain continuum has overlapping telencephalic inputs and brainstem outputs which may represent loci for limbic/autonomic integration. Brain Res. 1994;667:175–191. doi: 10.1016/0006-8993(94)91495-8. [DOI] [PubMed] [Google Scholar]
- Schwaber JS, Kapp BS, Higgins GA, Rapp PR. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci. 1982;2:1424–1438. doi: 10.1523/JNEUROSCI.02-10-01424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, McCann UD, Meaney MJ, Blanchard DC, Davis M, Frey KA, et al. Summary of a national institute of mental health workshop: developing animal models of anxiety disorders. Psychophamacology (Berl) 2001;157:327–339. doi: 10.1007/s002130100859. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- Shimada S, Inagaki S, Kubota Y, Kito S, Funaki H, Takagi H. Light and electron microscopic studies of calcitonin gene-related peptide-like immunoreactive terminals in the central nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Exp Brain Res. 1989a;77:217–220. doi: 10.1007/BF00250584. [DOI] [PubMed] [Google Scholar]
- Shimada S, Inagaki S, Kubota Y, Ogawa N, Shibasaki T, Takagi H. Coexistence of peptides (corticotropin releasing factor/neurotensin and substance P/somatostatin) in the bed nucleus of the stria terminalis and central amygdaloid nucleus of the rat. Neuroscience. 1989b;30:377–383. doi: 10.1016/0306-4522(89)90259-5. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Stout SC, Mortas P, Owens MJ, Nemeroff CB, Moreau J. Increased corticotropin-releasing factor concentrations in the bed nucleus of the stria terminalis of anhedonic rats. Eur J Pharmacol. 2000;401:39–46. doi: 10.1016/s0014-2999(00)00412-x. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: Brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychophamacology (Berl) 1986;88:147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Erickson K, Schulkin J, Rosen JB. Corticosterone facilitates retention of contextually conditioned fear and increases CRH mRNA expression in the amygdala. Behav Brain Res. 2004;149:209–215. doi: 10.1016/s0166-4328(03)00216-x. [DOI] [PubMed] [Google Scholar]
- Toufexis D. Region- and sex-specific modulation of anxiety behaviours in the rat. J Neuroendocrinol. 2007;19:461–473. doi: 10.1111/j.1365-2826.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Piechowski RA, Rittenhouse PA, Gray TS. Amygdaloid lesions: Differential effect on conditioned stress and immobilization-induced increases in cortiocosterone and renin secretion. Neuroendocrinology. 1991;15:89–95. doi: 10.1159/000125856. [DOI] [PubMed] [Google Scholar]
- Van Nobelen M, Kokkinidis L. Amygdaloid GABA, not glutamate neurotransmission or mRNA transcription controls footshock-associated fear arousal in the acoustic startle paradigm. Neuroscience. 2006;137:707–716. doi: 10.1016/j.neuroscience.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brain stem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Risbrough VB, Geyer MA, Caldwell S, Low MJ, Hauger RL. Role of dopamine D1 and D2 receptors in CRF-induced disruption of sensorimotor gating. Pharmacol Biochem Behav. 2007;86:550–558. doi: 10.1016/j.pbb.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Waddell J, Bouton ME, Falls WA. Central CRF receptor antagonist α-helical CRF9-41 blocks reinstatement of extinguished fear: the role of the bed nucleus of the stria terminalis. Behav Neurosci. 2008;122:1061–1069. doi: 10.1037/a0013136. [DOI] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle paradigm. Biol Psychiatry. 1997a;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in light-enhanced versus fear-potentiated startle. J Neurosci. 1997b;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Light-enhanced startle: further pharmacological and behavioral characterization. Psychophamacology (Berl) 2002a;159:304–310. doi: 10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Quantifying fear potentiated startle using absolute versus percent increase scoring methods: implications for the neurocircuitry of fear and anxiety. Psychophamacology (Berl) 2002b;164:318–328. doi: 10.1007/s00213-002-1213-0. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. The role of glutamate receptors within the amygdala in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002c;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Walker DL, Yang Y, Ratti E, Corsi M, Trist D, Davis M. Differential effects of the CRF-R1 antagonist GSK876008 on fear-potentiated, light- and crf-enhanced startle suggest preferential involvement in sustained vs phasic threat responses. Neuropsychopharmacology. 2008;34:1533–1542. doi: 10.1038/npp.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Mai JK, Lanta L, Gorcs T. Differential distribution of immunohistochemical markers in the bed nucleus of the stria terminalis in the human brain. J Chem Neuroanat. 1991;4:281–298. doi: 10.1016/0891-0618(91)90019-9. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Region-specific regulation of neuropeptide mrnas in rat limbic forebrain neurones by aldosterone and corticosterone. J Physiol. 1995;484:721–736. doi: 10.1113/jphysiol.1995.sp020698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whim MD. Frequency-dependent release of peptide cotransmitters from identified cholinergic motor neurons in aplysia. Proc Natl Acad USA. 1989;86:9034–9038. doi: 10.1073/pnas.86.22.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: The central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams PL, Roberts GW, Polak JM, Crow TJ. Distribution of neuropeptides in the limbic system of the rat: the bed nucleus of the stria terminalis, septum and preoptic area. Neuroscience. 1983;8:677–703. doi: 10.1016/0306-4522(83)90003-9. [DOI] [PubMed] [Google Scholar]
- Wray S, Hoffman GE. Organization and interrelationship of neuropeptides in the central amygdaloid nucleus of the rat. Peptides. 1983;4:525–541. doi: 10.1016/0196-9781(83)90059-1. [DOI] [PubMed] [Google Scholar]
- Wynn PC, Hauger RL, Holmes MC, Millan MA, Catt KJ, Aguilera G. Brain and pituitary receptors for corticotropin releasing factor: localization and differential regulation after adrenalectomy. Peptides. 1984;5:1077–1084. doi: 10.1016/0196-9781(84)90174-8. [DOI] [PubMed] [Google Scholar]
- Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem. 2007;14:634–644. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]