Abstract
BACKGROUND
The addition of liposomal muramyl tripeptide phosphatidylethanolamine (L-MTPPE) to chemotherapy has been shown to improve overall survival in patients with non-metastatic osteosarcoma (OS). We report the results of L-MTP-PE addition to chemotherapy for patients with metastatic OS.
METHODS
Intergroup-0133 (INT-0133) was a prospective randomized phase III trial for the treatment of newly diagnosed patients with OS. We compared three drug chemotherapy with cisplatin, doxorubicin, and high-dose methotrexate (Regimen A) to the same three drugs with the addition of ifosfamide (Regimen B). We evaluated the addition of L-MTP-PE to chemotherapy.
RESULTS
Five-year EFS for patients who received L-MTP-PE (n=46) was 42% versus 26% for those who did not (n=45) (relative risk for L-MTP-PE: 0.72, p=0.23, 95% CI: 0.42-1.2). The 5-year overall survival for patients who received MTP-PE versus no MTP-PE was 53% and 40% respectively (relative risk for L-MTP-PE: 0.72, p=0.27, 95% CI: 0.40-1.3). The comparison of Regimen A with regimen B did not suggest a difference for EFS (35% v. 34% respectively, relative risk for regimen B: 1.07, p=0.79, 95% CI: 0.62-1.8) or overall survival (52% v. 43% respectively, relative risk for regimen B: 1.1, p=0.75, 95% CI: 0.61-2.0).
CONCLUSIONS
When the metastatic cohort was considered in isolation, the addition of L-MTPPE to chemotherapy did not achieve a statistically significant improvement in outcome. However, the pattern of outcome is similar to the pattern in nonmetastatic patients.
Keywords: muramyl tripeptide, metastatic osteosarcoma, survival, Children's Oncology Group
INTRODUCTION
The prognosis for patients with metastatic osteosarcoma (OS) remains poor despite aggressive multi-modality therapy. In contrast to those with nonmetastatic disease who have a 5-year survival of approximately 70 - 75%, survival for metastatic patients is significantly lower.1-8 Surgical resectability, the number of metastases at diagnosis, and site(s) of metastases remain important determinants of long-term survival.3, 5, 9 Several approaches using conventional cytotoxic therapies have been used in an effort to enhance survival for patients who present with metastatic disease, but they have done little to improve the outcome of patients with metastatic OS in the last 30 years. Most recently, an upfront window trial of topotecan in newly diagnosed patients with metastatic OS reported only 1 partial response and 1 clinical response (decreased radiotracer uptake on bone scan and decrease in pain) in the cohort of 27 evaluable patients.1 New approaches are needed.
Muramyl tri-peptide phosphatidylethanolamine (MTP-PE) is a non-specific immune modulator that is a synthetic analogue of a component of bacterial cell walls. Incorporation of MTP-PE into liposomes (L-MTP-PE) has allowed targeted delivery of MTP-PE to monocytes and macrophages in areas such as the lungs.10 L-MTP-PE is able to activate these cells to become tumoricidal. Preclinical studies have already confirmed the anti-tumor effects of L-MTP-PE in rodent and canine OS models.10-21 In addition, it has been shown that concurrent administration of cytotoxic chemotherapy does not interfere with the anti-tumor effects of L-MTP-PE. 17
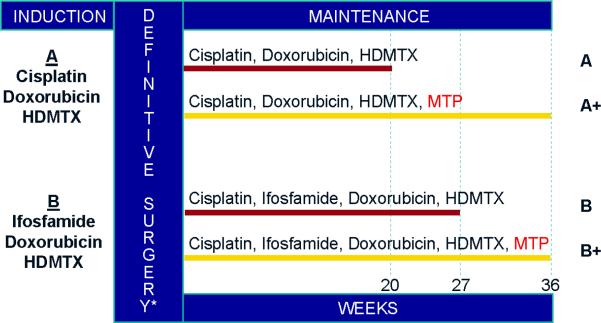
From November 1993 through November 1997 the Children's Cancer Group (CCG) and the Pediatric Oncology Group (POG) carried out Intergroup Study 0133 (INT-0133). This was a prospective, randomized phase III trial of treatment of newly diagnosed OS in patients 30 years old or younger. The study posed two questions in a 2-by-2 factorial design (Figure 1). First, there was a randomization to a three drug chemotherapy regimen (doxorubicin, cisplatin, and high-dose methotrexate) or a four drug regimen using these agents with ifosfamide. The second was a randomization to receive or not to receive L-MTPPE in addition to the randomly assigned chemotherapy. Outcome measures considered were event-free survival (EFS) or overall survival. The results of this trial for patients with non-metastatic OS demonstrated a survival advantage (statistically significant improvement in overall survival, and a trend towards improved event-free survival) for patients receiving L-MTP-PE.22 We report the results for patients with metastatic disease enrolled on INT-0133.
Figure 1.
Treatment Schema
Abbreviations: HDMTX, high dose methotrexate; MTP, muramyl tri-peptide phosphatidylethanolamine
*Resection of metastatic lesions, when possible, occurred after definitive surgery
PATIENTS AND METHODS
Patients
Patients enrolled on study had histologically confirmed high-grade intramedullary OS. Metastatic patients were defined to be those patients reported at least one site of metastasis at the time of enrollment and prior to any therapeutic measures. Histologic confirmation of metastasis was not required. Estimate of disease resectability was determined at study enrollment and was not subsequently revised after induction chemotherapy. Patients with clinically detectable metastatic disease were eligible at CCG institutions, but ineligible at POG institutions. Detailed eligibility requirements have previously been described.23
Treatment
Treatment details have also been previously described (Figure 1)23. Briefly, patients were assigned randomly to one of four regimens. There were two chemotherapy arms, regimens A (cisplatin, doxorubicin, and high-dose methotrexate [HDMTX]) and B (ifosfamide, doxorubicin, HDMTX, and cisplatin), and within those, patients were assigned randomly to receive L-MTP-PE or not. Even though L-MTP-PE treatment did not begin until week 12 of protocol therapy, randomization of treatment assignment was done at entry. This resulted in four treatment arms: A or B for chemotherapy and + or - whether or not they received L-MTP-PE.
Both regimens called for an initial period of chemotherapy, designated as induction, that lasted 10 weeks, followed by definitive resection of primary tumor. Maintenance was scheduled to begin at week 12, but did not begin until the surgeon determined that wound healing was adequate. Maintenance continued until week 31 in regimen A and week 38 in regimen B.
Half the patients were assigned randomly at entry to receive L-MTP-PE beginning at week 12 of protocol therapy. It was administered at a dose of 2 mg/m2. L-MTP-PE was administered twice weekly for 12 weeks beginning at week 12, and weekly for an additional 24 weeks beginning at week 24. MTP was not interrupted for delays in chemotherapy.
Approval from the institutional review board (IRB) was required at every institution before enrollment. Informed consent was obtained from all patients or their guardians, and the appropriate IRB-approved written informed consent was signed.
Statistical Methods
Patient status current to 31 August 2005 were considered in the intent-to-treat analysis. Analytic event-free survival (EFS) is defined to be the time from study entry to progression of disease, diagnosis of second malignancy, death or last follow-up, whichever occurred first. Local progression at the end of induction chemotherapy is specifically excluded as an event because we intended all patients on study to undergo surgery whenever possible. In prior Children's Oncology Group studies, patients with apparent radiographic progression of tumor after induction therapy often had significant necrosis observed in the primary tumor after resection. For this reason we did not consider apparent radiographic progression after induction to be an event; such patients remained on study. Patients who experienced disease progression, diagnosis of second malignancy or death were considered to have suffered an analytic event. Otherwise, the patient was censored at last follow-up.
Overall survival (S) is defined to be the time from study entry to death or last follow-up, whichever occurred first. Patients who died were considered to have experienced an event. Otherwise, the patient was censored at last follow-up.
The EFS and S survivor functions were estimated by the method of Kaplan and Meier.24 The statistical significance of the comparisons of risk for adverse event was assessed by means of the log-rank test,24 stratified by factors on which the randomization was balanced, viz., LDH at study enrollment. Relative risks and associated confidence intervals were estimated using a relative hazards model with the characteristic of interest as the only variable in the model.24
Interaction between assigned chemotherapy and assigned biological agent was assessed using the relative hazards regression. The hazard of an event (either analytic-event or death) was modeled as:
The following terms were included in the regression model: (1) c: chemotherapy, coded as 1 if the patient was assigned regimen B and 0 otherwise; (2) m: biological agent, coded as 1 if the patient was assigned to receive L-MTP-PE and 0 otherwise; and (3) interaction, coded as the product of c and m, that is, 1 if the patient received both regimen B and L-MTP-PE and 0 otherwise. A p-value associated with the test of hypothesis: β12 = 0 of 0.10 level or less was considered evidence of a significant interaction. If there was no evidence of interaction, the effect associated with L-MTP-PE was estimated by performing a stratified log-rank test, with chemotherapy randomization as the stratification factor.24
The prognostic significance of selected patient characteristics determined at study enrollment was assessed using a relative hazards model with the characteristic of interest as the only variable in the model.24 To explore the joint relationships between therapy assignment, patient characteristics and outcome, factors considered significantly related to outcome as single characteristics were incorporated into a relative risk regression model along with the randomized therapeutic assignment. Backwards stepwise regression was employed to evaluate whether therapeutic assignment was significantly related to outcome after adjustment for those previously identified important risk factors.
RESULTS
Patient Characteristics (Table 1)
Table 1.
Patient Characteristics
| No. of Patients | % | |
|---|---|---|
| Sex | ||
| Male | 56 | 62 |
| Female | 35 | 38 |
| Age at enrollment | ||
| 0 - 11 years | 25 | 28 |
| 12 - 14 years | 24 | 26 |
| 15 - 30 years | 42 | 46 |
| Race | ||
| White | 54 | 59 |
| Hispanic | 18 | 20 |
| Black | 13 | 14 |
| Asian | 1 | 1 |
| Other | 5 | 6 |
| Primary tumor site | ||
| Femur | 59 | 65 |
| Below Knee | 16 | 18 |
| Humerus | 7 | 8 |
| Pelvis | 5 | 5 |
| Other Axial | 3 | 3 |
| Not determined | 1 | 1 |
| Alkaline phosphatase* | ||
| < ULN | 43 | 47 |
| ≥ ULN | 48 | 53 |
| Lactate dehydrogenase | ||
| < ULN | 38 | 42 |
| ≥ ULN | 53 | 58 |
| Lung Involvement | ||
| Right lung only | 7 | 8 |
| Left lung only | 6 | 7 |
| Both lungs | 30 | 36 |
| Involved, but laterality not specified | 13 | 15 |
| No lung involvement | 28 | 33 |
| Not Reported | 7 | |
| Metastatic Bone Involvement | ||
| Yes | 20 | 24 |
| No | 62 | 76 |
| Not Reported | 9 | |
| Soft Tissue Involvement | ||
| Yes | 21 | 24 |
| No | 68 | 76 |
| Not Reported | 2 |
Abbreviations: ULN, upper limit of normal.
The ULN for this analysis was the ULN in the institution where the patient was treated.
Of the 777 eligible patients enrolled on INT-0133, 91 were found to have metastatic disease at initial diagnosis and are the subjects for this report. Characteristics determined at enrollment for these patients are described in Table 1. Median time of followup for patients who had not experienced an adverse analytic event was 89 months (range: 1 month-141 months).
Patient Outcomes - Event Free Survival (Figure 2)
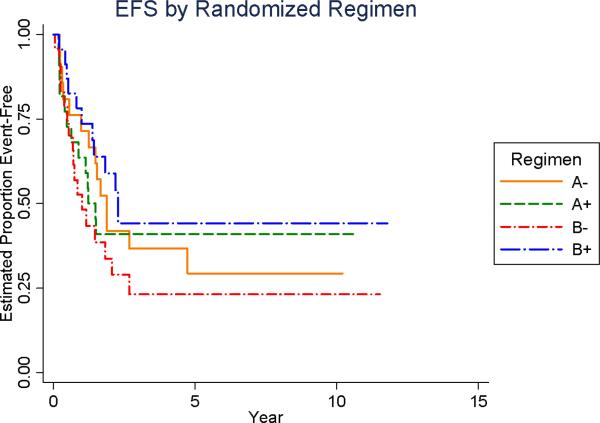
As of August 2005, the 5-year EFS for the entire cohort of 91 patients was 34% (95% confidence interval [95% CI]: 24%-45%). When analyzed according to chemotherapy regimen, the 5-year EFS for each of the regimens were as follows: 1) regimen A without MTP-PE = 29% (95%CI: 11%-51%); 2) regimen A with MTP-PE = 41% (95% CI: 21% - 60%); 3) regimen B without MTP-PE = 23% (95%CI: 8%-43%); and 4) regimen B with MTP-PE = 44% (95%CI = 23%-64%). There was no statistical difference among the regimens (log-rank =0.55, Figure 2A). Using the statistical test described above for risk for adverse analytic event, there was no evidence of an interaction between chemotherapy and L-MTP-PE assignment (p =0.20).
Figure 2A.
Event Free Survival (EFS) by Treatment Regimen Assignment
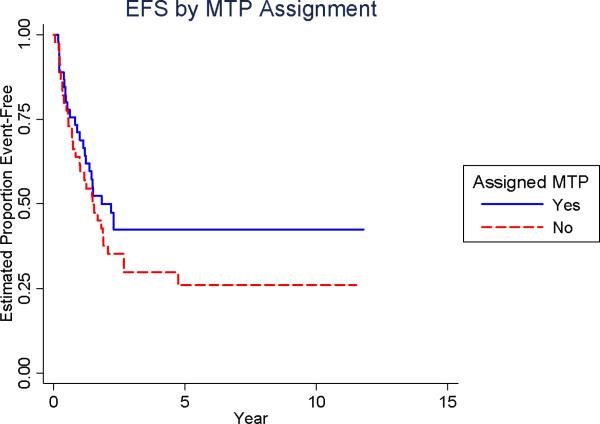
The relative risk for adverse analytic event associated with randomization to receive L-MTP-PE was 0.72 (p = 0.23; 95% CI: 0.42-1.2; Table 2). The EFS at 5-years was 42% for those randomized to receive MTP-PE versus 26% for those who were not (Figure 2B). The relative risk for adverse analytic event associated with randomization to receive chemotherapy regimen B was 1.07 (p = 0.79; 95% CI: 0.62-1.8, Table 2). The EFS at 5-years was 34% for those randomized to four-drug chemotherapy versus 35% for those randomized to three-drug chemotherapy (Figure 2C).
Table 2.
Relative Risks based on treatment regimens
| Relative Risk of Death | Relative Risk of Adverse Event | |
|---|---|---|
| L-MTP-PE Assigned (n = 46) | 0.72 | 0.72 |
| No L- MTP-PE Assigned (n = 45) | 1.0 | 1.0 |
| p-value | 0.27 | 0.23 |
| Regimen A (n = 43) | 1.0 | 1 |
| Regimen B (n = 48) | 1.1 | 1.07 |
| p-value | 0.75 | 0.79 |
Abbreviations: L-MTP-PE, liposomal Muramyl tri-peptide phosphatidylethanolamine
Figure 2B.
Event Free Survival (EFS) by L-MTP-PE assignment
Figure 2C.
Event Free Survival (EFS) by Chemotherapy Regimen Assignment
Local progression was specifically excluded as an event for this analysis as we intended all patients enrolled on study to undergo surgery. Only two patients in this current cohort had radiographic evidence of local progression at the end of induction chemotherapy: one patient had a concurrent new site of metastatic disease and was deemed a treatment failure; another patient was lost to followup and removed from protocol.
Patient Outcomes - Overall Survival (Figure 3)
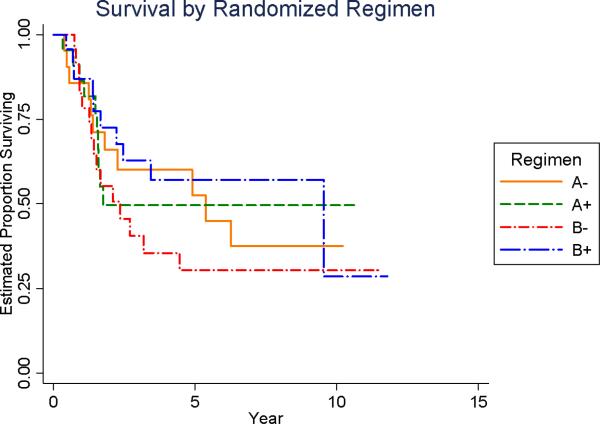
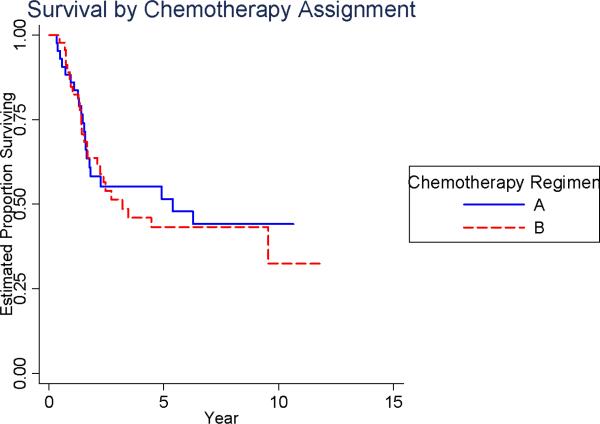
As of August 2005, the 5-year overall survival for the entire cohort of 91 patients was 47% (95% CI: 35%-58%). When analyzed according to chemotherapy regimen, the 5-year overall survival for each of the chemotherapy groups were as follows: 1) regimen A without MTP-PE = 53% (95%CI = 28%-73%); 2) regimen A with MTP-PE = 50% (95% CI = 26% - 69%); 3) regimen B without MTP-PE = 30% (95%CI = 13%-50%); and 4) regimen B with MTP-PE = 57% (95%CI = 33%-75%). There was no statistical difference among the regimens (log-rank =0.60, Figure 3A). Using the statistical test described above for risk for adverse analytic event, there was no evidence of an interaction between chemotherapy and L-MTP-PE assignment (p =0.39).
Figure 3A.
Overall Survival by Treatment Regimen Assignment
The relative risk for death associated with randomization to receive LMTP-PE was 0.72 (p = 0.27; 95% CI: 0.40-1.3, Table 2). The survival at 5-years was 53% for those randomized to receive MTP-PE versus 40% for those who were not (Figure 3B). The relative risk for death associated with randomization to receive chemotherapy regimen B was 1.1 (p = 0.75; 95% CI: 0.61-2.0, Table 2). The survival at 5-years was 43% for those randomized to Ifosfamide versus 52% for those who were not (Figure 3C).
Figure 3B.
Overall Survival by L-MTP-PE Assignment
Figure 3C.
Overall Survival by Chemotherapy Regimen Assignment
Impact of Clinical Factors on Survival (Tables 3-6)
Table 3.
Final relative risk regression incorporating prognostic factors1
| Event-Free Survival | |||
|---|---|---|---|
| Relative Risk | p-value | 95% CI | |
| Regimen B2 | 1.3 | 0.42 | 0.71 - 2.3 |
| L-MTP-PE3 | 0.67 | 0.13 | 0.37 - 1.13 |
| Overall Survival | |||
|---|---|---|---|
| Relative Risk | p-value | 95% CI | |
| Regimen B2 | 0.98 | 0.94 | 0.53 - 1.8 |
| L-MTP-PE3 | 0.77 | 0.41 | 0.43 - 1.41 |
Abbreviations: L-MTP-PE, liposomal Muramyl tri-peptide phosphatidylethanolamine
Adjusting for gender (male v. Female), race (white v. non-white) and serum alkaline phosphatase at enrolment (at or below ULN v. above ULN).
Risk relative to patients randomized to receive chemotherapy A
Risk relative to patients randomized not to receive L-MTP-PE
Table 6.
Characteristics of Patients Among the Treatment Arms
| Regimen | A | A + MTP | B | B + MTP |
|---|---|---|---|---|
| No. Patients | 21 | 22 | 24 | 24 |
| Age | ||||
| 0 - 11 | 8 | 7 | 5 | 5 |
| 12 - 14 | 2 | 5 | 7 | 10 |
| 15 - 30 | 11 | 10 | 12 | 9 |
| Gender | ||||
| Male | 12 | 11 | 17 | 19 |
| Female | 9 | 11 | 7 | 8 |
| Race | ||||
| White | 9 | 11 | 18 | 16 |
| Hispanic | 4 | 4 | 5 | 5 |
| Black | 4 | 6 | 0 | 3 |
| Other | 4 | 1 | 1 | 0 |
| Lactate Dehydrogenase | ||||
| ≤ ULN | 8 | 10 | 10 | 10 |
| < ULN | 13 | 12 | 14 | 14 |
| Alkaline Phosphatase | ||||
| ≤ ULN | 9 | 10 | 11 | 13 |
| < ULN | 12 | 12 | 13 | 11 |
| Tumor Site | ||||
| Distal Extremity | 1 | 5 | 5 | 5 |
| Proximal Extremity | 16 | 15 | 17 | 18 |
| Axial Skeleton | 3 | 2 | 2 | 1 |
| Not Determined | 1 | 0 | 0 | 0 |
| Lung Disease | ||||
| Unilateral | 11 | 8 | 8 | 4 |
| Bilateral | 5 | 9 | 8 | 13 |
| Missing | 5 | 5 | 8 | 7 |
| Sites of Disease | ||||
| Lung Only | 12 | 10 | 12 | 10 |
| Bone Only | 2 | 0 | 3 | 1 |
| Lung + Bone Only | 1 | 1 | 3 | 3 |
| Soft Tissue + Other | 4 | 8 | 4 | 5 |
| Not Reported | 2 | 3 | 2 | 5 |
Abbreviations: ULN, upper limit of normal; MTP, Liposomal Muramyl tri-peptide phosphatidylethanolamine
To explore joint relationships between therapy assignment and these prognostic factors, we performed an analysis of a relative risk regression model including these prognostic factors and therapy assignment. The relative risk for EFS and survival in this backwards stepwise regression analysis was not different from the univariate analysis of the relative risks of therapy assignment alone (Table 3).
Several clinical factors correlated with worse EFS and overall survival in this cohort of patients. Male patients, patients with high lactate dehydrogenase (LDH), patients with high alkaline phosphatase (AP), patients with metastatic bone involvement (either alone or in combination with other sites of metastatic disease), and non-Caucasian patients had consistently worse outcome. We identified gender, race and baseline AP as the strongest predictors of outcome for the patients who presented with metastatic disease (Tables 4-6).
Table 4.
Impact of clinical factors on event-free-survival
| Clinical Characteristic | No. of Patients (events) | Relative Risk of Event | 95% Confidence Interval | P-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 56 (42) | 1.00 | --- | 0.001 |
| Female | 35 (14) | 0.38 | 0.21 to 0.70 | |
| Age at diagnosis | ||||
| 0 - 11 years | 25 (12) | 1.00 | --- | 0.512 |
| 12 - 14 years | 24 (14) | 1.39 | 0.64 to 3.01 | |
| 15+ years | 42 (30) | 1.48 | 0.76 to 2.88 | |
| Race | ||||
| White | 54 (33) | 1.00 | --- | 0.0025 |
| Hispanic | 18 (9) | 3.05 | 1.55 to 6.00 | |
| Black | 13 (12) | 1.06 | 0.51 to 2.22 | |
| Other | 6 (2) | 0.47 | 0.11 to 1.96 | |
| Tumor Site | ||||
| Distal extremity | 16 (8) | 1.00 | --- | 0.423 |
| Proximal extremity | 66 (41) | 1.41 | 0.66 to 3.00 | |
| Axial skeleton | 8 (6) | 2.01 | 0.70 to 5.81 | |
| Not determined | 1 (1) | --- | --- | |
| Maximum tumor size + | ||||
| < 7 cm | 10 (4) | 1.00 | --- | 0.074* |
| ≥ 7 cm but < 9 cm | 12 (7) | 1.03 | 0.41 to 2.57 | |
| ≥ 9 cm but < 11 cm | 20 (13) | 1.17 | 0.54 to 2.51 | |
| ≥ 11 cm | 37 (23) | 1.33 | 0.67 to 2.64 | |
| Not determined | 12 (9) | --- | --- | |
| Lactate dehydrogenase | ||||
| Below ULN | 38 (18) | 1.00 | --- | 0.0011 |
| At or above ULN | 53 (38) | 2.50 | 1.42 to 4.40 | |
| Alkaline phosphatase | ||||
| Below ULN | 43 (17) | 1.00 | --- | < 0.0001 |
| At or above ULN | 48 (39) | 4.39 | 2.44 to 7.91 | |
| Lung Involvement | ||||
| Unilateral | 31 (19) | 1.00 | --- | 0.702 |
| Bilateral | 35 (20) | 0.88 | 0.47 to 1.66 | |
| Number of Lung Nodules | ||||
| 1 | 18 (11) | 1.00 | --- | 0.460 |
| 2 | 13 (9) | 1.22 | 0.50 to 2.95 | |
| 3+ | 35 (19) | 0.79 | 0.38 to 1.67 | |
| Site of Metastasis | ||||
| Lung Only | 44 (24) | 1.00 | --- | 0.0147 |
| Bone Only | 6 (6) | 3.49 | 1.40 to 8.67 | |
| Lung + Bone Only | 8 (6) | 2.62 | 1.07 to 6.46 | |
| Soft Tissue + Other | 21 (14) | 1.47 | 0.76 to 2.86 | |
| Unknown | 12 (6) | --- | --- |
Abbreviation: ULN, upper limit of normal.
Log-rank trend test
Maximal Tumor size determined by CT or MRI
Toxicity (Table 7)
Table 7.
Select grade 3 and grade 4 toxicity among treatment arms
| Regimen | All (N=91) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A no MTP (N=21) | A MTP (N=22) | B no MTP (N=24) | B MTP (N=24) | ||||||||||||||
| Grade | Grade | Grade | Grade | ||||||||||||||
| 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | ||||||||||
| Number | Percent | Number | Percent | Number | Percent | Number | Percent | Number | Percent | Number | Percent | Number | Percent | Number | Percent | Number | |
| Toxicity Code | 3 | 14.3 | 3 | 14.3 | 2 | 9.1 | 1 | 4.5 | 6 | 25.0 | 2 | 8.3 | 6 | 25.0 | 23 | ||
| Hematologic - WBC | |||||||||||||||||
| Hematologic - ANC | 1 | 4.8 | 9 | 42.9 | 3 | 13.6 | 5 | 22.7 | 6 | 25.0 | 3 | 12.5 | 8 | 33.3 | 35 | ||
| Hematologic - Platelet | 2 | 9.5 | 4 | 19.0 | 1 | 4.5 | 3 | 13.6 | 1 | 4.2 | 2 | 8.3 | 2 | 8.3 | 6 | 25.0 | 21 |
| Hematologic - HGB | 2 | 9.5 | 1 | 4.8 | 1 | 4.5 | 1 | 4.2 | 1 | 4.2 | 3 | 12.5 | 9 | ||||
| Hepatic - SGOT | 7 | 33.3 | 2 | 9.5 | 6 | 27.3 | 3 | 13.6 | 7 | 29.2 | 3 | 12.5 | 10 | 41.7 | 3 | 12.5 | 41 |
| Hepatic - SGPT | 6 | 28.6 | 5 | 23.8 | 6 | 27.3 | 4 | 18.2 | 12 | 50.0 | 4 | 16.7 | 11 | 45.8 | 6 | 25.0 | 54 |
| Hepatic - Alk Phos | 1 | 4.8 | 1 | 4.2 | 2 | ||||||||||||
| Hepatic - Total Bili | 2 | 9.5 | 1 | 4.8 | 2 | 8.3 | 2 | 8.3 | 7 | ||||||||
| Renal - Creatinine | 1 | 4.2 | 1 | ||||||||||||||
| Renal - CrCl | 1 | 4.5 | 1 | 4.2 | 2 | 8.3 | 4 | ||||||||||
| GI - Stomatitis | 2 | 9.5 | 5 | 23.8 | 4 | 18.2 | 3 | 13.6 | 5 | 20.8 | 2 | 8.3 | 2 | 8.3 | 7 | 29.2 | 30 |
| GI - Nausea & Vomiting | 1 | 4.8 | 3 | 14.3 | 4 | 18.2 | 1 | 4.5 | 2 | 8.3 | 4 | 16.7 | 1 | 4.2 | 16 | ||
| Cardiac - Rhythmn | 1 | 4.2 | 1 | 4.2 | 2 | ||||||||||||
| Nervous - Perph-Sensory | 1 | 4.8 | 1 | ||||||||||||||
| Nervous - Cent-Cerebellar | 1 | 4.8 | 1 | 4.2 | 1 | 4.2 | 3 | ||||||||||
| Skin | 1 | 4.2 | 1 | 4.2 | 2 | ||||||||||||
| Hearing - Objective | 1 | 4.5 | 2 | 8.3 | 1 | 4.2 | 4 | ||||||||||
| Infection | 4 | 19.0 | 3 | 13.6 | 1 | 4.5 | 5 | 20.8 | 4 | 16.7 | 1 | 4.2 | 18 | ||||
| Fever | 1 | 4.5 | 2 | 8.3 | 1 | 4.2 | 4 | ||||||||||
| Performance Status | 1 | 4.8 | 1 | ||||||||||||||
Toxicity data were collected for patients enrolled on protocol. Toxicity grading was based on the Children's Cancer Group Toxicity and Complications Criteria, a scale similar to but not identical to the National Cancer Institute's Common Toxicity Criteria (available upon request from the Children's Oncology Group Operations Center). No toxic deaths were reported. No significant differences were noted between the study arms. Select grade 3 and 4 toxicity data are presented in Table 7.
DISCUSSION
The poor prognosis of patients who present with metastatic OS highlights the need for new active agents to be identified. We have learned that dose-intensification of conventional cytotoxic agents, while leading to greater necrosis after pre-operative chemotherapy, does not alter the 20% long-term survival of patients with metastatic OS.25, 26 Addition of conventional cytotoxics that appear to have activity in the preclinical setting, such as topotecan, has also not led to improvement in patient survival.1
As an immune modulator, L-MTP-PE has been studied extensively and presents a “novel” avenue of therapy for these patients. The initial analysis of the non-metastatic cohort of INT-0133 detected interaction between the two factors under study.23 This led to a preliminary conclusion that the addition of LMTP-PE to traditional chemotherapy did not confer a survival advantage to these patients.23 However with longer followup of the same cohort, the test of the hypothesis of interaction no longer met a conventional level of significance, allowing us to perform the factorial analyses originally designed for this study. We identified a trend toward improved EFS and a statistically significant improvement in overall survival for those who received L-MTP-PE, independent of chemotherapy regimen.22 Specifically, in the non-metastatic cohort of patients (n=662), the relative risk of death for patients randomized to receive L-MTP-PE was 0.71 (95% confidence interval 0.52 - 0.96, p = 0.03) while the hazard ratio for EFS for patients who received L-MTP-PE was 0.80 (95% confidence interval 0.62 - 1.0, p = 0.08). Interestingly, the same pattern of survival enhancement seen in the non-metastatic, resectable group of patients was also seen in the metastatic cohort reported here: a trend toward improved EFS and overall survival with L-MTP-PE addition to chemotherapy. Additionally, there was no statistically significant difference between the two chemotherapy regimens with respect to EFS or overall survival in the metastatic cohort presented here. The study was not powered to detect differences between the four study arms. We cannot make firm conclusions about the apparent differences of survival between the study arms. A larger, adequately powered study would help to resolve potential differences between chemotherapy regimens.
Previous studies described clinical characteristics in the population of metastatic patients that correlate with outcome, including age, sites of metastases, number of pulmonary nodules, and levels of biochemical markers (AP, LDH).3, 5, 7, 9 Many of these studies were performed at single institutions or described a population of patients who were treated with different protocols over a span of decades. While this study was not powered to detect outcome differences in subpopulation of patients with metastatic disease, it does provide valuable clues as to potentially prognostic clinical characteristics. Several clinical factors in this particular cohort of patients were related to worse outcome, including gender (males), race (non-Caucasians), extent of disease involvement (bone metastases), and non-specific biochemical markers of tumor burden (high AP and LDH). When incorporated into a relative risk regression model, both gender and baseline alkaline phosphatase levels were related to overall and event-free survival. In this study, female patients had better EFS and overall survival. While this recapitulates the findings of published studies by some, but not all, investigators (most notably, the Scandinavian Sarcoma Group recently published data supporting the better prognosis of female OS patients)5, 7, 27, the reason(s) for this consistent observation remains unclear. Additionally, these results reinforce the notion that increased tumor burden (as implied by higher AP) leads to worse prognosis. Of interest is the lack of impact the number and location of lung nodules had on both EFS and overall survival. This is likely a reflection of the small sample size of the study population. A larger population would have provided an opportunity to detect the impact of lung tumor burden in these patients. Additionally, age at diagnosis did not correlate with outcome in this group of patients. In addition to these two characteristics, race was significantly related to risk of adverse analytic event, with non-Caucasian patients having worse EFS and overall survival.
Although the small number of patients in this cohort preclude precise estimation of treatment effects, it is still important to note that both EFS and overall survival appear to be improved for the patients who received L-MTP-PE. Additionally, the reductions in risk of adverse analytic event and death are very similar to the pattern in the much larger cohort of patients who presented without clinically detectable metastatic disease. These data suggest that L-MTP-PE might provide benefit when added to chemotherapy for the treatment of patients with osteosarcoma who present with metastatic disease. With the recent approval of L-MTP-PE by the European Medicines Agency (EMEA), the agent should become widely available for inclusion in additional, larger studies defining its efficacy in osteosarcoma.
Table 5.
Impact of clinical factors on overall survival
| Clinical Characteristic | No. of Patients (events) | Relative Risk of death | 95% Confidence Interval | P-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 56 (35) | 1.00 | --- | 0.0072 |
| Female | 35 (11) | 0.41 | 0.21 to 0.80 | |
| Age at diagnosis | ||||
| 0 - 11 years | 25 (9) | 1.00 | --- | 0.359 |
| 12 - 14 years | 24 (13) | 1.82 | 0.78 to 4.26 | |
| 15+ years | 42 (24) | 1.58 | 0.73 to 3.41 | |
| Race | ||||
| White | 54 (27) | 1.00 | --- | 0.0166 |
| Hispanic | 18 (8) | 3.22 | 1.46 to 7.10 | |
| Black | 13 (9) | 1.39 | 0.63 to 3.09 | |
| Other | 6 (2) | 0.69 | 0.16 to 2.90 | |
| Tumor Site | ||||
| Distal extremity | 16 (8) | 1.00 | --- | 0.678 |
| Proximal extremity | 66 (32) | 1.08 | 0.50 to 2.35 | |
| Axial skeleton | 8 (5) | 1.60 | 0.52 to 4.90 | |
| Not determined | 1 (1) | --- | --- | |
| Maximum tumor size + | ||||
| < 7 cm | 10 (3) | 1.00 | --- | 0.160* |
| ≥ 7 cm but < 9 cm | 12 (6) | 1.02 | 0.38 to 2.77 | |
| ≥ 9 cm but > 11 cm | 20 (9) | 0.86 | 0.36 to 2.08 | |
| ≥ 11 cm | 37 (20) | 1.16 | 0.55 to 2.41 | |
| Not determined | 12 (8) | --- | --- | |
| Lactate dehydrogenase | ||||
| Below ULN | 38 (14) | 1.00 | --- | 0.0008 |
| At or above ULN | 53 (32) | 2.84 | 1.51 to 5.35 | |
| Alkaline phosphatase | ||||
| Below ULN | 43 (11) | 1.00 | --- | < 0.0001 |
| At or above ULN | 48 (35) | 4.91 | 2.47 to 9.77 | |
| Lung Involvement | ||||
| Unilateral | 31 (15) | 1.00 | --- | 0.742 |
| Bilateral | 35 (15) | 0.89 | 0.43 to 1.82 | |
| Number of Lung Nodules | ||||
| 1 | 18 (9) | 1.00 | --- | 0.287 |
| 2 | 13 (8) | 1.48 | 0.57 to 3.85 | |
| 3+ | 35 (13) | 0.68 | 0.29 to 1.59 | |
| Site of Metastasis | ||||
| Lung Only | 44 (16) | 1.00 | --- | 0.0059 |
| Bone Only | 6 (6) | 3.49 | 1.40 to 8.67 | |
| Lung + Bone Only | 8 (5) | 2.62 | 1.07 to 6.46 | |
| Soft Tissue + Other | 21 (14) | 1.47 | 0.76 to 2.86 | |
| Unknown | 12 (5) | --- | --- |
Abbreviation: ULN, upper limit of normal.
Log-rank trend test.
Maximal Tumor size determined by CT or MRI
Acknowledgments
We thank the valuable contributions of Dr. Judith Sato and Dr. Helen Nadel to this study.
Research Support: NIH COG Chair's Grant U10 CA98543. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.htm
Research Funding: Paul A. Meyers, IDM Pharma
Footnotes
Expert Testimony: Eugenie S. Kleinerman, IDM Pharma (uncompensated); Paul A. Meyers, IDM Pharma (uncompensated)
Disclaimers:
Employment of Leadership Position: None
Consultant or Advisory Role: None
Honoraria: None
Stock Ownership: None
Preliminary results presented at the American Society of Clinical Oncology Annual Meeting, Chicago, Illinois, May 30 – June 3, 2008.
REFERENCES
- 1.Seibel NL, Krailo M, Chen Z, Healey J, Breitfeld PP, Drachtman R, et al. Upfront window trial of topotecan in previously untreated children and adolescents with poor prognosis metastatic osteosarcoma: children's Cancer Group (CCG) 7943. Cancer. 2007;109(8):1646–53. doi: 10.1002/cncr.22553. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17334983. [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106(5):1154–61. doi: 10.1002/cncr.21724. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16421923. [DOI] [PubMed] [Google Scholar]
- 3.Daw NC, Billups CA, Rodriguez-Galindo C, McCarville MB, Rao BN, Cain AM, et al. Metastatic osteosarcoma. Cancer. 2006;106(2):403–12. doi: 10.1002/cncr.21626. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16353204. [DOI] [PubMed] [Google Scholar]
- 4.McTiernan A, Meyer T, Michelagnoli MP, Lewis I, Whelan JS. A phase I/II study of doxorubicin, ifosfamide, etoposide and interval methotrexate in patients with poor prognosis osteosarcoma. Pediatr Blood Cancer. 2006;46(3):345–50. doi: 10.1002/pbc.20562. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16206197. [DOI] [PubMed] [Google Scholar]
- 5.Mialou V, Philip T, Kalifa C, Perol D, Gentet JC, Marec-Berard P, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome--the French pediatric experience. Cancer. 2005;104(5):1100–9. doi: 10.1002/cncr.21263. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16015627. [DOI] [PubMed] [Google Scholar]
- 6.del Prever AB, Fagioli F, Berta M, Bertoni F, Ferrari S, Mercuri M. Long-term survival in high-grade axial osteosarcoma with bone and lung metastases treated with chemotherapy only. J Pediatr Hematol Oncol. 2005;27(1):42–5. doi: 10.1097/01.mph.0000150739.73067.f3. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15654278. [DOI] [PubMed] [Google Scholar]
- 7.Kager L, Zoubek A, Potschger U, Kastner U, Flege S, Kempf-Bielack B, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21(10):2011–8. doi: 10.1200/JCO.2003.08.132. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12743156. [DOI] [PubMed] [Google Scholar]
- 8.Bacci G, Briccoli A, Rocca M, Ferrari S, Donati D, Longhi A, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities with metastases at presentation: recent experience at the Rizzoli Institute in 57 patients treated with cisplatin, doxorubicin, and a high dose of methotrexate and ifosfamide. Ann Oncol. 2003;14(7):1126–34. doi: 10.1093/annonc/mdg286. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12853357. [DOI] [PubMed] [Google Scholar]
- 9.Meyers PA, Heller G, Healey JH, Huvos A, Applewhite A, Sun M, et al. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J Clin Oncol. 1993;11(3):449–53. doi: 10.1200/JCO.1993.11.3.449. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8445419. [DOI] [PubMed] [Google Scholar]
- 10.Asano T, Kleinerman ES. Liposome-encapsulated MTP-PE: a novel biologic agent for cancer therapy. J Immunother Emphasis Tumor Immunol. 1993;14(4):286–92. doi: 10.1097/00002371-199311000-00006. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8280710. [DOI] [PubMed] [Google Scholar]
- 11.MacEwen EG, Kurzman ID, Rosenthal RC, Smith BW, Manley PA, Roush JK, et al. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst. 1989;81(12):935–8. doi: 10.1093/jnci/81.12.935. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2733037. [DOI] [PubMed] [Google Scholar]
- 12.Kleinerman ES, Jia SF, Griffin J, Seibel NL, Benjamin RS, Jaffe N. Phase II study of liposomal muramyl tripeptide in osteosarcoma: the cytokine cascade and monocyte activation following administration. J Clin Oncol. 1992;10(8):1310–6. doi: 10.1200/JCO.1992.10.8.1310. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1634921. [DOI] [PubMed] [Google Scholar]
- 13.Kleinerman ES, Maeda M, Jaffe N. Liposome-encapsulated muramyl tripeptide: a new biologic response modifier for the treatment of osteosarcoma. Cancer Treat Res. 1993;62:101–7. doi: 10.1007/978-1-4615-3518-8_14. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8096724. [DOI] [PubMed] [Google Scholar]
- 14.MacEwen EG, Kurzman ID, Helfand S, Vail D, London C, Kisseberth W, et al. Current studies of liposome muramyl tripeptide (CGP 19835A lipid) therapy for metastasis in spontaneous tumors: a progress review. J Drug Target. 1994;2(5):391–6. doi: 10.3109/10611869408996814. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7704483. [DOI] [PubMed] [Google Scholar]
- 15.Kleinerman ES. Biologic therapy for osteosarcoma using liposome-encapsulated muramyl tripeptide. Hematol Oncol Clin North Am. 1995;9(4):927–38. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7490249. [PubMed] [Google Scholar]
- 16.Kleinerman ES, Gano JB, Johnston DA, Benjamin RS, Jaffe N. Efficacy of liposomal muramyl tripeptide (CGP 19835A) in the treatment of relapsed osteosarcoma. Am J Clin Oncol. 1995;18(2):93–9. doi: 10.1097/00000421-199504000-00001. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7900714. [DOI] [PubMed] [Google Scholar]
- 17.Kleinerman ES, Meyers PA, Raymond AK, Gano JB, Jia SF, Jaffe N. Combination therapy with ifosfamide and liposome-encapsulated muramyl tripeptide: tolerability, toxicity, and immune stimulation. J Immunother Emphasis Tumor Immunol. 1995;17(3):181–93. doi: 10.1097/00002371-199504000-00007. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7613644. [DOI] [PubMed] [Google Scholar]
- 18.Kurzman ID, Shi F, Vail DM, MacEwen EG. In vitro and in vivo enhancement of canine pulmonary alveolar macrophage cytotoxic activity against canine osteosarcoma cells. Cancer Biother Radiopharm. 1999;14(2):121–8. doi: 10.1089/cbr.1999.14.121. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10850295. [DOI] [PubMed] [Google Scholar]
- 19.Anderson P. Liposomal muramyl tripeptide phosphatidyl ethanolamine: ifosfamide-containing chemotherapy in osteosarcoma. Future Oncol. 2006;2(3):333–43. doi: 10.2217/14796694.2.3.333. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16787112. [DOI] [PubMed] [Google Scholar]
- 20.Anderson PM, Pearson M. Novel therapeutic approaches in pediatric and young adult sarcomas. Curr Oncol Rep. 2006;8(4):310–5. doi: 10.1007/s11912-006-0038-0. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17254532. [DOI] [PubMed] [Google Scholar]
- 21.Nardin A, Lefebvre ML, Labroquere K, Faure O, Abastado JP. Liposomal muramyl tripeptide phosphatidylethanolamine: Targeting and activating macrophages for adjuvant treatment of osteosarcoma. Curr Cancer Drug Targets. 2006;6(2):123–33. doi: 10.2174/156800906776056473. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16529542. [DOI] [PubMed] [Google Scholar]
- 22.Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children's Oncology Group. J Clin Oncol. 2008;26(4):633–8. doi: 10.1200/JCO.2008.14.0095. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18235123. [DOI] [PubMed] [Google Scholar]
- 23.Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23(9):2004–11. doi: 10.1200/JCO.2005.06.031. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15774791. [DOI] [PubMed] [Google Scholar]
- 24.Kalbfleisch J, Prentice R. The statistical analysis of failure time data. John Wiley and Sons; New York: 1980. [Google Scholar]
- 25.Gorlick R, Meyers PA. Osteosarcoma necrosis following chemotherapy: innate biology versus treatment-specific. J Pediatr Hematol Oncol. 2003;25(11):840–1. doi: 10.1097/00043426-200311000-00003. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14608191. [DOI] [PubMed] [Google Scholar]
- 26.Meyers PA, Gorlick R, Heller G, Casper E, Lane J, Huvos AG, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16(7):2452–8. doi: 10.1200/JCO.1998.16.7.2452. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9667263. [DOI] [PubMed] [Google Scholar]
- 27.Smeland S, Muller C, Alvegard TA, Wiklund T, Wiebe T, Bjork O, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39(4):488–94. doi: 10.1016/s0959-8049(02)00747-5. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12751380. [DOI] [PubMed] [Google Scholar]