Abstract
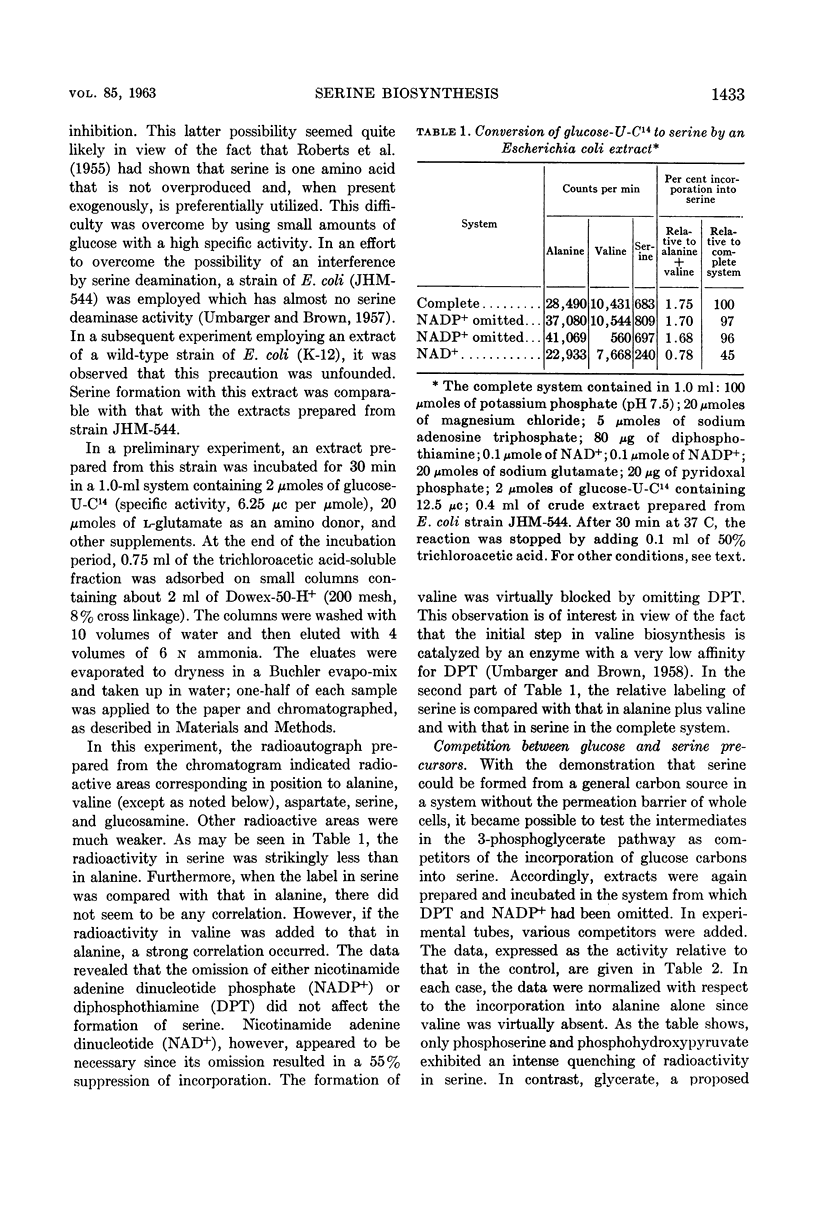
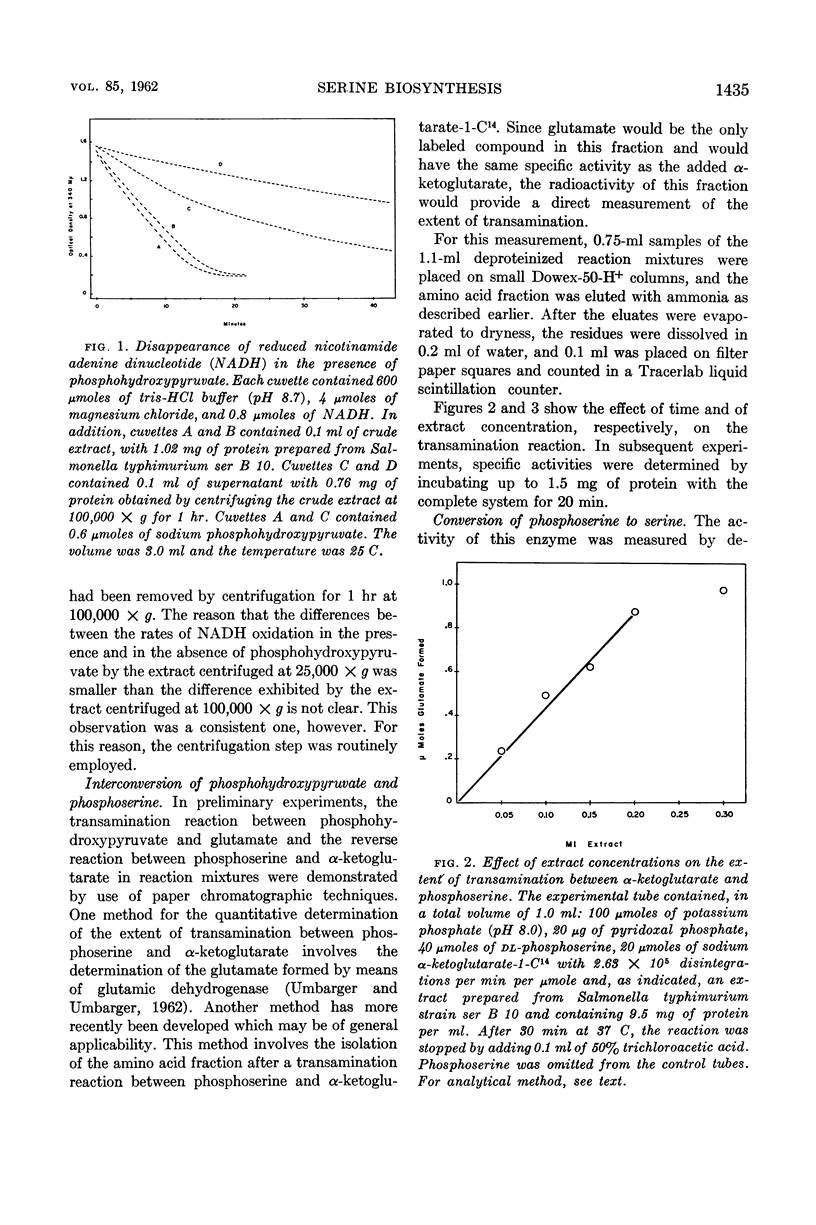
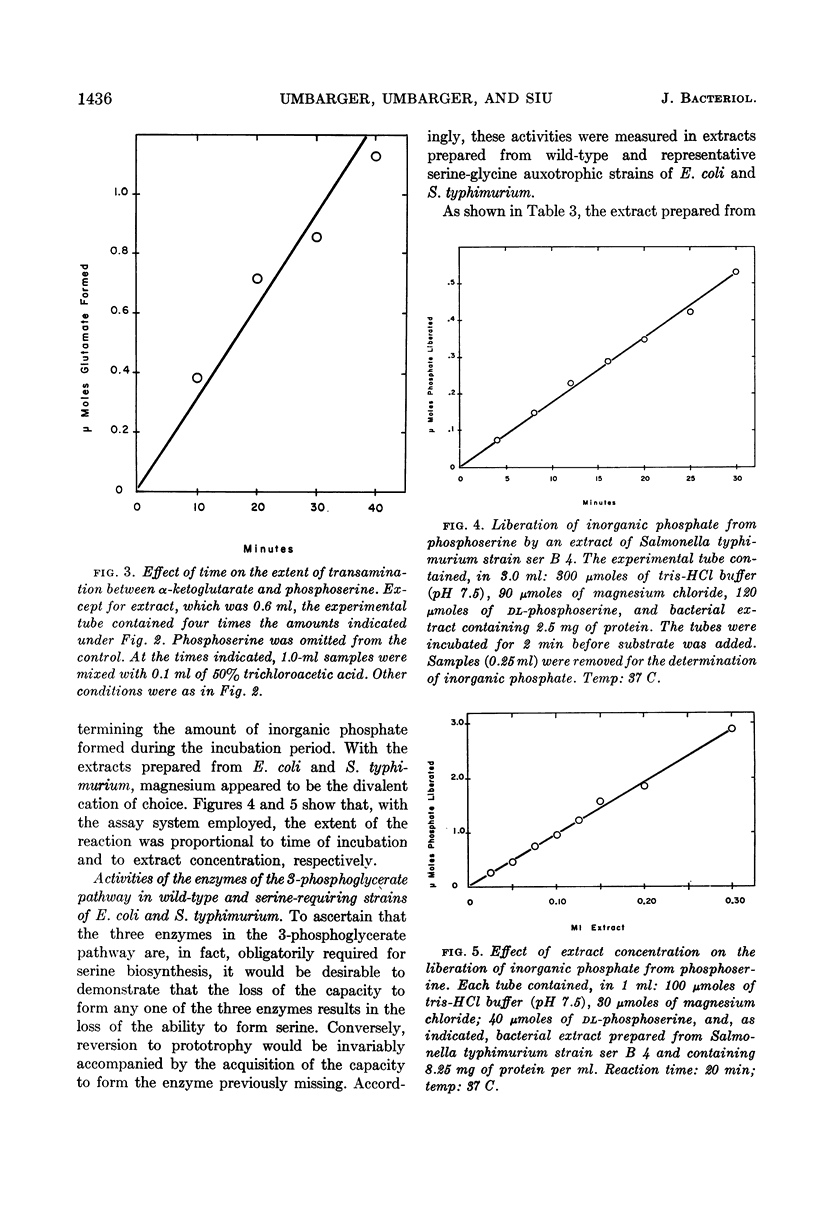
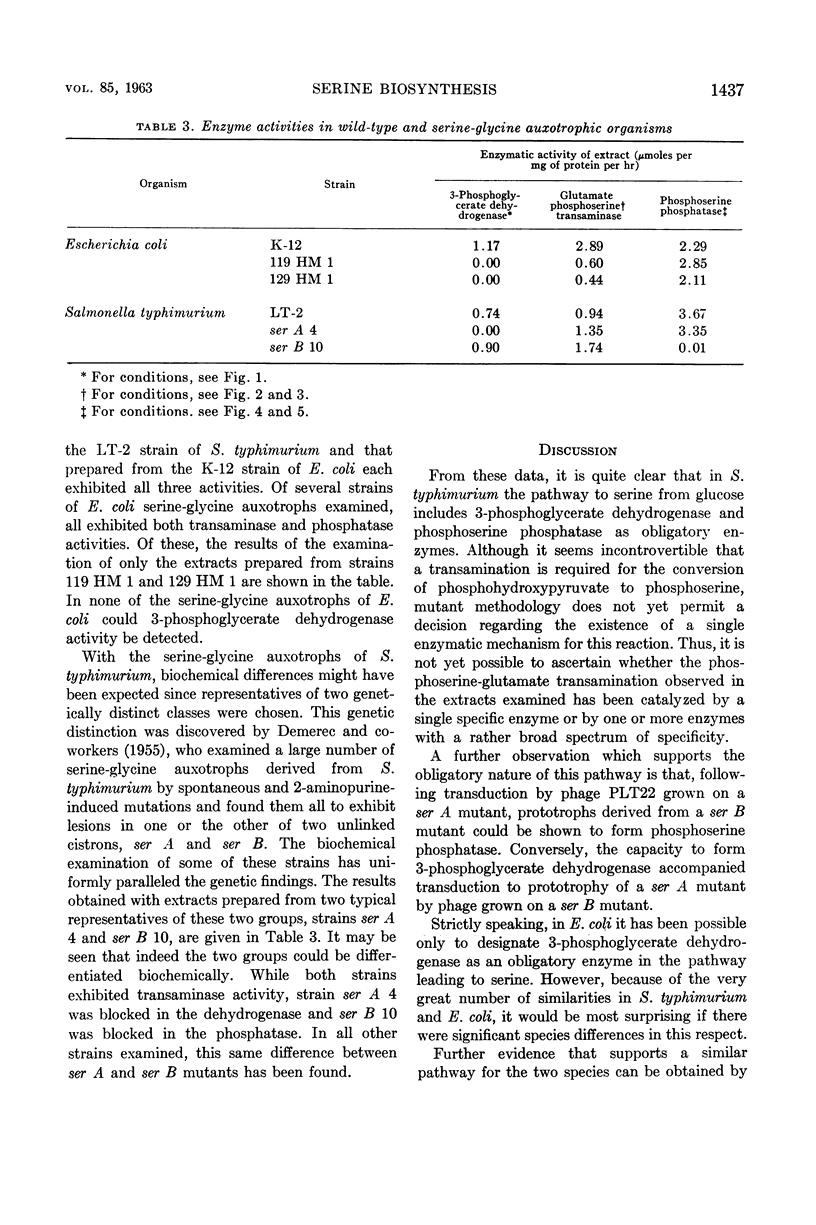
Umbarger, H. E. (Long Island Biological Association, Cold Spring Harbor, N.Y.), Merle A. Umbarger, and Patrick M. L. Siu. Biosynthesis of serine in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 85:1431–1439. 1963.—Evidence for the operation in extracts of Escherichia coli of a pathway from glucose to serine involving 3-phosphoglycerate, phosphohydroxypyruvate, and phosphoserine as intermediates was obtained by the technique of isotopic competition. The steps of the pathway were demonstrated in extracts of E. coli and Salmonella typhimurium. The first reaction was studied in the reverse of the biosynthetic direction by observing the disappearance of reduced nicotinamide adenine dinucleotide in the presence of phosphohydroxypyruvate. The enzyme catalyzing this reaction was missing in two E. coli mutants that required serine or glycine for growth and in a representative of one of two genetically distinct classes of S. typhimurium serine-glycine auxotrophs. The second reaction, the amination of phosphohydroxypyruvate, was also studied in the reverse of the biosynthetic direction using α-ketoglutarate as the amino acceptor in a transamination reaction with phosphoserine. The final step, the cleavage of phosphoserine, could not be catalyzed by extracts prepared from cells of S. typhimurium serine-glycine auxotrophs of the second genetic class. It has been concluded that these three reactions provide the only significant pathway to serine in these organisms.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORKENHAGEN L. F., KENNEDY E. P. The enzymic equilibration of L-serine with O-phospho-L-serine. Biochim Biophys Acta. 1958 Apr;28(1):222–223. doi: 10.1016/0006-3002(58)90463-3. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG D. M., ICHIHARA A. Further studies on the pathway of serine formation from carbohydrate. J Biol Chem. 1957 Jan;224(1):331–340. [PubMed] [Google Scholar]
- Glanville E V, Demerec M. Threonine, Isoleucine, and Isoleucine-Valine Mutants of Salmonella Typhimurium. Genetics. 1960 Oct;45(10):1359–1374. doi: 10.1093/genetics/45.10.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUHAUS F. C., BYRNE W. L. O-Phosphoserine phosphatase. Biochim Biophys Acta. 1958 Apr;28(1):223–224. doi: 10.1016/0006-3002(58)90464-5. [DOI] [PubMed] [Google Scholar]
- RUDMAN D., MEISTER A. Transamination in Escherichia coli. J Biol Chem. 1953 Feb;200(2):591–604. [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Isoleucine and valine metabolism in Escherichia coli. VIII. The formation of acetolactate. J Biol Chem. 1958 Nov;233(5):1156–1160. [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., UMBARGER M. A. The biosynthetic pathway of serine in salmonella typhimurium. Biochim Biophys Acta. 1962 Jul 30;62:193–195. doi: 10.1016/0006-3002(62)90515-2. [DOI] [PubMed] [Google Scholar]
- WILLIS J. E., SALLACH H. J. Evidence for a mammalian D-glyceric dehydrogenase. J Biol Chem. 1962 Mar;237:910–915. [PubMed] [Google Scholar]


