Abstract
Fluorous displaceable linker-facilitated synthesis of 1,4-benzodiazepine-2,5-dione library has been developed. Perfluorooctanesulfonyl protected 4-hydroxy benzaldehydes were used as the limiting agent for Ugi four-component reactions to form condensed products. Post-condensation reactions of the Ugi products generated 1,4-benzodiazepine-2,5-dione ring skeleton. Microwave-assisted Suzuki coupling reactions removed the fluorous tag and introduced biaryl functionality to the benzodiazepine ring. The library scaffold has four points of substitution diversities. The fluorous tag facilitated the intermediate purifications using fluorous solid-phase extraction (F-SPE), and had no negative impact on the reactivity of the Ugi reactions and post-condensation reactions.
Keywords: 1,4-Benzodiazepine-2,5-diones; Fluorous synthesis; Microwave reactions; Perfluorooctanesulfonate; Suzuki coupling; Ugi multicomponent reactions
Introduction
1,4-Benzodiazepines have a broad range of biological utilities and have been employed as anxiolytic,1 anticonvulsant,2 antitumor,3 and anti-HIV agents.4 Among the family of benzodiazepines, 1,4-benzodiazepine-2,5-diones (BZDs) have been identified as inhibitors of platelet aggregation to mimic the arginine-glycine-aspartic acid (RGD) peptide sequence,5 as precursors of benzodiazepines,6,7 as anxiolytic agents,8,9 and as Hdm2 antagonists to disrupt the p53-Hdm2 protein-protein interaction and induce cell growth arrest and apoptosis.10–12 The development of new synthetic protocols for BZDs and preparation of BZD analog libraries for biological screening are topics of continuous interest. Over the years, syntheses of BDZs on solid-supported,13–18 in ionic-liquid,19 and conventional solution phase reactions20,21 have been developed. When the BDZs were synthesized on solid-supported, high yields were obtained and the product separation was easier. However, the selection of linkers and the reaction condition optimization required significant amount of work. When BDZs were synthesized in ionic-liquid or solution phase, high yields were obtained, but the separation was always difficult. Introduced in this paper is a microwave-assisted fluorous approach for the synthesis of BDZs to accelerate intermediate separation and facilitate product synthesis.
In recent years, fluorous chemistry has gained increasing popularity in the synthesis of small molecule libraries.22–24 Fluorous linkers are employed as the “phase tag” for fluorous solid-phase extraction (F-SPE).25 The fluorous linker used in this project is perfluorooctanesulfonyl. It is different from the common protecting groups such as Boc, Cbz, Fmoc and trityl, and has following functions in multistep library synthesis: 1) as a protection group for phenol,26 2) as a phase tag for F-SPE, and 3) as a triflate alternative for Pd-catalyzed reactions to introduce aryl, amine, thiol and other functionalities to aryl and heteroaryl rings.27
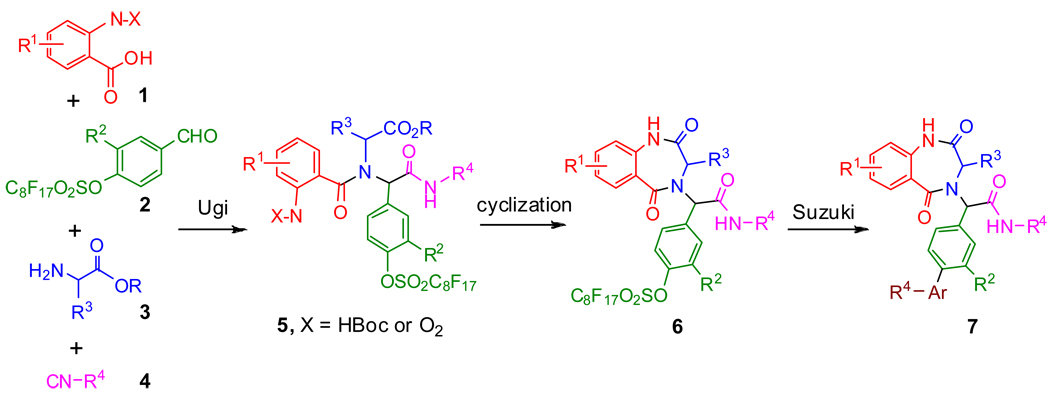
Multicomponent reaction (MCR) such as Ugi four-component reaction is a powerful way to make library scaffolds containing a high number of substitution diversities.28 Conducting post condensation reactions can lead to the generation of more complicated molecules. The advantage of using MCRs for construction of structurally diversified molecules can be enhanced through the incorporation of microwave and fluorous technologies.29–31 Combinatorial techniques involving MCR, fluorous linker, and microwave heating have been applied for the synthesis of BDZ libraries. It was designed based on following three major transformations: 1) Ugi MCRs invloving benzaldehyde 2 as a fluorous component to form 5, 2) cyclization of the Ugi products to form BDZs 6, and 3) formation of by microwave-assisted Suzuki 7 reactions to cleave the F-linker and introduce the biaryl functionality to BDZs.
Results and Discussion
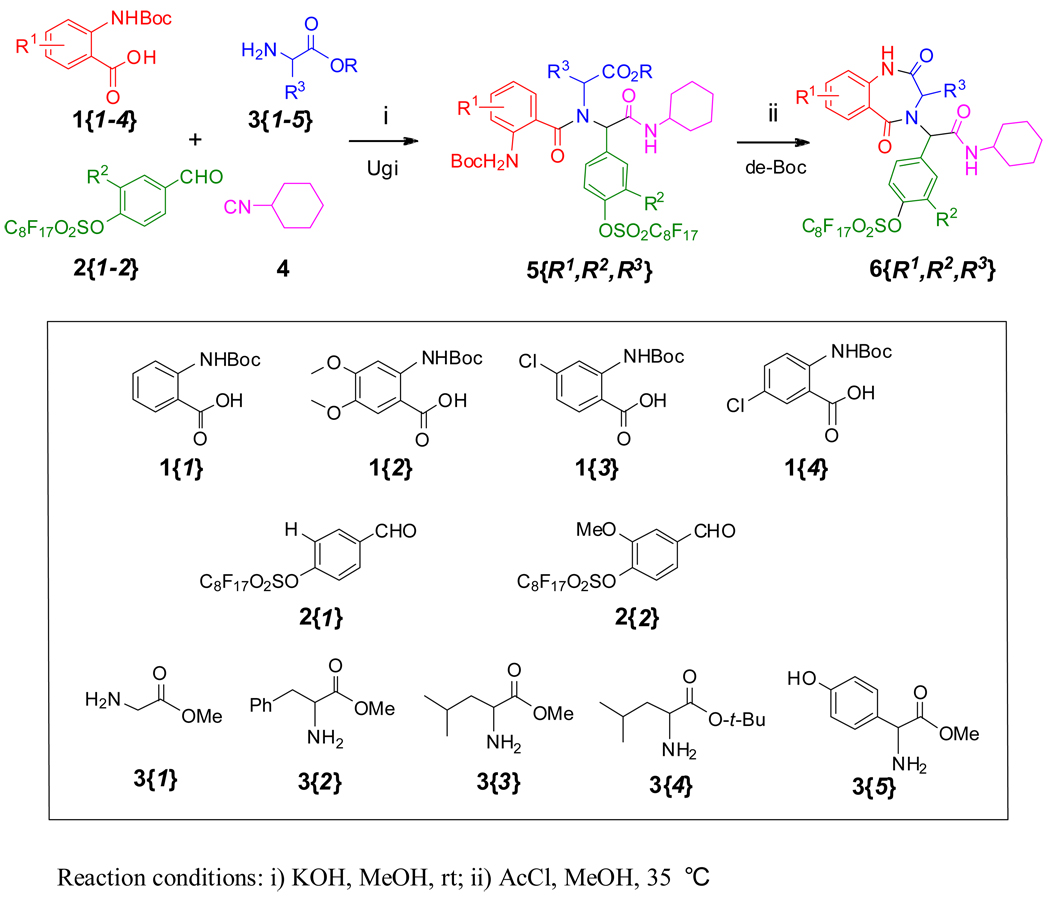
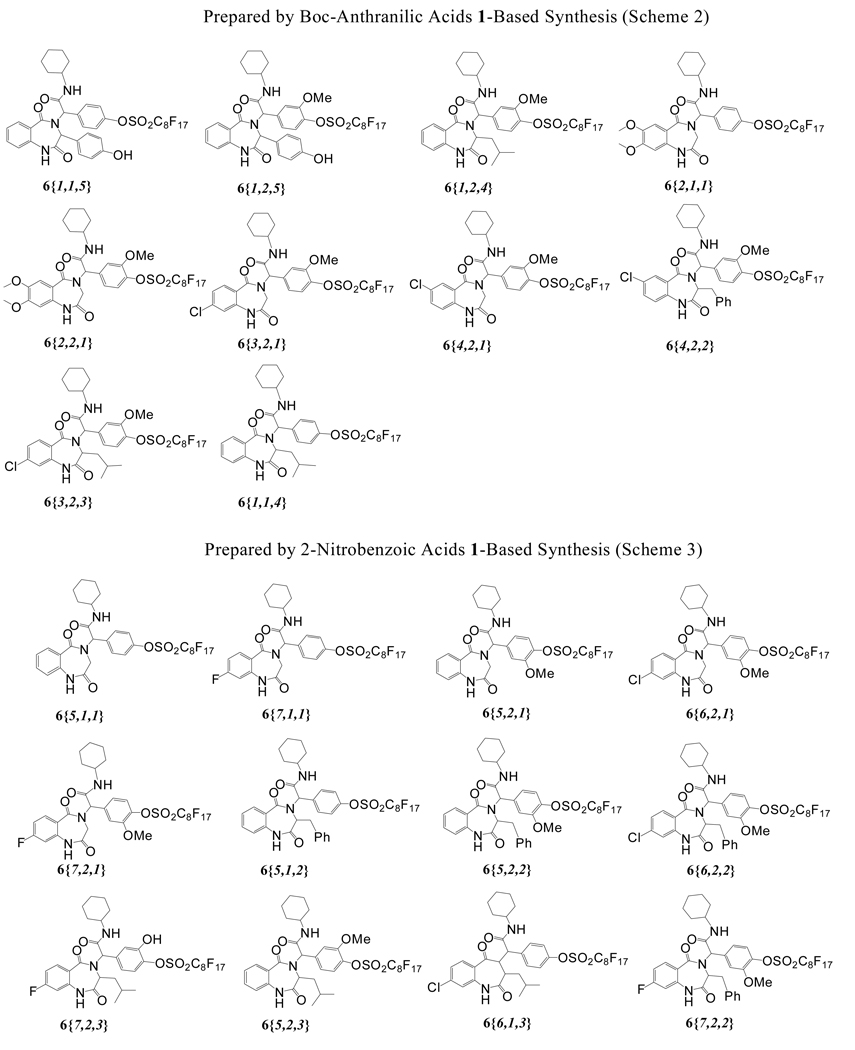
We developed two approaches for the synthesis of BDZs 6 using different benzoic acids 1 for the Ugi reactions. The first approach involving Boc-protected anthranilic acids 1{1–4} is shown in Scheme 2. The fluorous benzaldehydes 2 were prepared by coupling of perfluorooctanesulfonyl fluoride with corresponding 4-hydroxybenzaldehydes. Two fluorous benzaldehydes 2{1–2}, four Boc-protected anthranilic acids 1{1–4}, five amino esters 3{1–5}, and one cyclohexyl isocyanide 4 were used for Ugi reactions. As a demonstration of a feasible library synthesis, we didn’t carry out the full combination of the building blocks. Instead, we produced twenty-eight representative F-Ugi products 5{R1,R2,R3}. The F-Ugi products were then converted to the BDZs 6{R1,R2,R3} by de-Boc/cyclizations (Scheme 2). In the non-fluorous synthesis of BDZs, equal molar amounts of four reaction components were used for the Ugi reactions.20,32–34 In the fluorous synthesis, two equivalents of the non-fluorous reactants 1, 3, and 4 were used to completely consume the fluorous component 2. Reactions were promoted by KOH in MeOH at room temperature. The excess non-fluorous components were easily removed by F-SPE and twenty-eight F-Ugi products 5 were obtained with an average yield of 80% and an average purity of 86%. F-Ugi products 5 were isolated as a mixture of diastereomers, and no further attempt has been made to separate the diastereomers. All twenty-eight targeted products were obtained (Table 1). Twelve of twenty-eight products 5 were selected randomly for the de-Boc/cyclization reactions which were performed using 10% acetyl chloride in methanol to afford twelve F-BDZs 6 after purification by F-SPE35 (Table 2). The structures of ten F-BDZs 6 which were randomly selected and used in Suzuki reaction are listed in the top section of Scheme 4.
Scheme 2.
Boc-anthranilic acids 1-based synthesis of F-BDZs 6{R1,R2,R3}
Table 1.
Characterization of the Representative Compounds 5{R1,R2,R3} of Scheme 2
| Entry | Compound | Yielda | Purityb | MW(found)c |
|---|---|---|---|---|
| 1 | 5{3,2,3} | 71% | 85% | 1142 |
| 2 | 5{2,1,1} | 77% | 93% | 1082 |
| 3 | 5{2,2,1} | 88% | 89% | 1112 |
| 4 | 5{3,2,1} | 83% | 97% | 1086 |
| 5 | 5{4,2,1} | 91% | 97% | 1086 |
| 6 | 5{4,2,2} | 95% | 59% | 1176 |
| 7 | 5{1,1,5} | 99% | 74% | 1114 |
| 8 | 5{1,2,5} | 97% | 96% | 1144 |
| 9 | 5{1,1,4} | 99% | 90% | 1120 |
| 10 | 5{1,2,4} | 94% | 94% | 1150 |
| 11 | 5{1,1,3} | 92% | 92% | 1078 |
| 12 | 5{2,1,3} | 65% | 54% | 1138 |
| 13 | 5{3,1,3} | 71% | 90% | 1112 |
| 14 | 5{4,1,3} | 68% | 96% | 1112 |
| 15 | 5{1,2,3} | 73% | 85% | 1108 |
| 16 | 5{2,2,3} | 75% | 85% | 1168 |
| 17 | 5{4,2,3} | 52% | 85% | 1142 |
| 18 | 5{1,1,1} | 77% | 93% | 1022 |
| 19 | 5{3,1,1} | 74% | 93% | 1056 |
| 20 | 5{4,1,1} | 73% | 89% | 1056 |
| 21 | 5{1,2,1} | 81% | 90% | 1052 |
| 22 | 5{1,1,2} | 76% | 86% | 1112 |
| 23 | 5{2,1,2} | 70% | 76% | 1172 |
| 24 | 5{3,1,2} | 90% | 90% | 1146 |
| 25 | 5{4,1,2} | 71% | 57% | 1146 |
| 26 | 5{1,2,2} | 83% | 98% | 1142 |
| 27 | 5{2,2,2} | 82% | 98% | 1202 |
| 28 | 5{3,2,2} | 84% | 67% | 1176 |
The yield (%) was calculated by the weight of the solid obtained after F-SPE.
The purity (%) was based on the integration area of HPLC peaks detected at 214 nm.
MW (found) was determined by HPLC/ESI MS. Compounds in lines 11–28 were not used in the de-Boc/cyclization reactions.
Table 2.
Characterization of the Representative Compounds 6{R1,R2,R3} of Scheme 2
| Entry | Compound | Yielda | Purityb | MW(found)c |
|---|---|---|---|---|
| 1 | 6{1,1,5} | 76% | 90% | 982 |
| 2 | 6{1,2,5} | 76% | 90% | 1012 |
| 3 | 6{1,1,4} | 84% | 93% | 946 |
| 4 | 6{1,2,4} | 84% | 90% | 976 |
| 5 | 6{2,1,1} | 85% | 98% | 950 |
| 6 | 6{2,2,1} | 79% | 99% | 980 |
| 7 | 6{4,2,1} | 85% | 91% | 954 |
| 8 | 6{3,2,1} | 83% | 90% | 954 |
| 9 | 6{4,2,2} | 91% | 99% | 1044 |
| 10 | 6{3,2,3} | 96% | 95% | 1010 |
| 11 | 6{2,1,2} | 76% | 94% | 1040 |
| 12 | 6{2,2,3} | 94% | 94% | 1036 |
The yield (%) was calculated by the weight of the solid obtained after F-SPE.
The purity (%) was based on the integration area of HPLC peaks detected at 214 nm.
MW (found) was determined by HPLC/ESI MS. Compounds in lines 11 and 12 were not used in the Suzuki coupling reactions.
Scheme 4.
Structures of twenty-two F-BDZs 6{R1,R2,R3}
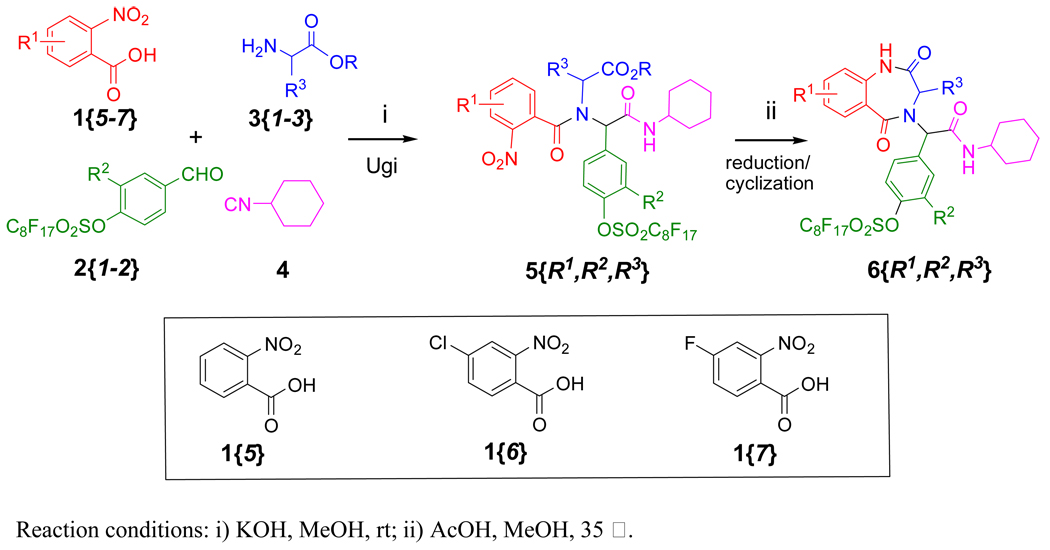
The second approach to synthesize F-BDZs 6 was using 2-nitrobenzoic acids 1{5–7} to replace anthranilic acids 1{1–4} for the Ugi reactions (Scheme 3). In this case, an optimized condition for Ugi reactions was 1:2:3:4 in a ratio of 2:1:2:1.6. Once the TLC showed the reaction was completed, the reaction mixture was purified by F-SPE to afford all eighteen F-Ugi products 5 in an average yield of 93% and an average purity of 97% as a mixture of diastereomers (Table 3). F-Ugi products 5 were then undergone zinc-promoted nitro reductions/cyclizations to yield eighteen F-BDZs 6 after F-SPE (Table 4). The structures of ten F-BDZs 6 which were randomly selected for Suzuki reaction are listed in the lower part of Scheme 4.
Scheme 3.
2-Nitrobenzoic acids 1-based synthesis of F-BDZs 6
Table 3.
Characterization of the Representative Compounds 5{R1,R2,R3} of Scheme 3
| Entry | Compound | Yielda | Purityb | MW(found)c |
|---|---|---|---|---|
| 1 | 5{6,1,3} | 84% | 99% | 1043 |
| 2 | 5{5,2,3} | 95% | 99% | 1039 |
| 3 | 5{7,2,3} | 90% | 99% | 1057 |
| 4 | 5{5,1,1} | 78% | 97% | 952 |
| 5 | 5{7,1,1} | 91% | 99% | 970 |
| 6 | 5{5,2,1} | 95% | 98% | 982 |
| 7 | 5{6,2,1} | 98% | 97% | 1017 |
| 8 | 5{7,2,1} | 99% | 97% | 1000 |
| 9 | 5{5,1,2} | 84% | 99% | 1042 |
| 10 | 5{5,2,2} | 99% | 98% | 1072 |
| 11 | 5{6,2,2} | 96% | 96% | 1107 |
| 12 | 5{7,2,2} | 96% | 92% | 1090 |
| 13 | 5{5,1,3} | 77% | 96% | 1008 |
| 14 | 5{7,1,3} | 98% | 99% | 1027 |
| 15 | 5{6,2,3} | 92% | 89% | 1072 |
| 16 | 5{6,1,1} | 97% | 98% | 987 |
| 17 | 5{6,1,2} | 100% | 91% | 1077 |
| 18 | 5{7,1,2} | 96% | 96% | 1060 |
The yield (%) was calculated by the weight of the solid obtained after F-SPE.
The purity (%) was based on the integration area of HPLC peaks detected at 214 nm.
MW (found) was determined by HPLC/ESI MS. Compounds in lines 13–18 were not used in the nitro reductions/cyclizations.
Table 4.
Characterization of the Representative Compounds 6{R1,R2,R3} of Scheme 3
| Entry | Compound | Yielda | Purityb | MW(found)c |
|---|---|---|---|---|
| 1 | 6{6,1,3} | 83% | 69% | 980 |
| 2 | 6{5,2,3} | 91% | 89% | 976 |
| 3 | 6{7,2,3} | 89% | 83% | 994 |
| 4 | 6{5,1,1} | 76% | 92% | 890 |
| 5 | 6{7,1,1} | 91% | 82% | 908 |
| 6 | 6{5,2,1} | 84% | 67% | 920 |
| 7 | 6{6,2,1} | 53% | 67% | 954 |
| 8 | 6{7,2,1} | 66% | 68% | 938 |
| 9 | 6{5,1,2} | 74% | 28% | 980 |
| 10 | 6{5,2,2} | 86% | 98% | 1010 |
| 11 | 6{6,2,2} | 95% | 93% | 1044 |
| 12 | 6{7,2,2} | 93% | 62% | 1028 |
| 13 | 6{5,1,3} | 91% | 88% | 946 |
| 14 | 6{7,1,3} | 79% | 87% | 964 |
| 15 | 6{6,2,3} | 78% | 84% | 1010 |
| 16 | 6{6,1,1} | 84% | 82% | 924 |
| 17 | 6{6,1,2} | 86% | 92% | 1014 |
| 18 | 6{7,1,2} | 91% | 83% | 998 |
The yield (%) was calculated by the weight of the solid obtained after F-SPE.
The purity (%) was based on the integration area of HPLC peaks detected at 214 nm.
MW (found) was determined by HPLC/ESI MS. Compounds in lines 13–18 were not used in the Suzuki coupling reactions.
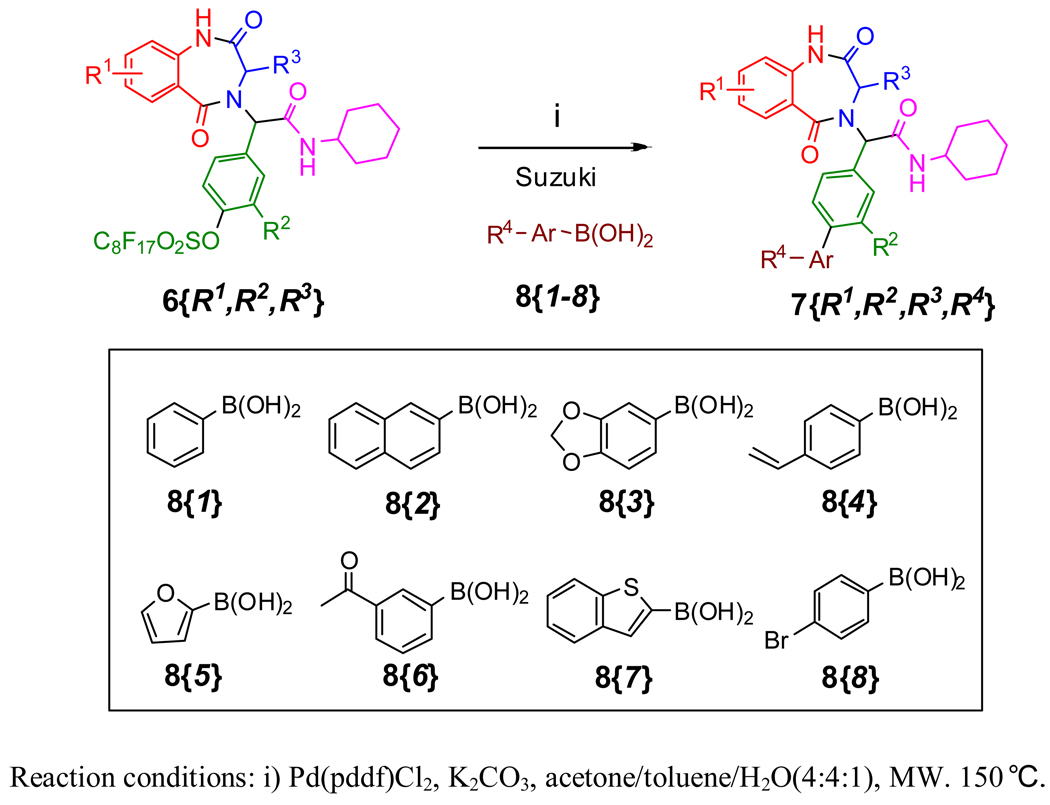
One of the major advantages of F-sulfonyl linker is that it is displaceable and can be removed by Pd-catalyzed coupling reactions.27 This “two birds with one stone” strategy combines the linker cleavage and introduction of another diversity group in a single operation. In this project, Suzuki reactions were used for F-linker cleavage and introduction of biaryl functionality to BDZs (Scheme 5). Eight boronic acids 8{1–8} were selected for the coupling reactions. The Suzuki reactions were carried out under microwave heating using Pd(dppf)Cl2 as a catalyst, K2CO3 as a base, and 4:4:1 acetone/toluene/water as a co-solvent.27 We didn’t carry out all the reactions between the selected 6s and eight boronic acids 8. To demonstrate the general feasibility, we used randomly selected compounds 6 to react with compounds 8{1–8}. The final products 7{R1,R2,R3,R4} were isolated from the reaction mixtures by F-SPE. No reagent impurities were found from the final product by LC-MS and 1H NMR analyses. However, Suzuki reactions between 6 and 8{8} failed. Finally, thirty six final products 7 were produced, and their yields, purities (an average of UVTWC and ELSD purities), and MS are displayed in Table 5 and Table 6. All products existed as a mixture of diastereomers. The diastereomers and selected compounds were further characterized by HRMS and 1H and 13C NMR (Supporting Information).
Scheme 5.
Fluorous linker cleavage by Suzuki coupling reactions
Table 5.
Characterization of the Representative Compounds 7{R1,R2,R3,R4} (Scheme 2)
| Entry | Compound | Yielda | Purityb | MW(found)c |
|---|---|---|---|---|
| 1 | 7{2,2,1,1} | 10% | >90% | 558 (MH+) |
| 2 | 7{4,2,1,1} | 26% | >90% | 532 (MH+) |
| 3 | 7{1,2,4,1} | 17% | >90% | 554 (MH+) |
| 4 | 7{3,2,1,1} | 21% | >90% | 532 (MH+) |
| 5 | 7{3,2,4,1} | 15% | 98% | 588 (MH+) |
| 6 | 7{1,1,4,5} | 10% | 87% | 514 (MH+) |
| 7 | 7{1,1,4,6} | 76% | 93% | 566 (MH+) |
| 8 | 7{2,1,1,7} | 36% | 100% | 584 (MH+) |
| 9 | 7{2,1,1,2} | 42% | 100% | 578 (MH+) |
| 10 | 7{2,2,1,3} | 53% | 97% | 602 (MH+) |
| 11 | 7{3,2,1,4} | 19% | 89% | 558 (MH+) |
| 12 | 7{4,2,1,5} | 17% | 90% | 522 (MH+) |
| 13 | 7{4,2,1,6} | 35% | 91% | 574 (MH+) |
| 14 | 7{4,2,2,2} | 23% | 98% | 672 (MH+) |
| 15 | 7{3,2,4,7} | 21% | 100% | 644 (MH+) |
| 16 | 7{1,1,5,6} | 49% | 99% | 602 (MH+) |
| 17 | 7{1,1,5,7} | 54% | 99% | 616 (MH+) |
| 18 | 7{1,2,5,3} | 15% | 86% | 634 (MH+) |
| 19 | 7{1,2,5,4} | 12% | 89% | 616 (MH+) |
| 20 | 7{4,2,2,8} | 0% | ||
| 21 | 7{1,2,4,8} | 0% |
The yield (%) was calculated by the weight of the solid obtained after F-SPE.
The purity (%) was an average of UVTWC and ELSD purities.
MW (found) was determined by HPLC/ESI MS. Compounds in lines 20 and 21 were not obtained.
Table 6.
Characterization of the Representative Compounds 7{R1,R2,R3,R4} (Scheme 3)
| Entry | Compound | Yielda | Purityb | MW(found)c |
|---|---|---|---|---|
| 1 | 7{5,1,2,1} | 23% | >90% | 558 (MH+) |
| 2 | 7{5,2,2,1} | 20% | >90% | 588 (MH+) |
| 3 | 7{6,2,2,1} | 15% | >90% | 622 (MH+) |
| 4 | 7{7,2,2,1} | 18% | >90% | 606 (MH+) |
| 5 | 7{5,1,1,1} | 25% | >90% | 468 (MH+) |
| 6 | 7{6,1,3,2} | 38% | 95% | 608 (MH+) |
| 7 | 7{6,1,3,6} | 28% | 91% | 600 (MH+) |
| 8 | 7{5,2,3,3} | 24% | 94% | 598 (MH+) |
| 9 | 7{7,2,3,4} | 19% | 96% | 598 (MH+) |
| 10 | 7{7,2,3,7} | 47% | 94% | 628 (MH+) |
| 11 | 7{7,1,1,2} | 50% | 99% | 536 (MH+) |
| 12 | 7{7,1,1,5} | 24% | 96% | 476 (MH+) |
| 13 | 7{5,2,1,3} | 47% | 99% | 542 (MH+) |
| 14 | 7{5,2,1,6} | 44% | 17% | 540 (MH+) |
| 15 | 7{6,2,1,4} | 49% | 87% | 558 (MH+) |
| 16 | 7{7,2,1,5} | 47% | 99% | 506 (MH+) |
| 17 | 7{7,2,1,7} | 32% | 99% | 572 (MH+) |
| 18 | 7{5,2,3,8} | 0% | ||
| 19 | 7{5,1,1,2} | 0% |
The yield (%) was calculated by the weight of the solid obtained after F-SPE.
The purity (%) was an average of UVTWC and ELSD purities.
MW (found) was determined by HPLC/ESI MS. Compounds in lines 18 and 19 were not obtained.
Conclusions
Thirty-six 1,4-benzodiazepine-2,5-diones derivatives were synthesized by a combinatorial approach involving MCRs, fluorous linkers, and microwave heating. Ugi four-component reactions and sequential cyclizations quickly assemble the BDZ core bearing four diversity points. F-SPE simplified the intermediate purification process. Microwave-assisted Suzuki reactions cleaved the F-linker and introduced the biaryl group to the 1,4-benzodiazepine-2,5-dione core simultaneously.
Experimental Section
The chemical reagents were purchased from Aldrich-Sigma (St. Luis, MO) and used without further purification. LC-MS were performed on a Shimadzu system. A C18 column (2.0 µm, 2.0 × 50 mm) was used for the separation. The mobile phases were acetonitrile and water both containing 0.05% formic acid. A linear gradient was used to increase from 10:90 v/v acetonitrile/water to 100% acetonitrile over 8.0 min at a flow rate of 0.5 mL/min. The routine UV detection was at 214 nm and the purity of compounds was determined using an average of values from ELSD and UVTWC detections.36 Mass spectra were recorded in positive and negative ion mode using electrospray ionization. NMR spectra were recorded on a Bruker 300 MHz NMR spectrometer using d-chloroform as solvent.
General procedures for F-SPE
A mixture containing fluorous and non-fluorous compounds in minimum amount of DMF was loaded onto a Fluor Flash@ cartridge preconditioned with 80:20 MeOH/H2O. The cartridge was eluted with 80:20 MeOH/H2O for the non-fluorous fraction followed by the same amount of MeOH for the fluorous fraction. The vacuum was used to elute samples. The fluorous fraction was dried under reduced pressure. The cartridge was washed thoroughly with acetone/methanol, and followed with 80:20 MeOH/H2O and reused.
General procedure for preparation of compound 2 shown in Scheme 2
To a magnetically stirred solution of 4-hydroxybenzaldehyde (or 4-hydroxy-3-methoxybenzaldehyde) (1.1 mmol) in DMF (5.0 mL) was added K2CO3 powder (1.2 mmol) at room temperature. The mixture was stirred for about 10 min before perfluorooctanesulfonyl fluoride (1.0 mmol) was added. The mixture was heated at 70°C for 8 hrs until TLC showed the disappearance of starting materials. The cooled reaction mixture was filtered and the solid was washed with EtOAc. The filtrate was extracted between EtOAc and water three times and the combined organic phase was washed with brine and dried over anhydrous Na2SO4 overnight. After concentrated under reduced pressure, the crude product was purified by F-SPE as described above.
General procedure for preparation of compounds 1{1–4}
To a magnetically stirred solution of anthranilic acid (1.0 mmol) in acetone (5.0 mL) was added NaOH powder (2.0 mmol) at room temperature and then di-tert-butyl dicarbonate (3.0 mmol) was added. The mixture was stirred at room temperature for 5 hrs until TLC showed the disappearance of anthranilic acid. The reaction mixture was added 2 mL water and distilled under reduced pressure to remove the acetone. The residue was washed with petroleum ether three times. The aqueous phase was added HCl (1 N) until the pH was less than 2. The mixture was extracted between EtOAc and water three times and the combined organic phase was washed by HCl (1 N), water and brine in turn. The organic phase was dried by anhydrous Na2SO4 overnight and distilled under reduced pressure to obtain compounds 1{1–4}.
General procedure for preparation of compound 5 of Scheme 2
The potassium hydroxide (2.0 equiv) and fluorous benzaldehydes 2 (1.0 equiv) were dissolved in methanol to a concentration of 1 M, then the glycine methyl ester hydrochloride 3 (2.0 equiv) was added. This solution was allowed to stand for 1 hr, and then the di-tert-butyl protected anthranilic acid 1{1–4} (2.0 equiv) was added, followed by the addition of cyclohexyl isocyanide 4 (2.0 equiv). The resulting solution was shaken on a parallel reactor bed at room temperature for 24 hrs. When TLC showed the reaction was completed, the reaction mixture was purified by F-SPE using a standard procedure.
General procedure for preparation of compound 6
The compounds 5 were dissolved in a 10% solution of acetyl chloride (AcCl) in MeOH to a concentration of 1 M. The solution was shaken on a parallel reactor at 35°C for 12 hrs. When TLC showed the reaction was completed, the reaction mixture was purified by F-SPE.
4-(2-(Cyclohexylamino)-1-(3-isobutyl-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-oxoethyl)phenyl perfluorooctylsulfonate 6{1,1,4}
yield 84%; 1H NMR (400 MHz, CDCl3) δ 8.22 (d, J = 21.6, 1H), 7.91 (dd, J = 18.3, 8.0, 1H), 7.58 (d, J = 8.7, 1H), 7.52 – 7.33 (m, 3H), 7.32 – 6.95 (m, 5H), 6.84 (d, J = 8.0, 1H), 6.47 (d, J = 132.6, 1H), 5.90 (dd, J = 137.0, 8.0, 1H), 4.14 (dd, J = 21.9, 8.7, 1H), 3.78 (s, 2H), 1.87 (s, 3H), 1.60 (dd, J = 39.1, 13.0, 5H), 1.43 – 0.90 (m, 11H), 0.89 – 0.72 (m, 3H), 0.72 – 0.49 (m, 3H), 0.39 (dd, J = 22.7, 15.5, 2H); 13C NMR (101 MHz, CDCl3) δ 171.73, 171.68, 167.22, 167.07, 149.68, 135.03, 133.20, 131.25, 125.80, 124.78, 122.06, 121.94, 119.74, 61.92, 59.27, 59.22, 48.91, 48.70, 41.24, 39.30, 38.16, 32.90, 32.75, 25.85, 25.44, 25.08, 24.75, 24.70, 23.05, 21.40, 21.03; ESI-MS m/z 946 (MH+).
4-(2-(Cyclohexylamino)-1-(3-isobutyl-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-oxoethyl)-2-methoxyphenyl perfluorooctylsulfonate 6{1,2,4}
yield 84%; 1H NMR (400 MHz, CDCl3) δ 8.13 – 7.85 (m, 3H), 7.54 – 7.28 (m, 2H), 7.26 – 7.04 (m, 5H), 7.03 – 6.88 (m, 1H), 6.87 – 6.73 (m, 2H), 6.58 (s, 0H), 6.26 (s, 1H), 5.96 (d, J = 8.0, 1H), 5.63 (s, 0H), 4.19 (dd, J = 15.9, 11.5, 2H), 3.95 – 3.67 (m, 7H), 1.87 (s, 4H), 1.60 (dd, J = 37.3, 13.2, 10H), 1.44 – 0.93 (m, 13H), 0.84 (dd, J = 6.5, 3.7, 1H), 0.77 (d, J = 6.3, 3H), 0.61 (d, J = 6.4, 3H), 0.39 (d, J = 6.6, 2H); 13C NMR (101 MHz, CDCl3) δ 171.76, 167.22, 167.10, 151.79, 138.96, 135.96, 134.96, 133.21, 132.01, 131.76, 125.85, 124.81, 122.73, 121.76, 119.61, 114.19, 62.36, 59.27, 56.57, 56.35, 48.91, 48.73, 39.37, 38.23, 32.91, 32.73, 25.82, 25.43, 25.11, 24.71, 23.08, 22.68, 22.47, 21.38, 21.10; ESI-MS m/z 976 (MH+).
4-(2-(Cyclohexylamino)-1-(7,8-dimethoxy-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-oxoethyl)phenyl perfluorooctylsulfonate 6{2,1,1}
yield 85%; 1H NMR (400 MHz, CDCl3) δ 7.82 (s, 1H), 7.45 (d, J = 8.5, 2H), 7.35 (s, 1H), 7.32 – 7.10 (m, 3H), 6.30 (d, J = 19.3, 2H), 5.78 (d, J = 7.7, 1H), 4.19 – 3.54 (m, 9H), 1.88 (s, 2H), 1.61 (s, 8H), 1.27 (s, 2H), 1.20 – 0.87 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 169.99, 167.88, 167.31, 152.96, 149.78, 146.45, 135.12, 131.38, 130.69, 122.13, 116.92, 113.17, 103.33, 89.89, 61.04, 56.33, 56.28, 49.01, 47.97, 32.92, 32.79, 25.39, 24.82, 24.74; ESI-MS m/z 950 (MH+).
4-(2-(Cyclohexylamino)-1-(7,8-dimethoxy-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-oxoethyl)-2-methoxyphenyl perfluorooctylsulfonate 6{2,2,1}
yield 79%; 1H NMR (400 MHz, CDCl3) δ 8.04 (s, 1H), 7.35 (s, 1H), 7.24 – 7.09 (m, 1H), 7.05 (s, 1H), 6.96 (d, J = 8.4, 1H), 6.33 (s, 1H), 6.23 (s, 1H), 5.78 (d, J = 8.0, 1H), 4.02 – 3.66 (m, 13H), 1.87 (s, 2H), 1.59 (d, J = 12.4, 7H), 1.22 (d, J = 32.2, 3H), 1.14 – 0.98 (m, 3H); ESI-MS m/z 980 (MH+).
4-(1-(8-Chloro-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-(cyclohexylamino)-2-oxoethyl)-2-methoxyphenyl perfluorooctylsulfonate 6{3,2,1}
yield 83%; 1H NMR (400 MHz, CDCl3) δ 8.15 – 7.71 (m, 2H), 7.49 – 6.74 (m, 8H), 6.23 (s, 1H), 5.64 (s, 1H), 4.23 – 3.16 (m, 9H), 1.98 (d, J = 95.9, 3H), 1.55 (s, 8H), 1.35 – 0.86 (m, 8H); ESI-MS m/z 955 (MH+).
4-(1-(7-Chloro-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-(cyclohexylamino)-2-oxoethyl)-2-methoxyphenyl perfluorooctylsulfonate 6{4,2,1}
yield 85%; 1H NMR (400 MHz, CDCl3) δ 8.28 (s, 1H), 7.90 (d, J = 2.4, 1H), 7.37 (dd, J = 8.5, 2.4, 1H), 7.18 (d, J = 8.2, 1H), 7.05 (s, 1H), 6.97 (d, J = 8.4, 1H), 6.83 (d, J = 8.6, 1H), 6.24 (s, 1H), 5.69 (d, J = 8.0, 1H), 3.97 – 3.62 (m, 7H), 1.87 (s, 2H), 1.75 – 1.46 (m, 6H), 1.37 – 1.16 (m, 3H), 1.15 – 0.93 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 170.12, 166.99, 166.88, 152.01, 139.20, 135.58, 134.66, 133.14, 131.83, 130.71, 126.40, 122.97, 122.05, 121.85, 114.38, 61.44, 56.47, 49.07, 47.52, 32.92, 32.76, 25.36, 24.80, 24.74; ESI-MS m/z 955 (MH+).
4-(1-(3-Benzyl-7-chloro-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-(cyclohexylamino)-2-oxoethyl)-2-methoxyphenyl perfluorooctylsulfonate 6{4,2,2}
yield 91%; 1H NMR (400 MHz, CDCl3) δ 9.09 (s, 1H), 7.89 (s, 1H), 7.21 (d, J = 7.5, 1H), 7.14 – 6.94 (m, 5H), 6.92 – 6.63 (m, 4H), 6.43 – 6.12 (m, 3H), 5.73 (t, J = 112.7, 1H), 4.46 – 4.10 (m, 1H), 3.69 (d, J = 31.1, 5H), 3.15 (d, J = 9.8, 1H), 2.47 (t, J = 13.0, 1H), 2.01 (dd, J = 62.3, 40.5, 2H), 1.58 (t, J = 40.0, 5H), 1.26 (s, 2H), 1.17 – 0.98 (m, 4H), 0.93 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 171.12, 167.05, 165.85, 151.83, 138.95, 135.86, 135.67, 133.95, 133.75, 133.37, 131.55, 131.18, 130.42, 129.01, 128.77, 128.66, 127.24, 126.97, 122.83, 121.93, 121.30, 113.71, 62.34, 62.09, 56.29, 48.95, 38.13, 35.55, 32.92, 32.74, 25.40, 24.76; ESI-MS m/z 1044 (MH+).
4-(1-(8-Chloro-3-isobutyl-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-(cyclohexylamino)-2-oxoethyl)-2-methoxyphenyl perfluorooctylsulfonate 6{3,2,3}
yield 96%; 1H NMR (400 MHz, CDCl3) δ 8.43 (d, J = 52.1, 1H), 7.86 (dd, J = 15.6, 8.5, 1H), 7.27 – 6.69 (m, 7H), 6.36 (d, J = 149.6, 1H), 5.87 (t, J = 90.2, 1H), 4.19 (d, J = 11.1, 1H), 3.98 – 3.54 (m, 7H), 2.85 (d, J = 30.1, 1H), 1.88 (s, 2H), 1.73 – 1.46 (m, 7H), 1.44 – 0.91 (m, 9H), 0.90 – 0.72 (m, 3H), 0.64 (d, J = 6.3, 3H), 0.40 (dd, J = 6.5, 3.0, 2H); 13C NMR (101 MHz, CDCl3) δ 171.70, 167.14, 166.47, 151.87, 139.05, 137.33, 136.11, 135.75, 133.46, 125.03, 124.19, 122.85, 121.77, 119.51, 114.11, 62.79, 59.11, 56.34, 48.79, 46.14, 38.27, 37.79, 32.86, 32.79, 32.72, 31.64, 25.90, 25.41, 25.34, 25.17, 24.76, 24.70, 23.08, 22.68, 21.30, 21.05; ESI-MS m/z 1010 (MH+).
General procedure for preparation of compound 5 following Scheme 3
The potassium hydroxide (2.0 equiv) and 2-Nitrobenzoic acid 1{5–7} (2.0 equiv) were dissolved in methanol to a concentration of 2 M. The solution was allowed to stand for 1 hr. Then the L-Phenylalanine methyl ester hydrochloride 3{1–3} (2.0 equiv), cyclohexyl isocyanide 4 (1.6 equiv) and fluorous benzaldehydes 2 (1.0 equiv) were added, the solution was shaken on a parallel reactor at room temperature for 24 hrs. When TLC showed the reaction was completed, the reaction mixture was purified by F-SPE.
General procedure for preparation of compound 6
The compounds 5 (1.0 equiv) were dissolved in a 50% solution of acetic acid (AcOH) in MeOH to an approximate concentration of 1 M in each and were treated with zinc powder (25 equiv). The solution were shaken on a parallel reactor at 35°C for 12 hrs. When TLC showed the reaction was completed, the reaction mixture was filtrated to remove the unreacted zinc powder. The filtrate was distilled under reduced pressure and purified by F-SPE.
4-(2-(Cyclohexylamino)-1-(2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-oxoethyl) phenyl perfluorooctylsulfonate 6{5,1,1}
yield 76%; 1H NMR (400 MHz, CDCl3) δ 8.15 (s, 1H), 7.92 (d, J = 7.8, 1H), 7.44 (dd, J = 22.1, 7.8, 3H), 7.22 (dd, J = 18.4, 6.9, 4H), 6.87 (d, J = 7.8, 1H), 6.34 (s, 1H), 5.82 (d, J = 7.8, 1H), 4.03 – 3.66 (m, 4H), 1.87 (s, 2H), 1.58 (dd, J = 35.0, 12.7, 5H), 1.36 – 1.15 (m, 4H), 1.15 – 0.90 (m, 4H); ESI-MS m/z 890 (MH+).
General procedure for preparation of compounds 7
To a reaction tube with a stirring bar was added compound 7 (1.0 mmol), 8 (0.9 mmol), Pd(pddf)Cl2 (0.04 mmol), and K2CO3 (2.0 mmol) in 0.6 mL of a 4:4:1 acetone/toluene/H2O solvent. The reactions took place automatically in a monomode microwave cavity (150 °C, 20 min) of a Biotage InitiatorTM single mode microwave reactor. HPLC was used to monitor the reaction. After the reaction, the reaction mixture was washed with 0.8 mL of water, and the organic layer was loaded onto a 2 g FluoroFlash cartridge directly and washed with 80:20 MeOH/H2O. The nonfluorous fractions were collected and concentrated. Finally, the fluorous fraction was eluted by methanol for the reuse of cartridge.
N-Cyclohexyl-2-(7,8-dimethoxy-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-(2-methoxybiphenyl-4-yl)acetamide 7{2,2,1,1}
yield 10%; 1H NMR (400 MHz, CDCl3): δ 7.53 (dd, J = 8.4, 1.1, 2H), 7.46 (s, 1H), 7.41 (dd, J = 13.6, 6.4, 3H), 7.34 (dd, J = 13.3, 7.4, 2H), 7.08 (d, J = 7.8, 1H), 7.01 (d, J = 9.3, 1H), 6.38 (s, 1H), 6.34 (s, 1H), 5.64 (d, J = 8.4, 1H), 4.00 – 3.92 (m, 5H), 3.92 – 3.84 (m, 4H), 3.80 (s, 3H), 1.98 (t, J = 12.9, 2H), 1.71 (d, J = 9.7, 3H), 1.37 (ddd, J = 22.1, 13.4, 3.8, 3H), 1.15 (dd, J = 22.8, 10.3, 3H); ESI-MS m/z 558 (MH+).
2-(Biphenyl-4-yl)-N-cyclohexyl-2-(2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)acetamide 7{5,1,1,1}
yield 25%; 1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 7.9, 1H), 7.95 (s, 1H), 7.62 (dd, J = 16.6, 7.6, 4H), 7.56 – 7.32 (m, 7H), 7.32 – 7.18 (m, 4H), 6.88 (d, J = 8.0, 1H), 6.45 (s, 1H), 5.67 (d, J = 7.9, 1H), 4.02 – 3.78 (m, 3H), 1.96 (t, J = 11.5, 2H), 1.79 – 1.64 (m, 3H), 1.44 – 1.26 (m, 3H), 1.13 (dd, J = 21.9, 10.2, 3H); 13C NMR (101 MHz, CDCl3) δ 170.66, 167.99, 167.88, 141.87, 136.07, 133.30, 132.82, 132.30, 129.98, 128.86, 127.82, 127.72, 127.14, 125.44, 124.91, 120.31, 77.35, 77.03, 76.71, 61.89, 48.90, 47.64, 32.98, 32.89, 25.46, 24.85, 24.79, 0.02; ESI-MS m/z 468 (MH+); HR-MS calcd for C29H30N3O3 (M+H)+ 468.2287, found 468.2310.
2-(8-Chloro-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-N-cyclohexyl-2-(2-methoxybiphenyl-4-yl)acetamide 7{3,2,1,1}
yield 21%; 1H NMR (400 MHz, CDCl3) δ 8.15 – 7.81 (m, 2H), 7.53 (d, J = 7.6, 2H), 7.47 – 7.29 (m, 3H), 7.29 – 7.17 (m, 4H), 7.17 – 6.88 (m, 2H), 6.37 (d, J = 5.0, 1H), 5.63 (s, 1H), 4.23 – 3.32 (m, 6H), 1.96 (s, 3H), 1.87 – 1.43 (m, 7H), 1.35 (d, J = 12.1, 2H), 1.27 – 0.82 (m, 4H); ESI-MS m/z 532 (MH+).
2-(8-Chloro-3-isobutyl-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-N-cyclohexyl-2-(4-(naphthalen-2-yl)phenyl)acetamide 7{6,1,3,2}
yield 38%; 1H NMR (400 MHz, CDCl3) δ 8.04 (s, 1H), 7.93 (dd, J = 19.8, 11.8, 6H), 7.79 (d, J = 8.3, 3H), 7.72 (dd, J = 8.5, 1.8, 1H), 7.61 (d, J = 8.3, 2H), 7.52 (s, 3H), 7.19 (dd, J = 8.5, 1.9, 1H), 6.91 (d, J = 1.9, 1H), 6.70 (s, 1H), 5.55 (d, J = 8.2, 1H), 4.37 – 4.22 (m, 1H), 4.02 – 3.83 (m, 1H), 1.93 (dd, J = 36.3, 20.1, 3H), 1.76 – 1.62 (m, 4H), 1.41 – 1.25 (m, 4H), 1.25 – 1.01 (m, 5H), 0.82 – 0.58 (m, 2H), 0.44 (dd, J = 10.5, 6.6, 6H); ESI-MS m/z 608 (MH+).
2-(3'-Acetylbiphenyl-4-yl)-2-(8-chloro-3-isobutyl-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-N-cyclohexylacetamide 7{6,1,3,6}
yield 28%; 1H NMR (400 MHz, CDCl3) δ 8.17 (s, 1H), 7.95 (t, J = 7.2, 2H), 7.78 (d, J = 7.8, 1H), 7.70 (dd, J = 12.4, 7.1, 3H), 7.63 – 7.50 (m, 3H), 7.19 (dd, J = 8.5, 1.9, 1H), 6.89 (d, J = 1.8, 1H), 6.67 (s, 1H), 5.54 (d, J = 8.1, 1H), 4.25 (dd, J = 10.5, 5.8, 1H), 4.00 – 3.82 (m, 1H), 2.73 – 2.58 (m, 3H), 1.95 (t, J = 12.8, 2H), 1.68 (d, J = 13.3, 3H), 1.43 – 1.25 (m, 4H), 1.14 (dt, J = 34.0, 10.3, 5H), 0.75 – 0.58 (m, 2H), 0.43 (dd, J = 16.0, 6.6, 6H); Isomer 1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 33.5, 1H), 8.04 – 7.86 (m, 2H), 7.86 – 7.72 (m, 2H), 7.72 – 7.57 (m, 2H), 7.51 (td, J = 8.1, 4.2, 3H), 7.39 (dd, J = 25.6, 8.5, 1H), 7.23 – 6.97 (m, 1H), 6.97 – 6.61 (m, 2H), 6.34 (s, 1H), 5.88 (d, J = 6.3, 1H), 4.32 (dd, J = 11.0, 3.4, 1H), 3.88 (dd, J = 11.4, 7.2, 1H), 2.73 – 2.56 (m, 3H), 2.09 – 1.89 (m, 2H), 1.88 – 1.66 (m, 3H), 1.50 – 1.29 (m, 4H), 1.28 – 1.04 (m, 4H), 1.02 – 0.79 (m, 3H), 0.73 (dd, J = 13.4, 7.5, 3H), 0.49 – 0.17 (m, 1H); ESI-MS m/z 600 (MH+).
2-(4-(Benzo[b]thiophen-2-yl)-3-methoxyphenyl)-N-cyclohexyl-2-(8-fluoro-3-isobutyl-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)acetamide 7{7,2,3,7}
yield 47%; 1H NMR (400 MHz, CDCl3) δ 8.38 (s, 1H), 7.95 – 7.54 (m, 6H), 7.43 – 7.24 (m, 5H), 7.18 – 6.97 (m, 3H), 6.87 (ddd, J = 13.2, 12.1, 7.2, 1H), 6.36 (d, J = 27.6, 1H), 6.00 (s, 1H), 4.51 – 4.23 (m, 1H), 4.15 – 3.80 (m, 5H), 1.99 (s, 2H), 1.88 – 1.67 (m, 3H), 1.52 – 1.29 (m, 4H), 1.29 – 1.05 (m, 4H), 0.99 – 0.56 (m, 6H), 0.51 – 0.26 (m, 1H); Isomer 1H NMR (400 MHz, CDCl3) δ 7.79 (s, 4H), 7.47 – 7.28 (m, 2H), 7.15 (s, 2H), 7.08 – 6.70 (m, 2H), 6.22 (d, J = 300.5, 1H), 5.53 (d, J = 8.4, 1H), 4.54 – 4.24 (m, 1H), 3.93 (d, J = 50.7, 4H), 2.34 – 2.10 (m, 1H), 2.04 – 1.72 (m, 3H), 1.61 (d, J = 43.0, 9H), 1.45 – 1.05 (m, 7H), 1.05 – 0.32 (m, 8H), 0.15 (s, 1H); ESI-MS m/z 628 (MH+).
N-Cyclohexyl-2-(8-fluoro-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-(4-(naphthalen-2-yl)phenyl)acetamide 7{7,1,1,2}
yield 50%; 1H NMR (400 MHz, CDCl3) δ 8.04 (s, 2H), 7.88 (s, 4H), 7.72 (d, J = 23.0, 5H), 7.58 – 7.34 (m, 6H), 7.03 (d, J = 23.7, 1H), 6.86 (s, 1H), 6.45 (s, 1H), 5.67 (s, 1H), 4.17 – 3.73 (m, 4H), 1.96 (d, J = 14.9, 2H), 1.66 (dd, J = 35.4, 9.9, 9H), 1.38 (s, 3H), 1.16 (dd, J = 16.0, 7.5, 4H); ESI-MS m/z 536 (MH+).
2-(4-(Benzo[d][1,3]dioxol-5-yl)-3-methoxyphenyl)-N-cyclohexyl-2-(2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)acetamide 7{5,2,1,3}
yield 47%; 1H NMR (400 MHz, CDCl3) δ 8.02 (d, J = 6.4, 2H), 7.44 (t, J = 7.6, 1H), 7.37 – 7.20 (m, 4H), 7.12 – 7.03 (m, 2H), 7.03 – 6.95 (m, 2H), 6.91 (d, J = 8.0, 1H), 6.85 (d, J = 8.0, 1H), 6.40 (s, 1H), 5.99 (s, 2H), 5.70 (d, J = 7.9, 1H), 4.05 – 3.83 (m, 3H), 3.80 (s, 3H), 1.97 (t, J = 13.1, 2H), 1.74 – 1.56 (m, 4H), 1.35 (dd, J = 16.8, 7.6, 2H), 1.24 – 1.04 (m, 3H); 13C NMR (101 MHz, CDCl3) δ 170.81, 168.07, 167.85, 156.82, 147.25, 146.87, 136.14, 134.45, 132.86, 132.25, 131.50, 131.22, 131.06, 125.43, 124.91, 122.97, 121.84, 120.38, 112.34, 110.18, 108.13, 101.06, 62.33, 55.75, 48.91, 47.71, 33.00, 32.84, 25.44, 24.85, 24.79, -13.05; ESI-MS m/z 542 (MH+); HR-MS calcd for C31H32N3O6 (M+H)+ 542.2291, found 542.2293.
2-(8-Chloro-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-N-cyclohexyl-2-(2-methoxy-4'-vinylbiphenyl-4-yl)acetamide 7{6,2,1,4}
yield 49%; 1H NMR (400 MHz, CDCl3) δ 8.05 – 7.69 (m, 2H), 7.48 (dt, J = 13.7, 8.3, 4H), 7.41 – 7.31 (m, 1H), 7.25 – 7.11 (m, 2H), 7.10 – 6.85 (m, 3H), 6.75 (ddd, J = 17.6, 10.9, 2.9, 1H), 6.36 (s, 0H), 5.91 – 5.73 (m, 1H), 5.65 (d, J = 7.7, 1H), 5.28 (dd, J = 10.9, 7.6, 1H), 4.05 – 3.71 (m, 6H), 1.98 (d, J = 15.3, 3H), 1.70 (d, J = 9.8, 3H), 1.32 (dd, J = 23.8, 11.4, 2H), 1.17 (dd, J = 24.2, 15.2, 4H); ESI-MS m/z 558 (MH+).
N-Cyclohexyl-2-(8-fluoro-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-(4-(furan-2-yl)-3-methoxyphenyl)acetamide 7{7,2,1,5}
yield 47%; 1H NMR (400 MHz, CDCl3) δ 8.24 (s, 1H), 7.87 (d, J = 8.0, 1H), 7.69 (dd, J = 8.9, 2.7, 1H), 7.49 (t, J = 10.2, 1H), 7.26 (s, 3H), 7.19 – 7.03 (m, 2H), 7.03 – 6.93 (m, 2H), 6.85 (dd, J = 8.5, 4.3, 1H), 6.50 (dd, J = 3.3, 1.8, 1H), 6.34 (s, 1H), 5.64 (d, J = 6.8, 1H), 4.09 – 3.75 (m, 6H), 2.64 (s, 1H), 1.95 (s, 3H), 1.68 (s, 3H), 1.46 – 1.26 (m, 2H), 1.13 (dd, J = 20.1, 9.2, 3H); ESI-MS m/z 506 (MH+).
2-(4-(Benzo[b]thiophen-2-yl)-3-methoxyphenyl)-N-cyclohexyl-2-(8-fluoro-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)acetamide 7{7,2,1,7}
yield 32%; 1H NMR (400 MHz, CDCl3) δ 8.08 (s, 1H), 7.90 – 7.63 (m, 6H), 7.33 (pd, J = 7.1, 1.3, 2H), 7.16 – 7.03 (m, 3H), 6.88 (dd, J = 8.8, 4.4, 1H), 6.35 (s, 1H), 5.65 (d, J = 7.9, 1H), 3.96 (s, 5H), 3.92 – 3.79 (m, 2H), 1.97 (t, J = 14.0, 3H), 1.75 – 1.66 (m, 4H), 1.36 (dd, J = 15.9, 7.6, 3H), 1.23 – 1.03 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 170.41, 167.48, 156.73, 140.03, 138.92, 134.90, 132.40, 129.94, 124.42, 124.31, 123.66, 123.22, 122.04, 121.88, 112.88, 107.38, 62.28, 55.88, 49.00, 45.11, 33.01, 25.41, 24.83; ESI-MS m/z 572 (MH+); HR-MS calcd for C32H31FN3O4S (M+H)+ 572.2019, found 572.2023.
2-(3'-Acetylbiphenyl-4-yl)-N-cyclohexyl-2-(3-(4-hydroxyphenyl)-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)acetamide 7{1,1,5,6}
yield 49%; 1H NMR (400 MHz, CDCl3) δ 8.11 (s, 1H), 7.92 (d, J = 7.8, 1H), 7.84 (s, 1H), 7.73 (d, J = 7.8, 1H), 7.70 – 7.63 (m, 3H), 7.60 (d, J = 8.0, 2H), 7.52 (t, J = 7.8, 1H), 7.18 (t, J = 7.2, 1H), 6.97 (dd, J = 15.5, 8.0, 1H), 6.86 – 6.54 (m, 4H), 6.41 (d, J = 8.4, 2H), 5.67 (d, J = 7.7, 1H), 5.41 (s, 1H), 5.06 (s, 1H), 3.93 (d, J = 8.0, 1H), 2.63 (d, J = 11.7, 4H), 1.96 (d, J = 12.0, 2H), 1.67 (d, J = 9.2, 3H), 1.33 (d, J = 9.3, 3H), 1.12 (d, J = 7.2, 3H); ESI-MS m/z 602 (MH+).
2-(4-(Benzo[d][1,3]dioxol-5-yl)-3-methoxyphenyl)-N-cyclohexyl-2-(3-(4-hydroxyphenyl)-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)acetamide 7{1,2,5,3}
yield 15%; 1H NMR (400 MHz, CDCl3) δ 7.86 – 7.62 (m, 2H), 7.62 – 7.35 (m, 3H), 7.34 – 7.26 (m, 2H), 7.23 – 7.06 (m, 2H), 7.06 – 6.87 (m, 3H), 6.87 – 6.71 (m, 3H), 6.71 – 6.52 (m, 2H), 6.41 (d, J = 8.1, 2H), 5.97 (s, 2H), 5.62 (d, J = 7.9, 1H), 5.42 (s, 1H), 5.19 – 4.90 (m, 1H), 3.76 (s, 5H), 2.62 (s, 1H), 1.96 (s, 2H), 1.67 (s, 3H), 1.34 (d, J = 9.1, 2H), 1.24 – 1.00 (m, 3H); ESI-MS m/z 634 (MH+).
2-(3'-Acetylbiphenyl-4-yl)-N-cyclohexyl-2-(3-isobutyl-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)acetamide 7{1,1,4,6}
yield 76%; 1H NMR (400 MHz, CDCl3) δ 8.17 (s, 1H), 7.97 (dd, J = 13.6, 7.2, 2H), 7.78 (d, J = 7.8, 1H), 7.69 (dd, J = 11.8, 6.5, 4H), 7.65 – 7.51 (m, 4H), 7.45 (dd, J = 12.1, 4.6, 2H), 7.23 (t, J = 7.7, 1H), 6.87 (d, J = 7.9, 1H), 6.68 (s, 1H), 5.62 (d, J = 8.1, 1H), 4.25 (dd, J = 10.0, 5.5, 1H), 3.99 – 3.83 (m, 1H), 2.73 – 2.56 (m, 4H), 1.95 (s, 3H), 1.68 (d, J = 13.1, 3H), 1.42 – 1.24 (m, 4H), 1.23 – 1.03 (m, 5H), 0.66 (ddd, J = 16.6, 11.1, 6.2, 2H), 0.42 (dd, J = 8.0, 6.7, 6H); ESI-MS m/z 566 (MH+); HR-MS calcd for C35H40N3O4 (M+H)+ 566.3019, found 566.3008.
2-(8-Chloro-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-N-cyclohexyl-2-(2-methoxy-4'-vinylbiphenyl-4-yl)acetamide 7{3,2,1,4}
yield 19%; 1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 8.5, 1H), 7.84 (s, 1H), 7.57 – 7.48 (m, 2H), 7.45 (d, J = 8.3, 2H), 7.34 (t, J = 12.3, 1H), 7.24 (dd, J = 8.5, 1.8, 1H), 7.07 (dd, J = 7.8, 1.4, 1H), 7.00 (d, J = 6.4, 1H), 6.95 (d, J = 1.8, 1H), 6.75 (dd, J = 17.6, 10.9, 1H), 6.37 (s, 1H), 5.79 (d, J = 17.6, 1H), 5.63 (d, J = 8.1, 1H), 5.27 (d, J = 10.9, 1H), 4.04 – 3.84 (m, 3H), 3.81 (s, 3H), 1.97 (t, J = 14.0, 2H), 1.67 (dd, J = 18.7, 15.0, 8H), 1.44 – 1.27 (m, 2H), 1.25 – 1.06 (m, 3H); 13C NMR (101 MHz, CDCl3) δ 170.21, 167.71, 167.27, 157.05, 138.75, 137.03, 136.56, 133.77, 131.29, 129.64, 125.94, 125.24, 123.90, 121.94, 120.15, 113.96, 112.42, 62.49, 55.79, 48.98, 47.55, 33.01, 32.87, 25.43, 24.79, 0.02; ESI-MS m/z 558 (MH+).
2-(7-Chloro-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-N-cyclohexyl-2-(4-(furan-2-yl)-3-methoxyphenyl)acetamide 7{4,2,1,5}
yield 17%; 1H NMR (400 MHz, CDCl3) δ 8.12 – 7.95 (m, 2H), 7.88 (d, J = 8.0, 1H), 7.47 (d, J = 1.3, 1H), 7.37 (dd, J = 8.6, 2.4, 1H), 7.06 (d, J = 9.3, 1H), 7.03 – 6.93 (m, 2H), 6.83 (d, J = 8.6, 1H), 6.50 (dd, J = 3.3, 1.8, 1H), 6.33 (s, 1H), 5.60 (d, J = 8.0, 1H), 4.06 – 3.75 (m, 7H), 1.96 (s, 3H), 1.61 (d, J = 16.7, 3H), 1.46 – 1.26 (m, 3H), 1.24 – 1.02 (m, 3H); ESI-MS m/z 522 (MH+).
2-(3'-Acetyl-2-methoxybiphenyl-4-yl)-2-(7-chloro-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-N-cyclohexylacetamide 7{4,2,1,6}
yield 35%; 1H NMR (400 MHz, CDCl3) δ 8.27 (s, 1H), 8.11 (s, 1H), 7.98 (d, J = 2.4, 1H), 7.93 (d, J = 7.8, 1H), 7.73 (d, J = 7.7, 1H), 7.50 (t, J = 7.8, 2H), 7.42 – 7.29 (m, 2H), 7.09 (dd, J = 7.8, 1.2, 1H), 7.04 (s, 1H), 6.87 (d, J = 8.6, 1H), 6.37 (s, 1H), 5.71 (d, J = 8.0, 1H), 3.96 (d, J = 1.8, 2H), 3.89 (t, J = 3.9, 1H), 3.80 (s, 3H), 2.72 – 2.56 (m, 4H), 1.97 (t, J = 11.3, 3H), 1.71 (dd, J = 9.1, 4.3, 3H), 1.61 (d, J = 13.3, 1H), 1.47 – 1.26 (m, 2H), 1.26 – 1.05 (m, 3H); 13C NMR (101 MHz, CDCl3) δ 198.15, 170.47, 167.58, 166.84, 156.94, 138.08, 137.06, 135.07, 134.71, 134.23, 132.89, 131.82, 131.38, 130.50, 129.36, 128.33, 127.31, 126.67, 122.00, 121.94, 112.42, 62.38, 55.81, 48.99, 47.56, 33.00, 32.84, 26.77, 25.42, 24.83, 24.78; ESI-MS m/z 574 (MH+); HR-MS calcd for C32H33N3O5Cl 574.2019 (M+H)+ found 574.2089.
2-(3-Benzyl-7-chloro-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-N-cyclohexyl-2-(3-methoxy-4-(naphthalen-2-yl)phenyl)acetamide 7{4,2,2,2}
yield 23%; 1H NMR (400 MHz, CDCl3) δ 8.09 (d, J = 2.5, 1H), 7.98 (s, 2H), 7.94 – 7.80 (m, 3H), 7.69 (dd, J = 8.6, 1.6, 1H), 7.60 – 7.35 (m, 4H), 7.19 – 7.03 (m, 3H), 7.03 – 6.93 (m, 1H), 6.86 (dd, J = 6.8, 5.1, 2H), 6.54 (dd, J = 9.5, 7.7, 2H), 5.41 (d, J = 8.3, 1H), 4.52 (t, J = 8.4, 1H), 4.02 – 3.64 (m, 5H), 2.62 (dd, J = 13.9, 8.5, 1H), 2.36 (dd, J = 13.6, 8.1, 1H), 1.92 (s, 2H), 1.64 (s, 3H), 1.38 – 1.18 (m, 3H), 1.10 (dd, J = 24.1, 12.1, 3H); ESI-MS m/z 672 (MH+); HR-MS calcd for C41H39N3O4Cl (M+H)+ 672.2629, found 672.2621.
2-(4-(Benzo[d][1,3]dioxol-5-yl)-3-methoxyphenyl)-N-cyclohexyl-2-(3-isobutyl-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)acetamide 7{5,2,3,3}
yield 24%; 1H NMR (400 MHz, CDCl3) δ 7.99 (dd, J = 7.9, 1.4, 1H), 7.61 – 7.48 (m, 2H), 7.43 (dt, J = 16.7, 7.1, 2H), 7.31 (d, J = 7.8, 2H), 7.23 (t, J = 7.6, 1H), 7.17 – 7.10 (m, 1H), 7.10 – 7.03 (m, 2H), 7.03 – 6.97 (m, 2H), 6.97 – 6.91 (m, 1H), 6.87 (dd, J = 14.8, 7.1, 3H), 6.65 (s, 1H), 6.00 (dd, J = 6.3, 2.7, 4H), 5.56 (d, J = 8.2, 1H), 4.37 – 4.17 (m, 1H), 4.00 – 3.74 (m, 5H), 1.96 (s, 2H), 1.68 (d, J = 9.0, 2H), 1.42 – 1.24 (m, 4H), 1.23 – 1.03 (m, 4H), 0.74 – 0.61 (m, 1H), 0.44 (dd, J = 6.5, 2.7, 5H); ESI-MS m/z 598 (MH+); HR-MS calcd for C35H40N3O6 (M+H)+ 598.2917, found 598.2926.
N-Cyclohexyl-2-(8-fluoro-3-isobutyl-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-(2-methoxy-4'-vinylbiphenyl-4-yl)acetamide 7{7,2,3,4}
yield 19%; 1H NMR (400 MHz, CDCl3) δ 7.69 (dd, J = 9.0, 3.0, 1H), 7.61 – 7.41 (m, 6H), 7.37 (d, J = 7.7, 2H), 7.17 (dd, J = 16.0, 8.7, 2H), 7.11 – 6.95 (m, 2H), 6.85 (dd, J = 8.8, 4.5, 1H), 6.81 – 6.67 (m, 2H), 6.64 (d, J = 7.9, 1H), 5.79 (d, J = 17.5, 1H), 5.52 (d, J = 7.9, 1H), 5.27 (d, J = 10.8, 1H), 4.35 – 4.20 (m, 1H), 3.92 (s, 1H), 3.86 – 3.76 (m, 3H), 1.96 (s, 2H), 1.66 (s, 2H), 1.29 (dd, J = 11.3, 8.4, 4H), 1.24 – 1.04 (m, 5H), 1.04 – 0.85 (m, 2H), 0.69 (dd, J = 18.6, 13.1, 1H), 0.46 (dd, J = 6.4, 5.0, 5H); ESI-MS m/z 598 (MH+).
2-(4-(Benzo[b]thiophen-2-yl)phenyl)-N-cyclohexyl-2-(3-(4-hydroxyphenyl)-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)acetamide 7{1,1,5,7}
yield 54%; 1H NMR (400 MHz, CDCl3) δ 7.94 – 7.60 (m, 7H), 7.55 (d, J = 6.9, 1H), 7.36 (td, J = 13.0, 6.8, 3H), 7.20 (d, J = 7.0, 1H), 7.06 – 6.89 (m, 1H), 6.75 (d, J = 7.3, 2H), 6.67 (d, J = 8.1, 2H), 6.42 (d, J = 8.6, 2H), 5.63 (s, 1H), 5.43 (s, 1H), 3.95 (s, 1H), 2.64 (s, 3H), 1.97 (s, 2H), 1.68 (s, 5H), 1.35 (s, 3H), 1.14 (d, J = 9.1, 3H); ESI-MS m/z 616 (MH+); HR-MS calcd for C37H34N3O4S (M+H)+ 616.2270, found 616.2274.
N-Cyclohexyl-2-(3-(4-hydroxyphenyl)-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-(2-methoxy-4'-vinylbiphenyl-4-yl)acetamide 7{1,2,5,4}
yield 12%; 1H NMR (400 MHz, CDCl3) δ 7.68 (dd, J = 22.7, 15.4, 3H), 7.58 – 7.37 (m, 6H), 7.32 (d, J = 7.8, 2H), 7.18 (dd, J = 20.0, 11.1, 3H), 6.97 (dd, J = 13.6, 6.0, 1H), 6.84 – 6.67 (m, 4H), 6.64 (d, J = 4.4, 2H), 6.41 (d, J = 8.7, 2H), 5.77 (d, J = 17.6, 1H), 5.60 (d, J = 8.3, 1H), 5.44 (s, 1H), 5.25 (d, J = 10.9, 1H), 4.63 (s, 1H), 3.97 (s, 1H), 3.77 (s, 3H), 1.96 (s, 2H), 1.66 (s, 3H), 1.34 (d, J = 9.4, 2H), 1.21 – 1.02 (m, 3H); ESI-MS m/z 616 (MH+).
2-(4-(Benzo[d][1,3]dioxol-5-yl)-3-methoxyphenyl)-N-cyclohexyl-2-(7,8-dimethoxy-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)acetamide 7{2,2,1,3}
yield 53%; 1H NMR (400 MHz, CDCl3) δ 8.27 (s, 1H), 7.54 – 7.41 (m, 1H), 7.37 – 7.24 (m, 2H), 7.01 (dd, J = 25.7, 12.3, 4H), 6.88 (t, J = 13.5, 1H), 6.39 (d, J = 16.5, 2H), 6.10 – 5.94 (m, 2H), 5.81 (s, 1H), 3.93 (d, J = 10.9, 5H), 3.81 (t, J = 17.9, 6H), 1.96 (d, J = 12.4, 2H), 1.66 (dd, J = 35.7, 11.2, 3H), 1.37 (d, J = 9.3, 2H), 1.27 – 1.03 (m, 3H); 13C NMR (101 MHz, CDCl3) δ 167.94, 156.79, 152.74, 147.26, 146.86, 146.28, 134.56, 131.46, 131.15, 130.93, 122.93, 121.80, 117.21, 113.13, 112.35, 110.13, 108.12, 103.27, 101.06, 56.25, 56.17, 55.74, 48.88, 33.00, 32.83, 25.44, 24.85, 24.79; ESI-MS m/z 602 (MH+); HR-MS calcd for C33H36N3O8 (M+H)+ 602.2502, found 602.2507.
N-Cyclohexyl-2-(8-fluoro-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-(4-(furan-2-yl)phenyl)acetamide 7{7,1,1,5}
yield 24%; 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 8.1, 4H), 7.68 (s, 2H), 7.52 (d, J = 15.9, 3H), 7.45 (d, J = 8.3, 3H), 7.19 (s, 2H), 6.89 (dd, J = 8.7, 4.4, 2H), 6.72 (d, J = 3.2, 1H), 6.51 (dd, J = 3.2, 1.7, 1H), 6.38 (s, 1H), 5.59 (d, J = 7.9, 1H), 3.95 (s, 5H), 1.98 (s, 4H), 1.70 (s, 8H), 1.39 (d, J = 12.8, 5H), 1.17 (d, J = 11.4, 6H); Isomer 1H NMR (400 MHz, CDCl3) δ 8.03 – 7.87 (m, 4H), 7.70 (dd, J = 27.4, 11.7, 6H), 7.40 (d, J = 7.5, 1H), 7.10 (dd, J = 8.7, 4.5, 1H), 6.94 (d, J = 3.3, 1H), 6.72 (dd, J = 3.4, 1.8, 1H), 6.60 (s, 1H), 5.76 (d, J = 7.5, 1H), 4.16 (s, 4H), 2.19 (s, 3H), 1.93 (s, 3H), 1.59 (d, J = 9.5, 4H), 1.38 (d, J = 11.8, 5H); ESI-MS m/z 476 (MH+).
2-(8-Chloro-3-isobutyl-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-N-cyclohexyl-2-(2-methoxybiphenyl-4-yl)acetamide 7{3,2,4,1}
yield 15%; 1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 8.5, 1H), 7.61 (s, 1H), 7.52 (dd, J = 9.9, 8.3, 2H), 7.44 (t, J = 7.5, 2H), 7.37 (t, J = 7.5, 2H), 7.22 (dd, J = 8.5, 1.9, 1H), 7.17 (d, J = 7.8, 1H), 7.10 (s, 1H), 6.90 (d, J = 1.9, 1H), 6.68 (s, 1H), 5.53 (d, J = 8.6, 1H), 4.31 (dd, J = 10.7, 5.5, 1H), 4.10 – 3.75 (m, 6H), 2.06 – 1.89 (m, 3H), 1.68 (s, 4H), 1.44 – 1.27 (m, 5H), 1.27 – 1.07 (m, 5H), 0.79 – 0.66 (m, 2H), 0.49 (dd, J = 8.4, 6.6, 5H); ESI-MS m/z 588 (MH+).
2-(4-(Benzo[b]thiophen-2-yl)-3-methoxyphenyl)-2-(8-chloro-3-isobutyl-2,5-dioxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-4(5H)-yl)-N-cyclohexylacetamide 7{3,2,4,7}
yield 21%; 1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 8.5, 1H), 7.82 (ddd, J = 21.2, 12.9, 6.6, 7H), 7.42 – 7.29 (m, 6H), 7.25 – 7.08 (m, 4H), 6.92 (d, J = 1.7, 1H), 6.64 (s, 1H), 5.57 (d, J = 8.1, 1H), 4.33 (dd, J = 10.6, 5.6, 1H), 4.10 – 3.82 (m, 5H), 2.07 – 1.86 (m, 3H), 1.70 (d, J = 14.6, 4H), 1.33 (ddd, J = 22.9, 12.5, 7.4, 4H), 1.19 (ddd, J = 24.2, 13.1, 5.3, 5H), 1.01 (dd, J = 8.9, 6.4, 1H), 0.86 – 0.65 (m, 2H), 0.50 (d, J = 6.5, 7H); Isomer 1H NMR (400 MHz, CDCl3) δ 7.96 (t, J = 10.1, 1H), 7.92 – 7.75 (m, 5H), 7.72 (d, J = 7.7, 1H), 7.43 – 7.30 (m, 3H), 7.19 (d, J = 7.9, 1H), 7.13 – 7.02 (m, 2H), 6.89 (d, J = 1.8, 1H), 6.33 (s, 1H), 5.89 (s, 1H), 4.37 (dd, J = 11.6, 3.0, 1H), 4.05 – 3.82 (m, 5H), 2.00 (s, 3H), 1.90 – 1.79 (m, 1H), 1.79 – 1.69 (m, 3H), 1.52 – 1.31 (m, 5H), 1.29 – 1.10 (m, 4H), 0.91 (d, J = 6.4, 4H), 0.75 (d, J = 6.5, 4H); ESI-MS m/z 644 (MH+).
2-(3-Benzyl-8-chloro-2,3-dihydro-2,5-dioxo-1H-benzo[e][1,4]diazepin-4(5H)-yl)-N-cyclohexyl-2-(2-methoxybiphenyl-4-yl)acetamide 7{6,2,2,1}
yield 15%; 1H NMR (400 MHz, CDCl3) δ 8.40 (s, 1H), 8.05 (dd, J = 8.5, 6.2, 1H), 7.68 – 7.40 (m, 3H), 7.40 – 7.15 (m, 7H), 7.15 – 6.91 (m, 3H), 6.84 (dd, J = 9.8, 4.5, 1H), 6.48 (t, J = 24.7, 1H), 5.99 – 5.30 (m, 1H), 4.65 – 4.35 (m, 1H), 3.73 (d, J = 10.1, 3H), 3.45 (dt, J = 25.8, 11.7, 1H), 2.62 (s, 1H), 2.41 – 2.18 (m, 1H), 1.88 (s, 2H), 1.67 (d, J = 34.3, 6H), 1.49 – 0.90 (m, 6H); ESI-MS m/z 622 (MH+).
2-(3-Benzyl-2,3-dihydro-2,5-dioxo-1H-benzo[e][1,4]diazepin-4(5H)-yl)-N-cyclohexyl-2-(2-methoxybiphenyl-4-yl)acetamide 7{5,2,2,1}
yield 20%; 1H NMR (400 MHz, CDCl3) δ 8.21 – 7.89 (m, 2H), 7.66 – 7.41 (m, 3H), 7.41 – 7.34 (m, 1H), 7.34 – 7.24 (m, 5H), 7.19 (d, J = 7.2, 2H), 7.11 – 7.03 (m, 1H), 7.00 (d, J = 8.7, 1H), 6.86 (dd, J = 22.5, 6.9, 1H), 6.48 (d, J = 25.1, 1H), 6.03 – 5.34 (m, 1H), 4.52 (ddd, J = 50.5, 26.6, 18.1, 1H), 4.05 – 3.62 (m, 4H), 3.52 – 3.28 (m, 1H), 2.62 (s, 1H), 2.33 (dd, J = 13.6, 8.2, 1H), 2.20 – 1.80 (m, 2H), 1.57 (s, 8H), 1.49 – 1.15 (m, 4H), 1.15 – 0.88 (m, 1H); ESI-MS m/z 588 (MH+).
2-(3-Benzyl-8-fluoro-2,3-dihydro-2,5-dioxo-1H-benzo[e][1,4]diazepin-4(5H)-yl)-N-cyclohexyl-2-(2-methoxybiphenyl-4-yl)acetamide 7{7,2,2,1}
yield 18%; 1H NMR (400 MHz, CDCl3) δ 8.48 (d, J = 43.2, 1H), 7.79 (dd, J = 5.6, 3.4, 1H), 7.64 – 7.41 (m, 3H), 7.41 – 7.23 (m, 6H), 7.23 – 6.93 (m, 5H), 6.77 (d, J = 68.3, 2H), 6.61 – 6.32 (m, 2H), 5.59 (dt, J = 117.2, 21.9, 1H), 4.70 – 4.41 (m, 1H), 3.75 (dd, J = 15.4, 8.7, 4H), 3.55 – 3.19 (m, 1H), 2.74 – 2.49 (m, 1H), 2.29 (dd, J = 13.6, 7.8, 0H), 1.89 (s, 2H), 1.66 (d, J = 48.5, 7H), 1.47 – 0.85 (m, 6H); ESI-MS m/z 606 (MH+).
N-Cyclohexyl-2-(2,3-dihydro-3-isobutyl-2,5-dioxo-1H-benzo[e][1,4]diazepin-4(5H)-yl)-2-(2-methoxybiphenyl-4-yl)acetamide 7{1,2,4,1}
yield 17%; 1H NMR (400 MHz, CDCl3) δ 8.01 (dd, J = 15.7, 8.0, 1H), 7.83 (d, J = 19.5, 1H), 7.54 (d, J = 7.9, 1H), 7.50 – 7.30 (m, 4H), 7.29 – 7.12 (m, 4H), 7.07 (dd, J = 17.0, 9.2, 1H), 6.95 – 6.78 (m, 1H), 6.51 (d, J = 126.6, 1H), 5.73 (dd, J = 102.3, 7.6, 1H), 4.33 (d, J = 31.6, 1H), 4.12 (d, J = 7.2, 0H), 3.88 (d, J = 21.5, 1H), 3.85 – 3.64 (m, 2H), 2.01 (dd, J = 28.1, 7.3, 3H), 1.89 – 1.50 (m, 6H), 1.49 – 1.01 (m, 8H), 0.97 – 0.79 (m, 2H), 0.75 – 0.60 (m, 1H), 0.53 – 0.34 (m, 2H); ESI-MS m/z 554 (MH+).
Scheme 1.
General transformations for the preparation of a biaryl-substituted BDZ library
Acknowledgments
This work was supported by Shandong University, National Cancer Institute (P30CA021765), the American Lebanese Syrian Associated Charities (ALSAC), and St. Jude Children’s Research Hospital.
Reference and Notes
- 1.Anzini M, Braile C, Valenti S, Cappelli A, Vomero S, Marinelli L, Limongelli V, Novellino E, Betti L, Giannaccini G, Lucacchini A, Ghelardini C, Norcini M, Makovec F, Giorgi G, Fryer RI. Journal of Medicinal Chemistry. 2008;51:4730–4743. doi: 10.1021/jm8002944. [DOI] [PubMed] [Google Scholar]
- 2.DeSarro G, Gitto R, Rizzo M, Zappia M, DeSarro A. General Pharmacology. 1996;27:935–941. doi: 10.1016/0306-3623(95)02147-7. [DOI] [PubMed] [Google Scholar]
- 3.Thurston DE, Bose DS, Thompson AS, Howard PW, Leoni A, Croker SJ, Jenkins TC, Neidle S, Hartley JA, Hurley LH. Journal of Organic Chemistry. 1996;61:8141–8147. doi: 10.1021/jo951631s. [DOI] [PubMed] [Google Scholar]
- 4.Breslin HJ, Kukla MJ, Kromis T, Cullis H, De Knaep F, Pauwels R, Andries K, De Clercq E, Janssen MAC, Janssen PAJ. Bioorganic & Medicinal Chemistry. 1999;7:2427–2436. doi: 10.1016/s0968-0896(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 5.McDowell RS, Gadek TR, Barker PL, Burdick DJ, Chan KS, Quan CL, Skelton N, Struble M, Thorsett ED, Tischler M, Tom JYK, Webb TR, Burnier JP. Journal of the American Chemical Society. 1994;116:5069–5076. [Google Scholar]
- 6.Gu ZQ, Wong G, Dominguez C, Decosta BR, Rice KC, Skolnick P. Journal of Medicinal Chemistry. 1993;36:1001–1006. doi: 10.1021/jm00060a007. [DOI] [PubMed] [Google Scholar]
- 7.Ananthan S, Clayton SD, Ealick SE, Wong G, Evoniuk GE, Skolnick P. Journal of Medicinal Chemistry. 1993;36:479–490. doi: 10.1021/jm00056a008. [DOI] [PubMed] [Google Scholar]
- 8.Wright WB, Brabander HJ, Greenblatt EN, Day IP, Hardy RA. Journal of Medicinal Chemistry. 1978;21:1087–1089. doi: 10.1021/jm00208a017. [DOI] [PubMed] [Google Scholar]
- 9.Araujo AC, Nicotra F, Airoldi C, Costa B, Giagnoni G, Fumagalli P, Cipolla L. European Journal of Organic Chemistry. 2008:635–639. [Google Scholar]
- 10.Marugan JJ, Leonard K, Raboisson P, Gushue JM, Calvo R, Koblish HK, Lattanze J, Zhao SY, Cummings MD, Player MR, Schubert C, Maroney AC, Lu TB. Bioorganic & Medicinal Chemistry Letters. 2006;16:3115–3120. doi: 10.1016/j.bmcl.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 11.Leonard K, Marugan JJ, Raboisson P, Calvo R, Gushue JM, Koblish HK, Lattanze J, Zhao SY, Cummings MD, Player MR, Maroney AC, Lu TB. Bioorganic & Medicinal Chemistry Letters. 2006;16:3463–3468. doi: 10.1016/j.bmcl.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Leonard K, Marugan JJ, Koblish HK, Calvo R, Raboisson P, Gushue JM, Lattanze J, Zhao SY, Cummings MD, Lu TB, Player MR, Maroney A. Clinical Cancer Research. 2005;11:9152S–9153S. [Google Scholar]
- 13.Zhang JF, Goodloe WP, Lou BL, Saneii H. Molecular Diversity. 2000;5:127–130. doi: 10.1023/a:1016282115183. [DOI] [PubMed] [Google Scholar]
- 14.Migihashi C, Sato F. Journal of Heterocyclic Chemistry. 2003;40:143–147. [Google Scholar]
- 15.Ettmayer P, Chloupek S, Weigand K. Journal of Combinatorial Chemistry. 2003;5:253–259. doi: 10.1021/cc020100w. [DOI] [PubMed] [Google Scholar]
- 16.Verdie P, Subra G, Averland-Petit MC, Amblard M, Martinez J. Journal of Combinatorial Chemistry. 2008;10:869–874. doi: 10.1021/cc800085d. [DOI] [PubMed] [Google Scholar]
- 17.Boojamra CG, Burow KM, Thompson LA, Ellman JA. Journal of Organic Chemistry. 1997;62:1240–1256. [Google Scholar]
- 18.Mayer JP, Zhang JW, Bjergarde K, Lenz DM, Gaudino JJ. Tetrahedron Letters. 1996;37:8081–8084. [Google Scholar]
- 19.Xie HW, Lu CF, Yang GC, Chen ZX. Synthesis-Stuttgart. 2009:205–210. [Google Scholar]
- 20.Hulme C, Peng J, Tang SY, Burns CJ, Morize I, Labaudiniere R. Journal of Organic Chemistry. 1998;63:8021–8023. [Google Scholar]
- 21.Keating TA, Armstrong RW. Journal of Organic Chemistry. 1996;61:8935–8939. doi: 10.1021/jo961517p. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W. Tetrahedron. 2003;59:4475–4489. [Google Scholar]
- 23.Zhang W. Chemical Reviews. 2004;104:2531–2556. doi: 10.1021/cr030600r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W. Chemical Reviews. 2009;109:749–795. doi: 10.1021/cr800412s. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Curran DP. Tetrahedron. 2006;62:11837–11865. doi: 10.1016/j.tet.2006.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Chen CHT, Lu YM, Nagashima T. Organic Letters. 2004;6:1473–1476. doi: 10.1021/ol0496428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou HY, Liu AF, Li XF, Ma XF, Feng W, Zhang W, Yan B. Journal of Combinatorial Chemistry. 2008;10:303–312. doi: 10.1021/cc700164u. [DOI] [PubMed] [Google Scholar]
- 28.Strocker AM, Keating TA, Tempest PA, Armstrong RW. Tetrahedron Letters. 1996;37:1149–1152. [Google Scholar]
- 29.Zhang W, Tempest P. Tetrahedron Letters. 2004;45:6757–6760. [Google Scholar]
- 30.Zhang W. Combinatorial Chemistry & High Throughput Screening. 2007;10:219–229. doi: 10.2174/138620707780126697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W. Microwave Methods in Organic Synthesis. 2006;266:145–166. [Google Scholar]
- 32.Hulme C, Ma L, Romano J, Morrissette M. Tetrahedron Letters. 1999;40:7925–7928. [Google Scholar]
- 33.Hulme C, Morrissette MM, Volz FA, Burns CJ. Tetrahedron Letters. 1998;39:1113–1116. [Google Scholar]
- 34.Hulme C, Peng J, Louridas B, Menard P, Krolikowski P, Kumar NV. Tetrahedron Letters. 1998;39:8047–8050. [Google Scholar]
- 35.Zhang W, Williams JP, Lu YM, Nagashima T, Chu QL. Tetrahedron Letters. 2007;48:563–565. doi: 10.1016/j.tetlet.2006.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemoff A, Yan B. Journal of Combinatorial Chemistry. 2008;10:746–751. doi: 10.1021/cc800100g. [DOI] [PubMed] [Google Scholar]