Abstract
Enterococcus faecium has emerged as an important cause of nosocomial infections over the last two decades. We recently demonstrated collagen type I as a common adherence target for some E. faecium isolates and a significant correlation was found to exist between acm-mediated collagen type I adherence and clinical origin. Here, we evaluated 60 diverse E. faecium isolates for their adherence to up to 15 immobilized host extracellular matrix and serum components. Adherence phenotypes were most commonly observed to fibronectin (20% of the 60 isolates), fibrinogen (17%) and laminin (13%), while only one or two of the isolates adhered to collagen type V, transferrin or lactoferrin and none to the other host components tested. Adherence to fibronectin and laminin was almost exclusively restricted to clinical isolates, especially the endocarditis-enriched nosocomial genogroup clonal complex 17 (CC17). Thus, the ability to adhere to fibronectin and laminin, in addition to collagen type I, may have contributed to the emergence and adaptation of E. faecium, in particular CC17, as a nosocomial pathogen.
Keywords: Enterococcus faecium, adherence, fibronectin, fibrinogen, laminin
Introduction
Enterococcus faecium, often found as part of the normal mammalian gut flora, has emerged in the last two decades as a significant nosocomial pathogen with high resistance to multiple antibiotics (Murray, 2000; Leavis et al., 2006). E. faecium can cause a broad range of serious infections, including bacteremia, surgical wound infections, urinary tract infections, meningitis and endocarditis (Murray & Weinstock, 1999). Recent surveillance data revealed a change in the epidemiology of enterococcal infections: prior to 1997, infections due to E. faecium were infrequent (~10% of enterococcal clinical isolates), but now, in the nosocomial environment, E. faecium accounts for ~38% of healthcare associated enterococcal infections (Hidron et al., 2008; Top et al., 2008). In addition to the acquisition of resistance to antibiotics (e.g., amipicillin, aminoglycosides, and vancomycin), hospital-associated E. faecium strains belonging to CC17 genogroup more often carry genes encoding the enterococcal surface protein (esp) (Willems et al., 2001), a putative hyaluronidase (hyl) (Rice et al., 2003), 9 of the 14 fms (E. faecium surface protein-encoding) genes reported by Sillanpaa et al. (2009) and a functional copy of the collagen adhesin gene (acm) (Nallapareddy et al., 2003).
Similar to several other gram-positive pathogens, adherence to exposed host extracellular matrix (ECM) is likely the first step in the infection process of E. faecium. ECM is a complex array of secreted molecules, including collagens and a number of other glycoproteins, proteoglycans, and glycosaminoglycans (GAGs) (Iozzo, 1997). Earlier reports on ECM protein adherence of E. faecium, including our previous pilot survey of 13 clinical E. faecium isolates, demonstrated the ability of occasional isolates to adhere to one or more ECM proteins, such as fibronectin (Fn), vitronectin and lactoferrin (Lf) (Zareba et al., 1997; Xiao et al., 1998; Styriak et al., 1999; Tyriak & Ljungh, 2003). Since relatively few isolates and adherence targets (except collagen type I (CI), see below) were included in these studies, the prevalence of adherence phenotypes to ECM components and their distribution among different populations of E. faecium, such as isolates associated with human infections versus non-clinical sources, remains unclear. Recently, Nallapareddy et al. assessed a diverse collection of 122 isolates for acm mediated adherence to CI and found a significant association between clinical (versus fecal or food) origin and CI adherence (Nallapareddy et al., 2003; Nallapareddy et al., 2008).
In this investigation, we examined the ability of 60 E. faecium strains, derived from human infections, community feces, and animals, to adhere to up to 15 ECM and serum components, including various glycoproteins, proteoglycans and GAGs. The possible association between adherence and clinical origin was also analyzed.
Materials and methods
Bacterial strains and growth conditions
A total of 60 E. faecium clinical and non-clinical isolates collected between 1990 and 2006 from diverse geographic locations (the United States, Argentina, China, Germany, and Spain) were included in this study. These isolates were derived from blood cultures of endocarditis patients (n = 20, collected between 1992 and 2006), other clinical infections (OCI group; isolated from blood of non-endocarditis patients, bile, catheters, sputum, urine and wounds; n = 19, collected between 1990 and 2006), healthy volunteer human feces from the community (n = 10, collected between 1994 and 2003), and animals (isolated from either feces or rumen contents of cattle; feces of chickens, turkeys, or swine; n = 11, collected between 1998 and 2002). Isolates known to be identical by pulsed-field gel electrophoresis to other isolates in this collection were previously excluded (Sillanpaa et al., 2009).
All of the E. faecium isolates were previously identified to species level by biochemical tests and confirmed by high stringency colony hybridization using intragenic efafm and aac(6′)-li probes (Singh et al., 1998; Duh et al., 2001). For routine growth, organisms were grown using brain heart infusion (BHI; Difco Laboratories, Detroit, MI) broth/agar and incubated overnight at 37 °C.
ECM components
The 15 host components used for adherence studies in this study can be classified into five groups. 1. Classic ECM proteins: CI (rat tail, Sigma, St. Louis, MO), collagen type V (CV, human placenta, Sigma), Fn (human plasma, Enzyme Research Laboratories, South Bend, IN), vitronectin (human placenta, Sigma) and laminin (Ln, natural mouse Ln from EHS cells, Invitrogen, Carlsbad, CA). 2. Plasma proteins: fibrinogen (Fg, human plasma, Enzyme Research Laboratories), Lf (human milk, Sigma), transferrin (Tf, human, Sigma) and plasminogen (human plasma, Enzyme Research Laboratories). 3. Small leucine-rich proteoglycans (SLRPs): decorin (bovine articular cartilage, Sigma) and biglycan (bovine articular cartilage, Sigma). 4. GAGs: heparan sulfate (bovine kidney, Sigma), heparin (porcine intestinal mucosa) and hyaluronic acid (human umbilical cord, Sigma). 5. Other: mucin (porcine mucosa, Sigma).
Adherence of radiolabeled cells of E. faecium isolates to immobilized ECM proteins
E. faecium cells were inoculated at an initial cell density of OD600 nm = 0.01 to 5 mL BHI broth containing 10 μCi mL−1 35S label, and were grown at 37°C for 8, 10 or 12 h. Adherence of bacteria to immobilized host proteins was tested by an assay described previously with minor modifications (Xiao et al., 1998; Nallapareddy & Murray, 2008). Briefly, 1 μg of CI, CV, and Ln in PBS, pH 7.4, and Fn and Fg in 50 mM carbonate-bicarbonate buffer, pH 9.6, was immobilized in the wells. Since we have not previously assessed the coating efficiencies of Lf, Tf, decorin, plasminogen, heparin, hyaluronic acid and mucin using this assay, we initially immobilized 1, 5 and 10 μg of each protein in PBS or in the above carbonate-bicarbonate buffer to the wells to test adherence of 8 E. faecium isolates. Peak adherence was observed by some of the isolates to Lf and Tf at 5 μg and without further increase at 10 μg, while the remaining isolates were non-adherent to any of the tested proteins at all three concentrations. No adherence to BSA was observed at these concentrations. Furthermore, coating with host proteins dissolved in the carbonate-bicarbonate buffer did not alter the binding of these test strains. Hence, 5 μg in PBS was used in subsequent experiments for coating these proteins. Adherence was determined from three independent experiments using triplicate wells. CI (1 μg) was included as a positive control (Nallapareddy et al., 2003; Nallapareddy et al., 2008) and BSA (5 μg, Sigma) as a negative control. The following formula was used for calculating the percentage of adhered bacteria: (radioactivity of adhered cells/total radioactivity of added cells) × 100. Isolates were classified as adhering to ECM proteins if ≥ 5% of total labeled cells bound to wells (Nallapareddy & Murray, 2008).
Sequencing of the purK allele
The allelic profile for purK was determined by sequencing for 25 of the 60 isolates, which had not previously been assessed for the presence of the purK1 allele (a marker for CC17 lineage (Willems et al., 2001; Nallapareddy et al., 2008)) using primer sequences available at http://efaecium.mlst.net/misc/info.asp.
Statistical analysis
The two-tailed Fisher's exact test was used to determine the statistical significance of the differences observed in adherence to various ECM molecules for different E. faecium isolates. P values less than 0.05 were considered as statistically significant.
Results and discussion
E. faecium adherence to CI, Fn and Fg at three different growth phases
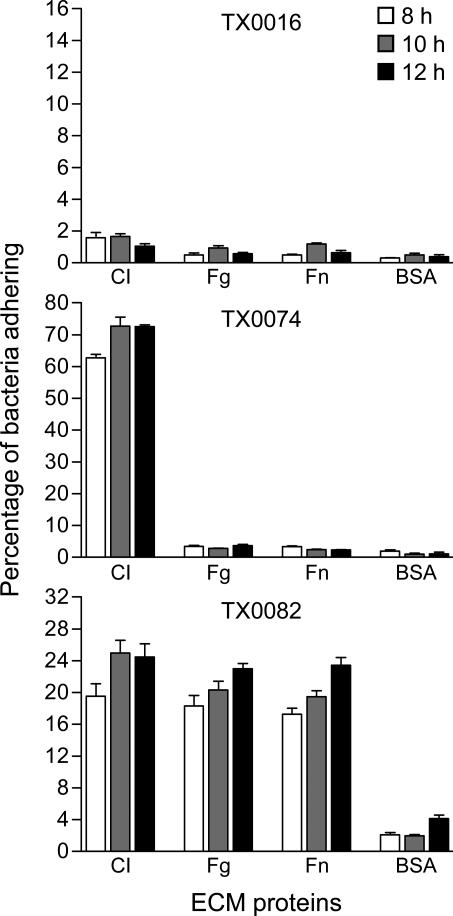
Previous studies on E. faecalis and Streptococcus bovis (Nallapareddy & Murray, 2008; Sillanpaa et al., 2008), revealed that the ability of bacterial strains to adhere to immobilized CI, Fn and Fg is often affected by their growth stage. To examine whether different growth phases influence the adherence phenotypes of E. faecium isolates, we selected three endocarditis strains: TX0016 (ST16; also known as strain DO and for which the genome sequence is available), TX0074 (ST337; non-CC17 strain) and TX0082 (ST17; founder sequence type of CC17), and compared their ability to adhere to CI, Fn, and Fg after growth in BHI for 8 h (approximately late exponential phase), 10 h (approximately the time of entry into the stationary phase), and 12 h (2 h after entry into stationary phase). Consistent with our earlier findings, there was no appreciable binding (< 5% of cells adhered) to CI, Fn, Fg, or BSA by TX0016 (Xiao et al., 1998; Nallapareddy et al., 2003), TX0074 showed a high level of adherence to CI, and TX0082 to CI and Fg (Nallapareddy et al., 2006) as well as Fn (Fig. 1). TX0074 and TX0082 showed higher adherence levels to tested proteins after growing for 10 h and 12 h compared to 8 h, while binding to BSA by both TX0074 and TX0082 was less at 10 h than at 12 h. Thus, for subsequent analyses, cells harvested at 10 h were used.
Fig. 1.
Time course of adherence of three endocarditis-derived E. faecium isolates to ECM and serum proteins. Cells were grown for 8, 10, or 12 h, as indicated in the figure. The results represent mean ± SD of 6 wells from two independent assays. CI, collagen type I; Fg, fibrinogen; Fn, fibronectin.
Adherence of 60 E. faecium isolates to ECM components
We initially examined the adherence of 8 E. faecium endocarditis isolates to all 15 ECM molecules. Since no binding was detected to vitronectin, biglycan and heparan sulfate in this screening assay, we limited our subsequent assays to 11 ECM molecules: CV, Fn, Ln, Fg, Lf, Tf, decorin, plasminogen, heparin, hyaluronic acid and mucin. Results of adherence from our first assay with all 60 isolates, using triplicate wells for each isolate, showed no binding by any of the isolates to plasminogen, heparin, hyaluronic acid, decorin and mucin. Since these proteins, along with vitronectin, biglycan and heparan sulfate above, appeared not to be significant adherence targets for E. faecium isolates, we limited our two replicative assays to Fn, Fg, Ln, CV, Tf and Lf, as at least one isolate exhibited adherence to these proteins in our first assay; hence, three independent assays with 9 wells in total were performed for Fn, Fg, Ln, CV, Tf and Lf. Despite the lack of adherence to the 9 proteins above in this study, we cannot rule out the possibility that adherence to some of these proteins could be elicited in other culture conditions, e.g., in the presence of host-derived material, or in vivo in the host.
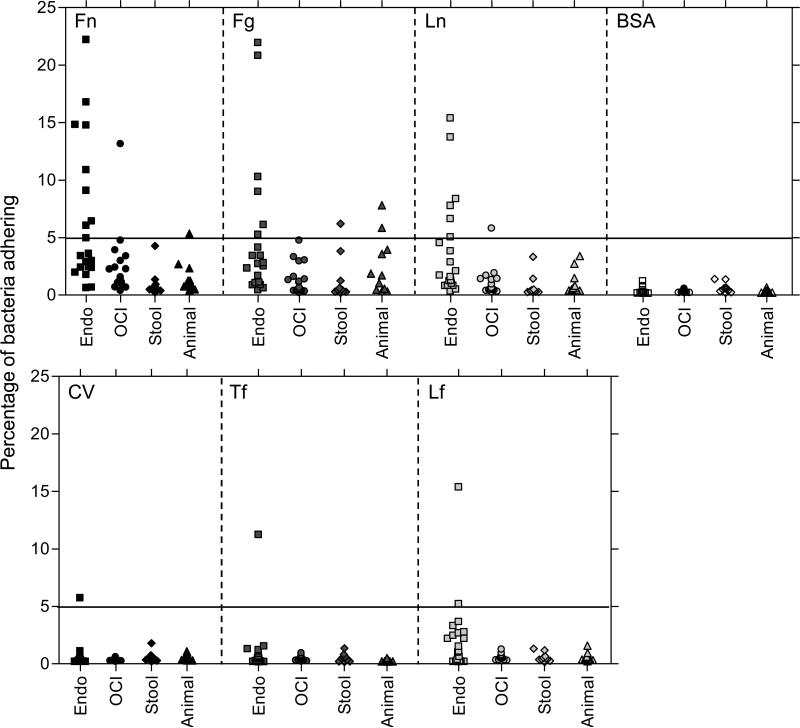
With cells grown in BHI for 10 h, 16 of the 60 isolates (27%) were considered adherent (≥ 5% of bacteria adhered) to one or more ECM molecules, while adherence to BSA was minimal (≤ 1.4%) for all 60 isolates (Fig. 2). Most of the 16 isolates exhibited adherence to one (6 isolates) or two (6 isolates) of the six ECM proteins, while only one isolate (TX0082, previously shown to express acm, scm and ebpfm (Nallapareddy et al., 2006; Sillanpaa et al., 2008)) was adherent to all six. Adherence to Fn was most commonly observed, with 12 of the 60 isolates (20%) showing more than 5% adherence and 6 of these showing adherence levels above 10% (Fig. 2). Adherence to Fg and Ln was slightly less common, with 10 of the 60 isolates (17%) exhibiting adherence to Fg and 8 (13%) to Ln. Two isolates were considered adherent to Lf; one of these was the only isolate showing adherence to CV and Tf. It is notable that these adherence phenotypes were clustered in some of the isolates, e.g., all eight isolates exhibiting adherence to Ln, with one exception, were also adherent to Fn and seven of the ten isolates that adhered to Fg also adhered to Fn. While yet to be investigated, this clustering of positive adherence within certain isolates could be a result of a generalized regulation mechanism, leading to expression of functional adhesins in a subset of the isolates during in vitro growth.
Fig. 2.
Adherence of 60 E. faecium isolates to immobilized ECM and serum proteins. Cells were grown in BHI for 10 h. Adherence was tested in wells coated with 1μg of collagen type V, fibronectin, laminin, fibrinogen or 5μg of transferrin, lactoferrin or BSA. Each data point represents the mean percentage of adherent cells of 9 wells from three assays. Endo, strains isolated from patients with E. faecium endocarditis; OCI, strains isolated from non-endocarditis E. faecium clinical infections; stool, isolates from the stool of healthy community volunteers; animal, isolates of animal origin.
Comparison of ECM protein adherence among clinical and non-clinical isolates
Ten of the 20 (50%) endocarditis-derived E. faecium isolates and three of the 19 (16%) other clinical isolates showed adherence to one or more ECM molecules, and the percentages of adherent cells among these strains ranged from 5% to 23%. In contrast, only one of 10 stool isolates from community volunteers (10%) adhered to at least one ECM molecule, while two of 11 animal-derived isolates (18%) adhered, and their adherence was at a lower level, as shown in Fig. 2. For Fn adherence, a statistically significant difference was noted between the percentages of adherent strains in clinical groups (endocarditis plus other clinical infections) and non-clinical groups (stool isolates from community volunteers and animal sources) (P = 0.04); this was driven by the higher percent of endocarditis-derived E. faecium isolates showing adherence to Fn compared to non-clinical isolates (stool isolates from community volunteers and animal sources) (P = 0.003) (Table 1). Similarly, there was a statistically significant difference between the percentages of adherent strains in clinical groups versus non-clinical groups for adherence to Ln (P = 0.04); again, more endocarditis-derived isolates adhered to Ln versus non-clinical isolates (P = 0.003) (Table 1). The percentage of Fg-adherent strains in clinical groups was found to be not significantly different (18%) from non-clinical groups (14%). One of the 20 endocarditis isolates adhered to CV and Tf and two to Lf, while none of the OCI isolates, stool isolates from healthy volunteers or animal sources showed adherence to these three ECM molecules. Taken together, these results indicate that in vitro-grown clinical E. faecium isolates, especially those derived from endocarditis, commonly exhibit adherence to the major protein components of the ECM, Fn, Ln and Fg, as well as CI (Nallapareddy et al., 2003; Nallapareddy et al., 2008), while adherence to the other ECM components tested is uncommon.
Table 1.
Adherence of 60 temporally and geographically diverse E. faecium isolates to six ECM proteins
| No. (%) of adherent isolates |
||||||
|---|---|---|---|---|---|---|
| ECM molecule | Clinical isolates |
Non-clinical isolates |
||||
| Endoa (n = 20)c | OCIb (n = 19)d | Total (n = 39) | Stool (community) (n = 10)e | Animal (n = 11)e | Total (n = 21) | |
| Fibronectin | 9 (45%)f | 2 (11%) | 11 (28%)g | 0 | 1 (9%) | 1 (5%) |
| Fibrinogen | 6 (30%) | 1 (5%) | 7 (18%) | 1 (10%) | 2 (18%) | 3 (14%) |
| Laminin | 7 (35%)f | 1 (5%) | 8 (21%)g | 0 | 0 | 0 |
| Collagen V | 1 (5%) | 0 | 1 (3%) | 0 | 0 | 0 |
| Transferrin | 1 (5%) | 0 | 1 (3%) | 0 | 0 | 0 |
| Lactoferrin | 2 (10%) | 0 | 2 (5%) | 0 | 0 | 0 |
Endo: isolates from E. faecium endocarditis patients
OCI: other clinical isolates from various tissues/infections (see Methods)
17 of 20 isolates had the CC17-associated purK1 allele
12 of 19 had purK1
none had purK1
P < 0.004, endocarditis isolates versus non-clinical isolates (stool isolates from community and animal sources)
P = 0.04, clinical isolates (endocarditis and OCI) versus non-clinical isolates (stool isolates from community and animal sources).
Adherence to ECM proteins in the E. faecium genogroup CC17
Our previous analysis of 35 of the 60 isolates studied here for their ancestral relationships identified 18 of them as CC17 and 17 as non-CC17 (Nallapareddy et al., 2008). Since the purK allele has been defined as an epidemic marker of the CC17 lineage by MLST studies (Willems et al., 2001), we sequenced the purK alleles of the 25 remaining isolates. Three of the 5 endocarditis and 8 of the 10 OCI isolates, but none of the four normal stool and 6 animal isolates were found to carry the CC17-specific purK1 allele. Thus, the overall combined results of MLST and purK allele analyses implicated 29 of the 39 clinical isolates and none of the 21 non-clinical isolates as being related to CC17.
For each of the six ECM molecules, a higher percentage of CC17 isolates exhibited adherence (ranging from 3 to 35%) compared to non-CC17 isolates (ranging from 0 to 13%). Statistically significant enrichment among CC17 isolates was detected for adherence to Fn (P = 0.009) and to Ln (P = 0.023) (Table 2). The percentage of Fg-adherent strains in the CC17 group was found to be not significantly different (21%) from the non-CC17 group (13%). The increased adherence to Fn and Ln among the endocarditis isolates of this study, of which most (17 of 20) were CC17-related, could be a “cause”, i.e., it might contribute to their ability to cause endocarditis, or an “effect”, i.e. it may have been elicited during the presence of the organism in the vegetation for a prolonged period.
Table 2.
Comparison of ECM protein adherence between CC17 and non-CC17 isolates
| No. (%) of adherent isolates |
||
|---|---|---|
| ECM molecule | CC17 n = 29 | Non-CC17 n = 31 |
| Fibronectin | 10 (35%)a | 2 (6%) |
| Fibrinogen | 6 (21%) | 4 (13%) |
| Laminin | 7 (22%)b | 1 (3%) |
| Collagen V | 1 (3%) | 0 |
| Transferrin | 1 (3%) | 0 |
| Lactoferrin | 2 (7%) | 0 |
P = 0.009 against the non-CC17 isolates of our study.
P = 0.023 against the non-CC17 isolates of our study.
Lack of correlation of the observed adherence phenotypes with the presence of fms genes encoding putative and characterized MSCRAMM and pilus family proteins
We recently evaluated the distribution of 14 fms genes encoding putative MSCRAMMs and/or pilus proteins (Hendrickx et al., 2007; Sillanpaa et al., 2008) among 433 isolates, including the 60 isolates of this study (Sillanpaa et al., 2009). Here, we tested for a possible association between gene presence and adherence phenotype and found no apparent differences in gene distribution profiles of adherent versus non-adherent isolates (to individual ECM and serum proteins). However, it is plausible that these genes are expressed at varying levels among different strains, resulting in adherence of some of the isolates but not others, as we recently found with acm (Nallapareddy et al., 2003; Nallapareddy et al., 2008), or that they are present as pseudogenes in many of the isolates, as shown for acm and three of the 14 genes, fms15, fms16 and fms19 (Nallapareddy et al., 2003; Hendrickx et al., 2007; Nallapareddy et al., 2008; Sillanpaa et al., 2008), or that some of them are functionally redundant, i.e., two or more of these genes code for adhesins that bind to the same ligand.
In summary, we have reported here a systematic evaluation of the ability of a large collection of E. faecium isolates from clinical and non-clinical sources to adhere to various host ECM and serum components and detected adherence to six ECM proteins: Fn, Fg, Ln, CV, Tf and Lf. Our observation of the more frequent adherence to Fn and Ln by clinical isolates, particularly endocarditis isolates, as compared to fecal isolates from community volunteers and animal isolates, and the significant association of these two adherence phenotypes with CC17-related isolates, suggests that these interactions may have contributed to the emergence of E. faecium in nosocomial infections. Our ongoing studies are aimed at determining the specific adhesins involved in E. faecium pathogenesis and their host ligands.
Acknowledgement
This work was supported by NIH grant R01 AI067861 from the Division of Microbiology and Infectious Diseases, NIAID, to Barbara E. Murray.
References
- Duh RW, Singh KV, Malathum K, Murray BE. In vitro activity of 19 antimicrobial agents against enterococci from healthy subjects and hospitalized patients and use of an ace gene probe from Enterococcus faecalis for species identification. Microb Drug Resist. 2001;7:39–46. doi: 10.1089/107662901750152765. [DOI] [PubMed] [Google Scholar]
- Hendrickx AP, van Wamel WJ, Posthuma G, Bonten MJ, Willems RJ. Five genes encoding surface-exposed LPXTG proteins are enriched in hospital-adapted Enterococcus faecium clonal complex 17 isolates. J Bacteriol. 2007;189:8321–8332. doi: 10.1128/JB.00664-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- Leavis HL, Bonten MJ, Willems RJ. Identification of high-risk enterococcal clonal complexes: global dispersion and antibiotic resistance. Curr Opin Microbiol. 2006;9:454–460. doi: 10.1016/j.mib.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Murray BE. Vancomycin-resistant enterococcal infections. N Engl J Med. 2000;342:710–721. doi: 10.1056/NEJM200003093421007. [DOI] [PubMed] [Google Scholar]
- Murray BE, Weinstock GM. Enterococci: new aspects of an old organism. Proc Assoc Am Physicians. 1999;111:328–334. doi: 10.1046/j.1525-1381.1999.99241.x. [DOI] [PubMed] [Google Scholar]
- Nallapareddy SR, Murray BE. Role played by serum, a biological cue, in the adherence of Enterococcus faecalis to extracellular matrix proteins, collagen, fibrinogen, and fibronectin. J Infect Dis. 2008;197:1728–1736. doi: 10.1086/588143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallapareddy SR, Weinstock GM, Murray BE. Clinical isolates of Enterococcus faecium exhibit strain-specific collagen binding mediated by Acm, a new member of the MSCRAMM family. Mol Microbiol. 2003;47:1733–1747. doi: 10.1046/j.1365-2958.2003.03417.x. [DOI] [PubMed] [Google Scholar]
- Nallapareddy SR, Singh KV, Murray BE. Construction of improved temperature-sensitive and mobilizable vectors and their use for constructing mutations in the adhesin-encoding acm gene of poorly transformable clinical Enterococcus faecium strains. Appl Environ Microbiol. 2006;72:334–345. doi: 10.1128/AEM.72.1.334-345.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallapareddy SR, Singh KV, Okhuysen PC, Murray BE. A functional collagen adhesin gene, acm, in clinical isolates of Enterococcus faecium correlates with the recent success of this emerging nosocomial pathogen. Infect Immun. 2008;76:4110–4119. doi: 10.1128/IAI.00375-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice LB, Carias L, Rudin S, Vael C, Goossens H, Konstabel C, Klare I, Nallapareddy SR, Huang W, Murray BE. A potential virulence gene, hylEfm, predominates in Enterococcus faecium of clinical origin. J Infect Dis. 2003;187:508–512. doi: 10.1086/367711. [DOI] [PubMed] [Google Scholar]
- Sillanpaa J, Prakash VP, Nallapareddy SR, Murray BE. Distribution of genes encoding MSCRAMMs and Pili in clinical and natural populations of Enterococcus faecium. J Clin Microbiol. 2009;47:896–901. doi: 10.1128/JCM.02283-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanpaa J, Nallapareddy SR, Singh KV, Ferraro MJ, Murray BE. Adherence characteristics of endocarditis-derived Streptococcus gallolyticus ssp. gallolyticus (Streptococcus bovis biotype I) isolates to host extracellular matrix proteins. FEMS Microbiol Lett. 2008;289:104–109. doi: 10.1111/j.1574-6968.2008.01378.x. [DOI] [PubMed] [Google Scholar]
- Sillanpaa J, Nallapareddy SR, Prakash VP, Qin X, Hook M, Weinstock GM, Murray BE. Identification and phenotypic characterization of a second collagen adhesin, Scm, and genome-based identification and analysis of 13 other predicted MSCRAMMs, including four distinct pilus loci, in Enterococcus faecium. Microbiology. 2008;154:3199–3211. doi: 10.1099/mic.0.2008/017319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KV, Coque TM, Weinstock GM, Murray BE. In vivo testing of an Enterococcus faecalis efaA mutant and use of efaA homologs for species identification. FEMS Immunol Med Microbiol. 1998;21:323–331. doi: 10.1111/j.1574-695X.1998.tb01180.x. [DOI] [PubMed] [Google Scholar]
- Styriak I, Laukova A, Fallgren C, Wadstrom T. Binding of selected extracellular matrix proteins to enterococci and Streptococcus bovis of animal origin. Curr Microbiol. 1999;39:327–0335. doi: 10.1007/s002849900467. [DOI] [PubMed] [Google Scholar]
- Top J, Willems R, van der Velden S, Asbroek M, Bonten M. Emergence of clonal complex 17 Enterococcus faecium in The Netherlands. J Clin Microbiol. 2008;46:214–219. doi: 10.1128/JCM.01351-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyriak I, Ljungh S. Binding of extracellular matrix molecules by enterococci. Curr Microbiol. 2003;46:435–442. doi: 10.1007/s00284-002-3879-2. [DOI] [PubMed] [Google Scholar]
- Willems RJ, Homan W, Top J, et al. Variant esp gene as a marker of a distinct genetic lineage of vancomycin-resistant Enterococcus faecium spreading in hospitals. Lancet. 2001;357:853–855. doi: 10.1016/S0140-6736(00)04205-7. [DOI] [PubMed] [Google Scholar]
- Xiao J, Hook M, Weinstock GM, Murray BE. Conditional adherence of Enterococcus faecalis to extracellular matrix proteins. FEMS Immunol Med Microbiol. 1998;21:287–295. doi: 10.1111/j.1574-695X.1998.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Zareba TW, Pascu C, Hryniewicz W, Wadstrom T. Binding of extracellular matrix proteins by enterococci. Curr Microbiol. 1997;34:6–11. doi: 10.1007/s002849900135. [DOI] [PubMed] [Google Scholar]