Abstract
Background
The aim of this study was to determine the risk factors for conversion from a normal to either a low or high ABI.
Methods
Participants in the Multi-Ethnic Study of Atherosclerosis who had two separate measurements of the ABI over a 3-year time period were assessed.
Results
At baseline, the mean age was 62 years and 50% were women, 28% African American, 12% Chinese, 22% Hispanic and 38% non-Hispanic White. Of the 5,514 participants with a baseline ABI between 0.90 and 1.40, 89 (1.6%) had an ABI ≤ 0.90 (“low ABI group”) and 71 (1.3%) had an ABI ≥ 1.40 (“high ABI group”) three years later. On multivariable analysis, the odds for having progressed into the low ABI group were significantly increased for higher baseline age, hypertension, diabetes, greater pack-years of cigarette smoking and homocysteine levels. The odds for progression into the high ABI group were increased for male gender and higher body mass index. Compared to non-Hispanic Whites, African Americans had a significantly higher odds for progression to the low ABI group (OR: 2.24, 95% CI: 1.29 – 3.88) while having a reduced odds for progression to the high ABI group (OR: 0.50, 95% CI: 0.24 – 1.00). Neither Chinese nor Hispanic ethnicity was significantly associated with progression to either ABI group.
Conclusions
The risk factors for progression to a low or high ABI were distinct and African Americans were at increased risk for progression to a low ABI but at decreased risk for progression into the high ABI group.
Introduction
The ankle-brachial index (ABI) is a valid1 and reproducible2, 3 method for detecting peripheral arterial disease (PAD). Since it is simple, inexpensive and non-invasive, the ABI is suitable for screening asymptomatic individuals and in community-based studies. Using an ABI value typically less than 0.90 as the criterion, many clinical and population-based studies have determined the prevalence of PAD.4-8 In these studies and compared to Whites, African Americans have repeatedly been shown to have a higher prevalence of PAD while Hispanic and Chinese Americans typically have prevalences similar to Whites.4, 7, 8 Moreover, this excess of PAD in African Americans appears independent of both traditional and novel cardiovascular disease (CVD) risk factors.9, 10
Recent evidence supports the importance of higher values of the ABI due in part to medial artery calcification as a marker of peripheral vascular disease.11 That is, individuals with an ABI > 1.40 have higher levels of certain CVD risk factors,12, 13 subclinical atherosclerosis14, 15 and risks for both incident total12 and CVD-related16 mortality while also having impaired quality of life in certain areas.13 Compared to a low ABI, studies on the prevalence of a ‘high ABI’ are more limited and therefore, the associations of ethnicity with a ‘high ABI’ are not as well characterized.
From a clinical perspective, if the initial ABI is normal, it is important to understand the incidence of and risk factors for progression to an abnormal value. However, there are few studies on determinants of changes in the ABI and we are unaware of any studies that evaluated the risk in multiple ethnic groups. Accordingly, the aim of this study was to determine the risk factors for longitudinal changes in the ABI to abnormal levels in the Multi-Ethnic Study of Atherosclerosis (MESA), including traditional and novel cardiovascular disease risk factors and an emphasis on ethnicity.
Methods
Subjects
Details about the MESA study design have been published elsewhere.17 In brief, between July 2000 and August 2002, 6,814 men and women who identified themselves as White, African American, Hispanic, or Chinese, were 45 to 84 years old and were free of clinically apparent cardiovascular disease (CVD) were recruited and participated in the baseline examination. Individuals with a history of physician-diagnosed heart attack, angina, heart failure, stroke or TIA, or having undergone an invasive procedure for cardiovascular disease were excluded from participation. The participants enrolled at baseline were eligible to participate in 3 subsequent study examinations occurring approximately every 2 years and referred to as clinic visits 2, 3 and 4. At clinic visit 3, subjects underwent repeat testing for the ankle brachial index.
Participants were recruited from the following 6 communities: Baltimore City and Baltimore County, Maryland; Chicago, Ill; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minn. Each field site recruited from locally available sources, which included lists of residents, lists of dwellings, and telephone exchanges. The institutional review boards at all participating centers approved the study and all participants gave informed written consent.
Data Collection
At the baseline examination and at visit 3, standardized questionnaires were used to obtain demographic information and level of education, annual household income, smoking history, and medication usage for high blood pressure, high cholesterol, or diabetes. Cigarette smoking was calculated in pack-years and also defined as current, former, or never. Height and weight were measured with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Resting blood pressure was measured 3 times in seated participants with a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon). The average of the last 2 measurements was used in the analysis. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or current use of an antihypertensive medication.
Laboratory
At the baseline examination and at visit 3, blood was collected after a 12-hour fast and stored at -70°C. Total and HDL cholesterol, triglycerides, glucose and creatinine were measured as previously reported17 LDL cholesterol was calculated by the Friedewald equation.18 Dyslipidemia was defined as a total-cholesterol/HDL-cholesterol ratio > 5.0 or use of cholesterol reducing medication. Diabetes was defined as fasting glucose ≥ 126 mg/dL or use of hypoglycemic medication. Impaired fasting glucose was defined as glucose 100 to 125 mg/dL.19 The estimated glomerular filitration rate (eGFR) was calculated using the Modified of Diet in Renal Disease (MDRD) study formula.20
Using baseline blood samples, measurements of the following classes of non-lipid biomarkers were conducted at either at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT) or the Biochemical Genetics Clinical Laboratory at Fairview-University Medical Center (Minneapolis, MN): inflammation (high-sensitivity plasma C-reactive protein, fibrinogen, interleukin-6), insulin concentration (fasting insulin) and hemostasis/fibrinolysis (factor VIII coagulant activity, plasmin-antiplasmin complex, D-dimer) as well as homocysteine.
Ankle-Brachial Index Protocol
At the baseline examination and clinic visit 3, systolic blood pressure measurements for calculation of the ABI were obtained using a hand-held Doppler instrument with a 5-mHz probe (Nicolet Vascular, Golden, Colorado). In brief, systolic blood pressures were measured in the bilateral brachial, dorsalis pedis, and posterior tibial arteries. Brachial artery pressures were averaged to obtain the ABI denominator. When the two brachial artery pressures differed by 10 mmHg or more, the highest brachial artery pressure was used as the denominator.21 For each lower extremity, the ABI numerator used was the highest pressure (dorsalis pedis or posterior tibial) from that leg. The lower of the right and left ABI values was used as the index ABI for that subject. In the analyses of prospective changes, the ABIs from the same leg were compared from baseline to visit 3. We defined progression to a “low ABI” as going from an ABI between 0.90 and 1.40 (a normal ABI) at baseline to an ABI ≤ 0.90 at visit 3. Similarly, we defined progression to a “high ABI” as going from a normal ABI at baseline to an ABI ≥ 1.40 at visit 3. Individuals with an incompressible ankle artery at either study visit were excluded from the study due to the inability to calculate the change in ABI for the affected vessel. A subset of 384 MESA participants had replicate ABI measurements at visit 3. The overall intraclass correlation coefficient (ICC) from these measurements was 0.93 while the intra-technician ICC was 0.95 and the inter-technician ICC was 0.92.
Statistical Analysis
Non-normally distributed variables were log transformed to achieve normality. Age, sex and race-adjusted means and percentages by ABI group at follow-up were computed using analysis of covariance. The p-values from these analyses compared those progressing into the low or high groups to those maintaining an ABI in the normal range. To estimate the associations of both CVD risk factors and ethnicity with ABI progression (high or low), separate logistic regression models were constructed to determine odds ratios (ORs) and 95% confidence intervals. The outcome for one set of models was progression to the low ABI group while the outcome for the other was progression into the high ABI group. We first analyzed the associations adjusting for age and sex. We then adjusted for BMI, hypertension dyslipidemia, pack-years of smoking, diabetes and family history of heart disease. Associations between changes in the ABI and changes in these same risk factors between visit 1 and visit 3 was modeled by adding the ‘change’ variables to the baseline variables in multivariable logistic regression models. The associations of non-lipid biomarkers with ABI progression were evaluated by separately adding each biomarker to the fully adjusted models. Those significantly associated with progression into the low or high ABI groups were retained in the final multivariable models. All analysis was done using Stata, 10.0 (StataCorp LP, College Station, TX). A two-tailed p-value of 0.05 was considered statistically significant.
Results
Baseline characteristics of the MESA cohort stratified by race are provided in Table 1. For all racial groups, the mean age was approximately 62 years and roughly 50% were women. African Americans had the largest body mass index while Chinese Americans had the smallest. African Americans also had the highest estimated glomerular filtration rate and prevalence of hypertension while Caucasians had the highest prevalence of dyslipidemia. Hispanic and African Americans had a similar prevalence of diabetes mellitus. Although African Americans also had the highest prevalence of current smokers, Caucasians had the highest amount of cigarette smoking when measured in pack-years. African Americans had the highest levels of homocysteine, C-reactive protein, interleukin-6, fibrinogen, D-dimer, factor VIII and plasmin-antiplasmin. Finally, the overall mean baseline ABI was 1.11, which was lowest in African Americans (1.07), highest in Hispanic Americans (1.12) and intermediate for non-Hispanic Whites and Chinese Americans (1.11 and 1.10, respectively).
Table 1. Cohort Characteristics by Ethnicity: The Multi-Ethnic Study of Atherosclerosis.
| Characteristic | African American (N = 1,894) |
Chinese (N = 803) |
Hispanic (N = 1,493) |
Caucasian (N = 2,624) |
|---|---|---|---|---|
| Age (years)* | 62.1 (10.0) | 62.3 (10.3) | 61.3 (10.3) | 62.6 (10.2) |
| Female† | 1050 (55.4) | 413 (51.4) | 775 (51.9) | 1363 (51.9) |
| Body Mass Index (kg/m2)* | 30.2 (5.9) | 24.0 (3.3) | 29.4 (5.1) | 27.7 (5.1) |
| Glomerular Filtration Rate (ml/min/1.73 m2) | 86.4 (19.3) | 82.3 (16.7) | 83.3 (18.4) | 75.9 (17.0) |
| Glucose (mg/dL)* | 107.2 (32.7) | 106.1 (28.8) | 110.7 (39.7) | 98.4 (22.1) |
| Insulin (mU/L)* | 7.4 (6.7) | 6.1 (5.7) | 8.1 (6.1) | 6.0 (4.5) |
| Diabetes† | 369 (19.6) | 122 (15.2) | 291 (19.5) | 189 (7.2) |
| Systolic Blood Pressure (mmHg)* | 132 (22) | 125 (21.6) | 127 (21.9) | 124 (20.4) |
| Diastolic Blood Pressure (mmHg)* | 74 (10.2) | 72 (10.3) | 72 (10.1) | 70 (10.0) |
| Hypertension† | 1127 (59.5) | 301 (37.5) | 618 (41.4) | 1012 (38.6) |
| Total Cholesterol (mg/dL)* | 190 (36.3) | 193 (31.8) | 198 (37.5) | 196 (35.1) |
| HDL Cholesterol (mg/dL)* | 52 (12.7) | 50 (12.7) | 48 (13.1) | 52 (15.7) |
| LDL Cholesterol (mg/dL)* | 117 (33.0) | 115 (29.0) | 120 (32.9) | 117 (30.1) |
| Triglycerides (mg/dL)* | 105 (69.0) | 143 (84.8) | 157 (101) | 133 (90.2) |
| Dyslipidemia† | 312 (16.5) | 118 (14.7) | 211 (14.1) | 460 (17.5) |
| Cigarette smoking (pack-years)* | 11.9 (19.5) | 4.9 (14.2) | 7.7 (16.5) | 15.1 (27.6) |
| Current Smoker† | 338 (18.0) | 45 (5.6) | 203 (13.6) | 301 (11.5) |
| Homocysteine (μmol/L)* | 9.7 (4.1) | 9.0 (2.9) | 9.1 (3.2) | 9.3 (4.0) |
| C-reactive Protein (mg/L)ξ | 2.53 (1.08 – 5.71) | 0.87 (0.47 – 1.79) | 2.45 (1.15 – 4.91) | 1.75 (0.26 – 4.02) |
| Interleukin-6 (pg/mL)* | 1.7 (1.3) | 1.2 (1.0) | 1.7 (1.2) | 1.5 (1.2) |
| Fibrinogen (mg/dL)* | 361 (80) | 329 (60) | 359 (75) | 334 (70) |
| D-dimer (μg/mL)* | 0.45 (1.0) | 0.28 (0.43) | 0.37 (0.82) | 0.35 (0.86) |
| Factor VIII (%)* | 178 (74.5) | 158 (57.0) | 162 (63.4) | 157 (64.6) |
| Plasmin-Antiplasmin (nM)* | 5.2 (2.5) | 4.1 (1.4) | 4.6 (2.0) | 4.8 (2.2) |
Mean (SD);
Frequency (%);
Median (Interquartile Range)
The mean time between visit 1 and visit 3 ABI measurements was 3.2 (SD 0.32) years. Nine-hundred and twenty-seven participants did not have their ABI measured at visit 3; 867 of these were because the participant did not attend visit 3. This group was similar to those who did have an ABI measured at visit 3 with respect to gender distribution, baseline ABI, body mass index, pack-years of cigarette smoking, homocysteine, cholesterol medication use and serum creatinine. Conversely, the non-visit 3 participants were older and had lower prevalence of hypertension and diabetes.
Correlations between risk factors and changes in the continuous ABI value from visit 1 to visit 3 were all very modest (r < 0.10). With adjustment for age, sex and ethnicity, there were essentially no risk factor associations for large (> 0.05/year) increases or decreases in the ABI except for cigarette smoking. Compared to those who did not exhibit a large increase or decrease in the ABI from visit 1 to visit 3, those who had a large decline had a higher prevalence of current smoking (17.0 vs. 10.1%, p = 0.03) while those who had a large increase in the ABI had a lower prevalence of current smoking (6.9 vs. 10.1%, p = 0.01).
Of the 5,514 MESA participants with a baseline ABI between 0.90 and 1.40, 89 (1.6%) had an ABI ≤ 0.90 and 71 (1.3%) had an ABI ≥ 1.40 at visit 3. Table 2 shows the age, sex and race-adjusted baseline risk factor distributions for those whose ABI changed from normal to 1) less than or equal to 0.90 [“low ABI group”], 2) greater than or equal to 1.40 [“high ABI group”], or 3) remained between 0.90 and 1.40 [“normal ABI group”]. The mean age, sex and ethnicity adjusted baseline ABI value for the low ABI group was 1.02. Compared to those in the normal ABI group and after adjustment for age, sex and race, significant baseline risk factors for progressing into the low ABI group included a lower baseline ABI but a higher fasting blood glucose, cholesterol, triglycerides, pack-years smoking and homocysteine, as well as higher prevalences of hypertension and current smoking. The mean age, sex and ethnicity adjusted baseline ABI value for the high ABI group was 1.19. Compared to those in the normal ABI group, the significant baseline risk factors for progressing into the high ABI group included a higher baseline ABI, body mass index and insulin but a lower HDL cholesterol. Baseline smoking was less prevalent among those who progressed into the high ABI group.
Table 2. Age, Sex and Race-Adjusted Baseline Risk Factor Levels by Category of ABI Progression over Three Years: The Multi-Ethnic Study of Atherosclerosis.
| Risk Factor | ABI Status at Year 3 | P-Values | |||
|---|---|---|---|---|---|
| Low ABI (≤ 0.9) [N = 89] |
Normal ABI (0.91 – 1.39) [N = 5,354] |
High ABI (≥1.4) [N = 71] |
Low vs. Normal | High vs. Normal | |
| Age (years)* | 68.3 (9.9) | 61.2 (9.9) | 60.0 (10.0) | <0.0005 | 0.33 |
| Gender (Female)† | 50 (56.2) | 2,824 (52.8) | 26 (36.6) | 0.56 | <0.01 |
| Baseline ABI* | 1.02 (0.08) | 1.11 (0.08) | 1.19 (0.08) | <0.01 | <0.01 |
| Body Mass Index (kg/m2)* | 28.2 (5.0) | 28.2 (5.0) | 29.8 (5.0) | 1.00 | <0.01 |
| Creatinine (mg/dL)* | 0.95 (0.17) | 0.95 (0.17) | 0.95 (0.17) | 0.72 | 0.97 |
| Glomerular Filtration Rate (ml/min/1.76 m2) | 80.8 (22.6) | 81.3 (16.9) | 80.4 (14.0) | 0.29 | 0.66 |
| Glucose (mg/dL)* | 112.3 (27.9) | 102.7 (27.8) | 102.2 (28.0) | <0.01 | 0.89 |
| Insulin (mU/L)* | 7.3 (5.3) | 6.7 (5.3) | 8.0 (5.3) | 0.30 | 0.05 |
| Diabetes† | 19 (21.3) | 652 (15.3) | 5 (7.0) | 0.12 | 0.25 |
| Hypertension† | 61 (68.5) | 2242 (41.1) | 22 (31.0) | 0.02 | 0.17 |
| HDL Cholesterol (mg/dL)* | 49.2 (13.5) | 51.2 (13.5) | 47.9 (13.5) | 0.16 | 0.04 |
| LDL Cholesterol (mg/dL)* | 119.2 (31.2) | 117.3 (31.0) | 116.4 (31.3) | 0.58 | 0.81 |
| VLDL Cholesterol (mg/dL)* | 5.4 (5.5) | 3.8 (5.4) | 4.8 (5.5) | <0.01 | 0.16 |
| Triglycerides (mg/dL)* | 155 (85) | 130 (85) | 135 (85) | >0.01 | 0.62 |
| Dyslipidemia† | 17 (19.1) | 848 (15.0) | 7 (9.9) | 0.75 | 0.19 |
| Total Cholesterol (mg/dL)* | 198 (35) | 194 (35) | 190 (35) | 0.29 | 0.27 |
| Cigarette smoking (pack-years)* | 17.9 (21.2) | 10.5 (21.1) | 7.9 (21.2) | <0.01 | 0.30 |
| Current Smoker† | 18 (20.2) | 645 (10.4) | 3 (4.2) | <0.01 | 0.05 |
| Homocysteine (μmol/L)* | 10.2 (3.3) | 9.1 (3.3) | 9.2 (3.3) | <0.01 | 0.88 |
| C-reactive Protein (mg/L)ξ | 2.44 | 2.23 | 2.49 | 0.65 | 0.12 |
| Interleukin-6 (mg/dL)* | 1.55 (1.1) | 1.47 (1.1) | 1.47 (1.1) | 0.50 | 0.99 |
| Fibrinogen (mg/dL)* | 351 (68) | 343 (68) | 352 (69) | 0.27 | 0.27 |
| D-dimer (μg/mL)* | 0.37 (0.74) | 0.34 (0.74) | 0.33 (0.75) | 0.74 | 0.90 |
| Factor VIII (%)* | 163 (64) | 161 (63) | 162 (64) | 0.75 | 0.84 |
| Plasmin-Antiplasmin (nM)* | 4.4 (1.9) | 4.7 (1.9) | 4.4 (1.9) | 0.20 | 0.24 |
Mean (SD);
Frequency (%);
Geometric Mean
In a logistic regression model containing age, sex, ethnicity, body mass index, hypertension, diabetes, dyslipidemia, smoking and family history of CHD (Table 3), the odds for having progressed into the low ABI group were significant for increasing baseline age [1-year increment] (Odds Ratio: 1.07, 95% Confidence Interval: 1.04 – 1.10), hypertension (OR: 1.75, 95% CI: 1.05 – 2.91), diabetes (OR: 1.81, 95% CI: 1.83 – 3.17) and pack-years of cigarette smoking [10 pack-year increment] (OR: 1.09, 95% CI: 1.04 – 1.15). Statin use was associated with a non-statistically significant reduction progression to a low ABI (OR = 0.64, 95% CI: 0.34 – 1.21). [The results for ethnicity are provided below]. When the 3-year changes in the CVD risk factors were included, both new hypertension (OR: 2.46, 95% CI: 1.11 – 5.44) and new dyslipidemia (OR: 1.74, 95% CI: 1.02 – 2.07) were associated with progression into the low ABI group. Using the same CVD risk factor variables listed above, the odds of progression into the high ABI group were only significant for male gender (OR: 1.78, 95% CI: 1.06 – 3.00), baseline body mass index [1-unit increment] (OR: 1.08, 95% CI: 1.03 – 1.13). Statin use was associated with a significant reduction in the odds for progression to a high ABI (OR = 0.29, 95% CI: 0.09 – 0.94). None of the 3-year changes in the CVD risk factor variables were significantly association with progression into the high ABI group.
Table 3. Risk Factor Associations for Progression to an Ankle Brachial Index (ABI) Less Than 0.90 or Greater Than 1.40.
| Variable | ABI < 0.90 | ABI > 1.40 | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p-value | Odds Ratio | 95% CI | p-value | |
| Age (10 years) | 1.91 | 1.47 - 2.48 | < 0.01 | 1.07 | 0.82 - 1.41 | 0.60 |
| Gender (Male) | 0.74 | 0.46 - 1.18 | 0.20 | 1.78 | 1.06 - 3.00 | 0.03 |
| BMI (3 units) | 0.97 | 0.83 - 1.09 | 0.58 | 1.25 | 1.09 - 1.45 | < 0.01 |
| Hypertension | 1.73 | 1.03 - 2.89 | 0.04 | 0.61 | 0.34 - 1.08 | 0.09 |
| Dyslipidemia | 0.72 | 0.40 - 1.31 | 0.29 | 0.56 | 0.24 - 1.32 | 0.19 |
| Smoking (10 pk-yrs) | 1.09 | 1.03 - 1.15 | <0.01 | 0.95 | 0.83 - 1.09 | 0.45 |
| Diabetes | 1.82 | 1.04 - 3.19 | 0.04 | 0.62 | 0.24 - 1.61 | 0.33 |
| Family History of CHD | 1.28 | 0.81 - 2.03 | 0.29 | 1.00 | 0.60 - 1.67 | 0.99 |
| Homocysteine (10 units) | 1.53 | 1.10 - 2.11 | 0.01 | - | - | - |
| New Hypertension | 2.46 | 1.11 – 5.44 | 0.03 | - | - | - |
| New Dyslipidemia | 1.74 | 1.02 – 2.97 | 0.04 | - | - | - |
Of the non-traditional CVD risk factors (e.g. CRP, fibrinogen, etc), only higher homocysteine [1 μmol/L] (1.04, 1.01 – 1.08) was significantly associated with progression to the low ABI group while there were no significant associations between any of the non-lipid biomarkers and progression to the high ABI group (results not shown).
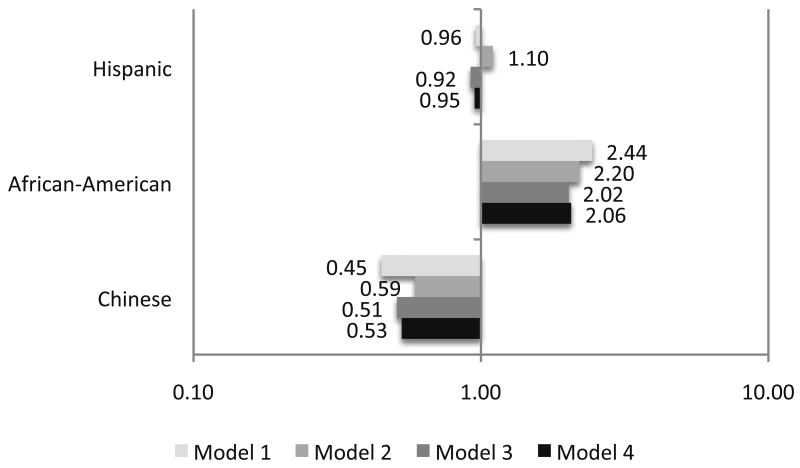
Using logistic modeling similar to that described above and with adjustment for age and sex and compared to non-Hispanic Whites, African Americans had a nearly 2.5-fold higher odds (p < 0.01) for progressing to the low ABI group (Figure 1). This association was modestly attenuated with further adjustment for body mass index, hypertension, diabetes, dyslipidemia and smoking (OR: 2.20, 95% CI: 1.27 – 3.82) and new hypertension and hypercholesterolemia (OR: 2.02, 95% CI: 1.21 – 3.37) but not when homocysteine was added to the model (OR: 2.06, 95% CI: 1.23 – 3.44). Compared to non-Hispanic Whites, the risk of progression to the low ABI group was not different for Chinese or Hispanics.
Figure 1. Ethnicity and the Odds of Progressing into the Low ABI Group* over 3 Years: The Multi-Ethnic Study of Atherosclerosis.
*Defined as an ABI ≤ 0.90 (non-Hispanic White Americans are the reference group).
Model 1 – Adjusted for age and sex
Model 2 – Adjusted for age, sex, body mass index, hypertension, diabetes, smoking and dyslipidemia
Model 3 – Adjusted for age, sex, body mass index, hypertension, diabetes, smoking, dyslipidemia, new hypertension and new dyslipidemia
Model 4 - Adjusted for age, sex, body mass index, hypertension, diabetes, smoking, dyslipidemia, family history of CVD new hypertension, new dyslipidemia and homocysteine
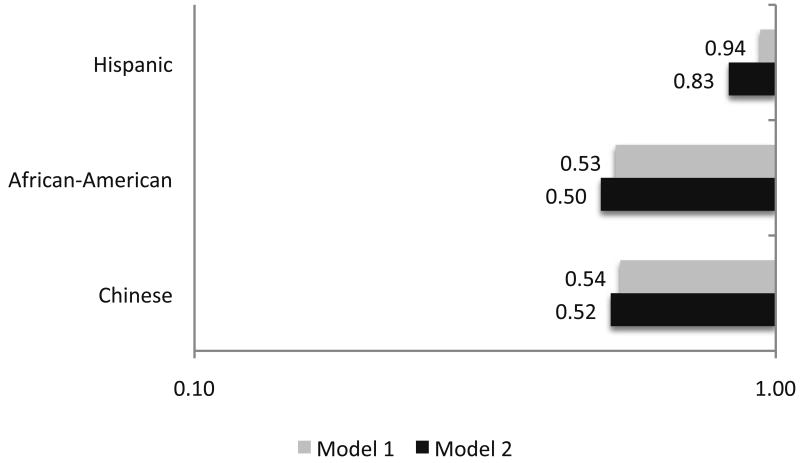
Figure 2 shows the associations between ethnicity and progressing to the high ABI group. With adjustment for age and sex and compared to non-Hispanic Whites, African Americans had a lower odds for progressing to the high ABI group (OR: 0.53, 95% CI: 0.28 – 1.03) that was slightly accentuated with adjustment for the traditional CVD risk factors (OR: 0.50, 95% CI: 0.24 – 1.00). Chinese and Hispanic participants were not at a different risk than non-Hispanic whites for progression to the high ABI group.
Figure 2. Race Specific Odds for Progressing into the High ABI Group* over 3 Years: The Multi-Ethnic Study of Atherosclerosis.
*Defined as an ABI ≥ 1.40 (non-Hispanic White Americans are the reference group).
Model 1 – Adjusted for age and sex
Model 2 – Adjusted for age, sex, body mass index, hypertension, diabetes, smoking and dyslipidemia
We conducted the same logistic regression analyses as those described above but utilizing ABI cut-points of 1.00 and 1.30. The results for these analyses were similar but, in general, the magnitudes of the associations were not as large as for those with ABI cut-points of 0.90 and 1.40. Also, there is controversy about whether analyses involving change variables as outcomes should include adjustment for the baseline values.22, 23 In sensitivity analyses, we ran multivariable logistic models that added the baseline ABI value to the final logistic models described above. This resulted in modest attenuation of the associations between the variables studied and progression into either the low or high ABI groups. Exceptions were the association between male gender and progression into the high ABI group diminishing dramatically to unity, and a reversal of the positive, albeit non-significant, coefficient for women for progression to a low ABI. These results suggest that after accounting for the differences in baseline ABI values, gender was not significantly related to ABI change.
Discussion
In this prospective study of a population-based multi-ethnic cohort, increasing age, hypertension, diabetes, cigarette smoking and homocysteine were significantly associated with progression to an ABI ≤ 0.90, while male gender and higher body mass index were significantly associated progression to an ABI ≥ 1.40. Additionally and compared to non-Hispanic Whites, African American ethnicity was significantly associated with an elevated odds for progression from a normal ABI to an ABI ≤ 0.90, independent of the levels of other risk factors. Conversely, the odds for converting from a normal ABI to an ABI ≥ 1.40 among African Americans was lower than among Whites but this difference was not statistically significant. These results suggest that there are factors that we did not measure that might predispose African Americans to developing a low ABI or protect them from developing a high ABI. Alternatively, there may be other, yet untested risk factors that may explain the differential associations between different ABI and racial groups.
Physiologically, at least fifty percent stenosis in the lower extremities is required to cause a sufficient reduction in blood flow to reduce blood pressure distal to an obstruction.24 Although there are other potential etiologies for luminal stenosis in the lower extremities, the predominant cause for significant flow-limiting disease is the accumulation of atherosclerosis.25, 26 Conversely, the physiologic mechanism resulting in an ABI above the normal range is assumed to be mechanical rigidity of the arteries in the lower extremities due to reduced arterial compliance or medial artery calcification.11, 27 We postulate that in a state of “arterial equilibrium”, these two processes could progress equally resulting in a stable ABI values over time in most people. Conversely, when one of the processes is predominant in a given individual, the ABI value would change in favor of that process.
In this context it is important that African Americans in our study were found to have a significantly increased risk for developing a “low ABI”, while at the same time, potentially having reduced risk for increasing the ABI above 1.40. Due to potential intrinsic differences in the elasticity and geometry of arteries in the lower extremities, African Americans have lower ABI values than those of other ethnic groups28 and are therefore more likely to have an ABI less than 0.90. Additionally, and forgoing differences in the rates of subclinical stenosis between different ethnic groups,29 the findings of increased odds for progressing below an ABI of 0.90, along with a probable reduction in the odds for progressing above an ABI greater than 1.40, suggests that African Americans, when compared to non-Hispanic Whites, may be more predisposed to developing significant atherosclerosis in the lower extremity resulting in flow-limiting stenosis. The alternative explanation is that in African Americans the progression of atherosclerosis is similar to that in non-Hispanic Whites but the progression of arterial rigidity is slower than in this ethnic group. This seems unlikely however since African Americans have been found to have lower arterial compliance than non-Hispanic Whites.30
Compared to African Americans, Hispanic Americans in our study had similar age and sex-adjusted prevalences of baseline diabetes and dyslipidemia, as well as levels of body mass index and essentially all of the non-lipid biomarkers. However, Hispanic Americans did not have an increased risk for changing their ABIs beyond either ABI threshold utilized in this study. Using the conceptual framework provided above, potential explanations for this result include no change in the extent of both atherosclerosis and arterial rigidity over the 3-year follow-up. Given that the natural history of atherosclerosis is for progression (rather than stability or regression) to more extensive disease,31 it follows that the arterial rigidity would have to progress at a similar rate to maintain the “equilibrium” between these two process seen in this ethnic group. This is supported by the finding of Hispanic Americans having high risk factor levels for both atherosclerosis (dyslipidemia, hypertension) and arterial rigidity (body mass index) than non-Hispanic Whites. Of note, African Americans also had high levels of risk factors for both atherosclerosis and arterial rigidity. Therefore, why was this ethnic group predisposed to a lower ABI while Hispanics were not? A possible explanation is that African Americans had higher levels of other atherosclerotic risk factors, such as hypertension and cigarette smoking, than Hispanics and therefore the “balance” was tipped towards this disease process.
We do not believe that the lack of association between Hispanic ethnicity and changes in the ABI is do to an inability of the ABI to detect PAD in this ethnic group. Rather, we believe it is the excess of diabetes in Hispanics, compared to other ethnic groups. Specifically, diabetes is a risk factor for both atherosclerosis and arterial rigidity and there is a strong concordance between atherosclerosis and arterial rigidity. That is, among those who have stiff arteries, a high proportion will also have PAD.32 However, 95% of those who have diabetes don't have stiff arteries and the finding of both conditions concomitantly is therefore a small proportion of Hispanics. Therefore, we believe the misclassification in our study is small.
Although not statistically significant, compared to non-Hispanic Whites, Chinese Americans in our study had reduced odds for both progression to the low and high ABI. Specifically, Chinese Americans had a clinically relevant odds ratio of approximately 0.60 for progression to either ABI group. Importantly, the magnitude of the odds for progressing into the high ABI group was comparable to that found in African Americans indicating that the lack of significance was due to the smaller sample size in the Chinese. Physiologically, these results suggest that Chinese Americans may be similar to Hispanic Americans in that they are not predisposed to developing more atherosclerosis than arterial rigidity and vice versa or perhaps less predisposed to either.
Individuals with diabetes have been shown to have a higher prevalence of stiff arteries and medial artery calcification.11 This would suggest that diabetes would be a significant risk factor for progressing to a high ABI. It is therefore curious that diabetes was not a significant risk factor for progressing to a high ABI in our study. We believe a plausible explanation for this finding is that our study was conducted in the context of a multi-ethnic cohort. In this regard and compared to non-Hispanic Whites, African and Hispanic American ethnicities were associated with a reduced risk for progressing to a high ABI and both had over twice the prevalence of diabetes as non-Hispanic Whites. Additionally, diabetes is a potent risk factor for the development and progression of atherosclerosis, as well as PAD.33, 34 Therefore, the effects of diabetes on arterial pathophysiology are extremely complex and make the use of the ABI in diabetics potentially problematic.
It is possible that those individuals who had a baseline ABI closer to the cut-points would be more likely to cross the threshold value due to measurement error. To examine this possibility, we determined the distribution of changes in the ABI across the spectrum of normal baseline ABI values. This analysis revealed that those who had a baseline value at the extremes of the normal range were much more likely to have a second ABI value that was closer to the normal ABI (i.e. regression to the mean). Therefore, for values farther to the extremes of the normal ABI spectrum, it appears random measurement error would have resulted in a smaller probability for crossing a threshold. This, combined with the high intraclass correlation for the measurement of the ABI in MESA, argue against measurement error being responsible for a significant proportion of subjects progressing beyond the threshold values.
Strengths of this study include a large sample size that includes ample representation of men and women, as well as multiple ethnic groups. All subjects underwent a standardized examination procedure over multiple study visits affording the opportunity to study prospective changes of several study variables. There were many novel biomarkers available for analysis. Finally, this study examined the associations for an increase or decrease in the ABI and the reproducibility of the ankle brachial index was high. On the other hand, within the context of changes in the ankle brachial index, this study is limited by a relatively short follow-up period (average: 3 years) and the absolute number of subjects who moved into the low or high ABI groups was small. However, since individuals with a PAD are at increased risk for incident CVD events in the relatively short term, the findings presented here are of clinical relevance. Also, there were approximately 900 subjects who did not have a follow-up ABI. This group was older than included participants but had lower levels of important risk factors, suggesting our results are not likely biased. Finally, the number of Chinese subjects in the low or high ABI categories was relatively small likely leading to the lack of significance for the associations in this ethnic group.
In conclusion, in this multi-ethnic cohort of men and women from across the United States and over an average of 3 years, age, hypertension, diabetes, pack-years of cigarette smoking and higher homocysteine were associated with developing an ABI ≤ 0.90, while male sex and increasing body mass index were associated with an increase in the ABI to ≥ 1.40. Importantly, African Americans had an increased risk for developing an ABI ≤0.90 but a reduced risk for developing an ABI ≥ 1.40. These results may inform clinicians on characteristics in their patients that may predispose them to developing peripheral arterial disease. Importantly, in exploratory analyses we found a significant association between change in the ABI and incident coronary artery bypass graft and other (non-coronary) revascularizations. Further work is warranted to confirm these findings.
Acknowledgments
This research was supported by a grant from the American Heart Association (Allison) and contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ouriel K, McDonnell AE, Metz CE, Zarins CK. Critical evaluation of stress testing in the diagnosis of peripheral vascular disease. Surgery. 1982;91:686–93. [PubMed] [Google Scholar]
- 2.Osmundson PJ, O'Fallon WM, Clements IP, Kazmier FJ, Zimmerman BR, Palumbo PJ. Reproducibility of noninvasive tests of peripheral occlusive arterial disease. J Vasc Surg. 1985;2:678–83. doi: 10.1067/mva.1985.avs0020678. [DOI] [PubMed] [Google Scholar]
- 3.Eguchi K, Yacoub M, Jhalani J, Gerin W, Schwartz JE, Pickering TG. Consistency of Blood Pressure Differences Between the Left and Right Arms. Arch Intern Med. 2007;167:388–93. doi: 10.1001/archinte.167.4.388. [DOI] [PubMed] [Google Scholar]
- 4.Allison MA, Ho E, Denenberg JO, et al. Ethnic-Specific Prevalence of Peripheral Arterial Disease in the United States. American Journal of Preventive Medicine. 2007;32:328–33. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Diehm C, Schuster A, Allenberg JR, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172:95–105. doi: 10.1016/s0021-9150(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 6.Gregg EW, Sorlie P, Paulose-Ram R, et al. Prevalence of Lower-Extremity Disease in the U.S. Adult Population >=40 Years of Age With and Without Diabetes: 1999-2000 National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:1591–7. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 7.Selvin E, Erlinger TP. Prevalence of and Risk Factors for Peripheral Arterial Disease in the United States: Results From the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–43. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 8.Collins TC, Petersen NJ, Suarez-Almazor M, Ashton CM. The prevalence of peripheral arterial disease in a racially diverse population. Arch Intern Med. 2003;163:1469–74. doi: 10.1001/archinte.163.12.1469. [DOI] [PubMed] [Google Scholar]
- 9.Allison MA, Criqui MH, McClelland RL, et al. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;48:1190–7. doi: 10.1016/j.jacc.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 10.Ix JH, Allison MA, Denenberg JO, Cushman M, Criqui MH. Novel Cardiovascular Risk Factors Do Not Completely Explain the Higher Prevalence of Peripheral Arterial Disease Among African Americans: The San Diego Population Study. J Am Coll Cardiol. 2008;51:2347–54. doi: 10.1016/j.jacc.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial Artery Calcification: A Neglected Harbinger of Cardiovascular Complications in NonñInsulin-Dependent Diabetes Mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–83. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 12.O'Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: Results from the Cardiovascular Health Study. Circulation. 2006;113:388–93. doi: 10.1161/CIRCULATIONAHA.105.570903. [DOI] [PubMed] [Google Scholar]
- 13.Allison MA, Hiatt WR, Hirsch AT, Coll JR, Criqui MH. A High Ankle-Brachial Index Is Associated With Increased Cardiovascular Disease Morbidity and Lower Quality of Life. J Am Coll Cardiol. 2008;51:1292–8. doi: 10.1016/j.jacc.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 14.Allison MA, Laughlin GA, Barrett-Connor E. Association Between the Ankle-Brachial Index and Carotid Intimal Medial Thickness in the Rancho Bernardo Study. The American Journal of Cardiology. 2006;98:1105. doi: 10.1016/j.amjcard.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 15.Allison MA, Laughlin GA, Barrett-Connor E, Langer R. Association Between the Ankle-Brachial Index and Future Coronary Calcium (The Rancho Bernardo Study) The American Journal of Cardiology. 2006;97:181–6. doi: 10.1016/j.amjcard.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: The Strong Heart Study. Circulation. 2004;109:733–9. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 20.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 21.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–83. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 22.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–78. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 23.Yanez ND, 3rd, Kronmal RA, Shemanski LR, Psaty BM. A regression model for longitudinal change in the presence of measurement error. Annals of epidemiology. 2002;12:34–8. doi: 10.1016/s1047-2797(01)00280-0. [DOI] [PubMed] [Google Scholar]
- 24.Ouriel K, Zarins CK. Doppler ankle pressure: an evaluation of three methods of expression. Arch Surg. 1982;117:1297–300. doi: 10.1001/archsurg.1982.01380340031008. [DOI] [PubMed] [Google Scholar]
- 25.Fowkes FG. Epidemiology of peripheral vascular disease. Atherosclerosis. 1997;131 Suppl:S29–31. doi: 10.1016/s0021-9150(97)06122-4. [DOI] [PubMed] [Google Scholar]
- 26.Spittell JA., Jr Peripheral arterial disease. Dis Mon. 1994;40:641–700. doi: 10.1016/0011-5029(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 27.Brooks B, Dean R, Patel S, Wu B, Molyneaux L, Yue DK. TBI or not TBI: that is the question. Is it better to measure toe pressure than ankle pressure in diabetic patients? Diabet Med. 2001;18:528–32. doi: 10.1046/j.1464-5491.2001.00493.x. [DOI] [PubMed] [Google Scholar]
- 28.Aboyans V, Criqui MH, McClelland RL, et al. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA) J Vasc Surg. 2007;45:319–27. doi: 10.1016/j.jvs.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 29.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: Prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–23. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 30.Din-Dzietham R, Couper D, Evans G, Arnett DK, Jones DW. Arterial stiffness is greater in African Americans than in whites: evidence from the Forsyth County, North Carolina, ARIC cohort. American Journal of Hypertension. 2004;17:304–13. doi: 10.1016/j.amjhyper.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Libby P. Vascular biology of atherosclerosis: overview and state of the art. Am J Cardiol. 2003;91:3A–6A. doi: 10.1016/s0002-9149(02)03143-0. [DOI] [PubMed] [Google Scholar]
- 32.Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg. 2008;48:1197–203. doi: 10.1016/j.jvs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Aboyans V, Criqui MH, Denenberg JO, Knoke JD, Ridker PM, Fronek A. Risk Factors for Progression of Peripheral Arterial Disease in Large and Small Vessels. Circulation. 2006;113:2623–9. doi: 10.1161/CIRCULATIONAHA.105.608679. [DOI] [PubMed] [Google Scholar]
- 34.Meijer WT, Grobbee DE, Hunink MGM, Hofman A, Hoes AW. Determinants of Peripheral Arterial Disease in the Elderly: The Rotterdam Study. Arch Intern Med. 2000;160:2934–8. doi: 10.1001/archinte.160.19.2934. [DOI] [PubMed] [Google Scholar]