Abstract
Taxol chemotherapy is one of the few therapeutic options for men with castration-resistant prostate cancer (CRPC). However, the working mechanisms for taxol are not fully understood. Here we demonstrated that treatment of 22Rv1, a PTEN-positive CRPC cell line, with paclitaxel and its semisynthetic analogue docetaxel decreases expression of the androgen receptor (AR)-activated genes prostate-specific antigen (PSA) and Nkx3.1 but increases expression of the AR repression gene maspin, suggesting that taxol treatment inhibits AR activity. This was further supported by the observation that the activity of AR luciferase reporter genes was inhibited by paclitaxel. In contrast, paclitaxel treatment failed to inhibit AR activity in the PTEN-null CRPC cell line C4-2. However, pretreatment of C4-2 cells with the PI3K inhibitor LY294002 restored paclitaxel inhibition of the AR. Treatment of 22Rv1 xenografts in mice with docetaxel induced mitotic arrest and a decrease in PSA expression in tumor cells adjacent to vascular vessels. We further demonstrated that paclitaxel induces nuclear accumulation of FOXO1, a known AR suppressive nuclear factor and increases the association of FOXO1 with AR proteins in the nucleus. FOXO1 knockdown with small interference RNA attenuated the inhibitory effect of paclitaxel on AR transcriptional activity, expression of PSA and Nkx3.1 and cell survival. These data reveal a previously uncharacterized, FOXO1-mediated, AR-inhibitory effect of taxol in CRPC cells that may play an important role in taxol-mediated inhibition of CRPC growth.
Introduction
The AR plays a central role in development and progression of prostate cancer (PCa). Because PCa cells rely on androgens for proliferation and survival, androgen deprivation therapy (ADT) is currently a standard treatment for advanced, metastatic PCa. However, the effectiveness of ADT is temporary, and tumors in the majority of patients eventually relapse and evolve into CRPC, from which most patients die (1, 2). Paradoxically, increasing evidence from both in vitro and in vivo studies suggest that prostate cancer at this stage is still dependent on the AR for growth and survival (3, 4). This notion is further supported by the recent findings that although serum testosterone levels are known to be significantly reduced following ADT, intra-prostatic androgens are reproducibly detected at concentrations sufficient to activate the AR (5). It is believed that such residual levels of intra-prostatic androgens following ADT are essential for continuous growth of CRPC cells, stressing that the AR remains the major target for the treatment of CRPC.
Improving and optimizing therapeutic strategies for men with CRPC is one of the most challenging aspects of PCa management today. Until recently, treatment options for such patients were limited and chemotherapy, in particular, provided response rates in the order of only 6%. Lately, however, due to recent success in the development of less toxic regimens, including taxol (paclitaxel and docetaxel), and effective markers for tumor response, such as PSA, chemotherapy can now be used to induce a regression in CRPC after failure of ADT. Randomized clinical trials have demonstrated that in addition to the improvement of the overall survival rate, treatment of CRPC patients with docetaxel/paclitaxel plus prednisone or estramustine resulted in at least 50% decline in PSA in 50% of the patients (6-8). Docetaxel is now approved by the United States Food and Drug Administration (FDA) for treatment of patients with CRPC. However, the exact mechanisms underlying the taxol-induced decline of serum PSA are not entirely clear.
Multiple mechanisms have been elucidated for taxol-mediated chemotherapy of human cancers. First, these compounds are known to inhibit the depolymerization of microtubules and thereby block the exit from mitosis and ultimately promote apoptotic cell death (9). They have also been shown to kill cancerous cells by inducing phosphorylation and inhibition of the anti-apoptotic protein Bcl-2 (10). Moreover, several studies have shown that paclitaxel induces apoptosis of breast and ovarian cancer cells by inducing nuclear localization of FOXO transcription factors and upregulation of their target gene Bim (11-13). Most recently, it has been shown that activation of FOXO1 inhibits androgen-independent activation of the AR and that this effect of FOXO1 requires its localization in the nucleus (14). In the present study, we sought to test the hypothesis that taxol inhibits AR activity via the nuclear function of FOXO1. We demonstrated that treatment of 22Rv1 CRPC cells with taxol inhibits AR activity and this effect of taxol is mediated primarily by increased association of FOXO1 with AR proteins.
Materials and Methods
Cell culture
The PCa cell line 22Rv1 was kindly provided by C. Y. Young (Mayo Clinic, Rochester, MN). LNCaP cells were purchased from the American Type Culture Collection. The C4-2 cell line was purchased from UroCor. All cell lines were cultured in RPMI 1640 medium containing 10% fetal bovine serum (Hyclone), 100 μg/ml streptomycin and 100 units/ml penicillin.
Plasmids, siRNAs and chemicals
The expression vector for FLAG-FOXO1 was described previously (15). The PSA luciferase reporter (PSA-Luc) that contains an ~5.8-kb genomic fragment from the promoter of the PSA gene, and the luciferase reporter construct containing three copies of the androgen responsive element (ARE) derived from the PSA gene (3×ARE-Luc) were obtained from C.Y. Young (Mayo Clinic, Rochester, MN). The renilla luciferase reporter vector was purchased from Promega. The FOXO1 gene-specific siRNA (5’-CCAGAUGCCUAUACAAACA-3’) and non-specific control siRNA (5’-UAGCGACUAAACACAUCAA-3’) were purchased from Dharmacon. Paclitaxel and docetaxel were purchased from Sigma-Aldrich. LY294002 was obtained from Invitrogen.
Cell transfection and luciferase reporter assay
Transient transfection of PCa cells was performed by electroporation as described (16). Approximately 75-90% transfection efficiencies were routinely achieved. For siRNA transfection, cells were transfected with 200 pmol of siGenome siRNAs specific for FOXO1 and non-specific control siRNA. For luciferase reporter assays, cells were transfected with plasmids for PSA-Luc or 3×ARE-Luc firefly and renilla luciferase reporter genes. At 24 h after transfection, cells were treated with paclitaxel. After 48 h of treatment, cells were harvested and firefly and renilla luciferase activities in cell lysates were measured using a dual luciferase kit (Promega). Renilla luciferase activities of cells were used as internal control.
Reverse transcription-polymerase chain reaction (RT-PCR) and quantitative real-time PCR
Total cellular RNAs were isolated from taxol or mock treated PCa cells or grafts using Trizol (Invitrogen), and cDNAs were synthesized using Superscript II reverse transcriptase (Invitrogen). PCR was performed using primers specific for PSA (FW: 5’-AGGCCTTCCCTGTACACCAA-3’; RV: 5’-GTCTTGGCCTGGTCATTTCC-3’), Nkx3.1 (FW: 5’-GTACCTGTCAGCCCCTGAAC-3’; RV: 5’-GGAGAGCTGCTTTCGCTTAG-3’), maspin (FW: 5’-CCCTATGCAAAGGAATTGGA-3’; RV: 5’-CAAAGTGGCCATCTGTGAGA-3’), AR (FW: 5’-TGTCCATCTTGTCGTCTTCG-3’; RV: 5’-CTGTCAGCTTCTGGGTTGTC-3’) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (FW: 5’-GAAGGTGAAGGTCGGAGTC-3’; RV: 5’-GAAGATGGTGATGGGATTTC-3’). PCR products were separated on a 2% agarose gel containing ethidium bromide (0.5μg/ml). For the quantitative real-time PCR, expression levels of genes examined were determined using a SYBR Green Supermix (Bio-Rad) on an iCycler iQ platform (Bio-Rad) according to the manufacturer's protocol. Reactions were carried out in triplicate and gene expression levels were normalized against GAPDH.
Nuclear extraction, immunoprecipitation, immunoblotting, and antibodies
Nuclear extraction was performed as described (17). Protein immunoprecipitations were carried out using an immunoprecipitation kit (Roche Applied Science) as described (15), and immunoblotting was performed as described (15). The antibodies used were: anti-FOXO1 and anti-FOXO1-S256-p (Cell Signaling Technology); anti-FLAG (M2) (Sigma-Aldrich); anti-Bim (Chemicon); anti-AR (C-19), anti-14-3-3ζ, and anti-Sam68 and anti-ERK2 (Santa Cruz Biotechnology).
Chromatin immunoprecipitation (ChIP) assays
22Rv1 cells were transfected with FLAG-tagged wild type FOXO1. At 24 h after transfection, cells were treated with or without paclitaxel for 48 h. Cells were crosslinked with 1% formaldehyde for 10 min, harvested, lysed in ChIP lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris, pH 8.0 and 1×protease inhibitor cocktail (Sigma-Aldrich)), and sonicated to produce soluble chromatin with the genomic DNA at an average size of 400-1000 bp. The soluble chromatin was pre-cleared by incubation with sheared salmon sperm DNA. One portion of the supernatant was used as a DNA input control, and the remainder was incubated with mouse IgG or M2 antibody overnight at 4°C. Immunoprecipitated complexes were washed successively with wash buffer (300 mM NaCl, 50 mM Tris pH 8.0, 2.7 mM KCl, 0.05% Tween 20 and 1% deoxycholate) and Tris-EDTA (TE) buffer. The bound protein-DNA immunocomplexes were eluted three times with 35 μl ChIP elution buffer (1% SDS, 0.1 M NaHCO3) and de-crosslinked at 65°C for 4 h. The DNA was extracted with a PCR purification kit (Invitrogen) and was subjected to PCR amplification using the primers specific for the region surrounding the ARE in the PSA promoter (FW: 5’-TCTGCCTTTGTCCCCTAGAT-3’; RV: 5’-AACCTTCATTCCCCAGGACT-3’). The PCR products were analyzed by 2% agarose gels and stained with ethidium bromide. The effect of paclitaxel on the association of FOXO1 with the PSA promoter was also quantified by real-time PCR. Ct values of immunoprecipitated samples were normalized to the corresponding value for input.
Apoptotic cell death assay and cell viability assay
22Rv1 cells were treated with different concentrations of paclitaxel. At 48 h after treatment, cells were collected and washed with 1xphosphate-buffered saline (PBS). After fixation with 70% ethanol, cells were washed twice with 1xPBS and stained with a solution containing 20 mg/ml propidium iodide (PI) and 50 mg/ml RNase A. Cells were incubated for 30 min at room temperature and sub-G1 cells were measured by flow cytometry using FACScan (Becton-Dickinson, San Jose, CA). Cell viability assay was performed as described (14).
Caspase 3 activity measurement
The activity of caspase 3 was measured by Caspase 3/CPP32 Colorimetric Protease Assay (Invitrogen). Briefly, cells were resuspended in 50 μl chilled lysis buffer and incubated on ice for 10 min. 100 μg of cellular proteins was diluted in 50 μl lysis buffer and 50 μl of reaction buffer (containing 10 mM DTT). 5 μl of the 4 mM DEVD-pNA substrate (200 μM final concentration) was added, and the reaction was carried out at 37°C for 2 h in the dark. The results were recorded in a microplate reader at 405 nm.
Xenografting and immunohistochemistry
8-week-old male nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice bred through the BC Cancer Research Centre (BC Cancer Agency, Vancouver, BC, Canada) were used in the experiments. 22Rv1 cells were grafted under renal capsule in NOD-SCID mice as described (18). After 4 weeks of grafting, mice were treated with docetaxel via i.p. injection at a dose of 15 mg/kg on days 1, 8 and 15. Control mice were sham treated with saline at the same schedule. At 24 h after the last treatment, mice were sacrificed and 22Rv1 renal grafts were collected. One portion of each graft was snap frozen for RNA extraction and the rest was fixed in formalin and paraffin embedded for histopathologic and immunohistochemical analyses as described (19). The mouse anti-AR (Santa Cruz Biotechnology) or the mouse anti-human PSA antibody (DAKO) was used for immunohistochemical staining. All sections used for immunohistochemistry were lightly counterstained with 5% (w/v) Harris hematoxylin.
Statistics
Experiments were carried out with three or more replicates. Statistical analyses were performed by Student's t test. Values with P < 0.05 are considered significant.
Results
Taxol inhibits AR transcriptional activity in 22Rv1 CRPC cells
Clinical studies show that administration of paclitaxel or docetaxel decreases serum levels of PSA in patients with CRPC (6-8). To test the possibility that taxol-induced decrease in PSA levels could be due to the inhibition of AR, we examined the effect of taxol on expression of several AR regulated genes in 22Rv1, a CRPC cell line that expresses both AR and PSA (20). Treatment of 22Rv1 cells with paclitaxel induced apoptosis in a dose-dependent manner (Fig. 1A). As demonstrated by RT-PCR, paclitaxel treatment decreased expression of AR transactivated genes PSA and Nkx3.1 at the messenger RNA level (Fig. 1B). No change was found in expression of the housekeeping gene GAPDH, suggesting that decreased expression of AR target genes is not likely mediated by paclitaxel-induced apoptotic cell death, but may be mediated by specific inhibition of AR activity by paclitaxel. This notion is further supported by the observation that paclitaxel treatment increased expression of the AR-repressed gene maspin in a dose-dependent fashion (Fig. 1B). These results were confirmed by quantitative real-time RT-PCR (Fig. 1C). Similar results were obtained in 22Rv1 cells treated with docetaxel (Supplementary Fig. 1A).
Figure 1.
Paclitaxel negatively regulates survival and expression of AR targeted genes in 22Rv1 CRPC cells. A, Effect of paclitaxel on apoptosis. 22Rv1 cells were treated with different concentrations of paclitaxel. After 48 h of treatment, cells were harvested, fixed, stained with propidium iodide (PI) and FACS analysis was performed and sub-G1 cells were considered dead. B, C, Effect of paclitaxel on expression of AR target genes. 22Rv1 cells were treated with different concentrations of paclitaxel. After 48 h of treatment, cells were harvested for mRNA and protein analysis. The expression levels of PSA, Nkx3.1 and maspin mRNA were examined by semiquantitative RT-PCR analysis (B, upper panel). GAPDH mRNA was used as an internal control (B, upper panel). The level of the AR protein was assessed by Western blot (B, lower panel). The relative mean mRNA expression level of AR regulated genes was measured by quantitative real-time RT-PCR in triplicate. Expression levels are relative to vehicle treatment, which was arbitrarily set to 1 (C).
To further explore whether paclitaxel inhibits AR activity in PCa cells, we examined the effect of paclitaxel on AR transcriptional activity using PSA promoter-based luciferase reporter assays. Paclitaxel treatment decreased promoter activity of the PSA gene in 22Rv1 cells (Fig. 2A). Importantly, treatment of 22Rv1 cells with paclitaxel also inhibited the activity of a composite AR reporter gene (3×ARE-Luc) that contains three copies of the ARE from the PSA proximal promoter (Fig. 2B), suggesting that the inhibitory effect of paclitaxel on PSA promoter activity is mediated through the AR. Thus, we conclude that taxol can inhibit AR transcriptional activity in 22Rv1 cells in culture. Notably, little or no change in AR protein levels was observed in 22Rv1 cells treated with 1 nM of paclitaxel for 48 h (Fig. 1B). Under the same conditions, however, expression of PSA and Nkx3.1 increased and maspin expression increased in comparison to mock-treated cells (Fig. 1B and 1C). Similarly, treatment of 22Rv1 cells with 2.5 nM of docetaxel resulted in a marked decrease in expression of PSA and Nkx3.1 but increase in expression of maspin (Supplementary Fig. 1A) whereas no change in AR protein levels was observed under this condition (Supplementary Fig. 1B). These data indicate that taxol-induced inhibition of AR activity is not simply caused by reduced AR protein levels.
Figure 2.
Paclitaxel inhibits the PSA promoter activity and the activity of a composite AR luciferase reporter gene that contains only three copies of the ARE derived from the promoter of the PSA gene (3×ARE-Luc). 22Rv1 cells were transfected with the renilla luciferase reporter gene and PSA-Luc (A) or 3×ARE-Luc (B). At 24 h after transfection, cells were treated with different concentrations of paclitaxel. After 48 h of treatment, cells were harvested and luciferase activity was measured. Error bars indicate SD among three individual experiments.
Effect of docetaxel on AR activity in 22Rv1 xenografts in mice
Next we sought to determine whether taxol could inhibit AR activity under in vivo conditions. 22Rv1 cells were grafted under the renal capsule of NOD/SCID mice. Because paclitaxel and docetaxel had a similar inhibitory effect on AR activity in PCa cells in vitro (Fig. 1 and Supplementary Fig. 1) and docetaxel is an approved chemotherapeutic agent for CRPC patients in the clinic, docetaxel was chosen for in vivo studies. After 4 weeks of grafting, mice were treated with docetaxel via i.p. injection at a dose of 15 mg/kg on days 1, 8 and 15. Control mice were mock treated with saline at the same schedule. At 24 h after the last treatment, mice were sacrificed. The docetaxel- (n = 8) or mock-treated (n = 6) grafts were collected. Real-time RT-PCR-based analysis with total RNA isolated from the entire frozen tissue demonstrated that there was a slight, but not significant, decrease in expression of PSA mRNA in grafts treated with docetaxel relative to those mock-treated grafts (Supplementary Fig. 2A). Similar analysis showed that there was only a modest, but not significant, increase in maspin expression in grafts treated with docetaxel in comparison to those treated with saline (Supplementary Fig. 2A). Analysis with the same RNA samples showed that no significant change in AR mRNA levels was observed in tumors treated with docetaxel (Supplementary Fig. 2B). However, histological analysis revealed that mitotic figures (abnormal microtubular bundles) were readily detected in tumor cells aligned with vascular vessels in xenografts treated with docetaxel but not with saline (Fig. 3A). In agreement with these observations, an apparent decrease in expression of AR and PSA proteins was detected in cells surrounding blood vessels in docetaxel-treated xenografts in comparison to sham-treated counterparts (Fig. 3B and 3C). These data indicate that the therapeutic effect of docetaxel, as indicated by the mitotic arrest, is correlated with decreased expression of PSA in tumor cells adjacent to vascular vessels, but not in the entire tumor.
Figure 3.
The effect of docetaxel on AR activity in 22Rv1 xenografts in mice. 22Rv1 cells were grafted under renal capsule in NOD/SCID mice. After 4 weeks of grafting, mice were treated with docetaxel via i.p. injection at a dose of 15 mg/kg on days 1, 8 and 15. Control mice were sham treated with saline at the same schedule. At 24 h after last treatment, mice were sacrificed and 22Rv1 renal grafts were collected. The tissues were fixed in formalin and paraffin embedded. Hematoxylin and eosin (H&E) staining (A), PSA (B) and AR (C) immunohistochemistry were performed. Photographs of representative tumor sections were taken at 200x magnification. Insets, cells surrounding blood vessels in higher magnification. Arrowheads point to mitotic figures.
Paclitaxel induces expression and nuclear localization of FOXO1 protein and increases its association with the AR in PCa cells
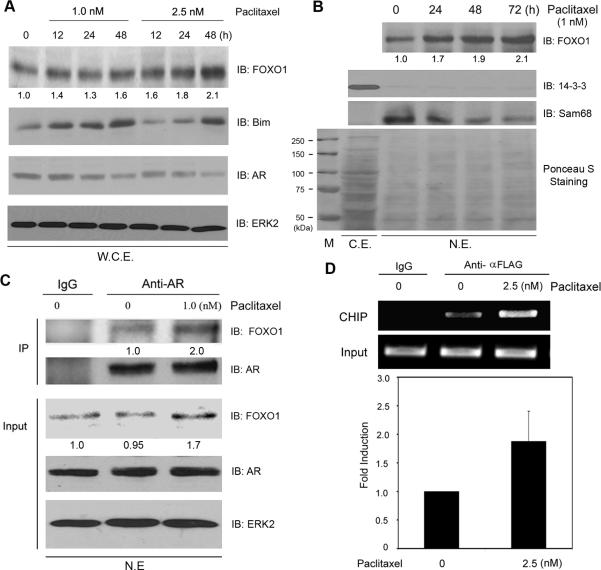
To define the molecular mechanism by which taxol promotes the inhibition of AR activity, we focused our attention on FOXO1 due to the following reasons: (a) Paclitaxel induces increased expression and nuclear localization of FOXO proteins in breast and ovarian cancer cells (11-13) and (b) FOXO1 functions as a potent repressor of the AR in the nucleus (14). Treatment of 22Rv1 cells with 1 nM of paclitaxel resulted in a modest, but reproducible increase in FOXO1 protein as demonstrated by Western blot analysis with whole cell lysates (Fig. 4A). FOXO1 expression markedly increased in cells treated with a high dose (2.5 nM) of paclitaxel (Fig. 4A). Accordingly, expression of the pro-apoptotic gene Bim, a transcriptional target of FOXO1, markedly increased following paclitaxel treatment (Fig. 4A). Similar to the results seen in 22Rv1 cells treated with docetaxel (Supplementary Fig. 1B), little or no change in the levels of AR proteins was detected in cells treated with paclitaxel for 24 h (Fig. 4A). In contrast, treatment of paclitaxel longer than 24 h resulted in a moderate decrease in AR levels (Fig. 4A), which is consistent with the results observed from animal studies (Fig. 3C). Further analysis demonstrated that paclitaxel promotes accumulation of the FOXO1 protein in the nucleus (Fig. 4B). Since FOXO1 inhibition of the AR requires nuclear localization of FOXO1 (14) and this inhibition appears to be mediated by their interaction (21), we sought to determine whether paclitaxel treatment increases the association between FOXO1 and AR proteins in the nucleus. As demonstrated by coimmunoprecipitation assay, more FOXO1 proteins were detected in the AR complex in paclitaxel-treated cells relative to the mock-treated cells (Fig. 4C). Paclitaxel treatment failed to increase the interaction of AR proteins with the ectopically expressed nuclear form of FOXO1 (Supplementary Fig. 3), suggesting that increased association of FOXO1 with the AR is likely due to the increased expression and nuclear localization of FOXO1 proteins induced by paclitaxel. Moreover, consistent with a previous report (21), FOXO1 was detected on the promoter of the PSA gene in paclitaxel-untreated cells as demonstrated by both regular and real-time PCR (Fig. 4D). Importantly, more FOXO1 proteins were detected in the PSA promoter in paclitaxel-treated cells in comparison to mock-treated cells (Fig. 4D). Thus, paclitaxel treatment elevates the level of FOXO1 protein in the nucleus and increases the association of FOXO1 with the AR protein and the PSA promoter in 22Rv1 cells.
Figure 4.
Paclitaxel induces expression and nuclear localization of the FOXO1 protein and increases its interaction with AR in 22Rv1 cells. A, Paclitaxel elevates FOXO1 protein level in 22Rv1 cells. 22Rv1 cells were treated with different concentrations of paclitaxel. Cells were harvested at the indicated time points. Cells were lysed and whole cell lysates (W.C.E.) were used for analysis of expression of FOXO1, Bim, and AR proteins by immunoblotting (IB). ERK2 was used as a loading control. The number underneath each band in the immunoblot indicates the relative intensity of the corresponding band (the same analysis can be seen in (B)). B, Paclitaxel induces the accumulation of FOXO1 protein in the nucleus of 22Rv1 cells. 22Rv1 cells were treated with 1.0 nM paclitaxel. At the indicated time points, cells were harvested and nuclear fractions were then analyzed for expression of FOXO1 by immunoblotting. 14-3-3ζ and Sam68 were employed as markers for cytoplasmic and nuclear fractions, respectively. C.E., cytosolic extraction. N.E., nuclear extraction. Ponceau S staining was used to monitor equal loading of different nuclear extractions. C, Paclitaxel increases the association of FOXO1 with AR proteins in the nucleus. 22Rv1 cells were treated with paclitaxel (1 nM) for 24 h. Nuclear fractions were lysed and cell lysates were subjected to immunoprecipitation (IP) with an anti-AR antibody or non-specific IgG. Immunoprecipitants were analyzed by immunoblotting (IB) using antibodies against FOXO1 and AR. The relative intensity of the band for immunoprecipitated FOXO1 was first normalized with the intensity of the AR band and then normalized against each other. For input FOXO1, the number underneath each band indicates the relative intensity of the corresponding band. D, Paclitaxel increases the association of FOXO1 with the PSA promoter. 22Rv1 cells were transfected with FLAG-tagged wild type FOXO1. At 24 h after transfection, cells were treated with paclitaxel and ChIP analysis was performed. Upper, representative ethidium bromide stained agarose gel showing amplification of promoter fragments. Lower, the effect of paclitaxel on the association of FOXO1 with the PSA promoter was quantified by real-time PCR. Results were normalized to the corresponding value for input.
Paclitaxel inhibition of the AR is mediated by FOXO1
Next, we sought to determine whether paclitaxel-induced expression of FOXO1 and FOXO1-AR association play a causal role in its inhibition of the AR. The FOXO1 gene was knocked down by a FOXO1-specific siRNA in 22Rv1 cells (Fig. 5A). As demonstrated by real-time RT-PCR, paclitaxel-induced inhibition of PSA and Nkx3.1 expression was completely abolished in cells treated with the FOXO1-specific siRNA (Fig. 5A). While paclitaxel induced a significant reduction in AR transcriptional activity in cells transfected non-specific siRNA, this effect was abrogated in cells transfected with FOXO1 siRNA (Fig. 5B). These findings suggest that paclitaxel-induced inhibition of AR activity is mediated by FOXO1. Consistently, paclitaxel-induced apoptosis of 22Rv1 cells was significantly diminished by knockdown of endogenous FOXO1 (Fig. 5C). This result was further confirmed by measuring the caspase 3 activity in 22Rv1 cells treated with control or FOXO1-specific siRNA in the presence of paclitaxel (Fig. 5D). Thus, FOXO1 not only contributes to paclitaxel-induced AR inhibition, it also plays an important role in paclitaxel-mediated apoptosis of CRPC cells.
Figure 5.
Paclitaxel-induced inhibition of the AR activity is mediated by FOXO1. A, FOXO1 knockdown abolishes paclitaxel-induced inhibition of expression of AR target genes. 22Rv1 cells were transfected with non-specific siRNA (NS siRNA) or FOXO1 siRNA. At 48 h after transfection, cells were treated with 1.0 nM paclitaxel. After 24 h of treatment, cells were harvested for mRNA and protein analysis. The expression levels of PSA and Nkx3.1 mRNAs were measured by quantitative real-time RT-PCR. GAPDH mRNA was used as an internal control. Expression of FOXO1 and AR proteins was analyzed by immunoblotting. ERK2 was used as a loading control. B, FOXO1 knockdown abolishes paclitaxel-induced inhibition of AR transcriptional activity. 22Rv1 cells were transfected with nonspecific control siRNA or FOXO1 siRNA in combination with the renilla luciferase reporter gene and 3×ARE-Luc. At 24 h after transfection, cells were treated with 1 nM of paclitaxel. After 24 h of treatment, cells were harvested and luciferase activity was measured. Error bars indicate SD among three individual experiments. C, D, Knocking down FOXO1 inhibits paclitaxel-induced apoptosis in 22Rv1 cells. 22Rv1 cells were transfected with NS siRNA or FOXO1 siRNA. At 48 h after transfection, cells were treated with 1 nM of paclitaxel. At 24 h after treatment, cells were harvested for FACS analysis (C) or measurement of caspase 3 activity (D). Results from one representative experiment are shown in C, left two panels. Quantitative results are shown in C, right panel. Error bars indicate SD among three individual experiments.
The AR inhibitory effect of paclitaxel is suspended in PTEN-mutated C4-2 CRPC cells
We demonstrated previously that FOXO1 inhibition of the AR requires the nuclear localization of FOXO1 (14). It has been well established that loss of PTEN results in Akt activation, which leads to phosphorylation and cytoplasmic localization of FOXO proteins (22). Given that accumulation of the FOXO1 protein in the nucleus is important for paclitaxel-induced inhibition of the AR (Figs. 4 and 5), we hypothesized that inactivation of PTEN would abolish paclitaxel inhibition of the AR in PCa cells. To test this hypothesis, we examined the effect of paclitaxel on PSA expression in PTEN-mutated LNCaP cells. Paclitaxel treatment failed to decrease PSA expression in this cell line as demonstrated by semiquantitative RT-PCR (Supplementary Fig. 4). As shown in Fig. 6A (lane 1 versus lane 2), paclitaxel treatment also failed to inhibit AR transcriptional activity in C4-2 cells, a castration-resistant metastatic cell line that was originally derived from LNCaP cells through serial xenografting in castrated mice (23). Similar to the previous report in Cos-7 cells (24), inhibition of Akt by treatment of C4-2 cells with the PI3K inhibitor LY294002 decreased AR transcriptional activity (Fig. 6A). LY294002 inhibition of Akt was evident by loss of FOXO1 phosphorylation at serine 256 residue (Fig. 6A), a known Akt phosphorylation site (22). Importantly, paclitaxel treatment resulted in a significant reduction in AR transcriptional activity in C4-2 cells pretreated with LY294002 in comparison to cells treated with LY294002 alone (Fig. 6A). This effect was reversed by knockdown of endogenous FOXO1 (Fig. 6A). Moreover, treatment of C4-2 cells with paclitaxel alone failed to decrease the cell viability (Fig. 6B). As expected, LY294002 treatment resulted in a marked inhibition of cell viability (Fig. 6B). Importantly, paclitaxel treatment also significantly decreased the viability of C4-2 cells when they were pretreated with the PI3K inhibitor and this effect was largely diminished by FOXO1 knockdown (Fig. 6B). Together, these data suggest that deregulation of the PTEN/FOXO1 pathway may play an important role in development of taxol resistance in castration-resistant prostate cancer.
Figure 6.
C4-2 cells are refractory to paclitaxel-induced inhibition of AR activity and loss of cell viability. A, C4-2 cells were transfected with NS siRNA or FOXO1 siRNA in combination with 3×ARE-Luc and the renilla luciferase reporter gene. At 24 h after transfection, cells were treated with 1 nM of paclitaxel and/or LY294002 as indicated. After 24 h of treatment, cells were harvested for measurement of luciferase activity. Protein expression was analyzed by immunoblotting using antibodies against FOXO1, phospho-FOXO1 at S256 (FOXO1-S256-p) and AR. ERK2 was used as a loading control. B, C4-2 cells were transfected with siRNAs as indicated and treated with paclitaxel and LY294002 as in (A). Cell viability was measured at 24 h after treatment of paclitaxel and/or LY294002. Error bars indicate SD among three individual experiments.
Discussion
Taxol is the first chemotherapy agent that increases the survival of patients with CRPC and hence has been approved by the US FDA for treatment of CRPC (1, 6-8). In addition, the effectiveness of this chemotherapeutic agent is further manifested by at least 50% decline of serum PSA in half of the CRPC patients (7). Although the taxol-induced decline in PSA level can be explained by the general effect of tumor regression, in the present study, we identified the inhibition of AR activity as a novel mechanism of taxol chemotherapy in CRPC. We provide evidence that both paclitaxel and docetaxel treatment resulted in a decrease in expression of AR transactivated genes, including PSA and Nkx3.1 in PTEN-positive 22Rv1 cells. The finding that expression of the AR repression gene maspin markedly increased following paclitaxel and docetaxel treatment further supports the conclusion that decreased expression of AR transactivated genes is not solely caused by taxol-induced cell death, but also through the inhibition of AR transcriptional activity.
The finding from cell culture studies is further supported by observations in animals. Cell-based analysis in tumor tissues indicates that docetaxel treatment not only causes cell cycle arrest at mitosis in tumor cells adjacent to blood vessels, but also decreases PSA expression in similar cell populations. These findings suggest that taxol can also inhibit AR function under in vivo conditions. It is worth noting that the anti-tumor and anti-AR activities of docetaxel were primarily detected in tumor cells immediately next to vascular vessels. These findings suggest that the drug bioavailability may be a critical factor that needs to be considered for better therapeutic effect of taxol in vivo.
Our data further shows that taxol-induced inhibition of the AR is mediated by the nuclear factor FOXO1. Similar to the findings in breast and ovarian cancer cells (11-13), paclitaxel treatment induces increased expression and nuclear localization of FOXO1 in 22Rv1 CRPC cells. The molecular mechanism underlying taxol-induced expression of FOXO1 in these types of cancer cells is unknown at present and further investigation is warranted. In addition, this study demonstrates that following paclitaxel treatment increased amounts of FOXO1 proteins were found to be associated with the AR and the PSA promoter. As reported (14, 21, 25, 26), FOXO1 functions as a potent repressor of the AR and this function of FOXO1 requires nuclear localization of FOXO1 (14). Thus, it is conceivable that treatment of CRPC cells with taxol inhibits AR activity by inducing nuclear accumulation of FOXO1 and increasing association of FOXO1 with the AR. In line with these findings, we further unraveled that there was no inhibitory effect of paclitaxel on AR activity in PTEN-mutated androgen-dependent LNCaP cell line and its androgen-refractory derivative C4-2. However, this can be reversed by the pretreatment of cells with the PI3K inhibitor LY294002. Thus, our findings suggest that the functional PTEN/FOXO1 pathway is an important factor in determining the anti-AR and anti-tumor efficacy of taxol in prostate cancer.
In summary, our studies demonstrate for the first time that taxol inhibits the AR activity in CRPC cells in culture and in animals. Mechanistically, we demonstrate that the AR inhibitory effect of taxol is mediated by accumulation of FOXO1 proteins in the nucleus and increased interaction between FOXO1 and the AR. Given that the suppressor gene PTEN, a key upstream regulator of FOXO1, is often mutated or deleted in advanced castration-resistant prostate cancer (27), deregulation of the PTEN/FOXO1 pathway may play an important role in the development of taxol resistance in prostate cancer.
Supplementary Material
Acknowledgements
We thank C. Y. Young for plasmids and cell lines and S. M. Dehm for critical reading of the manuscript. This work was supported in part by funds from the National Institutes of Health (CA134514), the Department of Defense (W81XWH-07-1-0137 and PC080591) and the Brainstorm Award from University of Minnesota Masonic Cancer Center (to H.H.), and from Cancer Research Society and Canadian Institutes of Health Research (to Y.Z.W.).
References
- 1.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–90. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 2.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–97. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 3.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62:1008–13. [PubMed] [Google Scholar]
- 4.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 5.Mohler JL, Gregory CW, Ford OH, 3rd, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–8. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 6.Smith DC, Pienta KJ. Paclitaxel in the treatment of hormone-refractory prostate cancer. Semin Oncol. 1999;26:109–11. [PubMed] [Google Scholar]
- 7.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 8.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 9.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–7. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 10.Haldar S, Chintapalli J, Croce CM. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res. 1996;56:1253–5. [PubMed] [Google Scholar]
- 11.Sunters A, Fernandez de Mattos S, Stahl M, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 12.Sunters A, Madureira PA, Pomeranz KM, et al. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–20. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 13.Goto T, Takano M, Hirata J, Tsuda H. The involvement of FOXO1 in cytotoxic stress and drug-resistance induced by paclitaxel in ovarian cancers. Br J Cancer. 2008;98:1068–75. doi: 10.1038/sj.bjc.6604279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu P, Li S, Gan L, Kao TP, Huang H. A transcription-independent function of FOXO1 in inhibition of androgen-independent activation of the androgen receptor in prostate cancer cells. Cancer Res. 2008;68:10290–9. doi: 10.1158/0008-5472.CAN-08-2038. [DOI] [PubMed] [Google Scholar]
- 15.Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–7. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Regan KM, Wang F, et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci USA. 2005;102:1649–54. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauter D, Himmelsbach K, Kriegs M, Carvajal Yepes M, Hildt E. Localization determines function: N-terminally truncated NS5A fragments accumulate in the nucleus and impair HCV replication. J Hepatol. 2009;50:861–71. doi: 10.1016/j.jhep.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Sudilovsky D, Zhang B, et al. A human prostatic epithelial model of hormonal carcinogenesis. Cancer Res. 2001;61:6064–72. [PubMed] [Google Scholar]
- 19.Verras M, Lee J, Xue H, Li TH, Wang Y, Sun Z. The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Cancer Res. 2007;67:967–75. doi: 10.1158/0008-5472.CAN-06-3552. [DOI] [PubMed] [Google Scholar]
- 20.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329–38. doi: 10.1074/jbc.M610447200. [DOI] [PubMed] [Google Scholar]
- 22.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–87. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 23.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994;57:406–12. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 24.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–27. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 25.Dong XY, Chen C, Sun X, et al. FOXO1A is a candidate for the 13q14 tumor suppressor gene inhibiting androgen receptor signaling in prostate cancer. Cancer Res. 2006;66:6998–7006. doi: 10.1158/0008-5472.CAN-06-0411. [DOI] [PubMed] [Google Scholar]
- 26.Ma Q, Fu W, Li P, et al. FoxO1 mediates PTEN suppression of androgen receptor N- and C-terminal interactions and coactivator recruitment. Mol Endocrinol. 2009;23:213–25. doi: 10.1210/me.2008-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertram J, Peacock JW, Fazli L, et al. Loss of PTEN is associated with progression to androgen independence. Prostate. 2006;66:895–902. doi: 10.1002/pros.20411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.