Abstract
Women with fragile X mental retardation (FMR1) gene premutations (55–200 CGG repeats) were until recently believed to be unaffected. It is now known that up to 8% of older female FMR1 premutation carriers develop fragile X-associated tremor/ataxia syndrome (FXTAS). Female carriers may also develop primary ovarian insufficiency, thyroid disease, hypertension, seizures, peripheral neuropathy, and fibromyalgia. We present a 60 year-old woman with FMR1 premutation who had depression, anxiety, and conversion disorder with seizures. The FMR1 premutation with its associated mRNA toxicity is postulated as an underlying neurobiological mechanism of conversion symptoms, through functional and structural neural dysconnectivity.
Keywords: conversion, psychogenic nonepileptic seizures, FMR1 premutation
INTRODUCTION
Premutation expansions (55–200 CGG repeats) of the fragile X mental retardation 1 (FMR1) gene are frequent in the general population, with estimated prevalence of 1 per 130–260 females and 1 per 250–800 males [Hagerman, 2008]. In the last decade, there have been tremendous advances in research related to carriers of the premutation, and a neurodegenerative movement disorder has been described, fragile X-associated tremor/ataxia syndrome (FXTAS).
FXTAS affects 17% of male carriers in their 50's and up to 75% of male carriers in their 80's [Jacquemont et al., 2004]. Clinical manifestations of FXTAS include: intention tremor, ataxia, parkinsonism, peripheral neuropathy, and autonomic dysfunction, as well as significant neurocognitive and behavioral changes [Leehey et al., 2007]. The presence of symmetrical regions of hyperintensity in the middle cerebellar peduncles (MCP) on T2 weighted MRI is a major radiologic criterion for FXTAS, although only 58% of males and 13% of female carriers may have a positive MCP sign [Brunberg et al., 2002; Adams et al., 2007]. Cerebellar cortical atrophy, white matter disease, and generalized cerebral atrophy have also been noted on imaging studies [Brunberg et al., 2002; Adams et al., 2007]. Functional magnetic resonance imaging (fMRI) studies in adult male premutation carriers have demonstrated deficits in facial expression recognition [Cornish et al., 2005], impaired amygdalar response to fearful faces [Hessl et al., 2007], and reduced hippocampal activation on recall tasks [Koldewyn et al., 2008].
The pathogenesis of FXTAS involves an increase in mRNA transcribed from the FMR1 gene, despite normal or borderline low FMR protein levels [Tassone et al., 2000]. The increased mRNA levels may lead to toxicity through dysregulation and sequestration of other molecules, forming eosinophilic ubiquitin-positive intranuclear inclusions present in neurons and astrocytes throughout the brain and the peripheral nervous system [Greco et al., 2006].
Until recently, women carrying FMR1 premutation alleles were believed to be unaffected except for primary ovarian insufficiency, which is present in up to 20% of female premutation carriers. FXTAS is now known to occur in approximately 8% of older female carriers [Coffey et al., 2008]. Women with the premutation have milder neurological involvement, develop FXTAS less often, and have less severe cognitive deficits and milder brain changes on MRI than males with FXTAS [Adams et al., 2007; Seritan et al., 2008]. Women with FXTAS may also develop thyroid disease, hypertension, seizures, peripheral neuropathy, and fibromyalgia, while female premutation carriers without FXTAS also report chronic muscle pain, persistent paresthesias in extremities, and a history of tremor more frequently than controls [Coffey et al., 2008].
Neuropsychiatric symptoms in FXTAS include: anxiety, depression, irritability, disinhibition, and socially inappropriate or reclusive behavior [Hessl et al., 2005; Bourgeois et al., 2006]. The FXTAS-associated dementia combining cortical and subcortical features has been described [Bacalman et al., 2006; Bourgeois et al., 2007; Seritan et al., 2008], with deficits in multiple cognitive domains and prominent executive dysfunction [Grigsby et al., 2007; Brega et al., 2008]. Psychiatric symptoms such as anxiety, social phobia, and depression are common in male and female premutation carriers without FXTAS [Hessl et al., 2007; Rodriguez-Revenga et al., 2008; Roberts et al., 2009]. Elevations of mRNA levels were positively correlated with obsessive-compulsive measures on the Symptom Checklist-90-Revised (SCL-90-R), suggesting that the mRNA toxicity is related to psychiatric symptoms in premutation carriers [Hessl et al., 2007]. Women with the premutation also had significantly higher somatization and depression measures on SCL-90-R than normative sample means. Here we present a woman with the FMR1 premutation who had depression, anxiety symptoms, and conversion disorder with seizures. The research protocol was approved by the Institutional Review Board at the University of California, Davis.
CLINICAL REPORT
The patient was a 60-year-old woman with the fragile X premutation, carrying an allele with 94 CGG repeats and a normal allele with 31 repeats. Since age 50, she had been experiencing bilateral limb weakness and numbness that progressed to burning sensation and pain in her extremities, significantly interfering with her activities of daily living. Around age 55, she also started having difficulty walking with occasional falls and deteriorating handwriting. She denied mood changes but became increasingly anxious over her functional decline. She also complained of worsening memory and at times her family observed her to be confused.
The patient described a series of spells starting around age 59, the first of which occurred during a plane flight, while returning home from vacation. The spells were characterized by feeling cold all over, becoming quiet and unresponsive, developing a “vacant look”, flushing of her face and ears, and shaking of her right hand, followed by intense fatigue. These episodes lasted between one and four hours and were initially occurring on a daily basis, but with no tonic or clonic activity, incontinence, or tongue biting. Upon presentation, these spells were occurring weekly, sometimes during medical procedures. She had an extensive work-up in a specialized academic center, which was negative. The EEG recorded during one of these episodes and an interictal EEG showed no epileptiform discharges.
The patient had a history of major depressive disorder, with two inpatient hospitalizations. Her first admission was at age 25, after her son’s diagnosis of fragile X syndrome. The second hospitalization occurred at age 45, after her son, at the time a young adult, died of complications of a viral illness. She underwent a trial of a selective serotonin reuptake inhibitor without benefit, and upon presentation was taking an anticonvulsant medication and a tricyclic antidepressant. She had received psychotherapy following sexual trauma in her late teenage years. At that time, she reported avoidance behavior along with recurrent and intrusive thoughts of the trauma, which later resolved. She had no history of suicide attempts, although she had an episode of self-mutilation by cutting on her arms, following the tragic loss of her son.
The patient’s medical history included hypertension, sensorimotor peripheral neuropathy diagnosed with electrical studies in 2001 and 2008, and hypothyroidism. She was taking a diuretic, a calcium channel blocker, thyroid supplementation, and occasional pain medication. In addition she took a low dose of levodopa at night for restless legs syndrome. Before being disabled by the seizure-like episodes, she had worked full-time as an artist. No recent life stressors were apparent prior to the onset of these spells, besides her neurological symptoms. She had been married for 40 years and had a 32-year-old daughter. Her daughter was not a premutation carrier. The patient had a brother with an unmethylated low end full mutation with 220 CGG repeats. Her brother had no known intellectual disability however he had anxiety, attention deficits, and emotional outbursts.
The patient was an overweight sad appearing, but cooperative woman. Her neurological exam demonstrated a mild to moderate terminal intention tremor on finger to nose testing bilaterally. She had subtle listing to the left on casual gait, and great difficulty performing tandem gait. She had decreased sensation to vibration in both lower extremities and depressed ankle reflexes. On sensory exam, there was a stocking pattern of loss of pain and temperature, vibration sense was absent at the toes and joint-position sense was poor at the toes. She had a mild snout reflex, a frontal release sign. The rest of her exam was normal, including rapid alternating movements.
Immediately following the MRI study, the patient had a seizure-like episode. She looked dazed, started hyperventilating, and was mute for about three hours. During the episode, she had global psychomotor slowing, and remained dazed looking but followed all commands, albeit slowly. She had a jerky irregular action tremor involving the distal arms that varied from moment to moment in direction and amplitude. Finger tapping and rapid alternating movements were performed with irregular jerky hesitations; each task appeared to require an extraordinary amount of effort. She had increased muscle tone in her lower extremities. The patient slowly regained her speech afterwards, first only to a whisper. She was emotional and spontaneously discussed her immense sadness related to loss of her son. A Structured Clinical Interview for DSM-IV (SCID) was completed.
A Mini-Mental State Examination (MMSE) was not performed at the time of this episode but the patient had scored 30/30 on the previous day. Additional neuropsychological testing revealed: full scale IQ of 111, within average range, with a verbal IQ of 107 and a performance IQ of 114. the patient generated a total of 47 words in 3 minutes on a verbal fluency test, normal for her age range. Verbal fluency measures executive functioning, as it involves the ability to generate information actively. The California Verbal Learning Test also showed no impairment. However, she did have mild deficits on other tests of executive function which require response inhibition, such as the “go-no-go” test. In the first task, she had to squeeze the experimenter’s hand whenever she heard the word “red”, and do nothing if she heard the word “green”. Within 15 repetitions of variable “go”/“no-go” ratio, she had two errors. In the second task, the patient had to tap twice if the experimenter tapped once and vice versa. Within 10 trials, she had two errors and a slow reaction time. Both results implicate a slight impairment in the inhibitory control system.
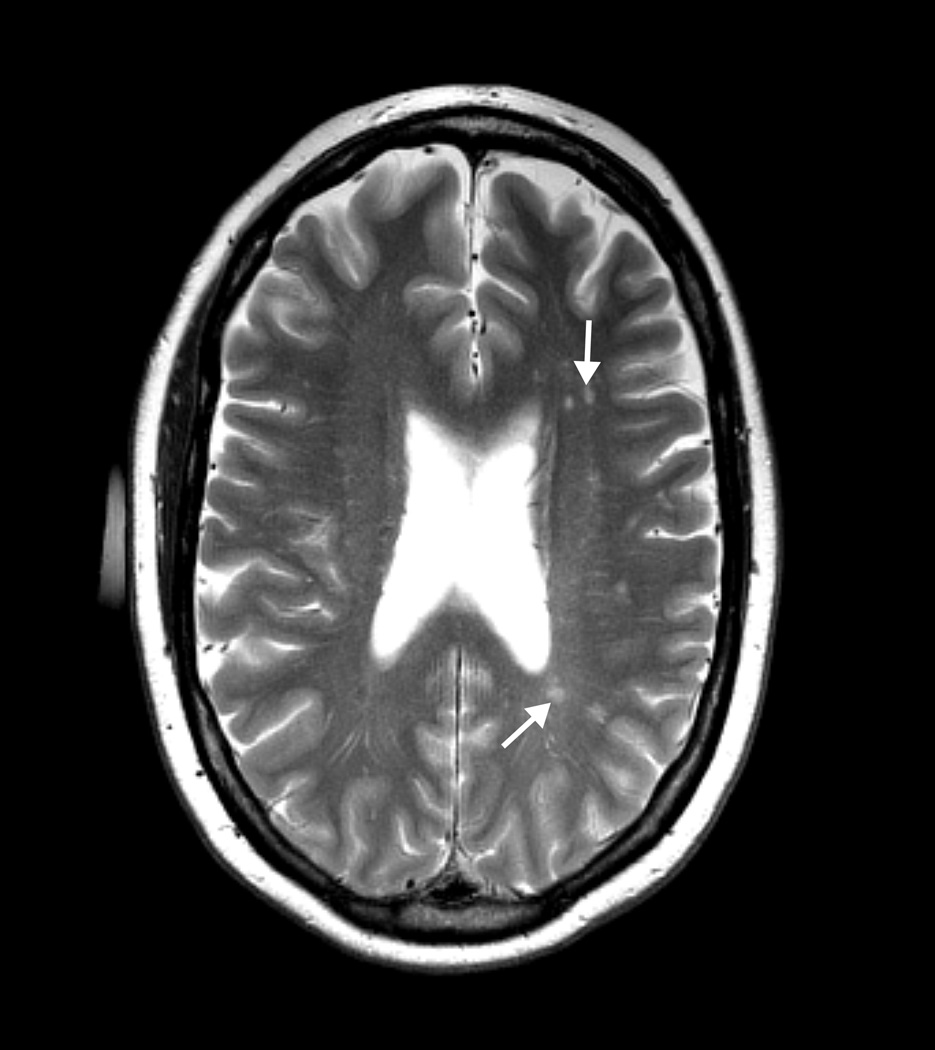
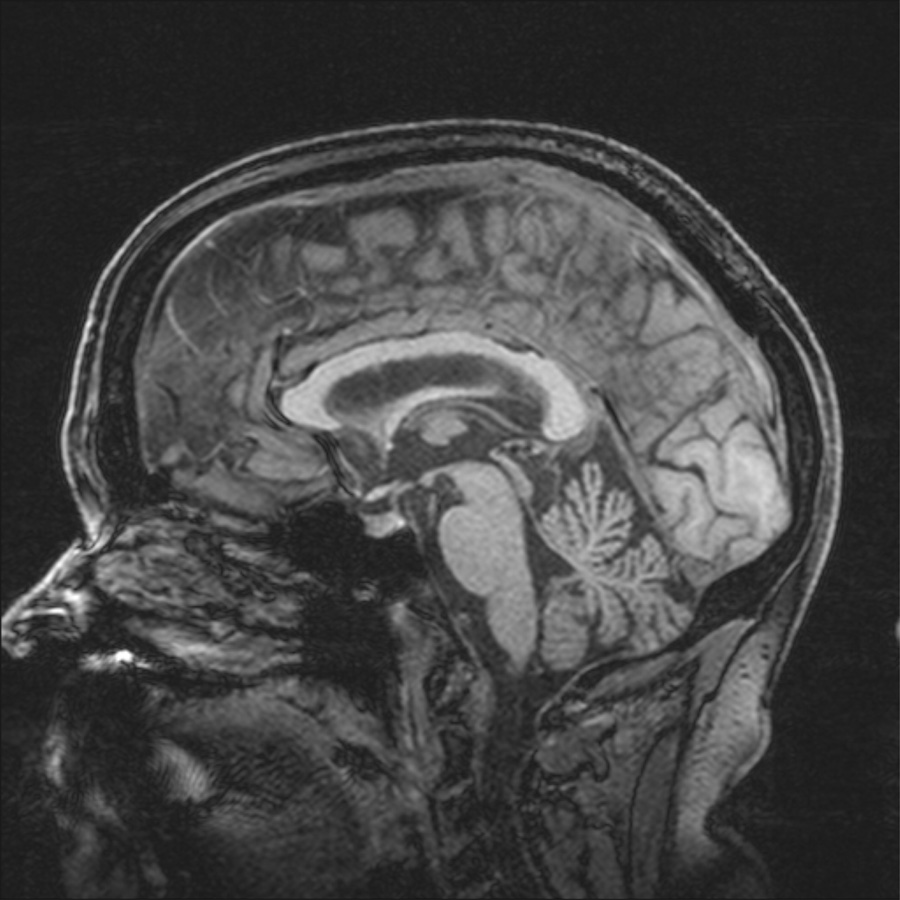
Laboratory data included routine hematological and chemistry studies and thyroid stimulating hormone, all within normal range. The patient’s brain MRI demonstrated mild generalized cerebral atrophy and enlarged subarachnoid space in the right parietal area. T2 weighted images showed scattered, tiny hyperintense foci within the hemispheric white matter. There was mild cerebellar atrophy but no MCP sign (Fig 1).
Figure 1.
Figure 1a. T2 weighted axial MRI image showing increased T2 signal intensity areas (arrows) and enlarged perivascular spaces in deep white matter.
Figure 1b. Magnetization-prepared rapid gradient echo (3D, T1 weighted) sagittal MRI image showing atrophy of cerebellar vermis.
Cognitive event-related potentials (ERPs), like evoked potentials, are averaged EEG responses. ERPs can also help elucidate which stages of cognitive operations are altered. ERPs were obtained in order to further evaluate auditory attention (N1, P3), central processing time (P3), working memory (P600), and language comprehension (N400). Semantic processing load was quantified with N400 amplitude, a negative deflection peaking at ~400 ms. The patient’s ERP tests revealed normal auditory N1 and P3 components. In addition, the P600 word repetition effect amplitude was normal and typical for older individuals with intact verbal memory. However, the N400 repetition effect, elicited by repeating semantically incongruous visual words 10–140 seconds later, was absent (new-old words, mean amplitude 300–550 ms post-stimulus onset at T6= 0.38 µV, indicating slightly greater negative voltage to old than new words which is opposite to the normal effect).
The patient met criteria for probable FXTAS with tremor, ataxia, cerebral atrophy and mild white matter changes, as well as cerebellar atrophy on MRI in addition to the fragile X premutation. A psychiatric diagnosis of major depressive disorder, severe, recurrent, and conversion disorder with seizures was established.
DISCUSSION
The common feature of somatoform disorders is the presence of physical symptoms that suggest a general medical condition, however are not fully explained by a medical illness, by the direct effects of a substance, or by another mental disorder. Additionally, the symptoms must cause clinically significant distress or impairment in social, occupational, or other areas of functioning. Conversion disorder is currently classified as a somatoform disorder and it involves unexplained symptoms or deficits affecting voluntary motor or sensory function that suggest a neurological condition. Psychological factors are judged to be associated with the symptoms or deficits, and history of early trauma is often correlated with development of a conversion disorder. Inconsistencies on neurological examination suggest this diagnosis, such as: fluctuating weakness, non anatomical sensory loss, and tunnel vision [Black et al., 2004]. Onset of conversion disorder is generally from late childhood to early adulthood, later onset suggesting a neurological or other medical etiology. A subsequent diagnosis of neurological disease in patients initially diagnosed with conversion disorders was believed to be common, but recent studies only show a 10–15% rate of misdiagnosis. [Mace et al., 1996]. In a dramatic presentation, a woman believed to have conversion disorder received a post-mortem diagnosis of Creutzfeldt-Jakob disease [Solvason et al., 2002].
The patient received extensive evaluations, including neurological exams which demonstrated some features of FXTAS. She was also at risk for developing a somatoform disorder, given her history of sexual abuse. Hypothyroidism could contribute to mood, cognitive, and anxiety symptoms. Another notable comorbidity was hypertension, which could lead to white matter microvascular ischemic changes. We postulate here that the FMR1 premutation with its associated RNA toxicity may also contribute to the development of conversion, as well as mood, anxiety, and cognitive symptoms, through a complex underlying mechanism involving white matter changes and dysfunctional neuronal connectivity.
The understanding of somatoform disorders has greatly advanced with the advent of modern functional imaging techniques. Altered activation in frontal inhibitory structures such as the anterior cingulate cortex (ACC) and orbitofrontal cortex has been reported in somatoform disorders [Roelofs et al., 2007]. An increasing number of studies support a neurobiological model of conversion disorder as the result of abnormal connectivity between specific cortical and subcortical areas, in particular the prefrontal and parietal cortices, the dorsal cognitive and rostral-ventral affective subdivisions of the anterior cingulate, the thalamus, and the basal ganglia [Ballmaier et al., 2005]. Simultaneous activation of frontal inhibitory areas and deactivation of primary motor and somatosensory cortex in conversion paralysis, hysterical anesthesia, and unexplained visual loss, points to an active inhibition of motor and sensory processing in conversion disorder [Roelofs et al., 2007]. An ERP study showed that hyperactive ACC action monitoring functions played a role in conversion paralysis [Roelofs et al., 2006]. These findings suggest disruption of sensory/motor processing, motivation, and attention at multiple nodes of a widely distributed network. Thus, conversion symptoms may be the result of dynamic reorganization of neural circuits linking volition, movement, and perception [Black et al., 2004].
Disordered neuronal connectivity due to white matter disease may account for conversion symptoms in FMR1 premutation carriers. There is neuropathological [Greco et al., 2006] and radiological [Brunberg et al., 2002] evidence of white matter involvement in patients with the premutation and FXTAS, and the patient’s MRI showed T2 hyperintensities. There is also evidence [Boorman et al., 2007] that individual white matter microstructure is related to functional connectivity within brain networks during action choice. White matter compromise leading to disruptions in neural connectivity (structural) and conductivity (physiological impairment in neural circuits) has been postulated as a salient pathophysiologic mechanism in other psychiatric disorders, including major depression, schizophrenia, and obsessive-compulsive disorder, in demyelinating diseases (multiple sclerosis, genetic white matter disorders), traumatic brain injury, as well as in normal aging [Kumar et al., 2002]. For example, a recent fMRI study of patients with schizophrenia showed significant impairment in functional connectivity between the dorsolateral prefrontal cortex and task-relevant brain regions (e.g., left premotor cortex and right inferior parietal lobule) in a version of the continuous performance task [Yoon et al., 2008]. Significant correlations were also found between the dorsolateral prefrontal cortex functional connectivity and the patients’ cognitive performance, behavioral disorganization, and global functioning. Abnormal functional connectivity during face processing has been reported in patients with autism spectrum disorders [Kleinhans et al., 2008], as well as in children with bipolar disorder [Rich et al., 2008]. In patients with Williams syndrome, altered functional connectivity between parahippocampal gyrus and parietal cortex and between the fusiform gyrus and a network of brain regions including amygdala and portions of the prefrontal cortex has been demonstrated [Sarpal et al., 2008]. Future neuroimaging research studies, such as diffusion tensor imaging in premutation carriers and patients with FXTAS will elucidate contributions of neuronal dysconnectivity to the associated cognitive and neuropsychiatric phenotype.
The cognitive ERP abnormalities of the N400 potential observed in our case may indicate impaired functional connectivity, particularly of the temporal neocortex, since the left superior temporal sulcus and bilateral anterior fusiform are thought to contain the main N400 generators [Halgren et al., 2002]. Abnormalities of the N400 have been reported in receptive aphasia, Alzheimer’s disease, left temporal lobe epilepsy, and in schizophrenia [Hagoort et al., 1996; Olichney et al., 2002; Olichney et al., 2004; Kiang et al., 2008]. While the N400 amplitude and latency elicited by new semantically incongruous words were normal in this patient, the usual decrement of amplitude with word repetition was absent, suggesting abnormal implicit memory processes and decreased ability to use the preceding semantically incongruous context [Olichney et al., 2000]. The absent or reduced N400 repetition effect is common in the FXTAS patients we have studied to date [Olichney et al., 2009].
Psychiatric treatment of conversion disorder is essential. A review of randomized controlled trials for treatment of somatoform disorders showed evidence of successful outcomes for cognitive-behavioral therapy, as well as for antidepressants in a small number of studies [Kroenke, 2007]. However, only one of three treatment studies showed benefit in conversion disorder suggesting that further research is needed in this direction.
In conclusion, women with the FMR1 premutation may present with a myriad of neuropsychiatric symptoms, including anxiety, depression, and conversion disorder. The frequency of conversion disorder among premutation carriers needs to be studied. FMR1 mRNA toxicity may cause white matter lesions resulting in neuronal dysconnectivity, which has been postulated as an underlying neurobiological mechanism for conversion symptoms. To date, a growing body of literature describes the molecular, clinical, and neuroimaging correlates of premutation carriers and FXTAS, along with symptomatic treatment approaches [Hagerman et al., 2008]. There is still much to be learned, especially in the therapeutic area. FMR1 premutation may constitute a template for understanding other neuropsychiatric illnesses and may help elucidate the neurobiological underpinnings of symptoms once-believed to be exclusively psychogenic.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health grants DE19583, AG032115, AG032119, HD036071, HD02274, AG18442, and State of California Department of Public Health (Alzheimer's Disease Program). We thank James Brunberg, MD and Patrick Adams, BS for the MRI images. Parts of this work were presented in poster format at the 2nd International Conference on Psychogenic Movement Disorders and Other Conversion Disorders, Washington, D.C., April 2009.
REFERENCES
- Adams JS, Adams PF, Nguyen D, Brunberg JA, Tassone F, Zhang W, Koldewyn K, Rivera SM, Grigsby J, Zhang L, DeCarli C, Hagerman PJ, Hagerman RJ. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS) Neurology. 2007;69:851–859. doi: 10.1212/01.wnl.0000269781.10417.7b. [DOI] [PubMed] [Google Scholar]
- Bacalman S, Farzin F, Bourgeois JA, Cogswell J, Goodlin-Jones B, Gane LW, Grigsby J, Leehey MA, Tassone F, Hagerman RJ. Psychiatric phenotype of the fragile X-associated tremor/ataxia syndrome (FXTAS) in males: newly described fronto-subcortical dementia. J Clin Psychiatry. 2006;67:87–94. doi: 10.4088/jcp.v67n0112. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Schmidt R. Conversion disorder revisited. Funct Neurol. 2005;20:105–113. [PubMed] [Google Scholar]
- Black DN, Seritan AL, Taber KH, Hurley RA. Conversion hysteria: Lessons from functional imaging. J Neuropsychiatry Clin Neurosci. 2004;16:245–251. doi: 10.1176/jnp.16.3.245. [DOI] [PubMed] [Google Scholar]
- Boorman ED, O’Shea J, Sebastien C, Rushworth MFS, Johansen-Berg H. Individual differences in white-matter microstructure reflect variation in functional connectivity during choice. Curr Biol. 2007;17:1426–1431. doi: 10.1016/j.cub.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Bourgeois JB, Farzin F, Brunberg JA, Tassone F, Hagerman P, Zhang L, Hessl D, Hagerman R. Dementia with mood symptoms in a carrier of the fragile X-associated tremor/ ataxia syndrome (FXTAS): clinical intervention with donepezil and venalafaxine. J Neuropsychiatry Clin Neurosci. 2006;18:171–177. doi: 10.1176/jnp.2006.18.2.171. [DOI] [PubMed] [Google Scholar]
- Bourgeois JA, Cogswell JB, Hessl D, Zhang L, Ono MY, Tassone F, Farzin F, Brunberg JA, Grigsby J, Hagerman RJ. Cognitive, anxiety and mood disorders in the fragile X-associated tremor/ataxia syndrome. Gen Hosp Psychiatry. 2007;29:349–356. doi: 10.1016/j.genhosppsych.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brega AG, Goodrich G, Bennett RE, Hessl D, Engle K, Leehey MA, Bounds MS, Paulich MJ, Hagerman RJ, Hagerman PJ, Cogswell JB, Tassone F, Reynolds A, Kooken R, Kenny M, Grigbsy J. The primary cognitive deficit among males with fragile X-associated tremor/ataxia syndrome (FXTAS) is a dysexecutive syndrome. J Clin Exp Neuropsychol. 2008;30:853–869. doi: 10.1080/13803390701819044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunberg JA, Jacquemont S, Hagerman RJ, Berry-Kravis EM, Grigsby J, Leehey MA, Tassone F, Brown WT, Greco CM, Hagerman PJ. Fragile X premutation carriers: Characteristic MR imaging findings in adult males with progressive cerebellar and cognitive dysfunction. Am J Neuroradiol. 2002;23:1757–1766. [PMC free article] [PubMed] [Google Scholar]
- Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan R, Bronsky HE, Yuhas J, Borodyanskaya M, Grigsby J, Doerflinger M, Hagerman PJ, Hagerman RJ. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet. 2008;146A:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Kogan C, Turk J, Manly T, James N, Mills A, Dalton A. The emerging fragile X premutation phenotype: evidence from the domain of social cognition. Brain Cogn. 2005;57:53–60. doi: 10.1016/j.bandc.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Greco C, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, Hessl D, Becker EJ, Papazian J, Leehey MA, Hagerman RJ, Hagerman PJ. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Leehey MA, Goodrich GK, Jaquemont S, Loesch DZ, Cogswell JB, Epstein J, Wilson R, Jardini T, Gould E, Bennett RE, Hessl D, Cohen S, Cook K, Tassone F, Hagerman PJ, Hagerman RJ. Impairment of executive cognitive functioning in males with fragile X-associated tremor/ataxia syndrome. Mov Disord. 2007;22:645–650. doi: 10.1002/mds.21359. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ. The fragile X prevalence paradox. J Med Genet. 2008;45:498–99. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Hall DA, Coffey S, Leehey M, Bourgeois J, Gould J, Zhang L, Seritan A, Berry-Kravis E, Olichney J, Miller JW, Fong AL, Carpenter R, Bodine C, Gane LW, Rainin E, Hagerman H, Hagerman PJ. Treatment of fragile X-associated tremor ataxia syndrome (FXTAS) and related neurological problems. Clin Interv Aging. 2008;3:251–262. doi: 10.2147/cia.s1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Dhond RP, Christensen N, Van Petten C, Marinkovic K, Lewine JD, Dale AM. N400-like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. Neuroimage. 2002;17:1101–1106. doi: 10.1006/nimg.2002.1268. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Brown CM, Swaab TY. Lexical-semantic event-related potential effects in patients with left hemisphere lesions and aphasia, and patients with right hemisphere lesions without aphasia. Brain. 1996;119:627–649. doi: 10.1093/brain/119.2.627. [DOI] [PubMed] [Google Scholar]
- Hessl D, Tassone F, Loesch DZ, Berry-Kravis E, Leehey MA, Gane LW, Barbato I, Rice C, Gould E, Hall DA, Grigsby J, Wegelin JA, Harris S, Lewin F, Weinberg D, Hagerman PJ, Hagerman RJ. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet. 2005;139:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- Hessl D, Rivera S, Koldewyn K, Cordeiro L, Adams J, Tassone F, Hagerman PJ, Hagerman RJ. Amygdala dysfunction in men with the fragile X premutation. Brain. 2007;130:404–416. doi: 10.1093/brain/awl338. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, Zhang L, Jardini T, Gane LW, Harris SW, Herman K, Grigsby J, Greco CM, Berry-Kravis E, Tassone F, Hagerman PJ. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291:460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- Kiang M, Kutas M, Light GA, Braff DL. An event-related brain potential study of direct and indirect semantic priming on schizophrenia. Am J Psychiatry. 2008;165:74–81. doi: 10.1176/appi.ajp.2007.07050763. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Koldewyn K, Hessl D, Adams J, Tassone F, Hagerman PJ, Hagerman RJ, Rivera S. Reduced hippocampal activation during recall is associated with elevated FMR1 mRNA and psychiatric symptoms in men with the fragile X premutation. Brain Imaging Behav. 2008;2:105–116. doi: 10.1007/s11682-008-9020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K. Efficacy of treatment for somatoform disorders: A review of randomized controlled trials. Psychosom Med. 2007;69:881–888. doi: 10.1097/PSY.0b013e31815b00c4. [DOI] [PubMed] [Google Scholar]
- Kumar A, Cook I. White matter injury, neural connectivity and the pathophysiology of psychiatric disorders. Dev Neurosci. 2002;24:255–261. doi: 10.1159/000066746. [DOI] [PubMed] [Google Scholar]
- Leehey MA, Berry-Kravis E, Min SJ, Hall DA, Rice CD, Zhang L, Grigsby J, Greco CM, Reynolds A, Lara R, Cogswell J, Jacquemont S, Hessl DR, Tassone F, Hagerman R, Hagerman PJ. Progression of tremor and ataxia in male carriers of the FMR1 premutation. Mov Disord. 2007;22:203–206. doi: 10.1002/mds.21252. [DOI] [PubMed] [Google Scholar]
- Mace CJ, Trimble TR. Ten-year prognosis of conversion disorder. Br J Psychiatry. 1996;169:282–288. doi: 10.1192/bjp.169.3.282. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Van Petten C, Paller KA, Salmon DP, Iragui VJ, Kutas M. Word repetition in amnesia. Electrophysiological measures of impaired and spared memory. Brain. 2000;9:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Riggins BR, Hillert DG, Nowacki R, Tecoma E, Kutas M, Iragui VJ. Reduced sensitivity of the N400 and late positive component to semantic congruity and word repetition in left temporal lobe epilepsy. Clin Electroencephalogr. 2002;33:111–118. doi: 10.1177/155005940203300307. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Hillert DG. Clinical applications of cognitive event-related potentials in Alzheimer's disease. Phys Med Rehabil Clin N Am. 2004;15:205–233. doi: 10.1016/s1047-9651(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Chan S, Schneider A, Niese A, Laird K, Nanakul R, Tassone F, Hagerman R. Reduced ERP word repetition effects in patients with Fragile X-associated tremor/ataxia syndrome. Cognitive Neuroscience Society Annual Meeting Program (Suppl. of J Cog Neurosci) 2009:134–135. [Google Scholar]
- Rich BA, Fromm SJ, Berghorst LH, Dickstein DP, Brotman MA, Pine DS, Leibenluft E. Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. J Child Psychol Psychiatry. 2008;49:88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Bailey D, Mankowski J, Ford A, Sideris J, Weisenfeld LA, Heath TM, Golden RN. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:130–139. doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Revenga L, Madrigal I, Montserrat A, Santos M, Montserrat M. Evidence of depressive symptoms in fragile-X syndrome premutated females. Psychiatr Genet. 2008;18:135–155. doi: 10.1097/YPG.0b013e3282f97e0b. [DOI] [PubMed] [Google Scholar]
- Roelofs K, de Brujin ERA, Van Galen GP An event-related potential study. Hypersensitivity to conflict in conversion paralysis. Biol Psychol. 2006;71:316–325. doi: 10.1016/j.biopsycho.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Roelofs K, Spinhoven P. Trauma and medically unexplained symptoms. Toward an integration of cognitive and neuro-biological accounts. Clin Psychol Review. 2007;27:798–820. doi: 10.1016/j.cpr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Sarpal D, Buchsbaum BR, Kohn PD, Kippenhan JS, Mervis CB, Morris CA, Meyer-Lindenberg A, Berman KF. A genetic model for understanding higher order visual processing: Functional interactions of the ventral visual stream in Williams syndrome. Cereb Cortex. 2008;18:2402–2409. doi: 10.1093/cercor/bhn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seritan AL, Nguyen DV, Farias ST, Hinton L, Grigsby J, Bourgeois JA, Hagerman RJ. Dementia in fragile X-associated tremor/ataxia syndrome (FXTAS): Comparison with Alzheimer's disease. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1138–1144. doi: 10.1002/ajmg.b.30732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solvason HB, Harris B, Zeifert P, Flores BH, Hayward C. Psychological versus biological clinical interpretation: a patient with prion disease. Am J Psychiatry. 2002;159:528–537. doi: 10.1176/appi.ajp.159.4.528. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in fragile X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Ursu S, Ryan Walter BS, Wendelken C, Ragland JD, Carter CS. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: Relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry. 2008;165:1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]