Abstract
Similarities in the pathologies of autoimmune diseases and cancer have been noted for at least 30 years. Inflammatory cytokines and growth factors mediate cell proliferation, and proteinases, especially the collagenase, Matrix Metalloproteinase-1 (MMP-1), contribute to disease progression by remodeling the extracellular matrix and modulating the microenvironment. This review focuses on two cancers (melanoma and breast) and on the autoimmune disorder, rheumatoid arthritis (RA), and discusses the activated stromal cells found in these diseases. MMP-1 was originally thought to function only to degrade interstitial collagens, but recent studies have revealed novel roles for MMP-1 involving the G protein-coupled receptors: the chemokine receptor, CXCR-4, and Protease Activated Receptor-1 (PAR-1). Cooperativity between MMP-1 and CXCR4/SDF-1 signaling influences the behavior of activated fibroblasts in both RA and cancer. Further, MMP-1 is a vital part of an autocrine/paracrine MMP-1/PAR-1 signal transduction axis, a function that amplifies its potential to remodel the matrix and to modify cell behavior. Finally, new therapeutic agents directed at MMP-1 and G protein-coupled receptors are emerging. Even though these agents are more specific in their targets than past therapies, these targets are often shared between RA and cancer, underscoring fundamental similarities between autoimmune disorders and some cancers.
Keywords: rheumatoid arthritis, collagenase, CXCR4, PAR-1, synovial fibroblasts, carcinoma associated fibroblasts, endothelial cells
As noted 30 years ago by Sporn and Harris [1], the pathologies of autoimmune diseases and cancer share the concepts of aberrant cell proliferation, remodeling of the extracellular matrix and perturbation of the tissue microenvironment. Specifically, they held that inflammatory cytokines and growth factors are important in initiating and maintaining proliferation, and that proteinases, especially the collagenase, Matrix Metalloproteinase-1 (MMP-1), contribute substantially to disease progression by remodeling the matrix and modulating the microenvironment. The authors also noted that as the biochemical mechanisms of disease pathogenesis become identified, new therapeutic targets will emerge. Although gaps in our knowledge of mechanism(s) remain, many of their insights are valid today, and we have a clearer picture of the molecular pathways mediating the pathogenesis of autoimmune disorders and cancers. Sporn and Harris considered seven proliferative diseases: cancer, atherosclerosis, rheumatoid arthritis, psoriasis, pulmonary fibrosis, scleroderma and cirrhosis of the liver.
In this review, we will concentrate on two of these: cancer (melanoma and breast) and the autoimmune disorder, rheumatoid arthritis (RA), and we will focus on the role of MMP-1 in activated fibroblasts and its effects on the behavior of endothelial cells. Many of our studies have centered on the mechanisms controlling MMP-1 expression in the activated synovial fibroblasts (synoviocytes) found in RA. In RA, increased production of MMP-1 is at the end of a cascade of inflammatory/immune mediators, and MMP-1 is now well established as a major mediator of irreversible connective tissue destruction. As our studies progressed, the similarities in the role of MMP-1 in RA and cancer became increasingly evident, leading us to investigate MMP-1 expression in two invasive cancers, breast and melanoma. Interestingly, this work has revealed novel roles for MMP-1 involving the G protein-coupled receptors, the chemokine receptor, CXCR-4, and Protease Activated Receptor-1 (PAR-1), roles which may also be applicable to RA. We will discuss how expression of these receptors on activated stromal cells conspires with MMP-1 to modulate cellular behavior in RA and cancer.
Matrix Metalloproteinase-1 (MMP-1), rheumatoid arthritis (RA), and cancer
MMPs are a family of zinc-dependent endopeptidases that function at neutral pH and that are responsible for the degradation of the extracellular matrix [2–4]. While low levels of these enzymes are essential for normal homeostasis, high levels are implicated in the pathology of several diseases. Although many MMPs are elevated in these conditions, MMP-1 has a particularly prominent role. Collagen (types I, II and III) is the body’s most abundant protein, comprising 30% of all protein, and collagen degradation is accomplished primarily by the interstitial collagenases, MMP-1, MMP-8, MMP-13 and MMP-14. The major collagen in joints is type II, which is almost unique to cartilage and which is preferentially degraded by MMP-13 produced by chondrocytes. MMP-8 is predominantly a product of neutrophils, although its expression by other cell types is now noted. MMP-14 is a membrane bound MMP and is constitutively expressed at low levels. In contrast, MMP-1 is expressed robustly by many cell types, and it degrades all three stromal collagens, making it the foremost player in collagen degradation in many diseases, including cancer and RA.
RA is an autoimmune disease, which affects numerous joints throughout the body. It is characterized by chronic inflammation of the synovial cells lining the joints and by irreversible destruction of the cartilage, tendons and bone that comprise joint structure [5,6]. Although the cause of RA remains elusive, a genetic predisposition related to HLA genotype and some inciting event are thought to trigger the disease by inducing an initial immune/inflammatory response in the joint tissues. This local reaction then recruits additional inflammatory cells (neutrophils and macrophages) to the inflamed joints, and these cells release cytokines [e.g., Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α)], exacerbating inflammation and the immune response. Further, these cytokines activate the synovial fibroblasts within the joint, increasing cell proliferation and expression of matrix-degrading enzymes, especially the MMPs. The increased cell proliferation produces a mass of activated fibroblasts (pannus), with ensuing destruction of type II collagen in cartilage and type I collagen in tendon and bone. This inflammatory/cytokine/MMP loop is self-sustaining until the connective tissues are destroyed, resulting in chronic pain and subsequent loss of joint function. Because of their singular ability to cleave the collagen triple helix, the over-expression of MMP-1 and MMP-13 by synovial fibroblasts and chondrocytes is critical in the pathogenesis of RA [5,6].
Similar to RA, the hallmark of cancer, cell proliferation, is also accompanied by the aberrant expression of MMPs, which are classically linked to invasion and metastasis due to their ability to degrade the extracellular matrix [2,7]. In particular, the destruction of type IV collagen in basement membrane by MMP-2 and MMP-9, and the degradation of the interstitial collagens, types I and III, in the stromal tissues by the collagenases, are essential components of metastasis. Although the tumor cells may mediate this tissue destruction, adjacent stromal cells (i.e., fibroblasts and endothelial cells) in the tumor microenvironment also degrade and remodel the matrix. However, these resident stromal cells must become activated through the engagement of signal transduction pathways. This occurs through interactions with the tumor cells, creating a population of stromal cells that shares many characteristics with the activated synovial fibroblasts in RA. In addition to degrading the extracellular matrix, MMPs can also mediate cell behavior by liberating peptides and growth factors anchored in the extracellular matrix, and by activating receptors to induce signal transduction pathways. Thus, by directly mediating cell behavior, MMPs can influence physiology and disease pathology, emphasizing the need to understand mechanisms regulating their expression.
Regulation of MMP-1 gene expression
MMP expression is tightly regulated and occurs primarily at the level of transcription [2–4,7]. In normal tissues, basal/constitutive expression is low, but is induced in response to growth factors and cytokines that activate signal transduction pathways (Figure 1). The molecular mechanisms of transcriptional activation of several MMPs, including MMP-1, at the promoter are well characterized and comprise the Activator Protein-1 (AP-1) element in the proximal region of the promoter ~ −70 bp, several ETS sites, and a Nuclear Factor kappa B (NFkB)-binding site located upstream. Chromatin remodeling is also an essential, often involving histone acetylation in response to inductive stimuli [8,9]. In many cancers increased expression of several MMPs, again including MMP-1, is constitutively high, due to either aberrant activation of signal transduction pathways and/or continuous stimulation of growth factor receptors, which target the MMP-1 promoter [7].
Figure 1. Major signaling pathways for cytokines and growth factors in synovial cells.
Upon binding to its receptor, cytokines, such as IL-1β, and growth factors, such as basic fibroblast growth factor, stimulate several signaling pathways: NFkB, p38, JNK and MEK/ERK. These pathways target DNA sequences in the promoter of the MMP-1 gene, increasing transcription. P50/p65 proteins bind to the NFkB site and cFos/cJun proteins bind to the AP-1 site. TNFα and other growth factors, such as VEGF, activate similar pathways, and target these same sites in the MMP promoters [3,4].
Although MMP expression is controlled primarily at the transcriptional level, mRNA stability also contributes. In addition, post-translational mechanisms are important, since MMPs are secreted as a zymogen, and require proteolytic cleavage for enzymatic activity. MMPs are subject to inhibition of their enzyme activity by a group of molecules called tissue inhibitors of metalloproteinases (TIMPs). TIMPs inhibit MMP enzyme activity on a 1:1 molar basis, but the high levels of MMPs seen in pathological conditions exceed TIMPs, and net tissue destruction continues [2–4].
Carcinoma Associated Fibroblasts (CAFs), MMP-1 and CXCR4
While cancer research has traditionally focused on the tumor cells, host/tumor cell interactions in the tumor microenvironment are increasingly recognized as critical components of tumor development and progression [10,11]. These interactions between the tumor and adjacent stromal cells result in the production of stromal factors, such as growth factors, chemokines, cytokines, proteases, and vascular-stimulating factors [12,13], all of which can contribute to the growth of the primary tumor and its dissemination to distant sites. The stromal fibroblasts found within carcinomas are termed carcinoma-associated fibroblasts (CAFs). Just as a tumor has been equated to a “wound that never heals” [14], CAFs have been compared to “activated fibroblasts” or myofibroblasts, which are present in inflammatory environments, including RA, where they are a major components of the proliferating pannus, and where they contribute to angiogenesis and matrix remodeling [12,13]. In breast cancer, CAFs also constitute the major cellular component of the tumor microenvironment [10,15]. As in RA, myofibroblasts, which selectively express alpha smooth muscle actin (αSMA), are believed to comprise the majority of CAFs [16,17]. However, others have suggested that not all CAFs within the tumor microenvironment are myofibroblasts [18]. Still others report that CAFs express elevated levels of the chemokine, stromal derived factor-1 (SDF-1), also known as CXCL12 [19,20]. The commonly used “CAF-markers”, such as αSMA, vimentin, and fibroblast activation protein (FAP) do not, therefore, necessarily overlap with each other. Consequently, CAFs can be considered as a heterogeneous population [21]. Although the importance of CAFs in tumor progression has been acknowledged, the identity, origin and common characteristics of CAFs have not been clearly defined.
Orimo and Weinberg have proposed three models for the derivation of CAFs in human carcinomas: (1) transdifferentiation of normal resident stromal fibroblasts, without genetic alterations; (2) clonal selection of a group of fibroblasts or progenitors with genetic alterations; and (3) differentiation of stromal fibroblasts recruited from bone marrow progenitor cells [22]. Based on their model, we have hypothesized that normal resident human mammary fibroblasts (HMFs) can transition to CAF-like cells or to bone fide CAFs by exposure to factors secreted by the breast cancer cells. We further hypothesized that as these normal quiescent cells transition to CAFs, they become activated and express many characteristics seen in the fibroblasts found at sites of inflammation, such as the synovium of RA joints (Figure 2) [20,23,24]. Indeed, we found that soluble factors produced by breast cancer cells induced expression of two tumor-enhancing and inflammatory related factors in HMFs: MMP-1 and the G protein-coupled receptor, CXCR4, [25] two markers linked to a poor prognosis in breast cancer and also seen in RA synoviocytes.
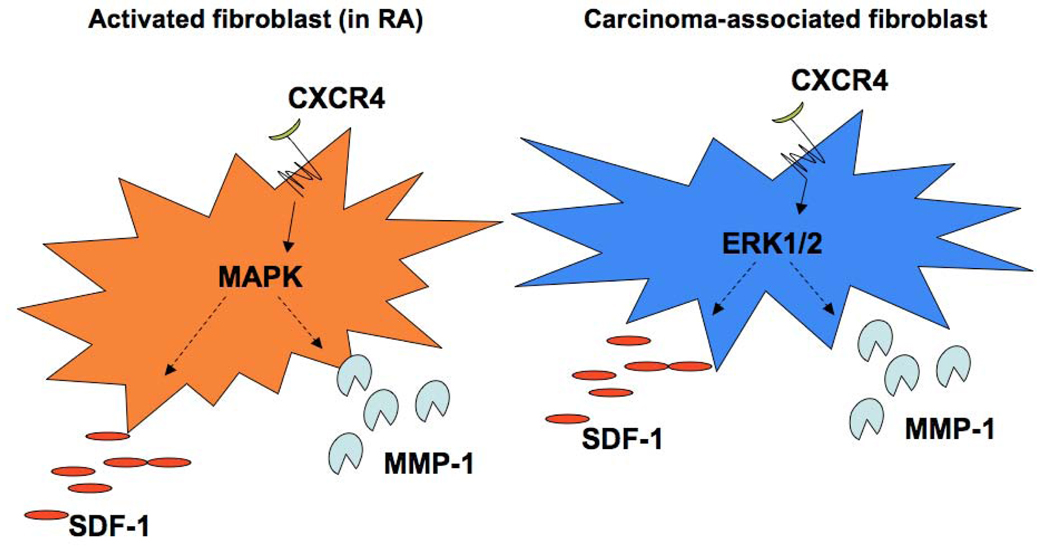
Figure 2. Similarities between the activated fibroblasts in Rheumatoid Arthritis and the Carcinoma-Associated Fibroblasts in cancer.
In both cell types, CXCR4, SDF-1, and MMP-1 are up-regulated compared to normal fibroblasts. Signaling through CXCR4 leads to activation of the ERK1/2 pathway, which is a component of the MAPK pathway, in CAFs and the MAPK pathway in activated fibroblasts. Both the MAPK and ERK1/2 pathways are known to stimulate MMP-1 and SDF-1 levels in other cell types [3,4,25].
Expression of the chemokine receptor, CXCR4, is well documented in breast cancer tumorigenesis [26]. CXCR4 is a seven-transmembrane G protein-coupled receptor with SDF-1 as its only known ligand. CXCR4 is often upregulated on breast cancer cells, and can induce targeted migration to distant organs containing cells that secrete SDF-1 [27], a process that may contribute to breast cancer metastasis. Importantly, elevated expression of CXCR4 has also been documented in synovial fibroblasts in RA. In tissue samples from RA patients, SDF-1 signaling through CXCR4 on fibroblasts results in their activation, migration and proliferation, as well as matrix remodeling due to increases in MMP-1 [28,29]. Since the breast cancer microenvironment has been compared to a site of chronic inflammation [14], our findings support the concept that similar to activated fibroblasts in autoimmune/inflammatory conditions such as RA, CXCR4 expression in CAFs identifies them as activated stromal cells.
In addition to cell migration, invasion of the extracellular matrix by malignant cells also requires degradation of the extracellular matrix [30]. We used short hairpin RNA (shRNA) technology to demonstrate that MMP-1 derived from breast cancer cells is necessary for tumor growth and invasion at the primary tumor site [31,32]. Since MMP-1 is a secreted protein, it is reasonable to propose that stromal-derived MMP-1 functions similarly to that produced by the breast cancer cells. For example, MMP-1 produced by stromal cells induced growth and invasion of breast cancer cells that did not express MMP-1 [32]. Furthermore, not all breast cancer cells produce MMP-1 [33], thereby increasing the significance of CAF-derived MMP-1 in tumor progression, and relating it to MMP-1 derived from synovial fibroblasts. Thus, MMP-1 and CXCR4 are phenotypically linked in activated HMFs in cancer and in synovial cells taken from RA pannus, where in both conditions, they are important mediators of disease pathology.
Endothelial cells, Protease Activated Receptor-1 (PAR-1) and MMP-1
The function of another G protein-coupled receptor, PAR-1, is directly linked to the enzymatic activity of MMP-1. Among all the MMPs, MMP-1 has the unique ability to cleave PAR-1 [34], with subsequent activation of signal transduction pathways and alterations in the expression of downstream genes. This unique ability to cleave PAR-1 gives MMP-1 a powerful role in controlling cell behavior.
As an example, we found that by silencing MMP-1 expression in a human melanoma cell line with shRNA technology reduced metastasis from the primary orthotopic (skin) site to the lung in a xenograft model [35]. In contrast, cells with a control shRNA (and, therefore, expressing high levels of MMP-1) readily metastasized. At first blush, the absence of metastatic ability would seem to be due to the absence of collagenolytic activity and the inability to degrade stromal collagens. However, successful metastasis requires to the coordinated expression of several genes and pathways in the both tumor cells and cells within the adjacent microenvironment [7,12,15]. Thus, the simple absence of MMP-1 was a naïve explanation. We noted a striking reduction of vasculature surrounding the primary melanomas in which MMP-1 expression was silenced, and this observation lead us to examine a potential link between MMP-1 expression and angiogenesis.
Indeed, when the human tumor cells were injected interdermally into nude mice, MMP-1 expression by the melanoma cells was associated with increased angiogenesis [35]. Since increased vessel formation has been linked to the ability of a tumor to metastasize [34–36],we hypothesized that MMP-1 was inducing angiogenesis, which is a critical aspect of neoplastic progression. Our work demonstrated that MMP-1 is a proangiogenic factor within the tumor microenvironment, and importantly, these proangiogenic activities resulted from the ability of MMP-1 to activate the G protein-coupled receptor, Protease Activator Receptor-1 (PAR-1) on the endothelial cells [35,36].
PAR-1 is one of four proteolytically activated PARs, which are expressed by several types of cells, including tumor cells, by endothelial cells, platelets, activated fibroblasts, and some types of tumor cells [37]. PAR receptors are unique in that their ligand is embedded and masked N-terminally under resting conditions. The serine protease, thrombin, is the classic activator of PAR-1, and when the ligand is exposed by proteolytic cleavage, it binds to the extracellular active site and activates PAR-1 intramolecularly [38,39]. Activated PAR initiates signal transduction pathways, with downstream consequences that lead to changes in cellular morphology and behavior under many circumstances, including tumor progression and inflammation [40]. Because the tumor microenvironment resembles chronic inflammation [14], the activation of PAR-1 on stromal cells in the tumor microenvironment may induce changes similar to those seen in stromal cells in RA [39,41]. PAR-1 is expressed on rheumatoid synovial fibroblasts [42,43] and on human chondrocytes [44]. Further, compared to wild type controls, experimental arthritis is ablated in PAR-1 −/− mice, and MMP-13 gene expression is reduced [45], thereby linking PAR-1 to MMP expression and indicating that PAR-1 plays a significant role in this model of arthritis.
Thus, it is potentially significant that MMP-1 and PAR-1 are highly expressed by both tumor and activated stromal cells in both tumor and inflammatory microenvironments. The first report of MMP-1 mediated activation of PAR-1 used MMP-1 derived from activated fibroblasts to cleave PAR-1 on tumor cells [34]. Subsequent studies demonstrated that MMP-1 could also activate PAR-1 on endothelial cells [46]. However, it was not known if PAR-1 cleavage by MMP-1 vs. thrombin on endothelial cells was redundant or resulted in distinct changes within the endothelial cells. A cell-free system demonstrated that MMP-1 and thrombin have different cleavage sites within the PAR-1 active site, with thrombin producing the hexapeptide, S42FLLRN47, and MMP-1 giving a truncated cleavage product, L44RN47, with low affinity for PAR-1 [47]. This differential cleavage of PAR-1 suggests that these proteases could have differential effects on endothelial cell gene expression [47]. To examine this possibility, we used the model system of stromal human microvessel endothelial cells (HMVECs) in which PAR-1 was activated by purified thrombin or MMP-1 [36].
We found that the MMP-1/PAR-1 signaling axis in endothelial cells activated Mitogen Activated Protein Kinases (MAPKs), resulting in the induction of a panel of pro-angiogenic genes, while thrombin activated another panel of genes. Consequently, PAR-1 activation by MMP-1 vs. thrombin could differentially influence cell behavior and pathological outcome. In addition, we proposed that the two proteinases could cooperate in the tissue microenvironments to promote both tumor progression and inflammation [36]. Since MMP-1 produced by activated fibroblasts in response to cytokines and growth factors is present in both RA and cancer, MMP-1 has the potential to activate PAR-1 on several types of cells, amplifying the role of fibroblast-derived MMP-1 as a collagenolytic enzyme and also as a critical signaling molecule.
Our studies with MMP-1 and endothelial cells describe a MMP-1/PAR-1 signaling axis that acts in a paracrine manner, whereby MMP-1 produced by tumor cells or activated fibroblasts modulates gene expression in endothelial cells [ 34,36] (Figure 3). Our more recent observations indicate that melanoma cells possess an autocrine MMP-1/PAR-1 signaling axis (Figure 3) in which tumor –produced MMP-1 signals through PAR-1 on the tumor cells to induce expression of a panel of genes associated with invasive behavior [48]. We propose a similar scenario for activated synovial fibroblasts in RA. Indeed, since PAR-1 is also expressed on RA synovial fibroblasts [42,43], it is reasonable to speculate that this signaling axis is operational in RA: MMP-1/PAR-1 signaling in activated synovial fibroblasts modulates gene expression in these cells and/or on neighboring chondrocytes. This would imply a broader and more significant role for MMP-1 in RA, one that mirrors that seen in tumor biology. Given the potential of MMP-1 for mediating joint destruction in RA, this new role would assign even more importance to defining therapeutic strategies that are targeted at reducing MMP-1 gene expression.
Figure 3. MMP-1 has multiple functions within the tumor microenvironment.
MMP-1 is secreted at high levels by melanoma cells, where it degrades collagen within the extracellular matrix surrounding the tumor cells to facilitate tumor cell invasion. MMP-1 also proteolytically activates PAR-1, expressed by multiple cell types within the microenvironment, to promote tumor progression. For example, MMP-1/PAR-1 signaling on endothelial cells induces pro-angiogenic gene expression, which may contribute to tumor metastasis, and autocrine MMP-1/PAR-1 signaling in melanoma cells induces pro-tumorigenic gene expression, which may contribute to several aspects of tumor progression, including proliferation, invasion and metastasis [34,36,48]. Both the collagenolytic and PAR-1 activating functions of MMP-1 also likely play a role in RA pathology.
Therapeutic strategies that target MMP-1 and G protein-coupled receptors
RA and cancer share several therapeutic strategies, including the use of chemotherapeutic agents such as methotrexate, antibodies directed at cytokines/growth factors and their receptors, and/or small molecule inhibitors of signal transduction pathways ([3,4,49–51]; www.Cancer.org). In cancers, these treatments are often successful, but they may also eventually fail, resulting in relapse as tumors mutate and become resistant. Since stromal cells are recognized as critical contributors cancer pathogenesis, and since these cells are more genetically stable than the tumor cells and are less likely to develop drug resistance, they have been suggested as appropriate therapeutic targets [52,53]. In arthritis, many of these same types of treatments with chemotherapeutic agents and targeted antibodies reduce inflammation and cytokine production, with the downstream effect of reducing joint destruction. However, despite their efficacy, there is the potential for serious side-effects, particularly since the antibodies directed at the cytokines/receptors are immunosuppressive, increasing the possibility of infection and even leukemias/lymphomas [51]. Therefore, in RA there is a need for therapies targeted at reducing joint destruction, and in cancer, for therapeutics that are directed at the stromal cells in the microenvironment.
Inhibiting MMP-1 gene expression
Directly inhibiting the effects of MMPs has been a therapeutic goal in the treatment of RA and cancer for a long time [2,3,49], since this approach has the potential to limit (or even prevent) invasion/metastasis and irreversible joint destruction. Many studies have focused on blocking enzymatic activity of MMPs with small molecule chemical inhibitors. Numerous compounds have been tested, including some phase I trials [2,3,54]. Unfortunately, these studies were fraught with difficulties due to lack of specificity and problems with drug delivery and toxicities.
Inhibition of MMP gene expression has been an alternative approach, and has focused on the retinoids, a class of vitamin A derivatives [2,4,49] that are ligands for a family of nuclear hormone receptors, the retinoic acid receptors (RARα, β, and γ). Retinoids inhibit MMP production primarily by affecting the levels of Activator Protein-1 (AP-1) proteins, i.e., members of the Fos and Jun families of transcription factors [55], but unfortunately, these compounds also lack specificity, affecting the expression of many genes and leading to undesirable side effects. Compounds targeting a related group of nuclear hormone receptors, the retinoid X receptors (RXRα, β, and γ) appear to be more selective and less toxic. These compounds, termed “rexinoids” [49] and bind as RXR:RXR homodimers to DNA in the promoter regions of responsive genes to reduce transcription. Among the new experimental rexinoids is LG268, which selectively inhibits MMP-1 and MMP-13 mRNA and protein [8].
Another group of nuclear hormone receptors are the peroxisome proliferator-activated receptors (PPAR) (α,δ and γ). Substantial data link specific ligands for PPARγ, the thiazolidinediones, with reduced MMP production, lower inflammation and therapeutic efficacy in cell cultures and in animal models of arthritis [57–61]. RXR is an obligate partner for PPARγ, resulting in RXR:PPARγ heterodimers that bind to specific PPARγ response elements (PPREs), also known as Direct Repeat-1 (DR-1 sites) in promoter DNA [9,49,59]. Rosiglitazone, one of the thiazolidinediones, inhibits IL-1β-induced MMP transcription in chondrocytic cells, and this inhibition is selective for MMP-1 and MMP-13 [9]. In keeping with reports describing cooperativity between RXR and PPARγ ligands [62–64], combined treatment with LG268 and rosiglitazone increases the inhibition of MMP-1 and MMP-13 [9], implying that doubly liganded RXR: PPARγ heterodimers increase efficacy.
In considering how the nuclear hormone receptors and their respective ligands exert their effects, it is noteworthy that the MMP-1 and MMP-13 promoters lack canonical DR-1 nuclear hormone response elements [59]. This suggests that LG268 and rosiglitazone maybe be reducing transcription through non-classical mechanisms to inhibit these genes. Since LG268 does not modulate IL-1β-induced activation of NF-κB or a major signal transduction cascade [8], the rexinoid may be acting through a mechanism other than suppression of a general inflammatory pathway. In contrast, rosiglitazone, and other thiazolidinediones, have anti-inflammatory effects that include suppression of signaling cascades and transcription factors such as AP-1 and NFκB [65]. Therefore, some of the additive/synergistic effects of LG268 and rosiglitazone may be mediated by different mechanisms.
It has been suggested that both RXR:PPARγ heterodimers (and possibly RXR:RXR homodimers) bind to a non-traditional DNA response element in the MMP-1 and MMP-13 promoters (Figure 4). This suggestion is supported by our work [8,9] and by the work of François et al. [59], both of which implicate the AP-1 site in the inhibitory mechanism of each drug. François et al. identified a degenerate DR-1 site [59] in the rabbit MMP-1 promoter, which overlaps the AP-1 site (Figure 4). Their model states that the rosiglitazone-liganded RXR:PPARγ dimer binds to this degenerate site and sterically prevents the binding of AP-1 proteins, thereby decreasing rabbit MMP-1 gene expression. A similar degenerate DR-1 site overlapping the proximal AP-1 site exists in both the human MMP-1 and MMP-13 promoters, thus providing a possible mechanism for some of the inhibitory effects of LG268 and rosiglitazone.
Figure 4. Proposed steric hindrance model for inhibiting MMP-1.
Overlapping degenerate DR-1 and AP-1 binding sites in promoter DNA. Numbers show distance from transcriptional start site in the rabbit MMP-1 gene [59]. In the model, IL-1β induces AP-1 proteins, which bind to their cognate element and increase transcription. However, if rosiglitzaone or LG268 is present, the liganded RXR:PPARγ dimer binds to the degenerate DR-1 site, and physically blocks AP-1 and transcriptional activation by IL-1β.
Chromatin remodeling is recognized as an essential component of gene regulation [66,67]. The acetylation of histones by histone-modifying enzymes and chromatin remodeling complexes mediate changes in transcription in response to inducers or repressors [66,67]. We found that changes in histone acetylation play an important role in up-regulating MMP-1 and MMP-13 gene expression [8,9]. IL-1β treatment leads to increases in the acetylation of histone subunit H4 at the proximal AP-1 site of MMP-1 and MMP13, while LG268 and rosiglitazone block this increase in acetylation [8,9]. Thus, these data show that the PPARγ ligand, rosiglitazone, and the experimental RXR ligand, LG268, inhibit MMP-1 (and MMP-13) gene expression in cytokine-stimulated cells. This inhibition appears to be mediated through multiple mechanisms operating at a transcriptional level.
Given the link between increased MMP-1 (and MMP-13) gene expression and RA, these classes of compounds could be useful as therapeutic agents. Since combined treatment with ligands for both RXR and PPARγ leads to additive inhibition of MMP-1 and MMP-13 production [9], perhaps these compounds could be used together to reduce or block joint destruction in arthritis, and in lower doses than single-drug treatment to thus minimize the risk of adverse side-effects. Importantly, clinical investigations with these classes of drugs are already underway in diseases such as diabetes and cancer. For example, rosiglitazone and another thiazolidinedione, pioglitazone, are currently in clinical use as insulin-sensitizing agents in diabetes [68]. In addition, the rexinoid, bexarotene (Targretin®), is a treatment for several cancers [69,70]. Finally, another study has reported the combined use of bexarotene and rosilgitazone in patients with refractory cancers [71]. There is, therefore, clinical precedent for the appropriate concentrations to be tested and for combinatorial use of these compounds in both cancer and RA, again illustrating some of the molecular similarities between RA and cancer.
G protein-coupled receptors
Our studies have documented links between expression of two G protein-coupled receptors, CXCR4 and PAR-1, and MMP-1 expression in RA and cancer. Although we have focused on activated stromal cells in the tumor microenvironment, our data are directly applicable to the activated stromal environment within RA joints. Other investigators have demonstrated the potential importance of therapies directed at blocking the signaling ability of these two G protein-coupled receptors.
The CXCR4/SDF-1 signaling axis is becoming a therapeutic target, as its roles in cancer and chronic inflammatory/autoimmune diseases are increasingly documented [72–74]. Most studies have concentrated on understanding the structure/function relationships between the receptor and putative antagonists, and new agents are emerging. For example, selective CXCR4 antagonists, T22 and T140, were initially developed as anti-HIV agents, but have been effective in blocking migration of several types of cancer cells [72], and a bio-stable form of T140 significantly repressed pulmonary metastases of breast cancer cells and melanoma cells in mice. Although cell culture experiments have established efficacy against the tumor cells, themselves, one wonders if some of the in vivo efficacy of these antagonists is due to their ability to modulate the behavior of activated stromal cells in the tumor microenvironment. An extension of this concept is the potential application of these inhibitors to the treatment of RA, since CXCR4 modulates the migration, proliferation and MMP-1 production by human synovial fibroblasts taken from patients with RA [28].
Likewise, the MMP-1/PAR-1 signaling axis is gaining interest as a therapeutic target. The initial studies on this topic have been in cancer models, where xenografts of advanced ovarian cancer delineated an MMP cascade, which culminated in MMP-1 activation of PAR-1 [75]. In this model, activated PAR-1 mediated angiogenesis, ascites formation and metastases, all of which were effectively inhibited by intraperitoneal administration of pepducins. These novel cell-permeable peptides, derived from the third intracellular loop of PAR-1 or PAR-4, disrupt signaling between the receptor and G proteins and inhibit activation of downstream pathways [75].
Several more classical PAR-1 antagonists have been described (reviewed in [76]). These act by interacting with the extracellular domain of PAR-1 and blocking its activation by proteolytic cleavage. Of these, SCH79797 is often used in laboratory experiments, both in vitro and in vivo, where it is anti-angiogenic. Other compounds, SCH53048 and E5555, are further along in development and clinical testing. SCH53048 is a reversible and orally administered compound derived from himbacine, which is found in the bark of the Australian magnolia tree. It is also anti-angiogenic, and in clinical trials, it has shown more than 80% inhibition of platelet aggregation within 60 minutes, with no apparent adverse effects. E5555 is currently undergoing phase 2 trials. These compounds may have a future as an important and effective anti-coagulants during some therapeutic procedures, and other application may well emerge [76].
Lastly, the emerging potential of RNA interference (RNAi) therapy cannot be ignored (reviewed in [77]). These short (~ 20 nt) of RNA are directed at specific sequences within the mRNA of choice, and upon binding, they efficiently silence expression of the target gene. Despite the difficulties associated with targeted delivery to the desired site and issues related to stability/clearance in vivo, these small molecules have exquisite specificity and are continuing to garner interest as therapeutics. Indeed, clinical trials with siRNAs against VEGF for age-related macular degeneration and against respiratory syncytial virus (RSV) infection are ongoing (reviewed in [77]). More immediately relevant to this discussion is the efficacy of stably expressed short hairpin RNAs (shRNA) against MMP-1 in breast cancer and melanoma in reducing primary growth and preventing metastasis [32,35]. Further, the successful in vivo delivery of PAR-1 siRNA in neutral liposomes in a melanoma model in nude mice [78] suggests that practical technical problems may be overcome [77].
Concluding Comments
As predicted by Sporn and Harris three decades ago, our knowledge of the precise molecular mechanisms that are driving non-malignant proliferative diseases (such as RA) and malignancies (such as breast cancer and melanoma) has grown enormously. This new information has re-enforced the original concept that these two seemingly disparate categories of diseases share many common properties, and the analogy between the reactive stroma in the tumor microenvironment and the pannus of RA has become solidified. We have focused on MMP-1, a collagenase once thought to function only as one of a few enzymes capable of initiating the degradation of the interstitial collagens. However, recent data document its role as a vital part of an autocrine/paracrine MMP-1/PAR-1 signaling axis, a function that amplifies its dual potential to remodel the extracellular matrix and to modulate cell behavior. In addition, the cooperativity between MMP-1 and the signaling of the chemokine receptor CXCR4/SDF-1 in modulating the behavior of activated fibroblasts again emphasizes the importance of MMP-1 as a pathological mediator in RA and cancer. Finally, also as predicted, new therapeutic agents are emerging. Interestingly, although they are more specific in the molecules that they target than past therapies, these targets are often shared between RA and cancer, again underscoring fundamental similarities between autoimmune disorders and some cancers. The similarity between autoimmune diseases and cancer have been noted for three decades and it is particularly noteworthy that Dr. Noel Rose has contributed throughout these same three decades and indeed even longer.
It is thus a pleasure to contribute to this special issue that recognizes Dr. Rose’s career, his work in serology, in epidemiology, and in multiple other areas of autoimmunity, including the development of the American Autoimmune Related Diseases Association [79–86]. This issue is part of the Journal’s series that recognizes the extraordinary contributions of scientists in the field of autoimmunity [87–89].
Acknowledgments
Supported by grants NIH-AR-25699 and NIH-CA-77267 to C.E.B., and by T32-CA-009658 to S.M.E., T32-AI-07363 to J.S.B., and T32-AR-07576 to A.C.S. and P.S.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sporn MB, Harris ED., Jr Proliferative diseases. Am J Med. 1981 Jun;70(6):1231–1235. doi: 10.1016/0002-9343(81)90832-9. [DOI] [PubMed] [Google Scholar]
- 2.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002 Mar;3(3):207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 3.Burrage PS, Brinckerhoff CE. Molecular targets in osteoarthritis: metalloproteinases and their inhibitors. Curr Drug Targets. 2007 Feb;8(2):293–303. doi: 10.2174/138945007779940098. [DOI] [PubMed] [Google Scholar]
- 4.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 5.Firestein GS. Etiology and pathogenesis of rheumatoid arthritis. Philadelphia: W.B. Saunders; 1997. [Google Scholar]
- 6.Martel-Pelletier J, Welsch DJ, Pelletier JP. Metalloproteases and inhibitors in arthritic diseases. Best Pract Res Clin Rheumatol. 2001 Dec;15(5):805–829. doi: 10.1053/berh.2001.0195. [DOI] [PubMed] [Google Scholar]
- 7.Fingleton B. Matrix metalloproteinases: roles in cancer and metastasis. Front Biosci. 2006;11:479–491. doi: 10.2741/1811. [DOI] [PubMed] [Google Scholar]
- 8.Burrage PS, Huntington JT, Sporn MB, Brinckerhoff CE. Regulation of matrix metalloproteinase gene expression by a retinoid X receptor-specific ligand. Arthritis Rheum. 2007 Mar;56(3):892–904. doi: 10.1002/art.22417. [DOI] [PubMed] [Google Scholar]
- 9.Burrage PS, Schmucker AC, Ren Y, Sporn MB, Brinckerhoff CE. Retinoid X receptor and peroxisome proliferator-activated receptor-gamma agonists cooperate to inhibit matrix metalloproteinase gene expression. Arthritis Res Ther. 2008;10(6):R139. doi: 10.1186/ar2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004 Nov 18;432(7015):332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002 Mar 29;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 12.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004 Nov;4(11):839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 13.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999 Aug 1;250(2):273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- 14.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 15.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48(5–6):509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 16.Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996 Jan;76(1):69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 17.Sappino AP, Skalli O, Jackson B, Schurch W, Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer. 1988 May 15;41(5):707–712. doi: 10.1002/ijc.2910410512. [DOI] [PubMed] [Google Scholar]
- 18.Holliday DL, Hughes S, Shaw JA, Walker RA, Jones JL. Intrinsic genetic characteristics determine tumor-modifying capacity of fibroblasts: matrix metalloproteinase-3 5A/5A genotype enhances breast cancer cell invasion. Breast Cancer Res. 2007;9(5):R67. doi: 10.1186/bcr1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008 Jun 1;68(11):4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005 May 6;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 21.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006 Dec;5(12):1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 22.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006 Aug;5(15):1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 23.Mueller L, Goumas FA, Affeldt M, Sandtner S, Gehling UM, Brilloff S, et al. Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment. Am J Pathol. 2007 Nov;171(5):1608–1618. doi: 10.2353/ajpath.2007.060661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato T, Sakai T, Noguchi Y, Takita M, Hirakawa S, Ito A. Tumor-stromal cell contact promotes invasion of human uterine cervical carcinoma cells by augmenting the expression and activation of stromal matrix metalloproteinases. Gynecol Oncol. 2004 Jan;92(1):47–56. doi: 10.1016/j.ygyno.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Eck S, Cote AL, Winkelman WD, Brinckerhoff CE. CXCR4 and MMP-1 are elevated in breast carcinoma-associated fibroblasts and in normal mammary fibroblasts exposed to factors secreted by breast cancer cells. Mol Can Res. 2009 doi: 10.1158/1541-7786.MCR-09-0015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jong JS, van Diest PJ, van der Valk P, Baak JP. Expression of growth factors, growth inhibiting factors, and their receptors in invasive breast cancer. I: An inventory in search of autocrine and paracrine loops. J Pathol. 1998 Jan;184(1):44–52. doi: 10.1002/(SICI)1096-9896(199801)184:1<44::AID-PATH984>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 27.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001 Mar 1;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Vicuna R, Gomez-Gaviro MV, Dominguez-Luis MJ, Pec MK, Gonzalez-Alvaro I, Alvaro-Gracia JM, et al. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum. 2004 Dec;50(12):3866–3877. doi: 10.1002/art.20615. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004 Jan;113(2):243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedl P, Wolf K. Proteolytic and non-proteolytic migration of tumour cells and leucocytes. Biochem Soc Symp. 2003;70:277–285. doi: 10.1042/bss0700277. [DOI] [PubMed] [Google Scholar]
- 31.Eck SM, Hoopes PJ, Petrella BL, Coon CI, Brinckerhoff CE. Matrix metalloproteinase-1 promotes breast cancer angiogenesis and osteolysis in a novel in vivo model. Breast Cancer Res Treat. 2008 Jul 3; doi: 10.1007/s10549-008-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyatt CA, Geoghegan JC, Brinckerhoff CE. Short hairpin RNA-mediated inhibition of matrix metalloproteinase-1 in MDA-231 cells: effects on matrix destruction and tumor growth. Cancer Res. 2005 Dec 1;65(23):11101–11108. doi: 10.1158/0008-5472.CAN-05-2446. [DOI] [PubMed] [Google Scholar]
- 33.Giambernardi TA, Grant GM, Taylor GP, Hay RJ, Maher VM, McCormick JJ, et al. Overview of matrix metalloproteinase expression in cultured human cells. Matrix Biol. 1998 Mar;16(8):483–496. doi: 10.1016/s0945-053x(98)90019-1. [DOI] [PubMed] [Google Scholar]
- 34.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005 Feb 11;120(3):303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Blackburn JS, Rhodes CH, Coon CI, Brinckerhoff CE. RNA interference inhibition of matrix metalloproteinase-1 prevents melanoma metastasis by reducing tumor collagenase activity and angiogenesis. Cancer Res. 2007 Nov 15;67(22):10849–10858. doi: 10.1158/0008-5472.CAN-07-1791. [DOI] [PubMed] [Google Scholar]
- 36.Blackburn JS, Brinckerhoff CE. Matrix metalloproteinase-1 and thrombin differentially activate gene expression in endothelial cells via PAR-1 and promote angiogenesis. Am J Pathol. 2008 Dec;173(6):1736–1746. doi: 10.2353/ajpath.2008.080512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004 Apr;84(2):579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 38.Rickles FR, Patierno S, Fernandez PM. Tissue factor, thrombin, and cancer. Chest. 2003 Sep;124(3 Suppl):58S–68S. doi: 10.1378/chest.124.3_suppl.58s. [DOI] [PubMed] [Google Scholar]
- 39.Tsopanoglou NE, Maragoudakis ME. Role of thrombin in angiogenesis and tumor progression. Semin Thromb Hemost. 2004 Feb;30(1):63–69. doi: 10.1055/s-2004-822971. [DOI] [PubMed] [Google Scholar]
- 40.Hollenberg MD, Compton SJ. International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol Rev. 2002 Jun;54(2):203–217. doi: 10.1124/pr.54.2.203. [DOI] [PubMed] [Google Scholar]
- 41.Maragoudakis ME, Tsopanoglou NE, Andriopoulou P. Mechanism of thrombin-induced angiogenesis. Biochem Soc Trans. 2002 Apr;30(2):173–177. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 42.Furuhashi I, Abe K, Sato T, Inoue H. Thrombin-stimulated proliferation of cultured human synovial fibroblasts through proteolytic activation of proteinase-activated receptor-1. J Pharmacol Sci. 2008 Sep;108(1):104–111. doi: 10.1254/jphs.08126fp. [DOI] [PubMed] [Google Scholar]
- 43.Hirano F, Kobayashi A, Hirano Y, Nomura Y, Fukawa E, Makino I. Thrombin-induced expression of RANTES mRNA through protease activated receptor-1 in human synovial fibroblasts. Ann Rheum Dis. 2002 Sep;61(9):834–837. doi: 10.1136/ard.61.9.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirilak Y, Pavlos NJ, Willers CR, Han R, Feng H, Xu J, et al. Fibrin sealant promotes migration and proliferation of human articular chondrocytes: possible involvement of thrombin and protease-activated receptors. Int J Mol Med. 2006 Apr;17(4):551–558. [PubMed] [Google Scholar]
- 45.Yang YH, Hall P, Little CB, Fosang AJ, Milenkovski G, Santos L, et al. Reduction of arthritis severity in protease-activated receptor-deficient mice. Arthritis Rheum. 2005 Apr;52(4):1325–1332. doi: 10.1002/art.21001. [DOI] [PubMed] [Google Scholar]
- 46.Goerge T, Barg A, Schnaeker EM, Poppelmann B, Shpacovitch V, Rattenholl A, et al. Tumor-derived matrix metalloproteinase-1 targets endothelial proteinase-activated receptor 1 promoting endothelial cell activation. Cancer Res. 2006 Aug 1;66(15):7766–7774. doi: 10.1158/0008-5472.CAN-05-3897. [DOI] [PubMed] [Google Scholar]
- 47.Nesi A, Fragai M. Substrate specificities of matrix metalloproteinase 1 in PAR-1 exodomain proteolysis. Chembiochem. 2007 Aug 13;8(12):1367–1369. doi: 10.1002/cbic.200700055. [DOI] [PubMed] [Google Scholar]
- 48.Blackburn JS, Lui I, Coon CI, Brinckerhoff CE. An autocrine Matrix Metalloproteinase/Protease Activated Receptor-1 signaling axis promotes melanoma invasion and metastasis. 2009 doi: 10.1038/onc.2009.272. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brinckerhoff CE, Sporn MB. Retinoids and rexinoids for the 21st century: a brave new world for arthritis. J Rheumatol. 2003 Feb;30(2):211–213. [PubMed] [Google Scholar]
- 50.Hansen IB, Ellingsen T, Hornung N, Poulsen JH, Lottenburger T, Stengaard-Pedersen K. Plasma level of CXC-chemokine CXCL12 is increased in rheumatoid arthritis and is independent of disease activity and methotrexate treatment. J Rheumatol. 2006 Sep;33(9):1754–1759. [PubMed] [Google Scholar]
- 51.Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med. 2000 Nov 30;343(22):1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 52.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006 Jul;116(7):1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smalley KS, Lioni M, Herlyn M. Targeting the stromal fibroblasts: a novel approach to melanoma therapy. Expert Rev Anticancer Ther. 2005 Dec;5(6):1069–1078. doi: 10.1586/14737140.5.6.1069. [DOI] [PubMed] [Google Scholar]
- 54.Milner JM, Cawston TE. Matrix metalloproteinase knockout studies and the potential use of matrix metalloproteinase inhibitors in the rheumatic diseases. Curr Drug Targets Inflamm Allergy. 2005 Jun;4(3):363–375. doi: 10.2174/1568010054022141. [DOI] [PubMed] [Google Scholar]
- 55.Schroen DJ, Brinckerhoff CE. Nuclear hormone receptors inhibit matrix metalloproteinase (MMP) gene expression through diverse mechanisms. Gene Expr. 1996;6(4):197–207. [PMC free article] [PubMed] [Google Scholar]
- 56.Beehler BC, Hei YJ, Chen S, Lupisella JA, Ostrowski J, Starrett JE, et al. Inhibition of disease progression by a novel retinoid antagonist in animal models of arthritis. J Rheumatol. 2003 Feb;30(2):355–363. [PubMed] [Google Scholar]
- 57.Cuzzocrea S, Mazzon E, Dugo L, Patel NS, Serraino I, Di Paola R, et al. Reduction in the evolution of murine type II collagen-induced arthritis by treatment with rosiglitazone, a ligand of the peroxisome proliferator-activated receptor gamma. Arthritis Rheum. 2003 Dec;48(12):3544–3556. doi: 10.1002/art.11351. [DOI] [PubMed] [Google Scholar]
- 58.Fahmi H, Pelletier JP, Di Battista JA, Cheung HS, Fernandes JC, Martel-Pelletier J. Peroxisome proliferator-activated receptor gamma activators inhibit MMP-1 production in human synovial fibroblasts likely by reducing the binding of the activator protein 1. Osteoarthritis Cartilage. 2002 Feb;10(2):100–108. doi: 10.1053/joca.2001.0485. [DOI] [PubMed] [Google Scholar]
- 59.Francois M, Richette P, Tsagris L, Raymondjean M, Fulchignoni-Lataud MC, Forest C, et al. Peroxisome proliferator-activated receptor-gamma down-regulates chondrocyte matrix metalloproteinase-1 via a novel composite element. J Biol Chem. 2004 Jul 2;279(27):28411–28418. doi: 10.1074/jbc.M312708200. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi T, Notoya K, Naito T, Unno S, Nakamura A, Martel-Pelletier J, et al. Pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, reduces the progression of experimental osteoarthritis in guinea pigs. Arthritis Rheum. 2005 Feb;52(2):479–487. doi: 10.1002/art.20792. [DOI] [PubMed] [Google Scholar]
- 61.Shiojiri T, Wada K, Nakajima A, Katayama K, Shibuya A, Kudo C, et al. PPAR gamma ligands inhibit nitrotyrosine formation and inflammatory mediator expressions in adjuvant-induced rheumatoid arthritis mice. Eur J Pharmacol. 2002 Jul 19;448(2–3):231–238. doi: 10.1016/s0014-2999(02)01946-5. [DOI] [PubMed] [Google Scholar]
- 62.A IJ, Tan NS, Gelman L, Kersten S, Seydoux J, Xu J, et al. In vivo activation of PPAR target genes by RXR homodimers. Embo J. 2004 May 19;23(10):2083–2091. doi: 10.1038/sj.emboj.7600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crowe DL, Chandraratna RA. A retinoid X receptor (RXR)-selective retinoid reveals that RXR-alpha is potentially a therapeutic target in breast cancer cell lines, and that it potentiates antiproliferative and apoptotic responses to peroxisome proliferator-activated receptor ligands. Breast Cancer Res. 2004;6(5):R546–R555. doi: 10.1186/bcr913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forman BM. The antidiabetic agent LG100754 sensitizes cells to low concentrations of peroxisome proliferator-activated receptor gamma ligands. J Biol Chem. 2002 Apr 12;277(15):12503–12506. doi: 10.1074/jbc.C200004200. [DOI] [PubMed] [Google Scholar]
- 65.Consoli A, Devangelio E. Thiazolidinediones and inflammation. Lupus. 2005;14(9):794–797. doi: 10.1191/0961203305lu2223oa. [DOI] [PubMed] [Google Scholar]
- 66.Henikoff S. Histone modifications: combinatorial complexity or cumulative simplicity? Proc Natl Acad Sci U S A. 2005 Apr 12;102(15):5308–5309. doi: 10.1073/pnas.0501853102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shia WJ, Pattenden SG, Workman JL. Histone H4 lysine 16 acetylation breaks the genome's silence. Genome Biol. 2006;7(5):217. doi: 10.1186/gb-2006-7-5-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004 Sep 9;351(11):1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 69.Dragnev KH, Petty WJ, Shah SJ, Lewis LD, Black CC, Memoli V, et al. A proof-of-principle clinical trial of bexarotene in patients with non-small cell lung cancer. Clin Cancer Res. 2007 Mar 15;13(6):1794–1800. doi: 10.1158/1078-0432.CCR-06-1836. [DOI] [PubMed] [Google Scholar]
- 70.Duvic M, Martin AG, Kim Y, Olsen E, Wood GS, Crowley CA, et al. Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Arch Dermatol. 2001 May;137(5):581–593. [PubMed] [Google Scholar]
- 71.Read WL, Baggstrom MQ, Fracasso PM, Govindan R. A phase I study of bexarotene and rosiglitazone in patients with refractory cancers. Chemotherapy. 2008;54(3):236–241. doi: 10.1159/000140468. [DOI] [PubMed] [Google Scholar]
- 72.Khan A, Greenman J, Archibald SJ. Small molecule CXCR4 chemokine receptor antagonists: developing drug candidates. Curr Med Chem. 2007;14(21):2257–2277. doi: 10.2174/092986707781696618. [DOI] [PubMed] [Google Scholar]
- 73.Taborga M, Corcoran KE, Fernandes N, Ramkissoon SH, Rameshwar P. G-coupled protein receptors and breast cancer progression: potential drug targets. Mini Rev Med Chem. 2007 Mar;7(3):245–251. doi: 10.2174/138955707780059826. [DOI] [PubMed] [Google Scholar]
- 74.Tamamura H, Fujii N. The therapeutic potential of CXCR4 antagonists in the treatment of HIV infection, cancer metastasis and rheumatoid arthritis. Expert Opin Ther Targets. 2005 Dec;9(6):1267–1282. doi: 10.1517/14728222.9.6.1267. [DOI] [PubMed] [Google Scholar]
- 75.Agarwal A, Covic L, Sevigny LM, Kaneider NC, Lazarides K, Azabdaftari G, et al. Targeting a metalloprotease-PAR1 signaling system with cell-penetrating pepducins inhibits angiogenesis, ascites, and progression of ovarian cancer. Mol Cancer Ther. 2008 Sep;7(9):2746–2757. doi: 10.1158/1535-7163.MCT-08-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smyth SS, Woulfe DS, Weitz JI, Gachet C, Conley PB, Goodman SG, et al. G-protein-coupled receptors as signaling targets for antiplatelet therapy. Arterioscler Thromb Vasc Biol. 2009 Apr;29(4):449–457. doi: 10.1161/ATVBAHA.108.176388. [DOI] [PubMed] [Google Scholar]
- 77.Gondi CS, Rao JS. Concepts in in vivo siRNA delivery for cancer therapy. J Cell Physiol. 2009 Apr 23; doi: 10.1002/jcp.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Villares GJ, Zigler M, Wang H, Melnikova VO, Wu H, Friedman R, et al. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Res. 2008 Nov 1;68(21):9078–9086. doi: 10.1158/0008-5472.CAN-08-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shoenfeld Y, Selmi C, Zimlichman E, Gershwin ME. The autoimmunologist: geoepidemiology, a new center of gravity, and prime time for autoimmunity. J Autoimmmun. 2008;31:325–330. doi: 10.1016/j.jaut.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 80.Mackay IR, Leskovsek NV, Rose NR. Cell damage and autoimmunity: a critical appraisal. J Autoimmun. 2008;30:5–11. doi: 10.1016/j.jaut.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eaton WW, Rose NR, Kalaydjian A, Pedersen MG, Mortensen PB. Epidemiology of autoimmune diseases in Denmark. J Autoimmun. 2007;29:1–9. doi: 10.1016/j.jaut.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rose NR. The year in immunology 2008. Preface. Ann N Y Acad Sci. 2008 Nov; doi: 10.1196/annals.1443.026. [DOI] [PubMed] [Google Scholar]
- 83.Cihakova D, Rose NR. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol. 2008;99:95–114. doi: 10.1016/S0065-2776(08)00604-4. [DOI] [PubMed] [Google Scholar]
- 84.Li HS, Ligons DL, Rose NR, Guler ML. Genetic differences in bone marrow-derived lymphoid lineages control susceptibility to experimental autoimmune myocarditis. J Immunol. 2008 Jun 1;180(11):7480–7484. doi: 10.4049/jimmunol.180.11.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burek CL, Rose NR. Autoimmune thyroiditis and ROS. Autoimmun Rev. 2008 Jul;7(7):530–537. doi: 10.1016/j.autrev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 86.Twarog FJ, Rose NR. Transfer of autoimmune thyroiditis of the rat with lymph node cells. J Immunol. 1970 Jun;104(6):1467–1475. [PubMed] [Google Scholar]
- 87.Whittingham S, Rowley MJ, Gershwin ME. A tribute to an outstanding immunologist - Ian Reay Mackay. J Autoimmun. 2008;31:197–200. doi: 10.1016/j.jaut.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 88.Gershwin ME. Bone marrow transplantation, refractory autoimmunity and the contributions of Susumu Ikehara. J Autoimmun. 2008;30:105–107. doi: 10.1016/j.jaut.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 89.Blank M, Gershwin ME. Autoimmunity: from the mosaic to the kaleidoscope. J Autoimmun. 2008;30:1–4. doi: 10.1016/j.jaut.2007.11.015. [DOI] [PubMed] [Google Scholar]