Summary
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
Keywords: Lupus susceptibility, Sex hormones, Interferon-inducible Ifi202, Estrogen receptor, Androgen receptor
Increased expression of interferon-inducible Ifi202 gene in certain strains of female mice is associated with susceptibility to systemic lupus erythematosus (SLE). Although, the development of SLE is known to have a strong sex bias, the molecular mechanisms remain unknown. Here we report that in vivo treatment of orchiectomized (NZB × NZW)F1 male mice with the female sex hormone 17β-estradiol (E2) significantly increased steady-state levels of Ifi202 mRNA in splenic cells whereas treatment with the male hormone dihydrotestosterone (DHT) decreased the levels. Moreover, increased expression of Ifi202 in B6.Nba2 B cells and reduced expression in T cells were associated with increased levels of estrogen receptor-α (ERα) and androgen receptor (AR), respectively. Furthermore, the steady-state levels of Ifi202 mRNA were higher in splenic cells from C57BL/6, B6.Nba2, NZB, and (NZB × NZW)F1 female mice as compared to males. E2-treatment of B cells and WT276 cells increased Ifi202 mRNA levels, whereas treatment with DHT decreased the levels. Interestingly, over-expression of ERα in WT276 cells increased the expression of Ifi202 and stimulated the activity of the 202-luc-reporter through the c-Jun/AP-1 DNA-binding site. Accordingly, ERα preferentially associated with the regulatory region of the Ifi202 gene in female B6.Nba2 B cells than males. Furthermore, Ifi202 mRNA levels were detectable in splenic cells of wild type (Esr1+/+), but not null (Esr1−/−), (NZB × NZW)F1 female mice. Together, our observations demonstrate that the female and male sex hormones differentially regulate the expression of Ifi202, thus, providing support for the role of Ifi202 in sex bias in SLE.
Introduction
Studies have demonstrated gender bias in the development of systemic lupus erythematosus (SLE), which occurs at a female-to-male ratio of 10:1 (1–4). The disease, which predominantly affects women of childbearing age, is characterized by the production of pathogenic autoantibodies to nuclear antigens and development of lupus nephritis (5–7). Studies in human SLE patients and in mouse models of SLE have provided evidence that SLE is a polygenic disease (5, 6, 8–11), which involves defects in a number of cell signaling pathways, resulting in increased survival of autoreactive cells (5, 12).
Clinical studies suggest that the gender bias in SLE is influenced by sex hormones, such as estrogen and androgen (2–4). It is well-documented that immune reactivity is more enhanced in female SLE patients than in males and lymphocytes and monocytes from female patients show higher antigen presenting activity (2, 3). In general female SLE patients exhibit higher levels of serum IgG than males and mount more robust humoral immune response. Therefore, it seems likely that enhanced activation of B cells in females contributes to lupus susceptibility. Moreover, female hormone estrogen is known to have immunostimulatory effects whereas male hormone androgen is known to have immunosuppressive effects (2–4).
Like SLE patients, in (NZB × NZW)F1 spontaneous mouse model of SLE disease, female mice develop the disease earlier and have shorter life spans than males (13, 14). Moreover, castrated male (NZB × NZW)F1 mice have earlier onset of lupus and shorter life span than their intact littermates (14). In addition, treatment of these mice with estrogen exacerbates disease activity and causes early mortality (13, 14). In contrast, administration of exogenous testosterone, when begun between 2 and 6 months of age, extends the lifespan of ovariectomized (NZB × NZW)F1 females (13, 14). These observations suggest that sex hormones, such as estrogen and testosterone, influence the pathogenesis of murine lupus.
Sex hormone estrogen classically functions by activating one of its two nuclear receptors, estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) (15–17). Although, both estrogen receptors are expressed in most immune cells, the ERα is shown to be the predominantly expressed (17). Several recent studies involving various mouse models of SLE have suggested a prominent role for ERα in the development of lupus disease (18–20). Interestingly, the ERα deficiency in (NZB × NZW)F1 female mice attenuated glomerulonephritis and increased survival of mice (20). Of note, the increased survival of ERα deficient female mice was associated with reduced development of anti-chromatin and anti-dsDNA antibodies as well as reduced serum levels of IFN-γ (20).
Binding of E2 to ERs results in activation of ERs and transcriptional activation of ER target genes (15–17). Many ER target genes contain a minimal estrogen responsive core element (ERE) sequence (GGTCANNNTGACC) in the 5′-regulatory or promoter region. The ERE sequence functions in an orientation and distance-independent manner, both of which are characteristics of an enhancer (21). Moreover, ER is also known to bind DNA through half ERE sites (GGTCAN) (15, 21). Because molecular mechanisms of the recruitment of ER to the promoter region of its target genes remain relatively complex, it remains an actively investigated research area. Importantly, proteins that are encoded by the ER target genes mediate many of the biological activities of female sex hormone estrogen (15–17).
Male sex hormone androgen signals via the intracellular androgen receptor (AR), a member of the super-family of nuclear hormone receptors (22). Androgen-dependent activation and nuclear translocation of the AR is followed by its binding to specific response elements in the promoter regions of target genes to modulate gene expression either positively or negatively (22). Interestingly, expression of AR mRNA has been reported in enriched populations of CD4+ T lymphocytes, CD8+ T lymphocytes, and macrophages (23). However, the enriched populations of B lymphocytes expressed only low levels of AR mRNA (23).
Interferon-inducible Ifi200-gene family includes structurally and functionally-related murine (for example, Ifi202a, Ifi202b, Ifi203, Ifi204, Ifi205 and Aim2) and human (for example, IFI16, MNDA, AIM2, and IFIX) genes (24–30). The Ifi202a and Ifi202b are highly homologous murine genes that encode p202a and p202b proteins, respectively (26, 27). These two proteins differ in only seven amino acids (out of 445 amino acids) (27). Because antibodies, which have been raised against the p202a protein (31), also detect p202b protein (26, 27), in this study, we have referred both p202a and p202b proteins as p202 protein.
Generation of B6.Nba2 congenic (congenic for NZB-derived Nba2 interval on C57BL/6 genetic background) mice and gene expression analyses identified Ifi202 (probably both Ifi202a and Ifi202b genes) gene as a lupus susceptibility gene (26, 32). Importantly, consistent with promoter polymorphisms contributing to differential expression of Ifi202a gene between C57BL/6 and NZB mice (26, 32, 33), increased steady-state levels of Ifi202a and Ifi202b mRNAs (as compared to C57BL/6 mice) are detectable in splenic cells from NZB and B6.Nba2 mice (33). Moreover, levels of Ifi202a mRNA are relatively higher than the Ifi202b mRNA (33). Interestingly, increased expression of p202 (probably both p202a and p202b proteins) protein in B6.Nba2 splenic B and T cells (more in B cells than in T cells) is associated with defects in apoptosis of B cells and increased susceptibility to develop lupus-like disease (26, 32). Furthermore, the B6.Nba2 congenic female mice produce higher levels of antinuclear autoantibodies than the age-matched male mice and (B6.Nba2 × NZW)F1 female mice develop severe proteinuria with much higher frequency (34). These observations prompted us to investigate whether sex hormones could regulate expression of the Ifi202 gene. Here, we report that female and male sex hormones differentially regulate the expression of Ifi202.
Materials and methods
Mice, orchiectomy, and sex hormone treatment
Spleens were isolated from wild-type (Esr1+/+) or null (Esr1−/−) (NZB × NZW)F1 female mice (20) (age ~10 weeks) that were housed in animal facilities of University of Nebraska Medical Center, Omaha, NE. Age-matched (6–8 weeks old) male and female non-autoimmune (C57BL/6J) and pre-autoimmune (B6.Nba2, NZB, and (NZB × NZW)F1) mice were purchased from The Jackson Laboratory (Bar Harbor, Main) and housed in the animal facilities of the University of Cincinnati. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the institution where animals were housed.
Male (NZB × NZW)F1 mice were orchiectomized at 3 months of age as described (35). After orchiectomy, pellets (Innovative Research of America, Sarasota, FL, USA) releasing E2, DHT, or placebo (P) for up to 3 weeks were inserted subcutaneously with a 10-gauge needle. Serum was collected on day 7, 8 or 9 and then analyzed by the Endocrine Laboratory at Colorado State University (Fort Collins, CO, USA) for luteinizing hormone (LH), estradiol and testosterone by radioimmunoassay as described (35). Orchiectomized male mice that were treated with E2 pellets exhibited serum E2 levels of 162 pg/ml, while levels in placebo-treated mice were not detectable (data not shown). Intact and orchiectomized mice treated with DHT had similarly low LH levels (less than 1 ng/ml), while orchiectomized mice treated with placebo had over 20 ng/ml of LH (data not shown). Orchiectomized male mice treated with placebo demonstrated testosterone levels less than 2 ng/ml, while intact (NZB × NZW)F1 male mice exhibited 12 ng/ml of testosterone (35).
Splenocyte isolation, purification of B or T cells, cell culture, and treatments
Total single cell splenocytes were prepared from age-matched male or female mice as described previously (36). After lysis of red blood cells, splenocytes were re-suspended in RPMI 1640 medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin/glutamate, and 1x minimal essential medium, non-essential amino acids/sodium pyruvate. Unless otherwise indicated, splenic cells from two or more age-matched male or female mice were pooled to purify B or T cells and to prepare total RNA or protein extracts. B or T cells were purified from splenic cells using magnetic beads (purification kit purchased from Miltenayi Biotech, Auburn, CA) allowing the positive selection of either B or T cells. The purified (>90–95% pure) T- and B-cells were used immediately for further experiments.
Estrogen-responsive mouse breast cancer cell line WT276(37) was generously provided by Dr. JoEllen Welsh, University of Notre Dame, Notre Dame, IN. Cells were maintained in DMEM medium supplemented with 10% fetal bovine serum and 1x antibiotic-antimycotic solution (Invitrogen, Carlsbad, CA). For treatment of WT276 cells with E2 or DHT, cells were cultured in phenol red-free RPMI 1640 medium (Invitrogen, Carlsbad, CA) and the medium was supplemented with 10% charcoal-stripped fetal bovine serum (Invitrogen, Carlsbad, CA). Cells were treated in vitro with either DHT (23) or E2 (38) at the concentration used previously.
Plasmids and expression vectors
Dr. Pierre Chambon (CNRS, France) generously provided ERα expression plasmid that allowed expression of ERα (39). The 202-luc-reporter (36) and the mutant 202AP-1mutCS1-luc reporter (40) plasmids have been described.
Reporter assays
For reporter assays, sub-confluent cultures of WT276 cells(in 6-well plates) were transfected with the 202-luc (2.5 μg) or the 202AP-1mutCS1-luc (with mutated AP-1CS1 site; see 40) reporter plasmid along with pRL-TK reporter plasmid (0.5 μg), using calcium phosphate transfection kit (Invitrogen, Carlsbad, CA), as suggested by the supplier. When indicated, cells were either treated with ethanol (vehicle) or the indicated concentration of E2 or DHT for 16–24 h. Unless otherwise indicated, cells were harvested between 40 and 45 h after transfections. Cells were lysed, and the firefly and Renilla dual luciferase activities were determined as described previously (36). Student’s t-test for paired samples was used to determine statistical significance of the reporter activity data. Differences were considered statistically significant at P ≤ 0.05.
Isolation of RNA from splenocytes and RT-PCR
Splenocytes (5–8 × 106 cells) were used to isolate total RNA using TRIzol (Invitrogen, Carlsbad, CA). Total RNA was digested with DNase I (to remove any contaminating genomic DNA in the preparation), and 0.5–2 μg of RNA was used for RT-PCR reaction using a pair of the Ifi202 primer (forward: 5′-ggtcatctaccaactcagaat-3′; reverse primer: 5′-ctctaggatg ccactgctgttg-3′). For RT-PCR, we used the Superscript one-step RT-PCR system from Invitrogen. Primers for Esr1 gene (forward: 5′-aattctgacaatcgacgccag- 3′; backward: 5′-ctctaggatgccactgctgttg-3′).
Quantitative real-time TaqMan PCR technology (7300 Real-Time PCR System, Applied Biosystems, Foster City, CA, USA) and commercially available real-time TaqMan gene expression assays were used to compare expression of genes between male and female mice. The PCR cycling program consisted of denaturation at 95°C for 10 min, 40 cycles at 95°C for 15 seconds, followed by annealing and elongation at 60°C for 1 min. The TaqMan assays for Ifi202 (Assay Id# Mm0304 8198_m1; the assay allowing the detection of both Ifi202a and Ifi202b mRNA levels), Esr1 (Assay Id# Mm00433149_m1), and the endogenous control β2-microglobulin (Assay Id# Mm00437762_ m1) were purchased from Applied Biosystems (Foster City, CA) and used as suggested by the supplier.
Chromatin immunoprecipitation assays
Splenic B cells were purified using magnetic beads and the purified splenic B cells (~2–4 × 106) were processed for chromatin immunoprecipitation assays (ChIPs) using ChampionChIP™ One-Day Kit (SABioscience, Frederick, MD) as suggested by supplier. In brief, cell lysates containing equal amounts of protein were immunoprecipitated with either an isotope antibody or anti-ERα antibodies (sc-542X; Santa Cruz Biotechnology, Santa Cruz, CA). The immunoprecipitates were collected using protein-A beads. Immune complexes were eluted from beads, proteins were digested, and DNA was collected. The isolated DNA was purified, precipitated, washed, and dissolved in water. Semi-quantitative PCR was performed with DNA samples for 28 cycles. PCR products were resolved in an agarose gel and visualized. The PCR primers that were used have been described previously (41). Quantitative PCR was performed using commercially available primer set (GPM041367, from SABioscience, Frederick, MD) corresponding to the genomic 5′-regulatory region of the Ifi202 gene and real-time PCR conditions as described above.
Immunoblotting
Total splenocytes, WT276, or NIH3T3 cells were collected in PBS and re-suspended in a modified radio-immune precipitation assay (RIPA) lysis buffer (50 mM Tris-HCl, pH 8.0, 250 mM NaCl, 1% NonidetP-40, 0.5% sodium deoxycholate, 0.1% SDS), supplemented with1X protease inhibitor (Roche Diagnostics, Mannheim, Germany) and incubated at 4° C for 30 min. Cell lysates were sonicated briefly before centrifugation at 14,000 rpm in a microcentrifuge for 10 min at 4 °C. The supernatants were collected, and the protein concentration was measured by Bio-Rad protein assay kit. Equal amounts of protein were processed for immunoblotting. Antiserum to p202 protein has been described previously (31). The p202 antiserum detects both p202a and p202b proteins in immunoblotting (27, 31). Antibodies to detect mouse ERα (sc-542; MC-20), AR (sc-816) and β2-mcroglobulin (sc-13565) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to detect β-actin (# 4967) were purchased from Cell Signaling Technology (Danvers, MA).
Statistical analyses
Data are presented as mean ± SEM. A p value <0.05 was considered statistically significant. These methods were performed using GraphPad Prizm 5.02 software for Windows (GraphPad Software).
Results
In vivo treatment of orchiectomized male mice with estrogen increased steady-state levels of Ifi202a mRNA whereas treatment with dihydrotestosterone reduced the mRNA levels
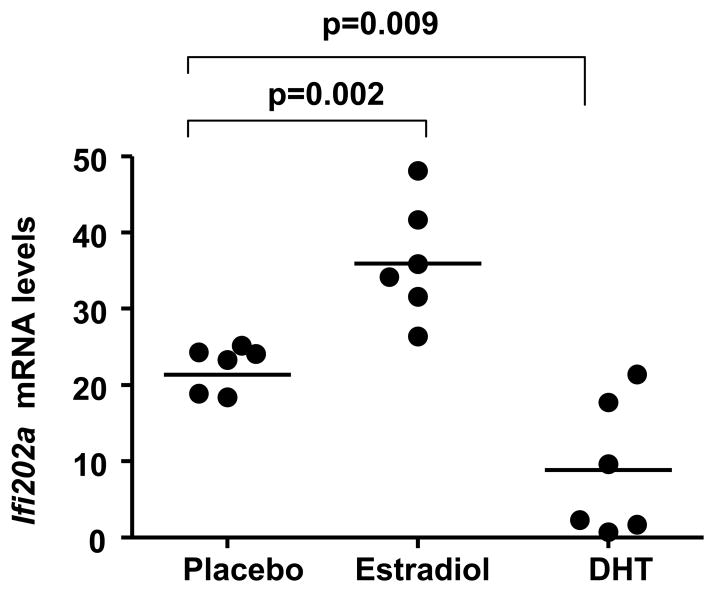
To investigate role of sex hormones in the regulation of Ifi202 gene, we chose to compare expression levels of Ifi202a mRNA (because splenic cells express both Ifi202a and Ifi202b genes and steady-state levels Ifi202a mRNA are more than Ifi202b; ref. 33) in (NZB × NZW)F1 male mice (age 12 weeks) that were orchiectomized and reconstituted with slow-releasing pellets releasing E2, DHT, or placebo. After three weeks of the reconstitution, splenic cells were analyzed for steady-state levels of Ifi202a mRNA by real-time PCR. As shown in Fig. 1, in vivo treatment of male mice with E2 releasing pellet, under the experimental conditions used (35), increased levels of Ifi202a mRNA measurably in the majority of E2 treated mice as compared to control mice. Interestingly, treatment of mice with DHT decreased the Ifi202 mRNA levels to a measurable extent in the majority of mice as compared to the control mice. These observations are consistent with the possibility that increased in vivo levels of female sex hormone E2 in orchiectomized (NZB × NZW)F1 male mice contribute to increased steady-state levels of Ifi202a mRNA. Moreover, our observations also indicated that increased levels of male sex hormone androgen in mice contribute to decreased steady-state levels of Ifi202a mRNA.
Figure 1. Ifi202 mRNA levels are regulated by sex hormones in splenocytes.
Male (NZB × NZW)F1 mice (age 3 months) were orchiectomized and reconstituted with slow-releasing pellets releasing E2 (n = 6), DHT (n = 6), or placebo (n = 6). Three weeks later, splenic total RNA was subjected to quantitative real-time PCR using primers specific for the Ifi202a gene. The ratio of Ifi202a mRNA to β2-microglobulin mRNA was calculated in units (one unit being the ratio of Ifi202a mRNA to β2-microglobulin mRNA in (NZB × NZW)F1 splenocytes). Units are indicated for an individual mouse in each group of mice.
Increased expression of Ifi202 in splenic B cells in female mice is associated with increased levels of ERα and reduced levels of AR
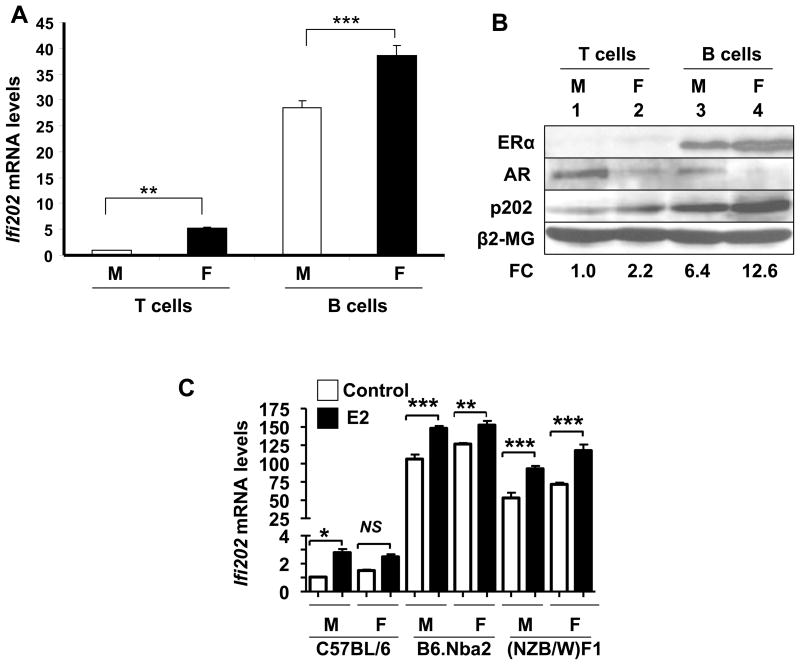
Our earlier studies had revealed that splenic B cells from pre-autoimmune (4 month-old) B6.Nba2 female mice express higher levels of Ifi202 mRNA as compared to T cells (32). Therefore, our above observations (Fig. 1) that in vivo treatment of orchiectomized (NZB × NZW)F1 male mice with estrogen increased Ifi202a mRNA levels whereas treatment with dihydrotestosterone reduced the mRNA levels prompted us to compare steady-state levels of Ifi202 mRNA in splenic B and T cells from male or female pre-autoimmune (age ~10 weeks) B6.Nba2 mice (32). We noted that steady-state levels of Ifi202 mRNA were significantly (~7-fold) higher in splenic B cells than T cells in the female mice (Fig. 2A). Interestingly, we found that levels of Ifi202 mRNA in female B and T cells were consistently higher than the age-matched male mice (Fig. 2A). Because sex hormones, such as E2 and DHT, regulate gene expression through binding to their respective receptors (15, 22), we also compared expression levels of ERα, AR, and p202 proteins in B and T cells from male and female B6.Nba2 mice. As shown in Fig. 2B, increased levels of p202 protein in splenic B cells from B6.Nba2 female mice as compared to age-matched male mice (compare lane 4 with 3) were associated with increased levels of ERα protein and reduced levels of AR protein. In contrast, reduced levels of p202 protein in T cells from male mice as compared to age-matched female mice (compare lane 1 with 2) were associated with increased levels of AR and reduced levels of ERα protein. Furthermore, consistent with our above observations (Fig. 2A and B), basal steady-state levels of Ifi202 mRNA were relatively high in B cells from C57BL/6 or (NZB × NZW)F1 female mice than the age-matched male mice and treatment of B cells with estrogen (10 nM for 24 h) resulted in further increases in the steady state levels of the mRNA (Fig. 2C). Together, these observations revealed that increased expression of the Ifi202 in splenic B cells from B6.Nba2 female mice is associated with increased levels of ERα and reduced levels of AR. Additionally, our observations revealed that the basal steady-state levels of the Ifi202 mRNA are relatively higher in B cells from non-autoimmune (C57BL/6) and pre-autoimmune (B6.Nba2 and (NZB × NZW)F1) female mice than age-matched males and the treatment with E2 can increase the mRNA levels further.
Figure 2.
Increased steady-state levels of Ifi202 mRNA and protein in splenic B cells from B6.Nba2 female mice are associated with increased levels of ERα and reduced levels of AR. Total splenocytes isolated from pre-autoimmune (age ~9 weeks) male or female mice (spleen cells pooled from three age-matched male or female mice) were subjected to purification of B or T cells using a kit (from Miltenayi Biotech) that allowed positive selection of either B or T cells. Total RNA and proteins were prepared from the purified (cells ~90% pure) T or B cells. (a) Steady state levels of Ifi202 mRNA were analyzed by quantitative TaqMan real-time PCR. The ratio of Ifi202 mRNA to β2-microglobulin mRNA was calculated in units (one unit being the ratio of Ifi202 mRNA to β2-microglobulin mRNA in B6.Nba2 splenocytes). The relative levels of Ifi202 mRNA in T cells from male mice are indicated as 1. A representative experiment is shown. Results are mean values of triplicate experiments and error bars represent standard deviation (** p <0.005; *** p <0.0005). (b) Total cell extracts prepared from T (lanes 1 and 2) or B (lanes 3 and 4) cells were analyzed by immunoblotting using antibodies specific to the indicated proteins. A representative experiment is shown. M, male; F, female. The numbers below the Figure indicate relative fold change (FC) in the p202 protein levels as compared to levels detected in T cells (indicated as 1.0) from male mice. (c) Steady-state levels of Ifi202 mRNA were analyzed in splenic B cells (isolated from male or female mice) without any or after E2 (10 nM, 24 h) treatment by quantitative TaqMan real-time PCR. The ratio of Ifi202 mRNA to β2-microglobulin mRNA was calculated in units (one unit being the ratio of Ifi202 mRNA to β2-microglobulin mRNA). The relative levels of Ifi202 mRNA in B cells from C57BL/6 male mice are indicated as 1. A representative experiment is shown. Results are mean values of triplicate experiments and error bars represent standard deviation (*p<0.03, ***p<0.005, NS, not significant).
Sex-dependent regulation of Ifi202 expression
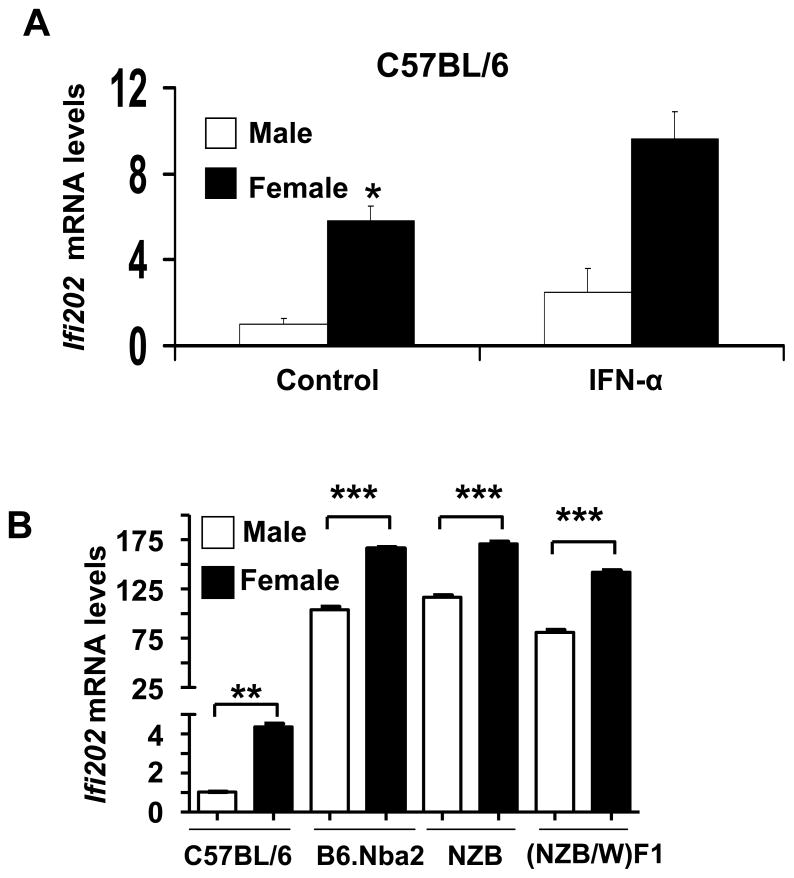
Our earlier studies had revealed that increased expression of Ifi202 in pre-autoimmune B6.Nba2 female mice (as compared to non-autoimmune C57BL/6 mice) is associated with increased lupus susceptibility (26, 32). Because steady-state levels of Ifi202 mRNA are very low (as compared to age-matched B6.Nba2 mice), but detectable in C57BL/6 mice (33), we decided to compare the steady-state levels of Ifi202 mRNA between male and female C57BL/6 mice. We noted that basal levels of Ifi202 mRNA were detectable in splenic cells from C57BL/6 male and female mice (age ~10 weeks) and interferon-treatment of cells increased the levels further (Fig. 3A). Interestingly, the basal levels of Ifi202 mRNA were about 5-fold higher in the female mice as compared to age-matched C57BL/6 males. This observation indicated that steady-state levels of Ifi202 mRNA are regulated in sex-dependent manner in C57BL/6 splenic cells.
Figure 3. Sex-dependent regulation of Ifi202 expression.
(a) Splenic cells were prepared from age-matched C57BL/6J male or female mice (age ~10 weeks; cells pooled from two age-matched male or female mice) and cells were either left untreated or treated with IFN-α (1,000 u/ml, for 14 h). Total RNA was isolated from control and IFN-treated cells and steady state levels of Ifi202 mRNA were analyzed by quantitative TaqMan real-time PCR. The ratio of Ifi202 mRNA to β2-microglobulin mRNA was calculated in units (one unit being the ratio of Ifi202 mRNA to β2-microglobulin mRNA in C57BL/6J splenocytes). The relative levels of Ifi202 mRNA in the male mice are indicated as 1. A representative experiment is shown. Results are mean values of triplicate experiments and error bars represent standard deviation (*p <0.05). (b) Splenic cells were prepared from age-matched non-autoimmune (C57BL/6J) or pre-autoimmune (B6Nba2, NZB and NZBWF1) male or female mice (age ~10 weeks; cells pooled from two age-matched male or female mice). Total RNA was isolated and steady state levels of the Ifi202 mRNA were analyzed by quantitative TaqMan real-time PCR. The ratio of Ifi202 mRNA to β2-microglobulin mRNA was calculated in units (one unit being the ratio of Ifi202 mRNA to β2-microglobulin mRNA in C57BL/6J). A representative experiment is shown. Results are mean values of triplicate experiments and error bars represent standard deviation (**p<0.005, ***p<0.0005).
Encouraged by the above observations, we also compared the steady-state levels of Ifi202 mRNA between young male and female lupus-prone mice (B6.Nba2, NZB, and (NZB/W)F1). As shown in Fig. 3B, basal steady-sate levels of the Ifi202 mRNA were consistently higher in splenic cells from the females than the age-matched males. Together, these observations suggested that the steady-state levels of Ifi202 mRNA in splenic cells are regulated in sex-dependent manner.
Treatment of WT276 cells with female or male sex hormone regulates Ifi202 expression
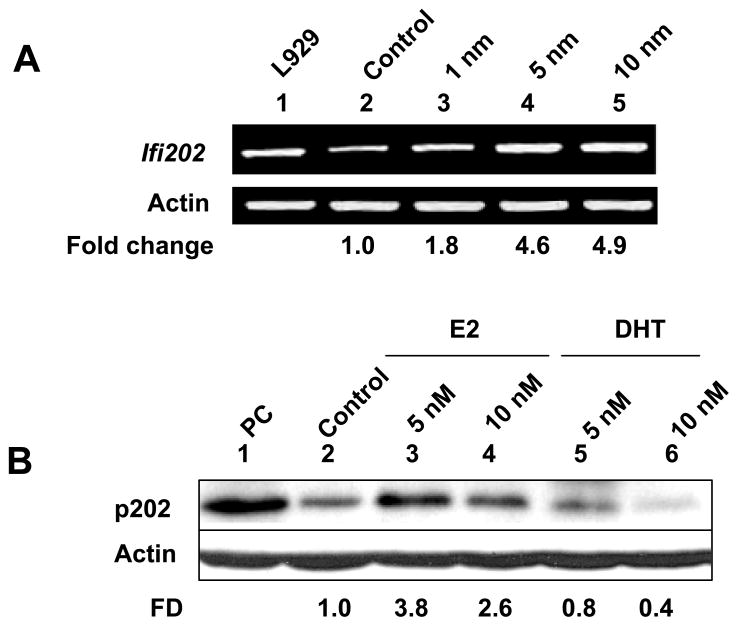
The mouse mammary tumor cell line WT276 has been reported to be estrogen-responsive (37). Therefore, to identify molecular mechanisms by which E2 treatment up-regulates expression of Ifi202 gene, we explored whether treatment of WT276 cells with sex hormones could regulate the expression of Ifi202 gene. For this purpose, we treated cells with increasing concentrations (1, 5 or 10 nM) of female sex hormone E2 (these concentrations were chosen based on an earlier studies, ref. 38) for 16 h. As shown in Fig. 4A, the treatment resulted in increases in steady-state levels of Ifi202 mRNA as determined by semi-quantitative RT-PCR. Moreover, consistent with E2-mediated up-regulation of Ifi202 expression by ERα, treatment of cells with tamoxifen (100 nM), a selective estrogen receptor modulator (42), which resulted in increases in ERα mRNA and protein levels (data not shown), abrogated the E2-mediated increases in Ifi202 mRNA levels (data not shown). Consistent with the above observations, we also noted increases in p202 protein levels after treatment of cells with E2 (Fig. 4B). Intrestingly, treatment of cells with 5 nM concentration of E2 resulted in increases in p202 protein levels (compare lane 3 with 2). However, treatment of cells with 10 nM E2 resulted in moderate decreases in p202 protein levels (compare lane 4 with 3). Furthermore, treatment of WT276 cells with the male sex hormone DHT decreased basal levels of p202 protein in a dose-dependent manner. Together, the above observations demonstrated that in vitro treatment of WT276 cells with female sex hormone E2 or male sex hormone DHT differentially regulated the levels of the p202 protein.
Figure 4. Treatment of WT276 cells with sex hormones regulates the Ifi202 expression.
(a) Sub-confluent cultures of WT276 cells were either treated with ethanol (vehicle) or increasing concentration of estrogen (1, 5, or 10 nM) for 16 h in phenol red-free medium. Total RNA isolated from cells was analyzed by semi-quantitative PCR for steady-state levels of Ifi202 or actin mRNA levels. (b) Sub-confluent cultures of WT276 cells were treated with ethanol (vehicle; lane 2), 5 (lane 3) or 10 nM (lane 4) concentration of E2, or 5 (lane 5) or 10 nM (lane 6) concentration of DHT for 16 h in phenol red-free medium. Total cell lysates from control and treated cells were analyzed by immunoblotting using antibodies specific to the indicated proteins. We also included a positive control for p202 protein in our experiment (lane 1). The numbers below the Figure indicate the fold difference (FD) in p202 protein levels as compared to control (lane 2).
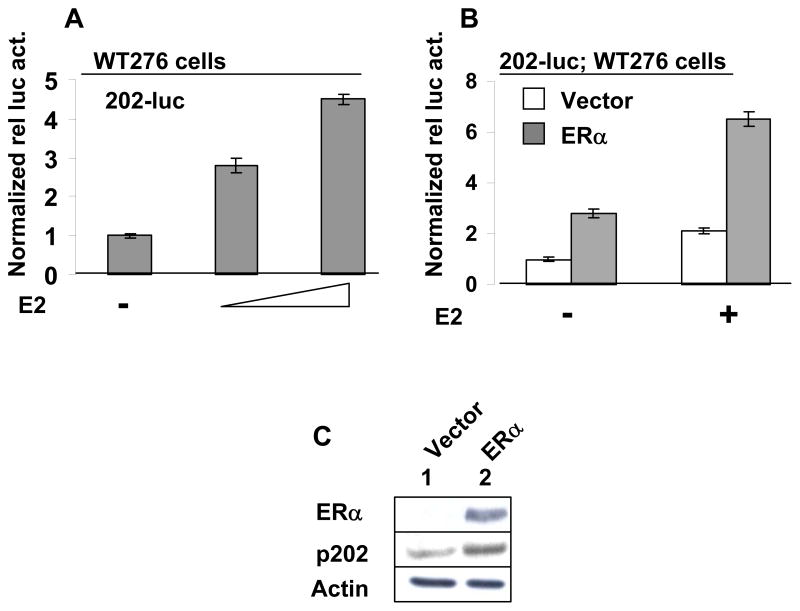
Estrogen through ERα up-regulates expression of the Ifi202 gene
To further investigate whether estrogen treatment of cells activates transcription of the Ifi202 gene through ERα, we first transfected estrogen-responsive WT276 cells with 202-luc-reporter plasmid and treated cells with increasing concentration of E2. As shown in Fig. 5A, treatment of cells with E2 stimulated the activity of the 202-luc-reporter in a dose-dependent manner. Next, we transfected WT276 cells with 202-luc-reporter along with either an empty vector or a plasmid encoding the ERα receptor. After transfections, cells were either treated with ethanol alone (vehicle) or 10 nM of E2. As shown in Fig. 5B, transfection of cells with the plasmid encoding ERα protein stimulated the activity of the 202-luc-reporter. Interestingly, transfection of cells with the plasmid encoding the ERα protein and subsequent treatment of the transfected cells with E2 strongly stimulated the activity of the reporter. To further test whether ERα regulates expression of the Ifi202 gene, we transfected NIH 3T3 mouse fibroblasts (we chose these cells because basal levels of p202 protein are detectable and these cells are not known to express ERα) with either an empty vector or the plasmid encoding ERα (cells treated with 5 nM E2 in phenol red-free medium) and analyzed the expression of p202 protein. As shown in Fig. 5C, ectopic expression of ERα protein in NIH 3T3 cells resulted in increases in p202 protein levels. Together, these observations suggested that activation of ERα by E2 in WT276 and NIH 3T3 cells up-regulates the expression of the Ifi202 gene.
Figure 5.
Treatment of estrogen-responsive WT276 cells with E2 or overexpression of ERα stimulated the activity of 202-luc-reporter. (a) Sub-confluent cultures of WT276 cells in a 6-well plate were transfected with 202-luc-reporter plasmid (2.5 μg) along with pRL-TK (0.5 μg) plasmid using calcium phosphate precipitation method. 24 h after transfections, cells were either treated with ethanol (vehicle) or E2 (5 or 10 nM). 40–45 h after transfections, cells were processed for dual luciferase activity. (b) Sub-confluent cultures of WT276 cells in a 6-well plate were transfected with 202-luc-reporter plasmid (2.5 μg), pRL-TK (0.5 μg) plasmid along with either empty vector or a plasmid encoding ERα using calcium phosphate precipitation method. 24 h after transfections, cells were either treated with ethanol (vehicle) or E2 (5 or 10 nM). 40–45 h after transfections, cells were processed for dual luciferase activity. (c) Sub-confluent cultures of NIH 3T3 cells in a 60 mm plate were transfected either empty vector or a plasmid encoding ERα using calcium phosphate precipitation method. 40–45 h after transfections, cells were processed for immunoblotting using antibodies specific to the indicated proteins.
Transcriptional activation of Ifi202 by ERα
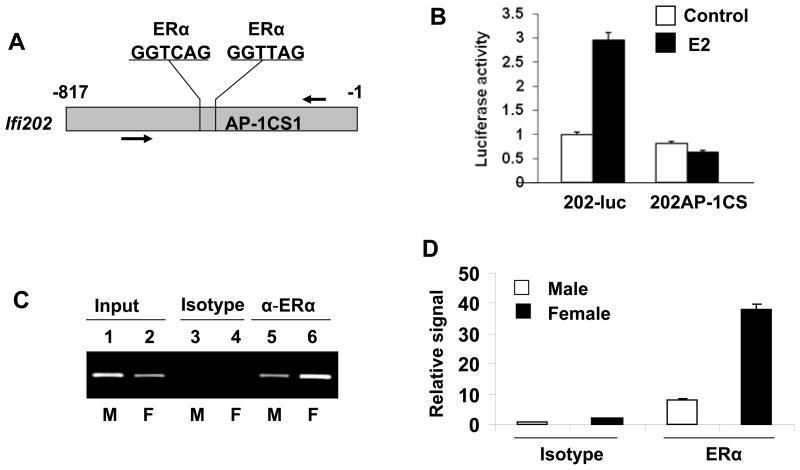
The 5′-regulatory region (~800 bp) of Ifi202 gene contains at least two potential ERE half-sites (Fig. 6A) and one of the sites is located next to an AP-1 DNA-binding site (AP-1CS1) that can bind to c-Jun/AP-1 (40) in gel-mobility shift assays. Molecular mechanisms through which the ERα regulates the transcription of its target genes through the half ERE sites are relatively complex (15, 16) and known to involve collaborations with other transcription factors, such as c-Jun/AP-1 (15, 16). Therefore, to investigate the role of ERα in the regulation of Ifi202 expression, we compared the activity of the wild type (202-luc) and the mutant (202-AP-1CS1-luc in which the AP-1CS1 site is mutated) reporters without or after E2 treatment of WT276 cells. As shown in Fig. 6B, the activity of the wild type reporter was stimulated ~2.5-fold by the treatment of cells with E2. However, the mutation in the AP-1CS1 site in the 5′-regulatory region of the Ifi202 gene abrogated the stimulation of the activity of the reporter after E2 treatment. This suggested that E2 treatment of WT276 cells stimulates transcription of the Ifi202 gene through the AP-1CS1 site. To further examine the role of ERα in the transcriptional activation of the Ifi202 gene by E2, we also compared in vivo association of ERα with the 5′-regulatory region of the Ifi202 gene in splenic B6.Nba2 B cells between female and age-matched males by chromatin immunoprecipitation assays (ChIPs). As shown in Fig. 6C, some binding of ERα to Ifi202 regulatory region was detected in male B cells (lane 5). Interestingly, relatively more ERα bound to the Ifi202 regulatory region in female B cells (compare lane 6 with 5). Moreover, a quantitative real-time pPCR revealed (Fig. 6D) that there was about 4-fold more binding of ERα to the Ifi202 regulatory region in the female B cells than males. These observations indicated that relatively higher levels of ERα associated with the regulatory region of the Ifi202 gene in female B6.Nba2 B cells than males.
Figure 6. ERα associates with the potential DNA-binding site in the 5′-regulatory region of Ifi202 gene in chromatin immunoprecipitation assays.
(a) Schematic representation of the 5′-regulatory region of the Ifi202 gene containing two potential ERα half DNA-binding sites, which are next to an AP-1 DNA-binding site. Relative location of PCR primers that were used to amplify the immunoprecipitated chromatin.
(b) Sub-confluent cultures of WT276 cells were either transfected with 202-luc reporter or 202AP1CS1-luc reporter plasmid along with pRL-TK plasmid. 24 h after transfections, cells were left untreated or treated with E2 (10 nM). 40 h after transfections, cells were processed for dual luciferase activity as described in methods. Normalized reporter activity is indicated.
(c) Soluble chromatin was prepared from B6.Nba2 male (lanes 1, 3, and 5) or female (lanes 2, 4, and 6) B cells. Chromatin was incubated with antibodies to ERα (lanes 5 and 6) or, as a negative control, with isotype IgG1 antibodies (lanes 3 and 4). DNA was extracted from immunoprecipitates and PCR amplified (30 cycles) using a pair of primers that covered ERα DNA-binding site in the 5′-regulatory region of the Ifi202 gene. As a positive control for PCR, we also amplified the input chromatin DNA from male (lane 1) and female (lane 2) B cells.
(d) Soluble DNA precipitated in (b) was also subjected to quantitative real-time PCR using PCR primers flanking the 5′-regulatory region of the Ifi202 gene as described in methods.
Basal steady-state levels of Ifi202 mRNA are not detectable in ERα-deficient mice
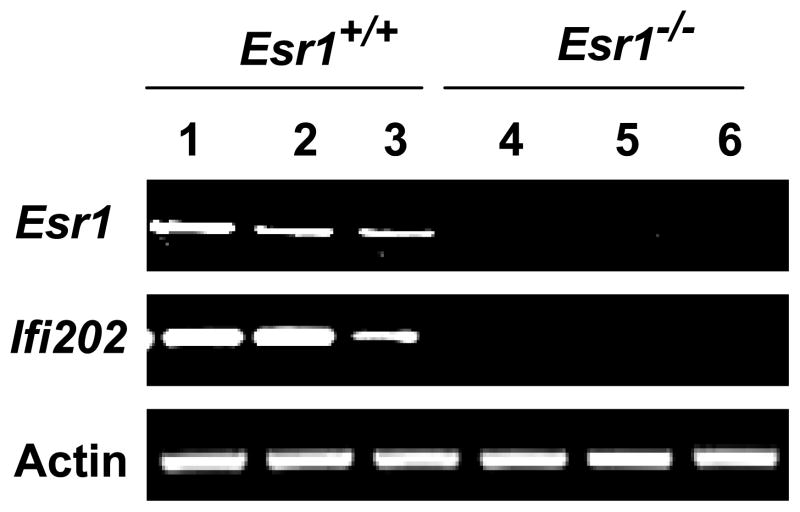
Because our above observations demonstrated that activation of ERα by E2 up-regulates the expression of the Ifi202 gene in WT276 cells, we compared steady-state levels of Ifi202 mRNA between ERα expressing wild type (Esr1+/+) and ERα-deficient (Esr1−/−) age-matched (NZB × NZW)F1 female mice (20). As shown in Fig. 7, we found that basal levels of Ifi202 mRNA were readily detectable in splenic cells from the wild-type mice but not the null mice. These observations indicated that the basal steady-state levels of the Ifi202 mRNA in (NZB × NZW)F1 splenic cells are regulated by expression levels of ERα.
Figure 7. Levels of Ifi202 mRNA in splenic cells depend on the ERα status.
Total RNA isolated from spleens of wild type (Esr1+/+; lanes 1–3) or ER-α-deficient (Esr1−/−; lanes 4–6) age-matched (NZB × NZW)F1 female mice was analyzed by semi-quantitative RT-PCR for expression levels of Esr1, Ifi202, and actin mRNA.
Discussion
Increased levels of estrogen in certain lupus-prone strains of female mice are known to activate and increase survival of autoreactive cells in naive repertoire (3, 4, 43–45). However, molecular mechanisms that contribute to increased cell survival in female mice remain to be elucidated. Therefore, identification of lupus-susceptibility genes whose expression is regulated by genetic factors and sex-hormones, and elucidation of the role of encoded proteins in cell survival is expected to provide insights into the molecular basis of sex bias in lupus susceptibility. Interestingly, estrogen treatment of female BALB/c transgenic (transgenic for R4A-γ2b H chain) mice results in up-regulation of Bcl-2 expression in splenic B cells (43). Furthermore, a recent study has identified a number of genes expression of which is regulated by sex hormones in splenic cells of (NZB × NZW)F1 lupus-prone mice (35). The study also identified Trp53 gene (encoding the p53 protein), whose expression is up-regulated in male mice (as compared to female mice). p53 represses transcription of Ifi202 gene (46). Therefore, our observation that expression of the Ifi202 gene is down-regulated by male sex hormone (DHT) in orchiectomized (NZB × NZW)F1 male mice (Fig. 1) makes it likely that increased levels of male sex hormone negatively regulate Ifi202 expression, in part, by up-regulating the p53 expression. Moreover, the study (35) also identified other genes that are known to encode proteins with immunomodulatory functions. However, none of the identified estrogen-responsive gene mapped within the NZB-derived Nba2 lupus susceptibility interval, which is syntenic to the human lupus susceptibility locus (26, 32).
Promoter polymorphisms-dependent increased expression of Ifi202 gene in certain strains of female mice before detection of auto-antibodies is associated with defects in apoptosis of B cells and the development of lupus-like disease (26, 32, 33). Because the development of lupus-like disease in B6.Nba2 mice has sex bias (34), we investigated whether sex hormones could regulate the expression of the Ifi202, an interferon-inducible lupus susceptibility gene within the Nba2 interval. Our experiments revealed that: (i) in vivo treatment of orchiectomized (NZB × NZW)F1 male mice with female sex hormone E2 increased steady-state levels of Ifi202 mRNA whereas treatment with male sex hormone DHT decreased the mRNA levels (Fig. 1); (ii) increased steady-state levels of Ifi202 mRNA and protein in splenic B cells from B6.Nba2 male and female mice were associated with increased levels of ERα and reduced levels of AR (Fig. 2); (iii) steady-state levels of Ifi202 mRNA were relatively higher in C57BL/6, B6.Nba2, NZB, and (NZB × NZW)F1 female mice than age-matched male mice (Fig. 3); (iv) treatment of E2-responsive WT276 cells with increasing concentrations of E2 increased the steady-state levels of Ifi202 mRNA and protein whereas treatment of cells with DHT decreased the p202 protein levels (Fig. 4); (v) treatment of WT276 cells with E2 or over-expression of ERα stimulated the activity of 202-luc-reporter plasmid (Fig. 5A and B) and over expression of ERα in NIH3T3 cells up-regulated the p202 protein levels (Fig. 5C); (vi) E2 treatment of WT276 cells activated transcription from 202-luc-reporter through the AP-1CS1 site and increased levels of ERα associated with the 5′-regulatory region of Ifi202 gene in B6.NBa2 female B cells than age-matched males (Fig. 6); and (vii) steady-state levels of Ifi202 mRNA were detectable in (NZB × NZW)F1 splenic cells of wild type (Esr1+/+), but not ERα-deficient (Esr1−/−), age-matched female mice (Fig. 7). Together, these observations demonstrated that female sex hormone and male sex hormone differentially regulate the expression of the Ifi202 gene in immune cells.
Of note, our observations revealed that basal and interferon-induced steady-state levels of Ifi202 mRNA were significantly higher in non-autoimmune C57BL/6 females than age-matched males (Fig. 3A). Moreover, steady-state levels of Ifi202 mRNA were also significantly higher in pre-autoimmune B6.Nba2, NZB, and (NZB × NZW)F1 female splenic cells than age-matched males (Fig. 3B). Similarly, steady-state levels of Ifi202 mRNA and protein were significantly higher in pre-autoimmune B6.Nba2 female splenic B or T cells than age-matched males (Fig. 2). Together, these observations are consistent with sex hormone-dependent (and disease-independent) regulation of Ifi202 expression.
ERα-deficient mice are reported to have elevated levels of estrogen and testosterone (47). Therefore, our observations that in vivo treatment of orchiectomized (NZB × NZW)F1 male mice with dihydrotestosterone reduced the mRNA levels (Fig. 1) and the lack of detection of Ifi202 mRNA in Esr1-null mice (Fig. 7) make it likely that increased levels of testosterone in ERα-deficient female mice through AR down-regulate the expression of Ifi202 gene. Further work will be needed to test this possibility.
A study(48) has revealed that treatment of BALB/c mice with ER-subtype-selective agonists that results in activation of ERα, but not ERβ, plays a major role in estrogen-induced thymic atrophy and thymic T cell and splenic B cell phenotype alterations. Moreover, the study also revealed that ERα, but not ERβ, mediates the estrogen-induced up-regulation of IFN-γ. Consistent with a role for ERα in IFN-γ production, our study (20) involving generation of ERα knockout (NZB × NZW)F1 mice and their characterization revealed that estrogen through ERα promotes lupus disease, at least in part, by inducing IFN-γ production. Moreover, estrogen is known to enhance IFN-γ production by CD11c+ cells (49). Together, these observations raise the possibility that activation of ERα by E2 in immune cells of certain strains of female mice up-regulates Ifi202 expression in part by increasing IFN-γ production. Therefore, further work will be needed to test this hypothesis.
Previous studies (43, 44, 50) have suggested that estrogen treatment of R4A-γ2b BALB/c mice (transgenic for the H chain of an anti-DNA antibody) with E2 leads to the survival and activation of autoreactive cells in naive repertoire. Moreover, studies(43) also revealed that estrogen treatment of B cells also up-regulates the expression of several genes, such as cd22, shp-1, and bcl-2, which are involved in B cell activation and survival. Interestingly, treatment of mice with tamoxifen, a selective ER modulator (42), blocked estrogen-induced B cell maturation but not survival (50). Because increased expression of p202 protein in splenic B cells from B6.Nba2 congenic mice is associated with defects of apoptosis of cells (26, 32, 33) and down-regulation of expression of p53 and E2F-responsive pro-apoptotic genes (36, 41), we compared basal transcriptional activity of NF-κB between B6.Nba2 female B cells and age-matched males. These experiments indicated that increased levels of p202 in female B cells (as compared to males) are associated with increased transcriptional activity of NF-κB (data not shown). Together, our observations support the idea that female sex hormone-dependent increased levels of p202 protein in B cells by increasing cell survival contribute to sex bias in lupus susceptibility (Fig. 8).
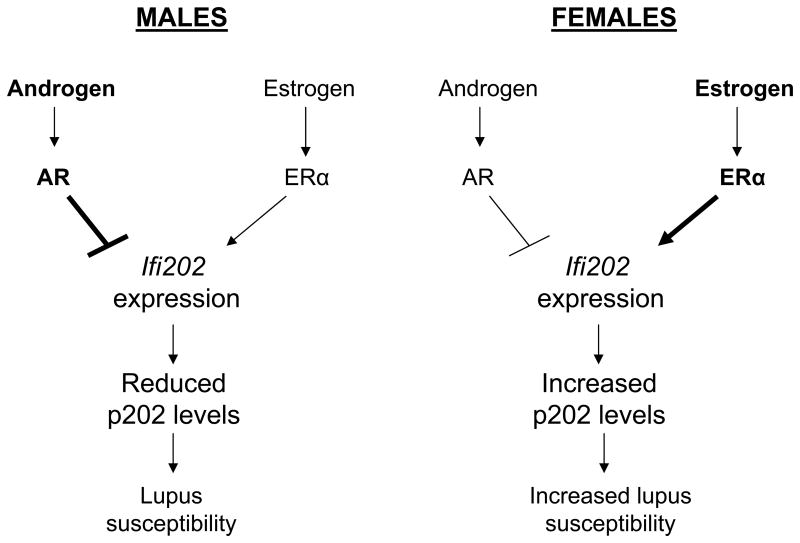
Figure 8.
Differential regulation of Ifi202 expression by sex hormones in male and female mice and the role of p202 protein in sex bias in lupus susceptibility.
The 5′-regulatory region (~800 bp) of Ifi202 gene contains at least two ERE half-sites (Fig. 6A). Interestingly, one of the ERE half-site is located next to an AP-1 consensus DNA-binding site (the AP-1CS1), which can bind to AP-1 and can stimulate the transcription of the Ifi202 gene (40). Because the ERα can regulate transcription of its target genes through the c-Jun/AP-1 DNA-binding sites (15, 16), we tested whether ERα regulates transcription of the Ifi202 gene through the AP-1CS1 site-dependent manner. Our experiments demonstrated that E2-mediated stimulation of the activity of 202-luc-reporter was abrogated due to a mutation in the AP-1CS1 site. Further work will be needed to investigate how ERα and c-Jun/AP-1 collaborate with each other to up-regulate the expression of the Ifi202 gene in B cells.
A search for an AR-responsive element (ARE) in the 5′-regulatory region of the Ifi202 gene did not result in identification of an ARE. Therefore, further work will be needed to determine whether 5′-regulatory region of Ifi202 gene, which is upstream to ~800-bp region contains an ARE. In this regards, it is worthwhile to note that bone marrow stromal cells mediate androgenic suppression of B lymphocytes development through up-regulation of TGF-β expression in response to DHT treatment (51). Consistent with these observations; we have noted that TGF-β treatment of splenic cells reduced steady-state levels of Ifi202 mRNA (data not shown). Therefore, further work will be noted to determine how androgens negatively regulate the expression of Ifi202 in T cells.
Interestingly, a recent study (29) has provided evidence that the p202 protein can recognize double-stranded DNA in cytoplasm (29). Moreover, the study proposed that increased levels of p202 protein in immune cells could inhibit the ability of the AIM2 protein, a pyrin domain containing member of the p200-family (28, 30), which can also sense DNA in cytoplasm and can form a caspase-1-activating inflammasome (30). In light of these observations it will be important to investigate whether the expression of the murine Aim2 is also differentially regulated by the female and male sex hormones in immune cells.
In summary, our observations provide support for our model (Fig. 8). The model predicts that increased levels of male hormone androgen through activation of AR down-regulate the expression of Ifi202 gene. In contrast, increased levels of female hormone estrogen through activation of ERα up-regulate the expression of Ifi202. Consequently, increased levels of p202 protein in immune cells of certain strains of female mice contribute to increased survival of autoreactive cells, resulting in increased susceptibility to lupus disease. Our observations will serve molecular basis to identify signaling pathways and molecules that contribute to sex bias in the development of SLE in human patients.
Acknowledgments
We thank Dr. JoEllen Welsh for generously providing WT276 cell line. We also thank Dr. Shuk-mei Ho for thoughtful suggestions concerning our manuscript.
This work was supported by a grant (AI066261) to D.C. A pre-doctoral award (# 0315287Z) from the American Heart Association supported M. G. B.
References
- 1.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 2.Rider V, Abdou NI. Gender differences in autoimmunity: molecular basis for estrogen effects in systemic lupus erythematosus. Int Immunopharmacol. 2001;1:1009–1024. doi: 10.1016/s1567-5769(01)00046-7. [DOI] [PubMed] [Google Scholar]
- 3.Cohen-Solal JF, Jeganathan V, Grimaldi CM, Peeva E, Diamond B. Sex hormones and SLE: influencing the fate of auto reactive B cells. Curr Top Microbiol Immunol. 2006;305:67–88. doi: 10.1007/3-540-29714-6_4. [DOI] [PubMed] [Google Scholar]
- 4.Zandman-Goddard G, Peeva E, Shoenfeld Y. Gender and autoimmunity. Autoimmun Rev. 2007;6:366–372. doi: 10.1016/j.autrev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Tsokos GC, Kammer GM. Molecular aberrations in human systemic lupus erythematosus. Mol Med Today. 2000;6:418–424. doi: 10.1016/s1357-4310(00)01798-6. [DOI] [PubMed] [Google Scholar]
- 6.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Jorgensen TN, Gubbels MR, Kotzin BL. New insights into disease pathogenesis from mouse lupus genetics. Curr Opin Immunol. 2004;16:787–793. doi: 10.1016/j.coi.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Zhu J, Mohan C. SLE 1, 2, 3…genetic dissection of lupus. Adv Exp Med Biol. 2007;601:85–95. doi: 10.1007/978-0-387-72005-0_9. [DOI] [PubMed] [Google Scholar]
- 10.Haywood MEK, Rose SJ, Horswell S, Lees MJ, Fu G, Walport MJ, Morley BJ. Overlapping BXSB congenic intervals, in combination with microarray gene expression, reveal novel lupus candidate genes. Gene Immun. 2006;7:250–263. doi: 10.1038/sj.gene.6364294. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Karypis G, Vegoe AL, Ruiz P, Gilkeson GS, Behrens TW. Genomic view of systemic autoimmunity in MRL/lpr mice. Genes Immun. 2006;7:156–168. doi: 10.1038/sj.gene.6364286. [DOI] [PubMed] [Google Scholar]
- 12.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferon (α/β) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 13.Roubinian JR, Papoian R, Talal N. Androgenic hormones modulate autoantibody response and improve survival in murine lupus. J Clin Invest. 1977;59:1066–1070. doi: 10.1172/JCI108729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roubinian JR, Talal N, Greenspan JS, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978;147:1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll JS, Brown M. Estrogen receptor target gene: an evolving concept. Mol Endocrinol. 2006;20:1707–1714. doi: 10.1210/me.2005-0334. [DOI] [PubMed] [Google Scholar]
- 16.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erlandsson MC, Ohlsson C, Gustafsson JA, Carlsten H. Role of oestrogen receptors-α and -β in immune organ development and in oestrogen-mediated effects on thymus. Immunology. 2001;103:17–25. doi: 10.1046/j.1365-2567.2001.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svenson JL, Eudaly J, Ruiz P, Korach KS, Gilkeson GS. Impact of estrogen receptor deficiency on disease expression in the NZM2410 lupus prone mouse. Clin Immunol. 2008;128:259–268. doi: 10.1016/j.clim.2008.03.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, McMurray RW. Effects of estrogen receptor subtype-selective agonists on autoimmune disease in lupus-prone NZB/NZW F1 mouse model. Clin Immunol. 2007;123:219–226. doi: 10.1016/j.clim.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Bynote KK, Hackenberg JM, Korach KS, Lubahn DB, Lane PH, Gould KA. Estrogen receptor-α deficiency attenuates autoimmune disease in (NZB × NZW)F1 mice. Genes Immun. 2008;9:137–152. doi: 10.1038/sj.gene.6364458. [DOI] [PubMed] [Google Scholar]
- 21.Kato S, Tora L, Yamauchi J, Masushige S, Bellard M, Chambon P. A far upstream estrogen response element of the ovalbumin gene contains several half-palindromic 5′-TGACC-3′ motifs acting synergistically. Cell. 1992;68:731–742. doi: 10.1016/0092-8674(92)90148-6. [DOI] [PubMed] [Google Scholar]
- 22.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 23.Liva SM, Voskuhl RR. Testosterone acts directly on CD4 T lymphocytes to increase IL-10 production. J Immunol. 2001;167:2060–67. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- 24.Choubey D, Snoddy J, Chaturvedi V, Toniato E, Opdenakker G, Thakur A, Samanta H, Engel DA, Lengyel P. Interferons as gene activators. Indications for repeated gene duplication during the evolution of a cluster of interferon-activatable genes on murine chromosome 1. J Biol Chem. 1989;264:17182–17189. [PubMed] [Google Scholar]
- 25.Choubey D. p202: an interferon-inducible negative regulator of cell growth. J Biol Regul Homeost Agents. 2000;14:187–192. [PubMed] [Google Scholar]
- 26.Choubey D, Kotzin BL. Interferon-inducible p202 in the susceptibility to systemic lupus. Front Biosc. 2002;7:e252–262. doi: 10.2741/A921. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Chatterjee G, Meyer JJ, Liu CJ, Manunath NA, Bray-Ward P, Lengyel P. Characteristics of three homologous 202 genes (Ifi202a, Ifi202b, and Ifi202c) from the murine interferon-activatable gene 200 cluster. Genomics. 1999;60:281–294. doi: 10.1006/geno.1999.5923. [DOI] [PubMed] [Google Scholar]
- 28.Ludlow LE, Johnstone RW, Clark CJ. The HIN-200 family: more than interferon-inducible genes? Exp Cell Res. 2005;308:1–17. doi: 10.1016/j.yexcr.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, Hume DA, Stacey KJ. Hin-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 30.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 31.Choubey D, Lengyel P. Interferon action: cytoplasmic and nuclear localization of the interferon-inducible 52-kD protein that is encoded by the Ifi202 gene from the gene 200 cluster. J Interferon Res. 1993;13:43–52. doi: 10.1089/jir.1993.13.43. [DOI] [PubMed] [Google Scholar]
- 32.Rozzo SJ, Allard JD, Choubey D, Vyse TJ, Izui S, Peltz G, Kotzin BL. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity. 2001;15:435–443. doi: 10.1016/s1074-7613(01)00196-0. [DOI] [PubMed] [Google Scholar]
- 33.Choubey D. Comment on: The candidate lupus susceptibility gene Ifi202a is largely dispensable for B-cell function. Rheumatology. 2008;47:558–559. doi: 10.1093/rheumatology/ken018. [DOI] [PubMed] [Google Scholar]
- 34.Gubbels MR, Jorgensen TN, Metzger TE, Menze K, Steele H, Flannery SA, Rozzo SJ, Kotzin BL. Effects of MHC and gender on lupus-like autoimmunity in Nba2 congenic mice. J Immunol. 2005;175:6190–6196. doi: 10.4049/jimmunol.175.9.6190. [DOI] [PubMed] [Google Scholar]
- 35.Gubbels MR, Jorgensen TN, Kotzin BL. Identification of candidate genes that influence sex hormone-dependent disease phenotypes in mouse lupus. Genes Immun. 2008;9:47–56. doi: 10.1038/sj.gene.6364447. [DOI] [PubMed] [Google Scholar]
- 36.Xin H, D’Souza S, Jorgensen TN, Vaughan AT, Lengyel P, Kotzin BL, Choubey D. Increased expression of Ifi202, an IFN-activatable gene, in B6.Nba2 lupus susceptible mice inhibits p53-mediated apoptosis. J Immunol. 2006;176:5863–5870. doi: 10.4049/jimmunol.176.10.5863. [DOI] [PubMed] [Google Scholar]
- 37.Zinser GM, McEleney K, Welsh J. Characterization of mammary tumor cell lines from wild type and vitamin D3 receptor knockout mice. Mol Cell Endocrinol. 2003;200:67–80. doi: 10.1016/s0303-7207(02)00416-1. [DOI] [PubMed] [Google Scholar]
- 38.Stefano GB, Prevot V, Beauvillain JC, Fimiani C, Welters I, Cadet P, Breton C, Pestel J, Salzet M, Bilfinger TV. Estradiol coupling to human monocytes nitric oxide release is dependent on intracellular calcium transients: evidence for an estrogen surface receptor. J Immunol. 1999;163:3758–3763. [PubMed] [Google Scholar]
- 39.Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P. The human estrogen receptor has two independent non-acidic transcriptional activation functions. Cell. 1989;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- 40.Xin H, Geng Y, Pramanik R, Choubey D. Induction of p202, a modulator of apoptosis, during oncogenic transformation of NIH 3T3 cells by activated H-Ras (Q61L) contributes to cell survival. J Cell Biochem. 2003;88:191–204. doi: 10.1002/jcb.10372. [DOI] [PubMed] [Google Scholar]
- 41.Panchanathan R, Xin H, Choubey D. Disruption of mutually negative regulatory feedback loop between interferon-inducible p202 protein and the E2F family of transcription factors in lupus-prone mice. J Immunol. 2008;180:5927–5934. doi: 10.4049/jimmunol.180.9.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nalbandian G, Paharkova-Vatchkova V, Mao A, Nale S, Kovats S. The selective estrogen receptor modulators, Tamoxifen and Raloxifene, impair dendritic cell differentiation and activation. J Immunol. 2005;175:2666–2675. doi: 10.4049/jimmunol.175.4.2666. [DOI] [PubMed] [Google Scholar]
- 43.Bynoe MS, Grimaldi CM, Diamond B. Estrogen up-regulates Bcl-2 and blocks tolerance induction of naive B cells. Proc Natl Acad Sci USA. 2000;97:2703–2708. doi: 10.1073/pnas.040577497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grimaldi CM, Michael DJ, Diamond B. Expansion and activation of a population of auto-reactive marginal zone B cells in a model of estrogen-induced lupus. J Immunol. 2001;167:1886–1890. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- 45.Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. 2002;109:1625–1633. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Souza S, Xin H, Walter S, Choubey D. The gene encoding p202, an interferon-inducible negative regulator of the p53 tumor suppressor, is a target of p53-mediated transcriptional repression. J Biol Chem. 2001;276:298–305. doi: 10.1074/jbc.M007155200. [DOI] [PubMed] [Google Scholar]
- 47.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 48.Li J, McMurray RW. Effects of estrogen receptor subtype-selective agonists on immune functions in ovariectomized mice. Int Immunopharmacol. 2006;6:1413–1423. doi: 10.1016/j.intimp.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 49.Siracusa MC, Overstreet MG, Housseau F, Scott AL, Klein SL. 17β-Estradiol alters the activity of conventional and IFN-producing killer dendritic cells. J Immunol. 2008;180:1423–1431. doi: 10.4049/jimmunol.180.3.1423. [DOI] [PubMed] [Google Scholar]
- 50.Peeva E, Venkatesh J, Diamond B. Tamoxifen blocks estrogen induced B cell maturation but not survival. J Immunol. 2005;175:1415–1423. doi: 10.4049/jimmunol.175.3.1415. [DOI] [PubMed] [Google Scholar]
- 51.Olsen NJ, Gu X, Kovacs WJ. Bone marrow stromal cells mediate androgenic suppression of B lymphocyte development. J Clin Invest. 2001;108:1697–1704. doi: 10.1172/JCI13183. [DOI] [PMC free article] [PubMed] [Google Scholar]