Abstract
Disuse typically causes an imbalance in bone formation and bone resorption, leading to losses of cortical and trabecular bone. In contrast, bears maintain balanced intracortical remodeling and prevent cortical bone loss during disuse (hibernation). Trabecular bone, however, is more detrimentally affected than cortical bone in other animal models of disuse. Here we investigated the effects of hibernation on bone remodeling, architectural properties, and mineral density of grizzly bear (Ursus arctos horribilis) and black bear (Ursus americanus) trabecular bone in several skeletal locations. There were no differences in bone volume fraction or tissue mineral density between hibernating and active bears or between pre- and post-hibernation bears in the ilium, distal femur, or calcaneus. Though indices of cellular activity level (mineral apposition rate, osteoid thickness) decreased, trabecular bone resorption and formation indices remained balanced in hibernating grizzly bears. These data suggest that bears prevent bone loss during disuse by maintaining a balance between bone formation and bone resorption, which consequently preserves bone structure and strength. Further investigation of bone metabolism in hibernating bears may lead to the translation of mechanisms preventing disuse induced bone loss in bears into novel treatments for osteoporosis.
Keywords: bear, trabecular bone, remodeling, architecture, disuse osteoporosis
Introduction
Reduced skeletal loading causes loss of cortical and trabecular bone in humans and other animals [1-8]. Trabecular bone, due to its greater surface to volume ratio, responds to disuse more rapidly [9, 10] and shows greater losses than cortical bone for a given period of inactivity [3, 11, 12]. Manifestations of disuse on trabecular bone include decreased histological measures of bone formation [13], increased histological measures of bone resorption [14], decreased bone mineral density [9, 12], and compromised bone architecture [7, 15]. These changes cause the mechanical properties of trabecular bone to deteriorate which can lead to an increased risk of bone fracture in chronically immobilized patients [16, 17]. In animal models of disuse, trabecular and cortical bone lost during inactivity may be fully recovered, but a remobilization period that is at least twice the length of the disuse period is required for complete restoration of bone [3, 18].
Bears hibernate for approximately 6 months of the year, and thus they experience annual periods of disuse and remobilization that are approximately equal in length [19]. However, the material and structural properties of black bear (Ursus americanus) cortical bone are not compromised with age [20-23]. Bears completely prevent cortical bone loss during hibernation; in the femoral diaphysis, hibernating grizzly bears (Ursus arctos) maintain bone geometry and strength, and surprisingly, porosity is lower and mineral content is higher in hibernating compared to active grizzly bears [24]. This is likely because bears demonstrate decreased, but balanced, intracortical bone remodeling in the femur during hibernation [24]. These findings suggest bears possess a unique biological mechanism to prevent disuse induced bone loss.
Hibernation is a mechanism to conserve energy when food is scarce; homeostatic, metabolically expensive processes are downregulated by the neuroendocrine system, and bears recycle, instead of excrete, catabolic products like urea and calcium [25-27]. Since bone turnover is a metabolically expensive process, decreasing intracortical bone turnover while maintaining a balance between bone formation and bone resorption probably helps bears conserve energy and maintain eucalcemia [24]. Trabecular bone, however, is typically more involved in mineral homeostasis and is more sensitive to changes in mechanical loading than cortical bone [3, 9, 12]. Trabecular bone mineral content, bone mineral density, and architectural properties in black bear forelimbs and in the ilium are not different before and after hibernation [28, 29], suggesting that hibernating bears prevent both cortical and trabecular bone loss. However, previous studies of seasonal changes in bear trabecular bone [28, 29] had small sample sizes (n ≤ 4 bears per season), and consequently low statistical power to detect changes between groups. Furthermore, it is unclear if site-specific remodeling responses in various skeletal locations (e.g., ilium compared to hindlimb) and bone types (i.e., trabecular versus cortical) respond similarly to the physical inactivity that occurs during hibernation. The goal of this study was to quantify the effects of hibernation on bear trabecular bone remodeling, architecture, and mineral density in several different skeletal locations. We hypothesized that trabecular bone architectural properties and mineral density would not be adversely affected in hibernating bears because histological indices of bone resorption and bone formation remain balanced during hibernation.
Materials & Methods
Grizzly bear bones
Sixteen grizzly bears (Ursus arctos horribilis) were housed at the Washington State University Bear Research, Education, and Conservation Facility (Pullman, WA) for this study. All handling and treatment procedures were approved by the Washington State University Institutional Animal Care and Use Committee. The hibernating bears (n = 5 male, 5 female; mean age = 8.3 ± 9.3 years) were sacrificed after 16-18 weeks of hibernation, and the active bears (n = 3 male, 3 female; mean age = 7.8 ± 8.8 years) were sacrificed after at least 14 weeks of physical activity following hibernation. Ages of the hibernating and active bears ranged from 1-21 years. The bears were administered an IV solution of calcein at 5 mg/kg body mass twice prior to death; injections were given 9 to 11 days apart, and 5 to 8 days passed after the second label was administered before animals were sacrificed (i.e., labeling schedules ranged from 1-9-1:5 to 1-11-1:8). Bears were euthanized by an injection of pentobarbital (10 mls/100 lbs body weight). After sacrifice, the hind legs and pelvis from each bear were removed, cleaned of soft tissue, and frozen at -20 °C.
Black bear bones
The grizzly bear samples described above provide an opportunity to study bone turnover in a unique animal model. However, a limitation of this model is the relatively low number of samples that are available. Therefore, trabecular bone architectural properties and mineral density were also quantified in femurs from wild black bears killed by hunters in Utah during fall (pre-hibernation) and spring (post-hibernation) hunting seasons. One left or right femur was obtained from each of 58 black bears, cleaned of soft tissue and stored at -20 °C. Twenty-one were from bears killed in the fall (15 male, 6 female; mean age = 5.5 ± 5.1 years), and thirtyseven were from bears killed in the spring (27 male, 10 female; mean age = 6.9 ± 4.0 years). Ages were determined by the Utah Department of Wildlife Resources from the dental cementum annuli [30], and ranged from 1 to 19 years. Bears in Utah begin denning in late October and emerge in late April; the spring bears were killed between April 26th and May 31st, and the fall bears were killed between August 26th and November 4th.
Grizzly bear trabecular bone samples for histological analyses
Trabecular bone cores (7.6 mm diameter) were removed from the ilium and distal femoral epiphysis of each grizzly bear using a diamond-plated bit (#160095, Starlite Industries, PA). Samples were histologically processed and sectioned longitudinally. Bone sections were left unstained for dynamic histomorphometry or were stained by a VonKossa/MacNeal's Tetrachrome protocol [31] for quantification of static bone formation and resorption parameters. All sections were digitized using a microscope and digital camera and were analyzed with image analysis software (Bioquant Osteo, Nashville, TN). Quantified indices for the stained slides included bone surface (BS), osteoid surface (OS/BS), osteoid thickness (O.Th), and eroded surface (ES/BS). The ratio of osteoid to eroded surface (OS/ES) was calculated to assess changes in the balance of bone remodeling. For the unstained slides, inter-label width (Ir.L.Wi) was measured at 5 μm intervals between the label midpoints of all double-labeled sites, and labeled surfaces (sLS, dLS) were quantified for all single and double-labeled sites. Mineralizing surface (MS/BS), mineral apposition rate (MAR), and adjusted apposition rate (Aj.AR) were calculated from the static and dynamic measurements [32].
Trabecular bone samples for μCT analyses
Trabecular cores (7.6 mm diameter) were removed from the ilium, distal femoral metaphysis, distal femoral epiphysis, and calcaneus of each grizzly bear and from the distal femoral metaphysis of each black bear. Bony landmarks were used to ensure that cores were removed from the same relative location in each bone. All trabecular bone samples were fixed in 70% ethanol at 4 °C.
Trabecular bone architecture was evaluated using a fan-beam micro-computed tomography (μCT) system (μCT40, Scanco Medical AG, Basserdorf, Switzerland). Samples were scanned transverse to the long axis of the core at 6 μm isotropic voxel size. Two hundred transverse slices (∼1.2 mm) centered at mid-core were evaluated for morphometric parameters. Energy settings for the scans were 70 kVP/114 uA with threshold = 205 and integration time = 300 ms. Bone volume fraction (BV/TV, %), trabecular number (Tb.N, mm-1), trabecular thickness (Tb.Th, mm), trabecular separation (Tb.Sp, mm), and trabecular tissue mineral density (Tb.M.Dn, mgHA/cm3) were computed using the manufacturer's software.
Statistics
All statistical analyses were completed with either JMP 7 or SAS 9.1 (SAS Institute Inc.; Cary, NC) statistical software. Bone properties were compared between hibernating and active grizzly bears at each skeletal site with ANOVA and Fisher's PLSD post-hoc test. The distribution of male and female grizzly bears was comparable between hibernating and active bear groups, and therefore male and female bears were pooled for analyses of grizzly bear data due to the small sample size. Different skeletal locations in the grizzly bears were pooled for combined analyses comparing hibernating to active bears using multivariate analysis of variance (MANOVA) to account for correlations between skeletal sites in the same bears. A 5% level of significance was used for all statistical tests. Post-hoc power analyses were conducted for grizzly bear data from each skeletal location to estimate the power to detect changes in bone properties that would be expected based on other animal models of disuse (Table 1).
Table 1.
Disuse-induced changes in bone properties observed in other models of physical inactivity. All percentage changes were statistically significant in their respective studies. These percentage changes were used to calculate statistical power for comparisons of active vs. hibernating and pre- vs. post-hibernation black bear bone properties. See text for explanations of bone property abbreviations. SF = spaceflight, TS = tail suspension, LI = limb immobilization, BR = bedrest.
| Bone Property | Disuse Model | Relative Percentage Change (Disuse: Control) |
Reference |
|---|---|---|---|
| OS/BS | Rats, 10 d SF (humerus) | -79.7% | [13] |
| ES/BS | Rats, 10 d SF (humerus) | +44.1% | [13] |
| MS/BS | Rats, 3 wk TS (tibia) | -54.3% | [47] |
| MAR | Rats, 3 wk TS (tibia) | -33.3% | [47] |
| BV/TV | Sheep, 12 wk LI (calcaneus) | -29.3% | [48] |
| Tb.N (mm-1) | Humans, 12 wk BR (ilium) | -16.8% | [49] |
| Tb.Th (mm) | Sheep, 12 wk LI (calcaneus) | -26.8% | [48] |
| Tb.Sp (mm) | Dogs, 8 wk LI (distal humerus) | +20.4% | [11] |
Pre- and post-hibernation black bears were compared with ANCOVA, treating age and sex as covariates. Bears reach skeletal maturity at approximately 8 years of age [33]; skeletally immature and skeletally mature bears were considered together for this study. Post-hoc power analyses were conducted for the black bear data using the regression mean squared error as an estimate of model variance.
Results
Trabecular bone remodeling indices – grizzly bears
Neither osteoid surface nor eroded surface was different between hibernating and active grizzly bears in the ilium or distal femur, or when both skeletal locations were analyzed together as a combined dataset (Table 2). Statistical power to detect changes in osteoid surface between hibernating and active bears ranged from 61-84% (i.e., 16-39% Type II error), and power to detect changes in eroded surface ranged from 32-62%. The ratio of osteoid to eroded surface (an indicator of the balance between bone formation and bone resorption) also was not different between hibernating and active grizzly bears at either skeletal location or in the combined dataset (Table 2).
Table 2.
Trabecular bone formation and resorption remained balanced in hibernating grizzly bears; there were no differences (p > 0.517) in osteoid surface, eroded surface, or the ratio of osteoid to eroded surface between hibernating (n = 10) and active (n = 6) grizzly bears. However, osteoid thickness, mineral apposition rate, adjusted apposition rate, and mineralizing surface were lower in the hibernating bears, suggesting a decrease in osteoblast activity levels during hibernation. Means and standard deviations (in parentheses) are presented. See text for explanations of abbreviations.
| Skeletal Location | Bone Property | Active | Hibernating | p-value |
|---|---|---|---|---|
| Ilium | O.Th (μm) | 7.8 (1.2) | 7.1 (1.5) | 0.324 |
| OS/BS (%) | 11.3 (6.1) | 10.1 (8.8) | 0.782 | |
| ES/BS (%) | 3.2 (1.9) | 2.8 (1.6) | 0.648 | |
| OS/ES (%) | 429 (212) | 355 (221) | 0.517 | |
| MS/BS (%) | 16.1 (10.0) | 7.7 (7.7) | 0.078 | |
| MAR (μm/day) | 1.86 (0.42) | 1.13 (0.46) | 0.007 | |
| Aj.AR (μm/day) | 2.77 (2.07) | 0.91 (0.68) | 0.018 | |
| Distal femoral epiphysis | O.Th (μm) | 8.9 (1.9) | 6.8 (1.0) | 0.009 |
| OS/BS (%) | 10.2 (4.3) | 8.4 (5.9) | 0.536 | |
| ES/BS (%) | 2.9 (1.0) | 2.8 (1.1) | 0.850 | |
| OS/ES (%) | 358 (97) | 328 (182) | 0.717 | |
| MS/BS (%) | 17.5 (6.0) | 5.4 (2.7) | < 0.0001 | |
| MAR (μm/day) | 1.47 (0.29) | 0.95 (0.41) | 0.018 | |
| Aj.AR (μm/day) | 2.78 (1.26) | 0.90 (0.74) | 0.002 | |
| Combined locations | O.Th (μm) | 8.4 (1.6) | 6.9 (1.2) | 0.037 |
| OS/BS (%) | 10.7 (5.1) | 9.3 (7.3) | 0.831 | |
| ES/BS (%) | 3.0 (1.5) | 2.8 (1.4) | 0.903 | |
| OS/ES (%) | 394 (162) | 341 (198) | 0.816 | |
| MS/BS (%) | 16.8 (7.9) | 6.5 (5.7) | 0.0004 | |
| MAR (μm/day) | 1.66 (0.40) | 1.04 (0.44) | 0.012 | |
| Aj.AR (μm/day) | 2.78 (1.63) | 0.90 (0.69) | 0.002 | |
Osteoid thickness was decreased (p = 0.009) in the distal femur of the hibernating grizzly bears, and was also lower in the hibernating bears for the combined dataset (p = 0.037), although it was not different between groups when the ilium was considered separately (Table 2). Mineral apposition was decreased in the hibernating bears in the ilium (p < 0.007) and distal femur (p = 0.018) and was also decreased when both locations were considered together (p = 0.012) (Table 2). Statistical power to detect changes in mineral apposition rate was 74% at either skeletal location. Similarly, adjusted apposition rate, representing the mineral apposition rate over the entire osteoid surface, was decreased in the hibernating bears in both skeletal locations (p < 0.018) and in the combined dataset (p = 0.002). Mineralizing surface in the hibernating bears was decreased in the distal femur (p < 0.0001) and approached a significant decrease in the ilium (p = 0.078), and was also decreased in the combined dataset (p = 0.0004) (Table 2). Statistical power to detect changes in mineralizing surface ranged from 45-98%.
Trabecular bone architectural properties and mineral density – grizzly bears
There were no differences in bone volume fraction between active and hibernating grizzly bears in the ilium, distal femoral metaphysis, distal femoral epiphysis, or calcaneus trabecular bone samples (Table 3, Figure 1); statistical power ranged from 48-82%. Trabecular number was not different between active and hibernating bears at any of the four skeletal sites; power ranged from 14-40%. Trabecular thickness was decreased (p = 0.046) in the ilium of the hibernating bears, but was not different between groups at any other skeletal site (Table 3); power for trabecular thickness ranged from 26-89%. Trabecular separation was lower (p = 0.047) in the calcaneus of hibernating compared to active bears, but was not different between groups at the other skeletal locations. Statistical power for trabecular separation ranged from 25-89%. Trabecular tissue mineral density was higher (p = 0.023) in the ilium of the hibernating compared to the active bears, but was not different between groups at the other skeletal locations (Table 3). When all four skeletal locations were pooled, there were no differences between hibernating and active bears for bone volume fraction, trabecular tissue mineral density, or any architectural properties (Table 3).
Table 3.
Trabecular architecture and mineralization were maintained in the hibernating (n = 10) compared to the active (n = 6) grizzly bears at four skeletal sites. Means and standard deviations (in parentheses) for each group are presented. See text for explanations of abbreviations.
| Skeletal Location | Bone Property | Active | Hibernating | p-value |
|---|---|---|---|---|
| Ilium | BV/TV | 0.20 (0.03) | 0.18 (0.04) | 0.224 |
| Tb.N (mm-1) | 1.7 (0.4) | 1.9 (0.4) | 0.440 | |
| Tb.Th (mm) | 0.15 (0.02) | 0.12 (0.02) | 0.046 | |
| Tb.Sp (mm) | 0.57 (0.11) | 0.54 (0.09) | 0.538 | |
| Tb.M.Dn (mgHA/cm3) | 902 (41) | 947 (29) | 0.023 | |
| Distal femoral metaphysis | BV/TV | 0.15 (0.05) | 0.16 (0.03) | 0.671 |
| Tb.N (mm-1) | 2.19 (1.00) | 2.10 (0.56) | 0.815 | |
| Tb.Th (mm) | 0.11 (0.02) | 0.12 (0.02) | 0.599 | |
| Tb.Sp (mm) | 0.52 (0.18) | 0.51 (0.13) | 0.831 | |
| Tb.M.Dn (mgHA/cm3) | 1005 (75) | 1038 (87) | 0.454 | |
| Distal femoral epiphysis | BV/TV | 0.33 (0.09) | 0.33 (0.10) | 0.924 |
| Tb.N (mm-1) | 2.50 (0.76) | 2.87 (0.96) | 0.437 | |
| Tb.Th (mm) | 0.16 (0.06) | 0.16 (0.06) | 0.837 | |
| Tb.Sp (mm) | 0.41 (0.10) | 0.39 (0.09) | 0.721 | |
| Tb.M.Dn (mgHA/cm3) | 918 (69) | 935 (57) | 0.616 | |
| Calcaneus | BV/TV | 0.33 (0.09) | 0.35 (0.05) | 0.436 |
| Tb.N (mm-1) | 3.23 (0.74) | 3.72 (0.44) | 0.115 | |
| Tb.Th (mm) | 0.15 (0.03) | 0.15 (0.03) | 0.907 | |
| Tb.Sp (mm) | 0.39 (0.06) | 0.34 (0.03) | 0.047 | |
| Tb.M.Dn (mgHA/cm3) | 941 (66) | 949 (60) | 0.803 | |
| Combined locations | BV/TV | 0.25 (0.10) | 0.25 (0.11) | 0.304 |
| Tb.N (mm-1) | 2.41 (0.89) | 2.65 (0.94) | 0.414 | |
| Tb.Th (mm) | 0.14 (0.04) | 0.14 (0.04) | 0.208 | |
| Tb.Sp (mm) | 0.47 (0.14) | 0.44 (0.12) | 0.256 | |
| Tb.M.Dn (mgHA/cm3) | 942 (72) | 967 (73) | 0.112 | |
Figure 1.
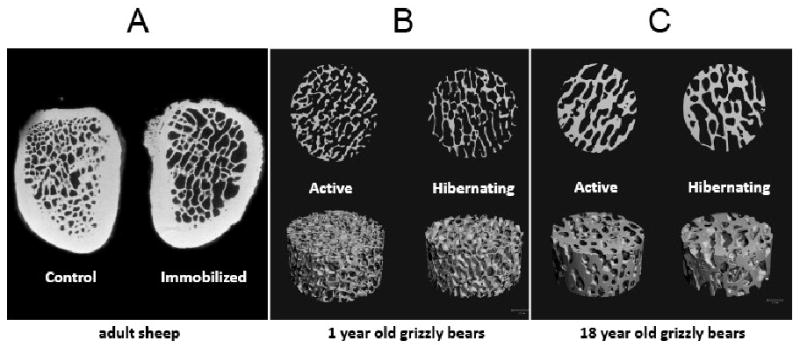
Trabecular bone does not respond similarly to disuse in bears and sheep. Trabecular bone volume fraction was decreased by 31% in sheep calcanei following 12 weeks of immobilization (A). Representative micro-computed tomography (μCT) scans from the distal femoral epiphysis of 1-year old (B) and 18-year old (C) grizzly bears demonstrate no bone loss after 17 weeks of disuse (hibernation). Sheep calcaneus images are reproduced with permission from: Calcif Tissue Int 42, Rubin, C. T. et al., “Ultrasonic measurement of immobilization-induced osteopenia: an experimental study in sheep,” pp. 309-312, copyright Springer-Verlag, New York Inc. (1988).
Trabecular bone architectural properties and mineral density – black bears
There were no differences in trabecular bone architecture or mineralization between pre- and post-hibernation black bears in the distal femoral metaphysis (Table 4). Statistical power to detect changes in trabecular bone properties between pre- and post-hibernation bears ranged from 87% for trabecular number to greater than 99% for trabecular separation. Bone volume fraction and trabecular number decreased with age (Table 4), whereas trabecular separation and trabecular tissue mineral density increased with age (Table 4). Trabecular thickness did not change with age. There were no statistically significant differences in bone properties between male and female bears (Table 4).
Table 4.
Trabecular bone architecture and mineral density in the distal femoral metaphysis were not different between pre-hibernation (n = 21) and post-hibernation (n = 37) black bears. Means and standard deviations (in parentheses) are presented. “Season p-value” is for the ANCOVA comparison between seasons. “Age p-value” and “Age r” (where “r” is the coefficient of correlation) are statistics describing age-related trends in bone properties, and “Sex p-value” describes differences between male and female bears. See text for explanations of abbreviations.
| Bone Property | Pre-hibernation | Post-hibernation | Season p-value | Age p-value | Age r | Sex p-value |
|---|---|---|---|---|---|---|
| BV/TV | 0.15 (0.05) | 0.13 (0.05) | 0.521 | 0.0004 | -0.504 | 0.930 |
| Tb.N (mm-1) | 2.4 (0.6) | 2.2 (0.5) | 0.539 | < 0.0001 | -0.614 | 0.195 |
| Tb.Th (mm) | 0.11 (0.02) | 0.11 (0.02) | 0.507 | 0.274 | +0.046 | 0.073 |
| Tb.Sp (mm) | 0.51 (0.10) | 0.55 (0.10) | 0.624 | < 0.0001 | +0.569 | 0.082 |
| Tb.M.Dn (mgHA/cm3) | 779 (117) | 791 (103) | 0.957 | 0.002 | +0.492 | 0.143 |
Discussion
Disuse typically causes an imbalance in bone formation and bone resorption [34, 35] that leads to trabecular bone loss. Decreased bone mineral density and compromised bone architecture are common in clinical cases of disuse such as spinal cord injury [36, 37] and spaceflight [12], and are problematic because they cause an increased risk of bone fracture [16, 17, 38]. Bears prevent cortical bone loss during disuse (hibernation) [24, 39], but the effects of hibernation on bear trabecular bone were unclear from previous studies [28, 29]. In contrast with other animal models of disuse, hibernating bears in this study demonstrated balanced trabecular bone formation and bone resorption (Table 2), which preserved trabecular bone architecture and mineral density in the hibernating bears (Tables 3-4, Figure 1). These results suggest that bears have evolved a mechanism to prevent disuse-induced losses of both cortical and trabecular bone.
Cortical bone properties improve with age in black bears; cortical bone geometry and ash fraction increased with age, and intracortical porosity decreased with age in black bear femoral diaphyses [21, 39]. In contrast, the current study suggests that black bears lose trabecular bone architecture with age; bone volume fraction and trabecular number decreased with age and trabecular separation increased with age in black bear distal femoral metaphyses (Table 4). Bone volume fraction also decreases with age in the black bear femoral neck [33]. Similar age-related changes occur in trabecular bone of many non-hibernating animals including horses [40], nonhuman primates [41, 42], and humans [43]. Rates of change with age were similar for bears and horses for trabecular number (horses: -0.035 mm-1 / year, bears: -0.079 mm-1 / year) and trabecular separation (horses: +0.015 mm / year, bears: +0.012 mm / year), suggesting that aging has a similar impact on trabecular bone in bears compared to a non-hibernating species [40]. Importantly, there were no differences in trabecular bone properties between pre- and post-hibernation black bears (Table 4), suggesting that bears prevent trabecular bone loss during disuse even though they do appear to lose trabecular bone with age.
Reduced skeletal loading usually causes an unbalanced increase in bone resorption over bone formation [7, 13, 14, 35] (Figure 2). In humans, for example, 12 weeks of bedrest increased bone resorption indices (eroded and osteoclast surfaces) by 100-250%, but did not change indices of bone formation (osteoid and osteoblast surfaces) in the ilium [14]. In contrast, trabecular bone remodeling remained balanced in hibernating grizzly bears; osteoid and eroded surfaces and the ratio of osteoid to eroded surface were unchanged during hibernation (Table 3). Importantly, eroded surface was not elevated in the hibernating bears, unlike the increase in eroded surface that can occur in disuse models like bedrest [14], spaceflight [13], and limb immobilization [7] (Figure 2). Eroded and osteoid surfaces were used as surrogate measures of bone cell surfaces in this study because cells were not preserved in the bear tissues. However, osteoid and eroded surfaces respond similarly to osteoblast and osteoclast surfaces, respectively, in other models of disuse [7, 14], and therefore these properties measured in bears are likely representative of bone cell surfaces.
Figure 2.
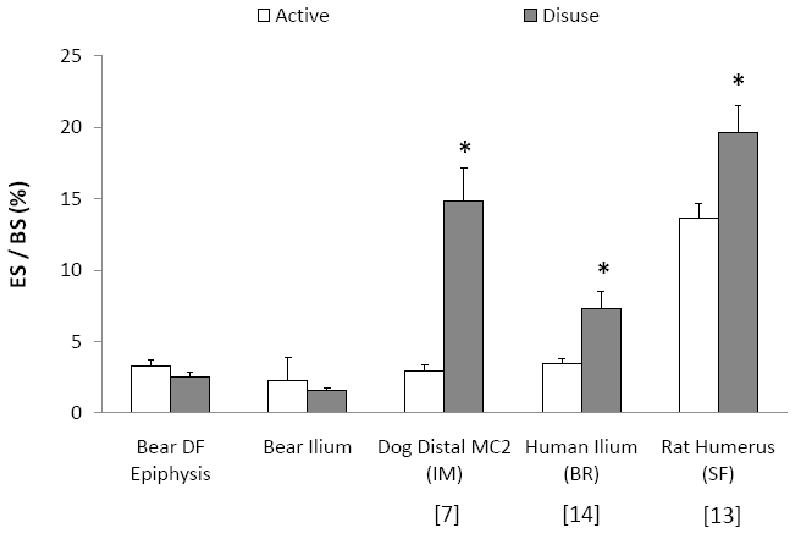
Bone resorption, measured by eroded surface (ES/BS), typically increases in models of disuse including immobilization (IM), bedrest (BR) and spaceflight (SF). Increases in eroded surface were statistically significant in subjects under disuse compared to active conditions in the studies represented below (*: p < 0.05 vs. active group in each study). In contrast, hibernating grizzly bears in the current study did not demonstrate increased indices of bone resorption compared to active bears (p > 0.648). MC2 = 2nd metacarpal, DF = distal femur. Means + SE bars are presented.
We previously found that hibernating grizzly bears experience decreased, but balanced, intracortical remodeling in the femoral diaphysis [24]. We recently found that intracortical remodeling in hibernating compared to active grizzly bears was decreased, but balanced, in other skeletal sites as well (resorption cavity density (p < 0.02): -71% proximal femur, -82% distal femur, -77% tibia, -84% 2nd metatarsal; refilling cavity density (p < 0.07): -69% proximal femur, -80% distal femur, -67% tibia, -52% 2nd metatarsal; n = 5 hibernating, 4 active bears [unpublished data]). Although the overall number of intracortical remodeling sites decreases in hibernating grizzly bears, rates of cellular activity in cortical bone are unchanged during hibernation (no difference in normalized mineral apposition rate (MARN) or filling period (FP) between hibernating and active bears) [24]. This is in contrast with the current study on trabecular bone, in which the total number of trabecular remodeling sites stayed constant (osteoid and eroded surfaces were not different between hibernating and active bears), but rates of cellular activity in trabecular bone decreased (decreased mineral apposition rate and osteoid thickness) (Table 2). It is possible that the differences between trabecular and cortical bone remodeling in hibernating bears are related to calcium demand from other body tissues. Bears drastically decrease physical activity and metabolic rate during hibernation, but essential physiological systems (e.g., cardiac and central nervous systems) continue to function. Calcium ions are essential for proper function of many body systems, but bears do not intake any calcium during hibernation [25]. Since trabecular bone plays a key role in mineral homeostasis, bears may maintain trabecular bone remodeling to maintain a mobile supply of calcium needed to sustain other body systems. Despite differences in the amount of bone remodeling, bone formation and resorption remained balanced in both cortical and trabecular bone of hibernating grizzly bears, meaning that any excess calcium not used elsewhere in the body can be deposited back into the skeleton to maintain eucalcemia [29]. This process likely preserves cortical and trabecular bone throughout the entire skeleton. For example, intracortical porosity was maintained at four diaphyseal skeletal sites in hibernating compared to active grizzly bears (porosity in hibernating vs. active bears (p > 0.260): proximal femur 6.2% vs. 5.5%, distal femur 5.6% vs. 6.7%, tibia 5.8% vs. 6.8%, 2nd metatarsal 7.4% vs. 7.6%; [unpublished data]), and trabecular bone volume fraction was not different between hibernating and active grizzly bears at four skeletal sites (p > 0.224) in the current study.
The mechanisms that maintain balanced bone remodeling and prevent cortical and trabecular bone loss in bears during hibernation are not yet known, but they are likely related to processes used to conserve metabolic energy and recycle calcium during hibernation. Hibernation is a mechanism to survive prolonged periods of famine when food is scarce; metabolic energy is conserved by decreasing many energy-expensive processes. It is possible that both cortical and trabecular bone remodeling mechanisms observed in hibernating grizzly bears contribute to energy conservation, since decreasing either the number of remodeling sites (cortical bone [24]) or the activity of the individual cells (trabecular bone (Table 2)) would decrease energy expensive processes and thus conserve metabolic energy. As mentioned above, the bears' ability to prevent disuse-induced bone loss (Tables 3-4) is probably a result of their ability to maintain balanced bone remodeling during hibernation. Since bears do not eat, drink, urinate, or defecate (i.e., intake or excrete calcium) during hibernation, they probably maintain a balance between bone formation and bone resorption to prevent lethal hypercalcemia; serum calcium levels are not different in hibernating and active bears [29]. Parathyroid hormone (PTH), which regulates serum calcium levels and promotes reabsorption of calcium from the kidneys, has been implicated in the ability of hibernating bears to maintain bone formation and prevent bone loss [24, 44, 45]. For example, PTH may reduce osteoblast apoptosis in the proapoptotic environment of hibernation. Bear PTH peptides have an enhanced ability (compared to human PTH peptides) to increase survival signals in osteoblastic cells [45], which could help preserve osteoblast number in hibernating bears and thus contribute to the bears' maintenance of bone formation during disuse. Serotonin regulation may also help hibernating bears maintain bone formation, since serotonin levels are decreased (-21%, p < 0.0001, [unpublished data]) in serum from hibernating compared to active bears, and decreased serotonin levels may promote osteoblast progenitor proliferation [46]. The roles of neuroendocrine control of bone remodeling and energy regulation in the ability of bears to prevent bone loss during disuse are also currently under investigation. Elucidating the biological mechanism that prevents disuse induced bone loss in bears will have important implications for treating osteoporosis in humans.
In conclusion, the current study provides strong support for the idea that bears prevent trabecular bone loss during hibernation. Thus, bears appear to have evolved the ability to prevent both cortical [24, 39] and trabecular bone loss during disuse. Balanced bone formation and resorption throughout the skeleton likely occurs to preserve calcium homeostasis during hibernation while the bears recycle (instead of excrete) catabolic waste products. Understanding the mechanism that maintains balanced bone remodeling and prevents bone loss in hibernating bears may lead to the development of improved treatments (e.g., novel PTH peptides) for osteoporosis.
Acknowledgments
This research was supported by Grant Number AR050420 from NIH. Additional funding was received from the National Science Foundation Graduate Research Fellowship Program, Michigan Space Grant Consortium, Michigan Technological University Department of Educational Opportunity, American Association of University Women, and Timothy Floyd, M.D. The authors thank Dr. David Burr for his advice on calcein labeling procedures, and Dr. Charles Turner and Peter O'Reilly for assistance with the micro-computed tomography bone scans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res. 1990;5:843–50. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- 2.Vailas AC, Zernicke RF, Grindeland RE, Kaplansky A, Durnova GN, Li KC, Martinez DA. Effects of spaceflight on rat humerus geometry, biomechanics, and biochemistry. Faseb J. 1990;4:47–54. doi: 10.1096/fasebj.4.1.2295378. [DOI] [PubMed] [Google Scholar]
- 3.Kaneps AJ, Stover SM, Lane NE. Changes in canine cortical and cancellous bone mechanical properties following immobilization and remobilization with exercise. Bone. 1997;21:419–23. doi: 10.1016/s8756-3282(97)00167-1. [DOI] [PubMed] [Google Scholar]
- 4.Takata S, Yasui N. Disuse osteoporosis. J Med Invest. 2001;48:147–56. [PubMed] [Google Scholar]
- 5.Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol. 2004;97:119–29. doi: 10.1152/japplphysiol.00741.2003. [DOI] [PubMed] [Google Scholar]
- 6.Garber MA, McDowell DL, Hutton WC. Bone loss during simulated weightlessness: a biomechanical and mineralization study in the rat model. Aviat Space Environ Med. 2000;71:586–92. [PubMed] [Google Scholar]
- 7.Li CY, Majeska RJ, Laudier DM, Mann R, Schaffler MB. High-dose risedronate treatment partially preserves cancellous bone mass and microarchitecture during long-term disuse. Bone. 2005;37:287–295. doi: 10.1016/j.bone.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 8.Rubin C, Gross T, Qin YX, Fritton S, Guilak F, McLeod K. Differentiation of the bone-tissue remodeling response to axial and torsional loading in the turkey ulna. J Bone Joint Surg Am. 1996;78:1523–33. doi: 10.2106/00004623-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Vico L, Collet P, Guignandon A, Lafage-Proust MH, Thomas T, Rehaillia M, Alexandre C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355:1607–11. doi: 10.1016/s0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 10.Collet P, Uebelhart D, Vico L, Moro L, Hartmann D, Roth M, Alexandre C. Effects of 1- and 6-month spaceflight on bone mass and biochemistry in two humans. Bone. 1997;20:547–51. doi: 10.1016/s8756-3282(97)00052-5. [DOI] [PubMed] [Google Scholar]
- 11.Grynpas MD, Kasra M, Renlund R, Pritzker KP. The effect of pamidronate in a new model of immobilization in the dog. Bone. 1995;17:225S–232S. doi: 10.1016/8756-3282(95)00296-p. [DOI] [PubMed] [Google Scholar]
- 12.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–12. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 13.Turner RT, Evans GL, Wakley GK. Spaceflight results in depressed cancellous bone formation in rat humeri. Aviat Space Environ Med. 1995;66:770–4. [PubMed] [Google Scholar]
- 14.Zerwekh JE, Ruml LA, Gottschalk F, Pak CY. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res. 1998;13:1594–601. doi: 10.1359/jbmr.1998.13.10.1594. [DOI] [PubMed] [Google Scholar]
- 15.Thomas T, Vico L, Skerry TM, Caulin F, Lanyon LE, Alexandre C, Lafage MH. Architectural modifications and cellular response during disuse-related bone loss in calcaneus of the sheep. J Appl Physiol. 1996;80:198–202. doi: 10.1152/jappl.1996.80.1.198. [DOI] [PubMed] [Google Scholar]
- 16.Zehnder Y, Luthi M, Michel D, Knecht H, Perrelet R, Neto I, Kraenzlin M, Zach G, Lippuner K. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004;15:180–9. doi: 10.1007/s00198-003-1529-6. [DOI] [PubMed] [Google Scholar]
- 17.Nazarian A, Stauber M, Zurakowski D, Snyder BD, Muller R. The interaction of microstructure and volume fraction in predicting failure in cancellous bone. Bone. 2006;39:1196–202. doi: 10.1016/j.bone.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Weinreb M, Patael H, Preisler O, Ben-Shemen S. Short-term healing kinetics of cortical and cancellous bone osteopenia induced by unloading during the reloading period in young rats. Virchows Arch. 1997;431:449–52. doi: 10.1007/s004280050122. [DOI] [PubMed] [Google Scholar]
- 19.Nelson RA. Winter sleep in the black bear. A physiologic and metabolic marvel. Mayo Clin Proc. 1973;48:733–7. [PubMed] [Google Scholar]
- 20.Harvey KB, Donahue SW. Bending properties, porosity, and ash fraction of black bear (Ursus americanus) cortical bone are not compromised with aging despite annual periods of disuse. J Biomech. 2004;37:1513–20. doi: 10.1016/j.jbiomech.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 21.McGee ME, Magic KW, Miller DL, Maki AJ, Donahue SW. Black bear femoral porosity decreases and mechanical properties increase with age despite annual periods of disuse (hibernation) Eng Frac Mech. 1952;74:1942. 2007. [Google Scholar]
- 22.McGee ME, Miller DL, Auger J, Black HL, Donahue SW. Black bear femoral geometry and cortical porosity are not adversely affected by ageing despite annual periods of disuse (hibernation) J Anat. 2007;210:160–9. doi: 10.1111/j.1469-7580.2006.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey KB, Drummer TD, Donahue SW. The tensile strength of black bear (Ursus americanus) cortical bone is not compromised with aging despite annual periods of hibernation. J Biomech. 2005;38:2143–50. doi: 10.1016/j.jbiomech.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 24.McGee ME, Maki AJ, Johnson SE, Nelson OL, Robbins CT, Donahue SW. Decreased bone turnover with balanced resorption and formation prevent cortical bone loss during disuse (hibernation) in grizzly bears (Ursus arctos horribilis) Bone. 2008;42:396–404. doi: 10.1016/j.bone.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folk GE. Physiological observations of subarctic bears under winter den conditions. In: Fisher KC, Dawe AR, Lyman CP, Schonbaum E, South FE Jr, editors. Mammalian Hibernation. New York: American: Elsevier Publishing Company, Inc.; 1967. pp. 75–85. [Google Scholar]
- 26.Nelson RA. Urea metabolism in the hibernating black bear. Kidney Int Suppl. 1978:S177–9. [PubMed] [Google Scholar]
- 27.Nelson RA, Wahner HW, Jones JD, Ellefson RD, Zollman PE. Metabolism of bears before, during, and after winter sleep. Am J Physiol. 1973;224:491–6. doi: 10.1152/ajplegacy.1973.224.2.491. [DOI] [PubMed] [Google Scholar]
- 28.Pardy CK, Wohl GR, Ukrainetz PJ, Sawers A, Boyd SK, Zernicke RF. Maintenance of bone mass and architecture in denning black bears (Ursus americanus) J Zool Lond. 2004;263:359–364. [Google Scholar]
- 29.Floyd T, Nelson RA, Wynne GF. Calcium and bone metabolic homeostasis in active and denning black bears (Ursus americanus) Clin Orthop. 1990;8:301–9. [PubMed] [Google Scholar]
- 30.Coy PL, Garshelis DL. Reconstructing reproductive histories of black bears from the incremental layering in dental cementum. Can J Zool. 1992;70:2150–2160. [Google Scholar]
- 31.Schenk RK, Olah AJ, Herrmann W. Preparation of calcified tissue for light microscopy. In: Dickson GR, editor. Methods of Calcified Tissue Preparation. New York: Elsevier; 1984. pp. 1–56. [Google Scholar]
- 32.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 33.Westwood S. Loss of Bone Mass With Aging and Femoral Sexual Dimorphism in the American Black Bear (Ursus americanus) Provo: Brigham Young University; 1996. p. 50. Department of Zoology. [Google Scholar]
- 34.Caillot-Augusseau A, Lafage-Proust MH, Soler C, Pernod J, Dubois F, Alexandre C. Bone formation and resorption biological markers in cosmonauts during and after a 180-day space flight (Euromir 95) Clin Chem. 1998;44:578–85. [PubMed] [Google Scholar]
- 35.Weinreb M, Rodan GA, Thompson DD. Osteopenia in the immobilized rat hind limb is associated with increased bone resorption and decreased bone formation. Bone. 1989;10:187–94. doi: 10.1016/8756-3282(89)90052-5. [DOI] [PubMed] [Google Scholar]
- 36.Reiter AL, Volk A, Vollmar J, Fromm B, Gerner HJ. Changes of basic bone turnover parameters in short-term and long-term patients with spinal cord injury. Eur Spine J. 2007;16:771–6. doi: 10.1007/s00586-006-0163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Modlesky CM, Majumdar S, Narasimhan A, Dudley GA. Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. J Bone Miner Res. 2004;19:48–55. doi: 10.1359/JBMR.0301208. [DOI] [PubMed] [Google Scholar]
- 38.Keyak J, Koyama A, LeBlanc A, Lu Y, Lang T. Reduction in proximal femoral strength after long-duration spaceflight. 53rd Annual Meeting of the Orthopaedic Research Society; San Diego, CA: Orthopaedic Research Society; 2007. p. 15. [Google Scholar]
- 39.McGee ME, Barlow LN, Simoni KJ, Wojda SJ, Auger J, Black HL, Donahue SW. North American Congress on Biomechanics. Ann Arbor, MI: American Society of Biomechanics; 2008. Post-hibernation black bears (Ursus americanus) do not demonstrate cortical bone loss compared to pre-hibernation bears despite 6 months of disuse. [Google Scholar]
- 40.Furst A, Meier D, Michel S, Schmidlin A, Held L, Laib A. Effect of age on bone mineral density and micro architecture in the radius and tibia of horses: an Xtreme computed tomographic study. BMC Vet Res. 2008;4:3. doi: 10.1186/1746-6148-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cerroni AM, Tomlinson GA, Turnquist JE, Grynpas MD. Bone mineral density, osteopenia, and osteoporosis in the rhesus macaques of Cayo Santiago. Am J Phys Anthropol. 2000;113:389–410. doi: 10.1002/1096-8644(200011)113:3<389::AID-AJPA9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 42.Jayo MJ, Jerome CP, Lees CJ, Rankin SE, Weaver DS. Bone mass in female cynomolgus macaques: a cross-sectional and longitudinal study by age. Calcif Tissue Int. 1994;54:231–6. doi: 10.1007/BF00301684. [DOI] [PubMed] [Google Scholar]
- 43.Ding M, Odgaard A, Linde F, Hvid I. Age-related variations in the microstructure of human tibial cancellous bone. J Orthop Res. 2002;20:615–21. doi: 10.1016/S0736-0266(01)00132-2. [DOI] [PubMed] [Google Scholar]
- 44.Donahue SW, Galley SA, Vaughan MR, Patterson-Buckendahl P, Demers LM, Vance JL, McGee ME. Parathyroid hormone may maintain bone formation in hibernating black bears (Ursus americanus) to prevent disuse osteoporosis. J Exp Biol. 2006;209:1630–8. doi: 10.1242/jeb.02185. [DOI] [PubMed] [Google Scholar]
- 45.McGee ME, Galley SA, Nelsen MP, Tsai CJ, Donahue SW. Synthetic black bear (Ursus americanus) PTH 1-34 upregulates c-fos and decreases the ratio of Bax/Bcl-2 in MC-3T3 osteoblastic cells. 53rd Annual Meeting of the Orthopaedic Research Society; San Diego, CA: Orthopaedic Research Society; 2007. pp. Poster #1289. [Google Scholar]
- 46.Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–37. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dehority W, Halloran BP, Bikle DD, Curren T, Kostenuik PJ, Wronski TJ, Shen Y, Rabkin B, Bouraoui A, Morey-Holton E. Bone and hormonal changes induced by skeletal unloading in the mature male rat. Am J Physiol. 1999;276:E62–9. doi: 10.1152/ajpendo.1999.276.1.e62. [DOI] [PubMed] [Google Scholar]
- 48.Thomas T, Skerry TM, Vico L, Caulin F, Lanyon LE, Alexandre C. Ineffectiveness of calcitonin on a local-disuse osteoporosis in the sheep: a histomorphometric study. Calcif Tissue Int. 1995;57:224–8. doi: 10.1007/BF00310263. [DOI] [PubMed] [Google Scholar]
- 49.Palle S, Vico L, Bourrin S, Alexandre C. Bone tissue response to four-month antiorthostatic bedrest: a bone histomorphometric study. Calcif Tissue Int. 1992;51:189–94. doi: 10.1007/BF00334546. [DOI] [PubMed] [Google Scholar]


