Abstract
Response expectancies, defined as expectations for nonvolitional responses, have been proposed to contribute to the experience of side effects of cancer and its treatment. To statistically evaluate this association, a systematic search of the published literature was conducted, resulting in fourteen studies appropriate for meta-analysis. Results revealed a significant (Z = 6.58, P < 0.001) medium-sized (r = 0.36) association between patients’ response expectancies for cancer treatment-related side effects and the experience of these side effects. Assessment of response expectancies with reference to the time the treatment-related side effect would occur resulted in larger effect sizes than when such temporal specificity in assessment was not included, Q(1) = 10.27, P < 0.01. AU: SHOULD THIS BE 0.01? In your original, it was just 01. Effect sizes were also moderated by patients’ prior experience with cancer treatment, Q(1) = 18.91, P = 0.001, such that prior experience led to stronger associations between response expectancies and side effects than no prior experience. Relationships between response expectancies and pain, fatigue, nausea and vomiting were explored. Effect sizes did not differ between side effects, with the exception that the relationship was significantly stronger for pain than for vomiting (P < 0.05). Overall, these results support the contribution of response expectancies to cancer treatment-related side effects. Additionally, the results support the conduct of research on interventions to alter response expectancies, with the goal of reducing side effects and improving patient quality of life.
Keywords: Cancer, cancer treatment, expectancy, response expectancies, side effects, pain, fatigue, nausea, vomiting
Introduction
While medical advances have improved cancer survival, there is an increasing emphasis on addressing the “human costs” of cancer treatment (1, p. 1110). These costs include the aversive side effects of often life-saving treatments. Side effects including pain, fatigue, nausea, and vomiting are not only commonly experienced; they can significantly negatively impact quality of life (2-4) and increase patients’ distress (5). Due to the clinical consequences of cancer treatment-related side effects, psychological predictors of such side effects have been explored. Among the most promising predictors are response expectancies.
Response expectancies (6,7) are specific expectations for nonvolitional outcomes, rather than expectations for behaviors or external events. For example, an expectation that one will become nauseated would be considered a response expectancy, while expectations that one will be able to lift one’s arm, or that it will rain today would not. Response expectancies are thought to reflect automatic (i.e., typically not under voluntary control) processes, and they are hypothesized to directly cause expected outcomes without further psychological mediators (6). Researchers in a range of clinical and experimental contexts have sought to determine if response expectancies predict subsequent nonvoltional experiences, and research has typically reported a significant association between these two constructs (8,9). The relationship between individuals’ response expectancies and experiences is especially pertinent to cancer patients because of the aversive side effects (e.g., pain, fatigue, nausea, and vomiting) commonly associated with cancer treatment regimens (e.g., surgery, chemotherapy, radiotherapy). That is, such cancer treatment-related side effects are nonvolitional outcomes, to which response expectancies may make a significant contribution. Therefore, a better understanding of the role of response expectancies in cancer patients’ experiences of side effects may not only be scientifically informative, but such understanding can also provide a stronger foundation for the development of future interventions for this population to reduce their overall side effect burden.
Empirical research has examined the relationship between response expectancies and cancer treatment-related side effects including pain (10,11), fatigue (11,12), nausea (13,14), and vomiting (15,16). Some of this research has reported significant effects of response expectancies on side effects (e.g., 12,15) whereas others have not (e.g., 17,18). A recent review of this literature generally supported the effects of response expectancies on outcomes (9). However, this review did not focus exclusively on cancer, did not statistically quantify the relationship between response expectancies and side effects, and did not explain why this relationship could potentially vary (i.e., an exploration of effect moderators). Therefore, it is of scientific interest to determine if the response expectancy effect exists in patients undergoing cancer treatments, and if it does, what is the overall magnitude of the relationship between response expectancies and side effects of cancer treatment?
Differences in the methodology used to assess response expectancies may help explain potential variations in the association between response expectancies and side effects. Response Expectancy Theory suggests that greater specificity in the assessment of both response expectancies and expected outcomes themselves should result in a stronger relationship between these variables (6,7). For example, studies that ask about the occurrence of a treatment side effect at a particular point in treatment (i.e., “In the clinic before your next chemotherapy infusion, how nauseated to you expect to be;” 12, p. 832), and then assess the side effect at that time should show stronger relations between response expectancies and the expected outcome than studies which take a more general or broader approach to response expectancy assessment (i.e., “I am certain I WILL have nausea;” 19). Therefore, we predict that studies with time-specific measures of response expectancies should show a stronger relationship between expectancies and side effects than studies with measures of expectancies that are not time-specific.
Response expectancies are also proposed to be self-reinforcing. That is, response expectancies should strengthen as a person gains experience with the specific event to which the expectancies correspond, but only if the event actually occurs following the expectancy (6,7,20). For example, one may expect nausea following a chemotherapy infusion. If one does indeed experience such post-treatment nausea, then the response expectancy for nausea will strengthen prior to the next chemotherapy infusion. The increase in response expectancy can subsequently increase the likelihood that nausea will occur following the next infusion. Therefore, we predict greater effect sizes among patients who have had prior experience with a cancer treatment than those who were treatment naïve.
The current study systematically evaluated the literature on the relationship between response expectancies and cancer-related side effects. Meta-analytic approaches were used to explore the hypothesis that this relationship was statistically significant across studies (Hypothesis 1), as well to determine the overall magnitude of the effect. Additionally, the study proposed two possible factors that could explain variance in the strength of this effect. The first factor proposed was that the assessment of time-specific expectancies would result in a stronger relationship between response expectancies and side effects than the assessment of expectancies for a side effect during treatment in general (Hypothesis 2). The second factor proposed was that patients with previous experience with treatments would report a stronger relationship between response expectancies and side effects than including patients undergoing a treatment for the first time (Hypothesis 3).
Methods
Study Selection
Studies included in the analysis were obtained by a systematic search of the databases PsycInfo, PubMed and CINAHL from inception through June 2008. All PsycInfo searches included the following string (mj = neoplasms)) not (mj = life-expectancy)) and (LA:PSYI = ENGLISH) and ((MD:PSYI = EMPIRICAL-STUDY) or (MD:PSYI = EXPERIMENTAL-REPLICATION) or (MD:PSYI = FOLLOWUP-STUDY) or (MD:PSYI = LONGITUDINAL-STUDY) or (MD:PSYI = PROSPECTIVE-STUDY) or (MD:PSYI = QUANTITATIVE-STUDY) or (MD:PSYI = TREATMENT-OUTCOME-CLINICAL-TRIAL)) and ((PT:PSYI = JOURNAL) or (PT:PSYI = PEER-REVIEWED-JOURNAL) or (PT:PSYI = PEER-REVIEWED-STATUS-UNKNOWN)). The search terms used were: expectancy, expectancies, expectations, expect*, mj = pain, mj = fatigue, mj = nausea, mj = vomiting, mj = chemotherapy, postchemotherapy, or side effects. Similar variations and limits in search terms and limitations were used (with the indicated modifications) in PubMed (“/psychology” was added to neoplasms, pain, fatigue, nausea, vomiting, and chemotherapy) and CINHAL (“/psychosocial-factors” was added to the same search terms). The resulting abstracts were reviewed to determine if they fit the inclusion criteria.
Inclusion criteria for studies in this review were that they: 1) were published in a peer-reviewed journal, 2) were in the English language, 3) used prospective methodology, 4) assessed expectations of the side effects pain, fatigue, nausea or vomiting and the following experience of the respective side effect, 5) included adult patients (over 18 years of age) who were involved in any cancer related treatment, 6) were not a case report, and 7) reported the statistical information needed to calculate an effect size.
The complete text was retrieved for abstracts that met the inclusion criteria. The reference sections of these studies, in addition to the reference sections of related articles and reviews (9, 21-24), were manually searched for further eligible studies. This resulting group of studies was evaluated further to determine if the inclusion criteria were met.
Study Coding
Two independent coders (S. J. S. and G. H. M.) developed a coding form and manual, which were used to systematically capture aspects of each study. All three authors reconciled any discrepancies encountered. The coding scheme included demographic variables of participants and characteristics of the effect size (e.g., if the effect size was reported directly or imputed, instrument used to assess expectancies, if the measurement of expectancies were time-specific, whether patients had previous experience with the cancer treatment).
Analyses
Pearson’s r effect sizes were determined for each study. Pearson’s rs were either directly reported in the articles or were imputed using established formulas (25). For any study where effect sizes were available at multiple time points or for multiple side effects, these effect sizes were averaged to determine the overall effect for that study. For studies which dichotomized response expectancies or side effect outcomes (e.g., experiencing nausea vs. not experiencing nausea; 13), a published formula to correct for the dichotomization of inherently continuous variables was applied (25). Following correction, these effect sizes were included in the primary analyses, using the macros made available by Lipsey and Wilson (25) for SPSS 15.0. To compute the overall effect size for the relationship between response expectancies and cancer treatment-related side effects, Pearson’s rs for each study were converted into Fisher’s zs. Each study effect size was weighted by its sample size (sample size was also adjusted for dichotomization, where applicable). After the average effect size was computed from these standardized and weighted effect sizes, the average Fisher’s z was converted back to a Pearson’s r for ease of interpretation. Moderator analyses were also calculated using the macros for SPSS. The fail-safe n (26,27) was calculated with Meta 5.3 (28).
Results
Included Studies
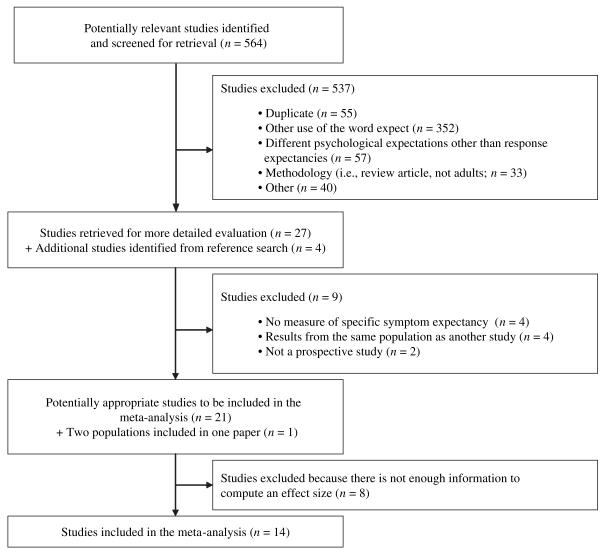
The search of electronic databases resulted in 120 abstracts from PsycInfo, 320 from PubMed and an additional 124 from CINAHL. The inclusion and exclusion of these 564 studies and the addition of studies found in the manual search are displayed in Figure 1, consistent with QUOROM guidelines (29).
Fig. 1.
Diagram of study inclusion and exclusion
Eight studies were excluded from the meta-analysis because effect sizes could not be extracted from the articles. Most commonly, this occurred when the relationship between expectancies and side effects was only reported within the context of a larger regression analysis that controlled for other variables (2, 30-32). The inclusion of other variables in the model (covariates) made it impossible to tease out direct relationships between response expectancies and side effects. Two additional studies were excluded because they did not report whether participants who expected side effects were the same participants who experienced the side effects (33,34). A single effect size was calculated for studies reporting on overlapping samples of participants. The systematic search and application of inclusion criteria resulted in the 14 studies, listed in Table 1.
Table 1.
Study Characteristics of Included Studies
| # | Study | n | Treatment | Time- Specific |
Previous Tx |
Cassileth Measure |
Symptoms | ES Imputed |
|---|---|---|---|---|---|---|---|---|
| 1 | Andrykowski et al., 1988 (35) | 55 | Chemotherapy | No | No | Yes | AN and PTN | Yes |
| Jacobsen et al., 1988 (36) | ||||||||
| Montgomery et al., 1998 (37) | ||||||||
| Tomoyasu, Bovbjerg, & Jacobsen, 1996 (38) | ||||||||
| 2 | Andrykowski & Gregg, 1992 (17) | 65 | Chemotherapy | No | No | Yes | PTN | No |
| Andrykowski, Redd, & Hatfield, 1985 (39) | ||||||||
| 3 | Haut et al., 1991 (13) | 36 | Chemotherapy | No | No | No | PTN (with Vomiting) | Yes |
| 4 | Hickok et al., 2001 (14) | 63 | Chemotherapy | No | No | Yes | AN, PTN, Vomiting | No |
| 5 | Higgins et al., 2007 (18) | 56 | Chemotherapy | Yes | No | Yes | PTN, DN | Yes |
| 6 | Montgomery & Bovbjerg, 2000 (20) | 26 | Biopsy Surgery | Yes | Yes | Yes | Pain | Yes |
| 7 | Montgomery & Bovbjerg, 2001 (12) | 52 | Chemotherapy | Yes | Yes | No | PTN | No |
| 8 | Montgomery & Bovbjerg, 2004 (11) | 60 | Chemotherapy | Yes | No | No | AN, Fatigue | No |
| 9 | Montgomery et al., 2002 (10) | 63 | Cancer Surgery | Yes | No | No | Pain, PTN, Fatigue | No |
| 10 | Rhodes et al., 1995 (15) | 299 | Chemotherapy | No | No | No | PTN, Vomiting | Yes |
| 11 | Roscoe, Hickok, & Morrow, 2000 (40) | 29 | Chemotherapy | No | No | Yes | PTN | Yes |
| 12 | Roscoe et al., 2004 (41) | 194 | Chemotherapy | No | No | Yes | PTN, Vomiting | No |
| 13 | Shelke et al., 2008 (19) | 322 | Chemotherapy | No | No | Yes | PTN | Yes |
| 14 | Zachariae et al., 2007 (16) | 125 | Chemotherapy | No | No | No | PTN, Vomiting, Fatigue | No |
| Zachariae et al., 2007 (42) |
AN = anticipatory nausea; PTN = post-treatment nausea, DN = delayed nausea.
Demographics
The mean age of the participants was 53.4 (standard deviation = 5.81, k = 13) years. They were primarily White (approximately 79%, 8% Black, 4% Hispanic, 1% Asian, and 9% other or not categorized, k = 8). A majority were female (89%, k = 14) and married (65%, k = 7) and 44% graduated college (k = 6). Twelve of the studies (86%) followed patients who were undergoing chemotherapy, while the other two studies were of patients undergoing breast cancer-related surgeries. No studies explored expectancy effects in radiotherapy. Eight of the studies (57%) included patients undergoing breast cancer-related procedures. One study focused exclusively on gynecologic cancers (40). The remaining studies were comprised of mixed groups of cancer patients, including breast cancer (k = 3), lung cancer (k = 3), lymphoma (k = 3), colorectal cancer (k = 2), gynecological cancers (k = 1), and other types of cancer (k = 4). Cancer type was not indicated in two studies (15,19). The five studies that indicated cancer stage were in samples diagnosed with early stage breast cancer. Stage of cancer was not indicated in the remaining studies. Of the studies that reported antiemetic use, all participants were prescribed antiemetics following medical centers’ standard procedures, resulting in a reported 62.4% taking antiemetics (k = 9).
Hypothesis 1: Main Effect of Response Expectancies on Cancer Treatment-Related Side Effects
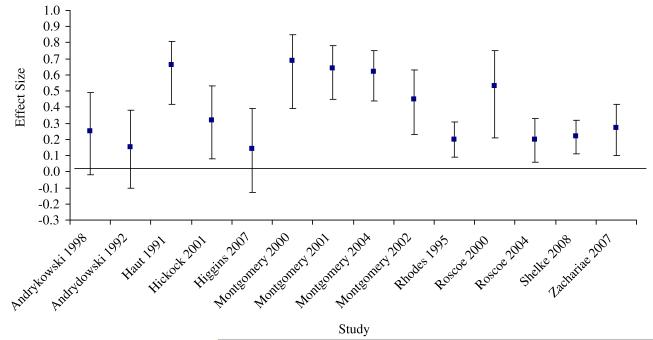
Hypothesis 1 was confirmed. There was a significant medium overall effect size for the relationship between response expectancies and corresponding side effects, r = 0.36, 95% confidence interval (CI) 0.26, 0.45, Z = 6.58, P < 0.001, adopting a random effects model (43). This effect was pooled from 14 studies with a total of 1,445 participants as presented in Figure 2. Additionally, fail-safe n analyses indicated that it would be necessary for 120 studies with null-results to reduce the present effect size of r = 0.36 to r = 0.05, and 53 studies to reduce it to a small effect size of r = 0.10.
Fig. 2.
Effect sizes and confidence intervals of included studies.
A homogeneity analysis was also significant, Q(13) = 38.69, P < 0.001, indicating that there was variance that could be potentially explained by moderating factors. As a check, moderator analysis confirmed that imputation did not systematically bias effect sizes, Q(1) = 0.06, P = 0.81.
Hypothesis 2: Time-Specific Assessment Moderation
With regard to Hypothesis 2, results confirmed that studies which assessed time-specific response expectancies (k = 5) had larger effect sizes, r = 0.52, CI 0.39, 0.63, than those studies (k = 9) that assessed response expectancies for side effects at any point during cancer treatment, r = 0.27, CI 0.17, 0.37, Q(1) = 10.24, P < 0.01. As a check, the potential effect of measurement method (i.e., particular scale used) was also analyzed. Measures used to assess response expectancies were either based on a Likert scale developed by Cassileth and colleagues (k = 8: 34), another form of a Likert scale (13,15), or a visual analog scale (10-12,16). There was no difference in effect sizes based on expectancy measure used Q(1) = 2.64, P = 0.10.
Hypothesis 3: Experience with Treatment Moderation
To test Hypothesis 3, we examined whether effect sizes differed based on patients’ experience with treatment. Only the studies of participants undergoing chemotherapy were included in this analysis due to the repeated nature of this treatment regimen (i.e., multiple infusions), as well as to hold the cancer treatment constant (k = 12). This sub-analysis resulted in an overall effect size of r = 0.33 (Z = 5.94, P < 0.001) with a significant homogeneity analysis, Q(11) = 29.61, P < 0.01, indicating that moderator analyses should be performed. Of the twelve chemotherapy studies, 10 assessed expectancies prior to the first chemotherapy infusion (i.e., before patients had any chemotherapy experience). Two studies assessed expectancies after patients had some experience with chemotherapy (e.g., assessed expectancies after the third chemotherapy infusion). Studies which examined the relationship between response expectancies and side effects among patients who had treatment experience were compared to studies that only assessed expectancies among patients with no prior treatment experience (e.g., prior to the first ever infusion in chemotherapy naïve participants). This moderator analysis revealed that the effect size for studies (k = 2) which included expectancy assessments after experience with chemotherapy infusions, r = 0.63, CI 0.48, 0.74) was significantly greater, Q(1) = 18.91, P < 0.01 than the effect size for studies (k = 10) which only measured expectancies prior to treatment experience, r = 0.24, CI 0.17, 0.30). Hypothesis 3 was thus confirmed, in that expectancies were more strongly related to side effects when they were based on direct treatment experience.
Post-Hoc Analyses
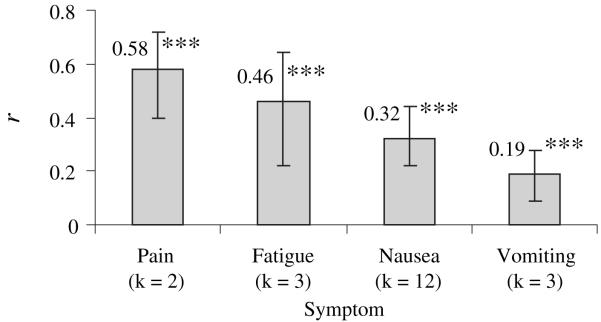
As effect sizes were derived from the association of response expectancies with a variety of side effects (e.g., pain, fatigue), we chose to explore whether type of side effect differentially influenced the effect size. Effect sizes by side effect are depicted in Figure 3. Response expectancies were significantly related to all of the side effects. Confidence intervals suggest that there were no differences between the magnitude of these effect sizes with the exception of pain and vomiting (P < 0.05). Response expectancies made a stronger contribution to pain than to vomiting.
Fig. 3.
Effect size for each side effect; ***P < 0.001. Note: All values are significantly different than zero.
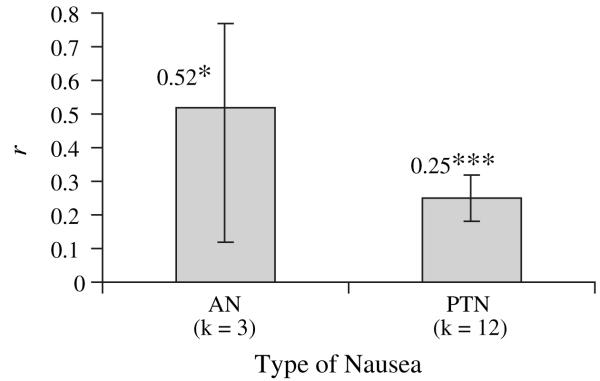
Additionally, nausea was the most commonly studied side effect. To explore if there was variability within type of nausea, the effect sizes for anticipatory nausea versus post-treatment nausea are displayed in Figure 4. Effect sizes for anticipatory nausea and post-treatment nausea were both significantly greater than zero (P < 0.05 and P < 0.001, respectively), and did not differ from each other.
Fig. 4.
Effect sizes for types of nausea; *P < 0.05, **P < 0.01, ***P < 0.001. Note: All values are significantly different than zero. AN = anticipatory nausea, PTN = post-treatment nausea.
Discussion
The present study established that there is a significant, positive, medium-sized effect of response expectancies on the experience of cancer treatment-related side effects. This meta-analytic result adds empirical support to the conclusions drawn from a previous narrative review (9). As hypothesized, the relationship between response expectancies and side effects was significantly moderated by how expectancies were measured (e.g., temporal specificity of the assessment). Response expectancy effects were stronger when they were assessed in a time-specific manner than when they referred to treatment in general. Also, previous experience with chemotherapy was a significant moderator of the expectancy effect of nausea in chemotherapy, such that having direct experience with chemotherapy treatment strengthened the association between response expectancies and nausea. Exploratory analyses suggested that the strength of response expectancy effects varied among different side effects, with response expectancies for pain having stronger effects than those for vomiting.
The result that the time-specific assessment of response expectancies had a stronger association with subsequent side effects than assessing patients’ expectancies for the occurrence of the side effect at any point during cancer treatment is consistent with theory (6,7). This result implies that future research should consider adopting time-specific methods for measuring response expectancies. Also, the result that there was a significant difference between the relationship of response expectancies and side effects based on patient experience with a treatment (i.e., before or after experience with chemotherapy) was also consistent with response expectancy theory (6,7). That is, expectancy effects were stronger following experience with cancer treatment. Based on modern conceptualizations of conditioned learning theory (e.g., 44), these results are not surprising. For example, previous trials of chemotherapy infusions may have led to expectancies for the outcome (in this case nausea), which in turn then contributed to the outcome. Learning trials, therefore, are one means by which response expectancies are formed (45). These results are similar to explanations describing psychological mechanisms underlying placebo effects following learning trials (45,46). Experimental studies have demonstrated that response expectancies following learning trials mediate subsequent conditioned placebo analgesic effects (47). However, the present results should be viewed with some caution, as only two studies measured expectancies following experience with chemotherapy.
The current results also suggest that there is variability in the strength of the relationship between response expectancies and side effects depending on the particular side effect under consideration. Although these results should be interpreted with caution, they are consistent with a study which examined response expectancy effects for pain, fatigue, and nausea within the same sample (11). In that study, strongest associations were between response expectancies and fatigue, followed by pain, and then by nausea in a sample of breast surgery patients. The present results are generally consistent with these data, as stronger effects were found for pain and fatigue relative to nausea. Weaker effects seen for vomiting in the present study may be due to the lower frequency of the occurrence of this side effect overall, perhaps restricting the variability to be explained. Furthermore, the relationship between expectancies and anticipatory nausea was of a similar magnitude to that of pain and fatigue. However, the effect size for anticipatory nausea was based on only three papers, and more work needs to be done in this area. In contrast, several papers (k = 12) examined the effects of response expectancies on post-treatment nausea in chemotherapy patients. The magnitude of this effect appears smaller than that for pain and fatigue. Perhaps the clear pharmacologic causes of post-treatment nausea make it more difficult to detect the psychological contribution of response expectancies.
Limitations and Future Directions
To standardize the relationship explored in the present analysis, the effect sizes reported did not control for other factors that may influence side effects such as age, sex, type of medications taken, or type of chemotherapy regimen (48), as the reporting of these factors varied widely between studies. Therefore, the present overall effect does not address the unique contribution of expectancies to these side effects when other potentially relevant factors are controlled. However, with the exception of a study that did not find an overall effect for the relationship between response expectancies and side effects (17), the relationship between response expectancies and side effects remained significant when controlling for other variables such as, age, sex, susceptibility to motion sickness, or the rating of emetic potential of the chemotherapeutic agents received (2,40). Thus, the robustness of this effect suggests that it will remain present when controlling for additional factors.
Another limitation of the present meta-analysis was the small number of studies used in moderator analyses. The subanalyses presented were designed to provide possible explanations for the variability in the relationship between response expectancies and side effects, and to guide future research. Future studies should inquire further about the consequences of assessing time-specific expectancies, if the expectancy effect is stronger after multiple treatments, which side effects are affected most by expectancies, and if anticipatory nausea is more affected by expectancies than post-treatment nausea. Understanding when response expectancies have their greatest effects on cancer treatment-related side effects would help to focus future research aiming to ameliorate such side effects by changing these expectancies.
The overall significant expectancy effect reported here supports the development of interventions that aim to change patients’ expectancies for cancer treatment-related side effects. One study has already demonstrated that manipulating response expectancies with hypnosis in patients undergoing excisional breast biopsies ultimately reduced their reports of distress and pain following the procedure (10). Hypnosis is an established method for changing expectations (7) and in turn reducing pain (49) and may be successfully applied to mitigating other side effects of cancer, as well (11,50). Other behavioral techniques explored to reduce side effects of cancer treatment, such the use of guided imagery (51) or acupressure wrist bands (52), may also be used to create an expectancy that targeted side effect will be reduced, in addition to benefiting patients through other mechanisms. Future research is needed to determine the most effective interventions for changing expectancies and in turn, reducing side effects and improving overall quality of life in the hundreds of thousands of patients who undergo cancer treatment each year.
Conclusion
In summary, response expectancies were significantly associated with side effects of cancer treatment. As for the methodological moderators, expectancies assessed with time-specific items were more likely to correspond with the presence of the expected side effect than expectancies asking about experiencing the side effect during treatment in general. Also, there was empirical support for the hypothesis that experience with cancer treatment would strengthen the relationship between response expectancies and side effects of that treatment. The overall expectancy effect indicated that response expectancies were related to a variety side effects including, pain, fatigue, nausea, and vomiting, although, the size of the effects for each side effect varied. Results suggest that it would be beneficial to continue to develop and use interventions to change expectancies, which will ultimately lessen the experience of these side effects and improve cancer patients’ quality of life.
Acknowledgments
This work was supported by the National Cancer Institute (5R25CA081137; K07CA131473) and the American Cancer Society (#RSGPB CPPB-108036). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- 1.National Institutes of Health National Institutes of Health state-of-the-science conference statement: Symptom management in cancer: pain, depression, and fatigue, July 15-17, 2002. J Natl Cancer Inst. 2003;95:1110–1114. doi: 10.1093/jnci/djg014. [DOI] [PubMed] [Google Scholar]
- 2.Colagiuri B, Roscoe JA, Morrow GR, et al. How do patient expectancies, quality of life, and postchemotherapy nausea interrelate? Cancer. 2008;113:654–661. doi: 10.1002/cncr.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta D, Lis CG, Grutsch JF. The relationship between cancer-related fatigue and patient satisfaction with quality of life in cancer. J Pain Symptom Manage. 2007;34:40–47. doi: 10.1016/j.jpainsymman.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Park KU. Assessment of change of quality of life in terminally ill patients under cancer pain management using the EORTC Core Quality of Life Questionnaire (QLQ-C30) in a Korean sample. Oncology. 2008;74:7–12. doi: 10.1159/000143212. [DOI] [PubMed] [Google Scholar]
- 5.Jim HS, Andrykowski MA, Munster PN, Jacobsen PB. Physical symptoms/side effects during breast cancer treatment predict posttreatment distress. Ann Behav Med. 2008;34:200–208. doi: 10.1007/BF02872674. [DOI] [PubMed] [Google Scholar]
- 6.Kirsch I. Response expectancy as a determinant of experience and behavior. Am Psychol. 1985;40:1189–1202. [Google Scholar]
- 7.Kirsch I. Changing expectations: a key to effective psychotherapy. Brooks/Cole Publishing Company; Pacific Grove, CA: 1990. [Google Scholar]
- 8.Kirsch I. Response expectancy theory and application: a decennial review. Appl Prev Psychol. 1997;6:69–79. [Google Scholar]
- 9.Roscoe JA, Jean-Pierre P, Shelke AR, et al. The role of patients’ response expectancies in side effect development and control. Curr Probl Cancer. 2006;30:40–98. doi: 10.1016/j.currproblcancer.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery GH, Weltz CR, Seltz M, Bovbjerg DH. Brief presurgery hypnosis reduces distress and pain in excisional breast biopsy patients. Int J Clin Exp Hypn. 2002;50:17–32. doi: 10.1080/00207140208410088. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery GH, Bovbjerg DH. Presurgery distress and specific response expectancies predict postsurgery outcomes in surgery patients confronting breast cancer. Health Psychol. 2004;23:381–387. doi: 10.1037/0278-6133.23.4.381. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery GH, Bovbjerg DH. Specific response expectancies predict anticipatory nausea during chemotherapy for breast cancer. J Consult Clin Psychol. 2001;69:831–835. doi: 10.1037//0022-006x.69.5.831. [DOI] [PubMed] [Google Scholar]
- 13.Haut MW, Beckwith BE, Laurie JA, Klatt N. Postchemotherapy nausea and vomiting in cancer patients receiving outpatient chemotherapy. J Psychosoc Oncol. 1991;9:117–130. [Google Scholar]
- 14.Hickok JT, Roscoe JA, Morrow GR. The role of patients’ expectations in the development of anticipatory nausea related to chemotherapy for cancer. J Pain Symptom Manage. 2001;22:843–850. doi: 10.1016/s0885-3924(01)00317-7. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes VA, Watson PM, McDaniel RW, Hanson BM, Johnson MH. Expectation and occurrence of postchemotherapy side effects: nausea and vomiting. Cancer Pract. 1995;3:247–253. [PubMed] [Google Scholar]
- 16.Zachariae R, Paulsen K, Mehlsen M, et al. Chemotherapy-induced nausea, vomiting, and fatigue--the role of individual differences related to sensory perception and autonomic reactivity. Psychother Psychosom. 2007;76:376–384. doi: 10.1159/000107566. [DOI] [PubMed] [Google Scholar]
- 17.Andrykowski MA, Gregg ME. The role of psychological variables in post-chemotherapy nausea: Anxiety and expectation. Psychosom Med. 1992;54:48–58. doi: 10.1097/00006842-199201000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Higgins SC, Montgomery GH, Bovbjerg DH. Distress before chemotherapy predicts delayed but not acute nausea. Support Care Cancer. 2007;15:171–177. doi: 10.1007/s00520-006-0113-y. [DOI] [PubMed] [Google Scholar]
- 19.Shelke AR, Roscoe JA, Morrow GR, et al. Effect of a nausea expectancy manipulation on chemotherapy-induced nausea: a University of Rochester Cancer Center Community Clinical Oncology Program Study. J Pain Symptom Manage. 2008;35:381–387. doi: 10.1016/j.jpainsymman.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery GH, Bovbjerg DH. Pre-infusion expectations predict post-treatment nausea during repeated adjuvant chemotherapy infusions for breast cancer. Br J Health Psychol. 2000;5:105–119. [Google Scholar]
- 21.Chvetzoff G, Tannock IF. Placebo effects in oncology. J Natl Cancer Inst. 2003;95:19–29. doi: 10.1093/jnci/95.1.19. [DOI] [PubMed] [Google Scholar]
- 22.Hofman M, Morrow GR, Roscoe JA, et al. Cancer patients’ expectations of experiencing treatment-related side effects: a University of Rochester Cancer Center--Community Clinical Oncology Program Study of 938 patients from community practices. Cancer. 2004;101:851–857. doi: 10.1002/cncr.20423. [DOI] [PubMed] [Google Scholar]
- 23.Mondloch MV, Cole DC, Frank JW. Does how you do depend on how you think you’ll do? A systematic review of the evidence for a relation between patients’ recovery expectations and health outcomes. CMAJ. 2001;165:174–179. [PMC free article] [PubMed] [Google Scholar]
- 24.Schnur JB, Hallquist MN, Bovbjerg DH, et al. Predictors of expectancies for post-surgical pain and fatigue in breast cancer surgical patients. Pers Individ Dif. 2007;42:419–429. doi: 10.1016/j.paid.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipsey MW, Wilson DB. Practical Meta-analysis. SAGE Publications, Inc.; Thousand Oaks, CA: 2001. [Google Scholar]
- 26.Orwin RG. A fail-safe N for effect size in meta-analysis. J Educ Statistics. 1983;8:157–159. [Google Scholar]
- 27.Rosenthal R. The “file drawer problem” and tolerance for null results. Psychol Bull. 1979;85:638–641. [Google Scholar]
- 28.Meta: Meta-analysis programs. Vers. 5.3 National Collegiate Software Clearinghouse; Raleigh, NC: 1989. [Google Scholar]
- 29.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 30.Molassiotis A, Yam BM, Yung H, Chan FY, Mok TS. Pretreatment factors predicting the development of postchemotherapy nausea and vomiting in Chinese breast cancer patients. Support Care Cancer. 2002;10:139–145. doi: 10.1007/s00520-001-0321-4. [DOI] [PubMed] [Google Scholar]
- 31.Olver IN, Taylor AE, Whitford HS. Relationships between patients’ pre-treatment expectations of toxicities and post chemotherapy experiences. Psychooncology. 2005;14:25–33. doi: 10.1002/pon.804. [DOI] [PubMed] [Google Scholar]
- 32.Watson M, Meyer L, Thomson A, Osofsky S. Psychological factors predicting nausea and vomiting in breast cancer patients on chemotherapy. Eur J Cancer. 1998;34:831–837. doi: 10.1016/s0959-8049(97)10146-0. [DOI] [PubMed] [Google Scholar]
- 33.Beisecker A, Cook MR, Ashworth J, et al. Side effects of adjuvant chemotherapy: perceptions of node-negative breast cancer patients. Psychooncology. 1997;6:85–93. doi: 10.1002/(SICI)1099-1611(199706)6:2<85::AID-PON247>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 34.Cassileth BR, Lusk EJ, Bodenheimer BJ, et al. Chemotherapeutic toxicity--the relationship between patients’ pretreatment expectations and posttreatment results. Am J Clin Oncol. 1985;8:419–425. doi: 10.1097/00000421-198510000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Andrykowski MA, Jacobsen PB, Marks E, et al. Prevalence, predictors, and course of anticipatory nausea in women receiving adjuvant chemotherapy for breast cancer. Cancer. 1988;62:2607–2613. doi: 10.1002/1097-0142(19881215)62:12<2607::aid-cncr2820621226>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 36.Jacobsen PB, Andrykowski MA, Redd WH, et al. Nonpharmacologic factors in the development of posttreatment nausea with adjuvant chemotherapy for breast cancer. Cancer. 1988;61:379–385. doi: 10.1002/1097-0142(19880115)61:2<379::aid-cncr2820610230>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 37.Montgomery GH, Tomoyasu N, Bovbjerg DH, et al. Patients’ pretreatment expectations of chemotherapy-related nausea are an independent predictor of anticipatory nausea. Ann Behav Med. 1998;20:104–108. doi: 10.1007/BF02884456. [DOI] [PubMed] [Google Scholar]
- 38.Tomoyasu N, Bovbjerg DH, Jacobsen PB. Conditioned reactions to cancer chemotherapy: percent reinforcement predicts anticipatory nausea. Physiol Behav. 1996;59:273–276. doi: 10.1016/0031-9384(95)02072-1. [DOI] [PubMed] [Google Scholar]
- 39.Andrykowski MA, Redd WH, Hatfield AK. Development of anticipatory nausea: a prospective analysis. J Consult Clin Psychol. 1985;53:447–454. doi: 10.1037//0022-006x.53.4.447. [DOI] [PubMed] [Google Scholar]
- 40.Roscoe JA, Hickok JT, Morrow GR. Patient expectations as predictor of chemotherapy-induced nausea. Ann Behav Med. 2000;22:121–126. doi: 10.1007/BF02895775. [DOI] [PubMed] [Google Scholar]
- 41.Roscoe JA, Bushunow P, Morrow GR, et al. Patient expectation is a strong predictor of severe nausea after chemotherapy: a University of Rochester Community Clinical Oncology Program study of patients with breast carcinoma. Cancer. 2004;101:2701–2708. doi: 10.1002/cncr.20718. [DOI] [PubMed] [Google Scholar]
- 42.Zachariae R, Paulsen K, Mehlsen M, et al. Anticipatory nausea: the role of individual differences related to sensory perception and autonomic reactivity. Ann Behav Med. 2007;33:69–79. doi: 10.1207/s15324796abm3301_8. [DOI] [PubMed] [Google Scholar]
- 43.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 46.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 47.Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72:107–113. doi: 10.1016/s0304-3959(97)00016-x. [DOI] [PubMed] [Google Scholar]
- 48.Morrow GR, Roscoe JA, Hickok JT, et al. Nausea and emesis: evidence for a biobehavioral perspective. Support Care Cancer. 2002;10:96–105. doi: 10.1007/s005200100294. [DOI] [PubMed] [Google Scholar]
- 49.Montgomery GH, DuHamel KN, Redd WH. A meta-analysis of hypnotically induced analgesia: how effective is hypnosis. Int J Clin Exp Hypn. 2000;48:138–153. doi: 10.1080/00207140008410045. [DOI] [PubMed] [Google Scholar]
- 50.Montgomery GH, Bovbjerg DH, Schnur JB, et al. A randomized clinical trial of a brief hypnosis intervention to control side effects in breast surgery patients. J Natl Cancer Inst. 2007;99:1304–1312. doi: 10.1093/jnci/djm106. [DOI] [PubMed] [Google Scholar]
- 51.Kwekkeboom KL. A pilot study to predict success with guided imagery for cancer pain. Pain Manage Nurs. 2003;4:112–123. doi: 10.1016/s1524-9042(02)54213-2. [DOI] [PubMed] [Google Scholar]
- 52.Roscoe JA, Morrow GR, Hickok JT, et al. The efficacy of acupressure and acustimulation wrist bands for the relief of chemotherapy-induced nausea and vomiting. A University of Rochester Cancer Center Community Clinical Oncology Program multicenter study. J Pain Symptom Manage. 2003;26:731–742. doi: 10.1016/s0885-3924(03)00254-9. [DOI] [PubMed] [Google Scholar]