Abstract
Proteolysis is used by all forms of life for shaping the proteome in response to adverse environmental conditions in order to ensure optimal survival. Here we will address the role of proteolysis in helping cells respond to environmental stress, with a focus on the impact of proteolysis under DNA-damaging conditions and in maintenance of cellular homeostasis in response to metal exposure in bacteria.
Keywords: ClpXP, Metal homeostasis, Proteolysis, SOS response
1. Overview of the bacterial protease machines
Proteolysis is a powerful mechanism used by cells to control adaptation and recovery after exposure to a variety of stress conditions. Escherichia coli has five ATP-dependent proteases: ClpAP, ClpXP, FtsH, HslUV and Lon [16,17,43]. Each of these enzymes contains ATPase and proteolytic components. These components can be encoded either as domains in the same polypeptide chain (Lon and FtsH) or as two separate subunits (ClpAP, ClpXP and HslUV). To degrade proteins, the hexameric AAA+ ATPases (ClpA, ClpX or HslU) or the AAA+ ATPase component of Lon or FtsH recognizes specific substrates, then unfolds these proteins and translocates the polypeptides into the proteolytic chamber (e.g., ClpP, HslV or the attached peptidase) where they are cleaved into short peptides [43]. The ATPase components select the substrates by recognizing short peptide motifs often near the N or C terminus in the substrate sequence. Adaptor or delivery proteins facilitate recognition and promote degradation of several substrates [3,43].
2. Proteolysis plays key roles in adjusting protein levels during the SOS response
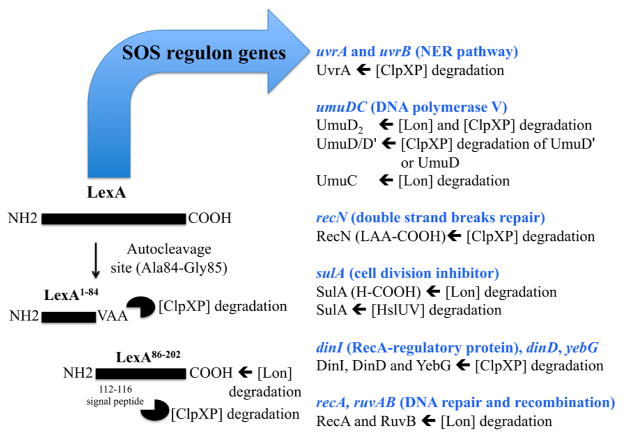
DNA damage, due to exposure to diverse environmental (chemical and physical) and endogenous (metabolic products) factors, induces the SOS response that, in turn, enables the cells to respond to damage and repair and replicate their DNA. The key regulator of the SOS response is the LexA repressor. In Escherichia coli, the LexA repressor undergoes a RecA and DNA damage-dependent autocleavage reaction that generates two LexA fragments that are degraded by the Lon and ClpXP proteases [26,36]. This LexA inactivation results in transcriptional activation of the approximately 40 SOS regulon genes [9]. Many of these genes encode proteins involved in repair of DNA damage or damage tolerance that enhance survival during exposure to DNA-toxic agents. These DNA repair proteins can be deleterious when present at inappropriate levels, especially in the absence of damage; therefore, their activity must be restricted both temporally and spatially to regions of DNA damage.
Survival under adverse environments that can damage DNA requires changes in the transcriptional and proteomic profiles. Over the past few years, it has become evident that many SOS regulon proteins are unstable and that changes in proteolytic stability are important for controlling their levels. E. coli uses three energy-dependent cytoplasmic proteases, ClpXP, Lon and HslUV, to degrade numerous SOS-regulated proteins: e.g., the LexA repressor, a component of the nucleotide excision repair (NER) pathway (UvrA), the components of the lesion-bypass DNA polymerase V (UmuD, UmuD′ and UmuC), RecN (double-strand DNA break-repair protein), and SulA (cell division inhibitor). A recent study showed that among SOS-response proteins, 25% were ClpXP substrates [38].
2.1. LexA degradation
E. coli LexA protein inhibits the transcription of genes belonging to the SOS regulon that are involved in DNA repair, replication and cell division. After DNA damage, the RecA protein is activated by binding to single-stranded DNA exposed in the cells, and stimulates LexA’s self-cleavage activity [28]. LexA’s hinge region connects the N-terminal DNA binding domain and the C-terminal dimerization domain and contains the Ala84-Gly85 autocleavage site. The resulting N- and C-terminal fragments of 84 and 118 amino acid residues, respectively, are then rapidly degraded in vivo [26]. The N-terminal fragment is degraded by the ClpXP protease, whereas the C-terminal fragment is a substrate for both Lon and ClpXP proteases [27,36].
A key to proper regulation of SOS induction is that the ClpXP and Lon proteases do not recognize the LexA repressor until after RecA-induced cleavage of the Ala84-Gly85 bond. ClpXP specifically recognizes the LexA fragments for destruction via newly created or exposed sequence motifs. The new C-terminal sequence on the N-terminal LexA fragment (VAA-coo−), that is very similar to the LAA-coo− of the ssrA degradation tag, targets it to ClpXP degradation [36]. SsrA is one of the best characterized degradation tags, an 11-amino acid peptide (AANDENYALAA-coo−) that is attached co-translationally to the C-terminus of nascent polypeptides when ribosomes stall [22]. LAA residues at the end of the ssrA tag are the principal recognition determinants of the ClpXP protease [11]. Furthermore, the C-terminal carboxylate makes an important contribution to the ClpX interaction, explaining why this sequence is specifically recognized when it is located at the precise C-terminal end of the protein [25]. Replacement of both alanines with aspartic acids in the LexA-DNA binding fragment inhibited the degradation by ClpXP, and cells expressing this variant were more sensitive to UV-irradiation [36]. Neher and colleagues showed that degradation of the LexA DNA binding fragment by ClpXP protease plays an important biological role by helping cells survive after DNA damage. Failure to degrade this fragment may result in incomplete derepression of the SOS response genes needed for optimal DNA repair.
The C-terminal LexA fragment is also unstable in wild-type cells and stabilized ~10-fold in Lon-defective cells [27], and is only modestly stabilized in the clpX − cells [36]. It has been proposed that autocleavage disrupts the structure of the C-terminal domain, exposing a peptide signal (located around residues 112–116) that is recognized by ClpX [36]. The sequence determinants that are recognized by Lon have not been investigated.
2.2. Degradation of the nucleotide excision repair (NER) protein UvrA
NER is a versatile DNA repair pathway that removes a wide range of DNA lesions through the concerted action of the UvrABC proteins. The uvrA and uvrB genes encode key components of this repair pathway and belong to the SOS network [9,23].
NER is comprised of two subpathways: global NER and transcription-coupled repair (TCR). Global NER repairs lesions throughout the genome via a multistep ATP-dependent process. The first step involves damage recognition by the UvrA2UvrB or UvrA2UvrB2 complex [30,47]. After the damaged is found, UvrA hydrolyzes ATP, dissociates from the complex, and the UvrB-DNA preincision complex is formed. The UvrC endonuclease then binds to this preincision complex and cleaves the damaged DNA strand on both sides of the lesion. This ssDNA segment is then removed to allow for repair DNA synthesis [49].
The TCR subpathway that preferentially repairs lesions from the transcribed strand of active genes requires Mfd, the transcription-coupling repair factor. Mfd recognizes stalled RNA polymerase (RNAP) at the damaged site and dissociates RNAP from the DNA, allowing repair proteins (UvrA and UvrB) access to the lesion [44]. As a consequence of the TCR subpathway, DNA lesions are repaired in the genome more efficiently from transcribed strands than from non-transcribed strands.
The UvrAB complex is thought to recognize distortions in the DNA. This idea is supported by the observation that pyrimidine-pyrimidone (6–4) photoproducts (6-4PPs), which distort the DNA backbone more than cyclobutane pyrimidine dimers (CPDs), are incised more efficiently [7]. CPDs and 6-4PPs are the major photoproducts formed in the genomic DNA upon exposure to UV irradiation. The NER machinery can also attack undamaged DNA and thus can be a source of spontaneous mutations [5,19]. Therefore, the levels and the activity of these repair proteins may need to be tightly controlled for optimal cell fitness.
Pruteanu and Baker showed that controlled proteolysis during the repair and recovery phases of a damage response is an elegant mechanism to restrict the activity of the NER protein UvrA to regions of DNA damage [41]. They showed that UvrA is induced upon UV irradiation as a result of increased protein stability and an induced rate of protein synthesis, in concert with increased transcription of uvrA. The UvrA levels peak between ~20 and 120 min post UV. During post-UV recovery, UvrA levels decrease principally as a consequence of ClpXP-dependent protein degradation. UvrA is degraded at rates that are correlated with the amount of repaired CPDs in the cells. CPDs are both induced by UV light and repaired with similar efficiences in wild-type, clpX or clpP mutant cells [41]. In wild-type cells, the maximum rate of UvrA degradation is reached by ~40 min post UV when ~80% of CPDs are removed. The rate of protein synthesis starts to decline gradually at this time point and enhanced protein degradation helps to keep UvrA from accumulating to higher levels. As repair progresses, UvrA levels fall due to decreased synthesis coupled with the maximum degradation rate to eventually restore UvrA to the pre-damaged level (by ~180 min post UV).
Undamaged DNA was shown to be a necessary cofactor for ClpXP-dependent degradation of UvrA in vitro [41]. This observation suggests that the UvrA bound to undamaged DNA is the form recognized by ClpXP. DNA binding thus may provide a mechanism by which the cells select the UvrA that should be degraded so as to prevent inappropriate targeting of NER to undamaged sites.
UvrA degradation is also influenced by protein-protein interactions. UvrB and Mfd share a region of homology that is believed to interact with UvrA [10]. Using genetic and in vitro degradation experiments Pruteanu and Baker showed that UvrA-UvrB protein-protein interactions contribute to increased UvrA stability during the initial phases of a DNA damage response, in addition to the damaged DNA-UvrA contacts. In contrast, Mfd appears to act as an enhancer of UvrA turnover, at least at later times during recovery [41].
These data unveil a complex network of interactions that contribute to tuning the level of UvrA in the cell in response to the extent of DNA damage, as well as striking parallels to the findings with excision repair proteins from eukaryotes. In E. coli, yeast and mammals, “distortion recognition factors” involved in critical early recognition step of repair (UvrA in E. coli, Rad4 in yeast, and XPC in mammals) are subjected to degradation and are stabilized by their interacting partner proteins (UvrB, Rad23 and HR23, respectively).
2.3. Degradation of the DNA polymerase V (UmuD′2C) components
DNA lesion bypass is an important cellular response to unrepaired damage in the genome during replication. The Umu proteins act in concert with RecA, Ssb and DNA polymerase III holoenzyme to facilitate the process of translesion synthesis. Translesion synthesis is a DNA damage tolerance process that enables the DNA replication machinery to replicate past DNA lesions. Because this repair pathway is error-prone by definition (it has poor fidelity and is often template-independent), the expression and activity of the Umu proteins is strictly controlled at several levels. The umuDC operon is under LexA control and both the UmuD and UmuC proteins are rapidly degraded in vivo.
During SOS induction, UmuD undergoes a similar RecA-activated self-cleavage reaction as does the LexA repressor. This cleavage removes the N-terminal 24 amino acids of UmuD to convert it into the UmuD′ form and activate it for its role in translesion synthesis. UmuD and UmuD′ can form both homodimers and a heterodimer, and these dimer species interact with different affinities to UmuC [4,21]. The UmuD′ dimer physically interacts with UmuC to form a heterotrimeric complex of UmuD′2C known as E. coli DNA polymerase V.
The Umu proteins are substrates for the Lon and ClpXP proteases and their stabilities are profoundly influenced by the interacting partners. Lon protease rapidly degrades UmuD before it is converted to the mutagenically active form known as UmuD′, and also degrades UmuC before it is stabilized by interaction with UmuD′. In contrast, UmuD′ has a much longer half-life in vivo and is poorly degraded by the Lon protease [13].
Alanine-block mutagenesis on umuD performed by Gonzalez and colleagues, followed by protein stability measurements in vivo, localized the sites within unprocessed UmuD responsible for Lon-mediated degradation [14]. Multiple alanine substitutions at residues 15-18 significantly stabilize UmuD. These substitutions, when combined with alanine replacements at residues 26–29, almost completely stabilize UmuD. However, multiple alanine substitutions spanning only residues 26–29 had no apparent effect on UmuD degradation [14]. Those data were interpreted to indicate that the primary Lon degradation signal is located between residues 15 and 18 (sequence: FPLF) with the auxiliary site between residues 26 and 29 (sequence: FPSP), of the amino terminal region of UmuD. The authors suggested that the auxiliary site may either stabilize the Lon-UmuD interaction at the putative Lon recognition site (FPLF) or may maintain the accessibility of the amino terminal region for Lon recognition. Fusion of the amino terminal 40 residues of UmuD to an otherwise stable protein was sufficient to convert it into a Lon substrate. This region contains the information necessary for binding and provides the foundation for substrate degradation by the Lon protease [14]. Interestingly, the residues necessary for Lon-mediated degradation span the UmuD SOS-induced cleavage site (Cys24-Gly25). Gonzalez and colleagues suggested that Lon might compete with RecA-mediated self-cleavage of UmuD, and that the targeting of the Lon protease to the amino terminal region of UmuD is a critical mechanism that ensures cell survival despite irreparable DNA lesions at minimal mutational cost [14].
ClpXP protease acts at a later stage (after activation of UmuD′) by specifically degrading UmuD′ in a UmuD/UmuD′ heterodimer [13]. Residues 9–12 (sequence: LREI) of unprocessed UmuD are necessary for UmuD′ degradation by ClpXP [15]. Alanine substitutions of these residues increased the stability of the UmuD protein and of the UmuD′ interacting subunit in the UmuD(mutant)/UmuD′ heterodimer. The LREI sequence is close to the putative Lon recognition signal (residues 15–18). However, the fact that in vivo degradation of the mutant UmuD (LREI-AAAA sequence substitution) homodimer by Lon occurred with the same efficiency as the wild-type UmuD homodimer suggests that the Lon degradation signal does not overlap with that used by ClpXP [14]. Neher and colleagues showed that this region in UmuD (sequence: LREI) is used to tether the protein to the N-terminal domain of ClpX, with UmuD behaving as a UmuD′ or UmuD delivery factor for ClpXP [37]. They proposed that low-affinity signals for ClpXP degradation in UmuD and UmuD′ are recognized efficiently only when the substrate is tethered to ClpX via a UmuD partner subunit. Using peptide binding studies and mutagenesis, the authors showed that, for example, a sequence around Arg-37 in UmuD may serve as such a degradation signal.
Regulated proteolysis of the Umu proteins provides a mechanism by which cells maintain the correct level of these proteins, both during and after exposure to DNA damage, enabling them to return to high-fidelity DNA synthesis. A recent study showed that Clp-mediated degradation plays an important role in preventing gratuitous mutagenesis [1]. Elevated dinB (error-prone DNA polymerase IV) expression and stabilization of the UmuD/UmuD′ heterodimer in clpXP double mutant cells both contribute to this elevated level of error-prone synthesis and mutagenesis.
2.4. Proteolytic degradation of other SOS proteins
The RecN protein involved in DNA double-strand-break repair has a short half-life; it is degraded by ClpXP (and to a lesser extent ClpAP) via recognition of a degradation signal at its C terminus [34,38]. The C-terminal sequence of RecN is very similar to the ssrA degradation tag (LAA-coo−). Substitution of both alanines with aspartic acids at the C-terminus of RecN results in an extremely stable protein [34,38]. DNA damage-induced RecN forms both nucleoid-associated and cytoplasmic foci. Degradation of the cytoplasmic RecN aggregates by ClpXP is important for cell viability in stressed cells with chronic DNA damage [34]. The toxic effect of RecN aggregates is specific to cells with DNA damage, since wild-type and ΔclpX cells had similar viability in the absence of DNA damage when RecN aggregates were induced from an arabinose-inducible expression vector [34]. The authors suggest that RecN aggregates might lead to sequestration of a de novo RecN protein and/or other DNA repair proteins, and specifically interfere with DNA repair pathways. These data again demonstrate the role of the ClpXP protease in efficient recovery from DNA damage by promoting turnover of a DNA damage-inducible protein (in this case RecN) that is crucial for cell homeostasis in cells stressed by DNA damage.
SulA, a cell division inhibitor and SOS-regulated protein, is degraded by Lon and HslUV to allow septation following recovery from DNA damage [33,50]. The degradation of SulA in vivo is predominantly due to Lon, with HslUV appearing to act as a backup. This conclusion is supported by the observation that SulA accumulation in an E. coli lon mutant during the SOS response inhibits cell division, but this effect can be suppressed by overexpression of HslUV [24]. Substitution of the extreme C-terminal histidine residue with another amino acid results in stabilization and accumulation of SulA [20]. The authors suggest that this C-terminal histidine residue is recognized specifically by Lon, leading to a high-affinity interaction. In vitro degradation of SulA revealed that Lon and HslUV cleave several sites in the functionally important regions of SulA (the central and C-terminal regions) despite the fact that the peptide bond specificities of the two enzymes were distinct from each other [39]. Degradation by both of these enzymes is generally processive; thereby, their action contributes to the efficient, rapid and accurate downregulation of the function of this substrate.
Neher and colleagues used mass spectrometry to analyze proteins trapped by ClpXPtrap or Lontrap after DNA damage. They found DinI, DinD and YebG as novel ClpXP substrates and RecA and RuvB as novel Lon substrates [38]. ClpPtrap and Lontrap are mutants of ClpP and Lon, respectively, in which the active sites are mutated; therefore, the substrates are trapped but not degraded [12,38].
Thus, present findings provide evidence that intracellular proteolysis is a critical component controlling many of the DNA repair pathways that permit bacteria to survive or recover from DNA damage (Fig. 1). However, further studies will be needed to advance our understanding and fully appreciate the complex interplay between proteolysis and the DNA damage response.
Fig. 1.
Proteolysis and the SOS response in E. coli.
3. Proteolysis in biological metal homeostasis
Metals play an integral role in many biochemical processes essential to life, as key structural and/or catalytic components of a large number of proteins. For example, numerous transcription factors and replication proteins contain zinc, and iron metalloproteins are necessary for DNA replication, repair and transcription.
Metals are essential nutrients for most organisms. However, excess zinc or free iron is toxic; therefore, their levels must be carefully balanced so that enough metal is present to sustain key metabolic processes but toxic effects are minimized. To achieve metal homeostasis, prokaryotes have evolved an elaborate system of transport, storage and regulatory proteins. The stability of some of these proteins is controlled by metal-ion binding and is therefore important in metal response pathways. Here we describe some examples of protein turnover in zinc and iron regulation that provide insight into how protein degradation can contribute to metal homeostasis.
3.1. ZntR degradation and zinc homeostasis
Zinc is an essential component of numerous enzymes and regulatory proteins [8]. In E. coli, the balance between the available intracellular zinc and its potential toxicity is maintained by regulating the uptake and efflux of zinc [18]. Zinc ions are transported into the cytoplasm via the primary zinc import system, ZnuABC, and other pathways [40]. Zinc import is regulated by Zur, a zinc-responsive homolog of the iron uptake regulator Fur, which binds available intracellular zinc and represses the znu operator, thus blocking function of this zinc import pathway. When zinc ions are in excess, they bind ZntR, a member of the MerR family of metal-responsive transcriptional regulators, and convert ZntR into a strong activator of the zinc-exporter ZntA, resulting in increased efflux of zinc [6]. The apo- and zinc-bound forms of ZntR both bind to DNA, but transcription is activated only by the zinc-bound form, because only this form adopts the proper conformation on the zntA promoter.
Besides the critical role of transcriptional control in zinc homeostasis, it has recently been shown that the proteolytic stability of ZntR also contributes to maintaining optimal intracellular zinc levels [42]. Their study demonstrates that ZntR is an in vivo substrate for both ClpXP and Lon proteases, and establishes that the protein is directly degraded by Lon in vitro. However, in the presence of high zinc concentrations, ZntR degradation is suppressed. Using side-directed mutants of ZntR defective in either zinc or DNA binding, the authors showed that this increased stability of ZntR in the presence of excess zinc depends on both the DNA and zinc binding capabilities of ZntR. Replacement of a conserved arginine (R19A) in the DNA binding domain both enhanced ZntR degradation and abolished zinc-induced transcriptional activation of zntA. On the other hand, mutants defective in zinc binding had a similar half-life as the wild-type protein, and this half-life did not change in the presence of zinc. Therefore, the stabilizing effect of zinc requires that the protein be able to bind DNA [42].
The increased stability of ZntR is likely due to conformational changes induced upon zinc and DNA binding. Pruteanu and colleagues concluded that the zinc-ZntR-operator ternary complex is most stable against proteolysis, due to its unique structure that likely occludes the protein determinants that must be recognized by the proteases for degradation. They proposed that increased stability of ZntR in the presence of toxic zinc concentrations, may be necessary to ensure high zinc export. The reduced half-life of apo-ZntR ensures that zinc efflux is activated only when intracellular zinc accumulates to high enough levels (to be potentially toxic), eliminating the premature activation of zntA expression.
3.2. Proteolysis in iron homeostasis
Iron is required for numerous biochemical processes as a cofactor for several enzymes and as a catalyst in electron transport processes. However, free iron in cells is extremely toxic. For example, in the presence of H2O2 (or oxygen as a poor alternative), iron catalyzes the generation of reactive oxygen species that damage DNA, proteins and membrane lipids. Bacterial iron homeostasis is achieved by tightly controlling its uptake, metabolism, and storage. When the intracellular iron level is high, the Fur protein (ferric uptake regulator) represses transcription of several iron uptake genes [2].
Iron is a transition metal existing in one of two interconvertible redox states, the reduced Fe2+ ferrous form and the oxidized Fe3+ ferric form. The intracellular concentration of free ferrous iron is controlled mainly by the iron storage proteins, which incorporate ferrous iron; however, once bound, the deposited iron in the central cavity of these proteins is thought to be largely in the oxidized ferric form. Bacteria have three types of iron storage proteins: ferritins, heme-containing bacterioferritins and Dps (DNA binding protein from starved cells). The iron storage proteins have a similar molecular architecture that provides them with iron-storing capability. They contain either 24 (ferritins and bacterioferritins) or 12 (Dps proteins) identical (or similar) subunits that assemble into a spherical shell surrounding the central cavity that then functions as the iron storage reservoir [2].
Dps levels are very low in exponentially growing cultures of Escherichia coli. Under conditions of nutritional stress or oxidative damage, Dps accumulates to high levels and binds DNA without apparent sequence specificity. Dps-DNA complexes are extremely stable and protect DNA during starvation and oxidative stress [31]. Accumulation of Dps is the result of increased gene expression and increased protein stability. ClpXP and ClpAP proteases are involved in growth phase-dependent degradation of Dps [12,45,46]. Dps is rapidly degraded by ClpXP in log-phase cells, and stabilized when cells experience starvation or oxidative stress [12,46]. Schmidt and colleagues have recently provided evidence for degradation of Dps by ClpS/ClpAP [45]. The Dps protein contains distinct ClpX and ClpS recognition motifs at its N terminus; Dps lacking the first five N-terminal residues is no longer a ClpXP substrate, and ClpS/ClpAP recognition and degradation of Dps requires removal of these residues by an unknown endopeptidase [12,45]. The N-terminally truncated Dps variant (Dps6-167) with the destabilizing Leu6 as N-terminal residue, if it proves to have a physiologically important role, would be one of the first discovered “physiological” N-end rule substrates in bacteria [45].
We suggest that increased stability of Dps in stationary phase is necessary for cell survival in E. coli, since the dps mutant cells are more sensitive than wild-type cells when exposed to high concentrations of iron or copper during stationary phase [35]. Although Dps is not a copper storage protein, cells lacking Dps have increased cellular copper concentrations. The copper concentrations were reduced by Dps overproduction in copper-stressed exponentially growing cells [48]. In Enterococcus hirae, copper-stimulated degradation of CopZ (a copper chaperone) fine-tunes copper homeostasis [29].
Another strategy to control iron homeostasis is incorporation of iron in iron-sulfur (Fe-S) clusters to prevent accumulation of iron to toxic levels. Biogenesis of cellular Fe-S proteins requires the iscSUA-hscBA-fdx gene cluster, which is regulated by IscR, a SoxS homologue, encoded by iscR gene in the iscRSUA operon. IscR contains a 2Fe-2S cluster and mediates repression of iscRSUA expression. IscR, IscS and IscU have all been identified as ClpXP-trapped proteins [12]. Using peptide arrays, Flynn and colleagues showed that IscR and IscS contain N-terminal ClpX recognition motifs. Fusion of these motifs to an otherwise stable protein resulted in rapid degradation of the fusion protein. Furthermore, substitutions of the conserved amino acids in IscS N-terminal motif converted the rapidly degraded fusion protein into a highly stable protein. However, the biological significance of these proteins as proteolytic substrates remains to be investigated.
Mettert and Kiley have recently demonstrated the physiological significance of FNR degradation by ClpXP in oxygen sensing [32]. FNR is a global regulatory protein that requires a (4Fe-4S)2+ cluster for its transcriptionally active dimeric form. E. coli cells efficiently sense and respond to oxygen via the iron-sulfur cluster in the N-terminal region of FNR. Under aerobic conditions the (4Fe-4S)2+ cluster is converted into a (2Fe-2S)2+ cluster, resulting in inactive, monomeric (2Fe-2S)2+-FNR. The (2Fe-2S)2+ cluster is further destroyed resulting in inactive, monomeric apo-FNR present in aerobically grown cells. Using various FNR mutants with altered dimerization properties and Fe-S cluster stability, the authors showed that loss of dimerization upon (4Fe-4S)2+ cluster destruction by oxygen targets FNR for degradation by the ClpXP protease. Degradation of FNR by ClpXP requires both the N- and the C-terminal ClpX recognition motifs present in the FNR protein sequence [32].
These examples give a flavor of how metals can influence protein structure and protein-interactions and in turn can lead to important changes in protein stability. Furthermore, metal-binding proteins are subject to other types of regulation as well, which can also influence stability. Future studies addressing regulated proteolysis of substrates with impact on metal homeostasis will allow for a deeper understanding of the dynamic nature of intracellular metal physiology.
Acknowledgments
Work in the Baker laboratory was supported by NIH grant GM49224. T.A.B. and M.P. are/were employees of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al Mamun AA, Humayun MZ. Spontaneous mutagenesis is elevated in protease-defective cells. Mol Microbiol. 2009;71:629–639. doi: 10.1111/j.1365-2958.2008.06551.x. [DOI] [PubMed] [Google Scholar]
- 2.Andrews SC, Robinson AK, Rodríguez-Quiñones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 3.Baker TA, Sauer RT. ATP-dependent proteases of bacteria: recognition logic and operating principles. Trends Biochem Sci. 2006;31:647–53. doi: 10.1016/j.tibs.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battista JR, Donnelly CE, Ohta T, Walker GC. Dominant negative umuD mutations decreasing RecA-mediated cleavage suggest roles for intact UmuD in modulation of SOS mutagenesis. Proc Natl Acad Sci USA. 1990;8:7190–7194. doi: 10.1073/pnas.87.18.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branum ME, Reardon JT, Sancar A. DNA repair excision nuclease attacks undamaged DNA. A potential source of spontaneous mutations. J Biol Chem. 2001;276:25421–25426. doi: 10.1074/jbc.M101032200. [DOI] [PubMed] [Google Scholar]
- 6.Brocklehurst KR, Hobman JL, Lawley B, Blank L, Marshall SJ, Brown NL, Morby AP. ZntR is a Zn(II)-responsive MerR-like transcriptional regulator of zntA in Escherichia coli. Mol Microbiol. 1999;31:893–902. doi: 10.1046/j.1365-2958.1999.01229.x. [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekhar D, Van Houten B. In vivo formation and repair of cyclobutane pyrimidine dimers and 6-4 photoproducts measured at the gene and nucleotide level in Escherichia coli. Mutat Res. 2000;450:19–40. doi: 10.1016/s0027-5107(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 8.Coleman JE. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- 9.Courcelle J, Khodursky A, Peter B, Brown PO, Hanawalt PC. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics. 2001;158:41–64. doi: 10.1093/genetics/158.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deaconescu AM, Chambers AL, Smith AJ, Nickels BE, Hochschild A, Savery NJ, Darst SA. Structural basis for bacterial transcription-coupled DNA repair. Cell. 2006;124:507–520. doi: 10.1016/j.cell.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 11.Flynn JM, Levchenko I, Sauer RT, Baker TA. Modulating substrate choice: the SspB adaptor delivers a regulator of the extracytoplasmic-stress response to the AAA+ protease ClpXP for degradation. Genes Dev. 2001;18:2292–301. doi: 10.1101/gad.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn JM, Neher SB, Kim YI, Sauer RT, Baker TA. Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol Cell. 2003;11:671–683. doi: 10.1016/s1097-2765(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 13.Frank EG, Ennis DG, Gonzalez M, Levine AS, Woodgate R. Regulation of SOS mutagenesis by proteolysis. Proc Natl Acad Sci USA. 1996;93:10291–10296. doi: 10.1073/pnas.93.19.10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez M, Frank EG, Levine AS, Woodgate R. Lon-mediated proteolysis of the Escherichia coli UmuD mutagenesis protein: in vitro degradation and identification of residues required for proteolysis. Genes Dev. 1998;12:3889–3899. doi: 10.1101/gad.12.24.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez M, Rasulova F, Maurizi MR, Woodgate R. Subunit-specific degradation of the UmuD/D′ heterodimer by the ClpXP protease: the role of trans recognition in UmuD′ stability. EMBO J. 2000;19:5251–5258. doi: 10.1093/emboj/19.19.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottesman S. Proteolysis in bacterial regulatory circuits. Annu Rev Cell Dev Biol. 2003;19:565–587. doi: 10.1146/annurev.cellbio.19.110701.153228. [DOI] [PubMed] [Google Scholar]
- 17.Hanson PI, Whiteheart SW. AAA+ proteins: have engine, will work. Nat Rev Mol Cell Biol. 2005;6:519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 18.Hantke K. Bacterial zinc transporters and regulators. Biometals. 2001;14:239249. doi: 10.1023/a:1012984713391. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa K, Yoshiyama K, Maki H. Spontaneous mutagenesis associated with nucleotide excision repair in Escherichia coli. Genes Cells. 2008;13:459–469. doi: 10.1111/j.1365-2443.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 20.Ishii Y, Amano F. Regulation of SulA cleavage by Lon protease by the C-terminal amino acid of SulA, histidine. Biochem J. 2001;358:473–480. doi: 10.1042/0264-6021:3580473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonczyk P, Nowicka A. Specific in vivo protein-protein interactions between Escherichia coli SOS mutagenesis proteins. J Bacteriol. 1996;178:2580–2585. doi: 10.1128/jb.178.9.2580-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keiler KC, Waller PR, Sauer RT. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science. 1996;271:990–993. doi: 10.1126/science.271.5251.990. [DOI] [PubMed] [Google Scholar]
- 23.Kenyon CJ, Walker GC. Expression of the E. coli uvrA gene is inducible. Nature. 1981;289:808–810. doi: 10.1038/289808a0. [DOI] [PubMed] [Google Scholar]
- 24.Khattar MM. Overexpression of the hslVU operon suppresses SOS-mediated inhibition of cell division in Escherichia coli. FEBS Lett. 1997;414:402–404. doi: 10.1016/s0014-5793(97)01024-7. [DOI] [PubMed] [Google Scholar]
- 25.Kim YI, Burton RE, Burton BM, Sauer RT, Baker TA. Dynamics of substrate denaturation and translocation by the ClpXP degradation machine. Mol Cell. 2000;5:639–648. doi: 10.1016/s1097-2765(00)80243-9. [DOI] [PubMed] [Google Scholar]
- 26.Little JW. The SOS regulatory system: control of its state by the level of RecA protease. J Mol Biol. 1983;167:791–808. doi: 10.1016/s0022-2836(83)80111-9. [DOI] [PubMed] [Google Scholar]
- 27.Little JW. Variations in the in vivo stability of LexA repressor during the SOS regulatory cycle. In: Friedberg EC, Bridges BA, editors. Cellular Responses to DNA Damage. Alan R. Liss, Inc; New York, N.Y: 1983. pp. 369–378. [Google Scholar]
- 28.Little JW. Autodigestion of lexA and phage lambda repressors. Proc Natl Acad Sci USA. 1984;81:1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu ZH, Solioz M. Copper-induced proteolysis of the CopZ copper chaperone of Enterococcus hirae. J Biol Chem. 2001;276:47822–47827. doi: 10.1074/jbc.M106218200. [DOI] [PubMed] [Google Scholar]
- 30.Malta E, Moolenaar GF, Goosen N. Dynamics of the UvrABC nucleotide excision repair proteins analyzed by fluorescence resonance energy transfer. Biochemistry. 2007;46:9080–9088. doi: 10.1021/bi7002235. [DOI] [PubMed] [Google Scholar]
- 31.Martinez A, Kolter R. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J Bacteriol. 1997;179:5188–5194. doi: 10.1128/jb.179.16.5188-5194.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mettert EL, Kiley PJ. ClpXP-dependent proteolysis of FNR upon loss of its O2-sensing [4Fe-4S] cluster. J Mol Biol. 2005;354:220–232. doi: 10.1016/j.jmb.2005.09.066. [DOI] [PubMed] [Google Scholar]
- 33.Mizusawa S, Gottesman S. Protein degradation in Escherichia coli: the lon gene controls the stability of sulA protein. Proc Natl Acad Sci USA. 1983;80:358–362. doi: 10.1073/pnas.80.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagashima K, Kubota Y, Shibata T, Sakaguchi C, Shinagawa H, Hishida T. Degradation of Escherichia coli RecN aggregates by ClpXP protease and its implications for DNA damage tolerance. J Biol Chem. 2006;281:30941–30946. doi: 10.1074/jbc.M606566200. [DOI] [PubMed] [Google Scholar]
- 35.Nair S, Finkel SE. Dps protects cells against multiple stresses during stationary phase. J Bacteriol. 2004;186:4192–4198. doi: 10.1128/JB.186.13.4192-4198.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neher SB, Flynn JM, Sauer RT, Baker TA. Latent ClpX-recognition signals ensure LexA destruction after DNA damage. Genes Dev. 2003;17:1084–1089. doi: 10.1101/gad.1078003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neher SB, Sauer RT, Baker TA. Distinct peptide signals in the UmuD and UmuD′ subunits of UmuD/D′ mediate tethering and substrate processing by the ClpXP protease. Proc Natl Acad Sci USA. 2003;100:13219–13224. doi: 10.1073/pnas.2235804100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neher SB, Villen J, Oakes EC, Bakalarski CE, Sauer RT, Gygi SP, Baker TA. Proteomic profiling of ClpXP substrates after DNA damage reveals extensive instability within SOS regulon. Mol Cell. 2006;22:193–204. doi: 10.1016/j.molcel.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Nishii W, Takahashi K. Determination of the cleavage sites in SulA, a cell division inhibitor, by the ATP-dependent HslVU protease from Escherichia coli. FEBS Lett. 2003;553:351–354. doi: 10.1016/s0014-5793(03)01044-5. [DOI] [PubMed] [Google Scholar]
- 40.Patzer SI, Hantke K. The zinc-responsive regulator Zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J Biol Chem. 2000;275:24321–24332. doi: 10.1074/jbc.M001775200. [DOI] [PubMed] [Google Scholar]
- 41.Pruteanu M, Baker TA. Controlled degradation by ClpXP protease tunes the levels of the excision repair protein UvrA to the extent of DNA damage. Mol Microbiol. 2009;71:912–924. doi: 10.1111/j.1365-2958.2008.06574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pruteanu M, Neher SB, Baker TA. Ligand-controlled proteolysis of the Escherichia coli transcriptional regulator ZntR. J Bacteriol. 2007;189:3017–3025. doi: 10.1128/JB.01531-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sauer RT, Bolon DN, Burton BM, Burton RE, Flynn JM, Grant RA, Hersch GL, Joshi SA, Kenniston JA, Levchenko I, Neher SB, Oakes ES, Siddiqui SM, Wah DA, Baker TA. Sculpting the proteome with AAA(+) proteases and disassembly machines. Cell. 2004;119:9–18. doi: 10.1016/j.cell.2004.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savery NJ. The molecular mechanism of transcription-coupled DNA repair. Trends Microbiol. 2007;15:326–333. doi: 10.1016/j.tim.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt R, Zahn R, Bukau B, Mogk A. ClpS is the recognition component for Escherichia coli substrates of the N-end rule degradation pathway. Mol Microbiol. 2009;72:506–517. doi: 10.1111/j.1365-2958.2009.06666.x. [DOI] [PubMed] [Google Scholar]
- 46.Stephani K, Weichard D, Hengge R. Dynamic control of Dps protein levels by ClpXP and ClpAP proteases in Escherichia coli. Mol Microbiol. 2003;49:1605–1614. doi: 10.1046/j.1365-2958.2003.03644.x. [DOI] [PubMed] [Google Scholar]
- 47.Theis K, Skorvaga M, Machius M, Nakagawa N, Van Houten B, Kisker C. The nucleotide excision repair protein UvrB, a helicase-like enzyme with a catch. Mutat Res. 2000;460:277–300. doi: 10.1016/s0921-8777(00)00032-x. [DOI] [PubMed] [Google Scholar]
- 48.Thieme D, Grass G. The Dps protein of Escherichia coli is involved in copper homeostasis. Microbiol Res 2006. 2009 doi: 10.1016/j.micres.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Truglio JJ, Croteau DL, Van Houten B, Kisker C. Prokaryotic nucleotide excision repair: the UvrABC system. Chem Rev. 2006;106:233–252. doi: 10.1021/cr040471u. [DOI] [PubMed] [Google Scholar]
- 50.Wu WF, Zhou Y, Gottesman S. Redundant in vivo proteolytic activities of Escherichia coli Lon and the ClpYQ (HslUV) protease. J Bacteriol. 1999;181:3681–3687. doi: 10.1128/jb.181.12.3681-3687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]