Abstract
2-(4-Aminophenyl)benzothiazoles related to 1 are potentially important pharmaceuticals. Metabolism apparently involves oxidation and esterification to 3. In water, hydrolysis and photolysis of 3 generates the nitrenium ion 4 that can be detected indirectly by N3− trapping and directly by UV-vis spectroscopy following laser flash photolysis. The transient, with λmax 570 nm, and a lifetime of 530 ns, reacts with N3− at a diffusion-controlled rate and generates the quinol 6 by reaction with water.
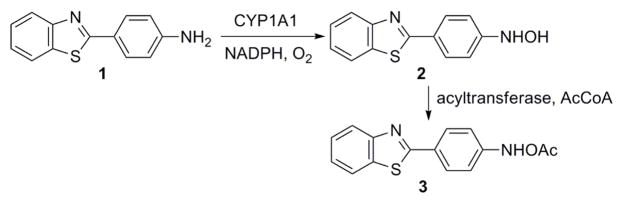
Benzothiazole derivatives such as 2-(4-aminophenyl)benzothiazole, 1, are under investigation as anti-tumor, antifungal, and antibacterial agents,1–3 and as radiopharmaceuticals for binding and in vivo imaging of Aβ-plaques, one of the earliest pathological processes in the development of Alzheimer’s disease.4 One anti-tumor derivative of 1 is currently in Phase 1 clinical trials in Great Britain.5 The use of 1 and its derivatives as anti-tumor agents requires biological activation.5,6 The proposed metabolism of 1 to form the active agent 3 is shown in Scheme 1, although neither 2 nor 3 had been isolated and characterized. It is presumed that 3 further decomposes into a reactive electrophile, but no direct evidence for this proposal has been presented.7
Scheme 1.
Proposed metabolism of 1.
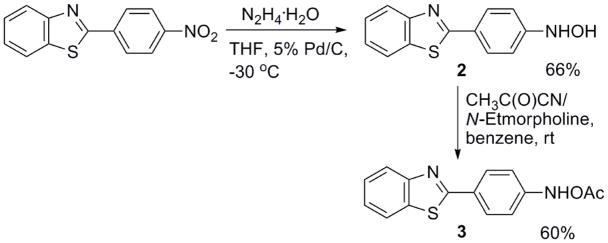
We have succeeded in synthesizing both 2 and 3 from 2-(4-nitrophenyl)benzothiazole using procedures we previously developed for making similar derivatives of carcinogenic aromatic amines (Scheme 2).8 Reduction of the nitro compound9 with hydrazine hydrate in the presence of 5% Pd/C catalyst generates 2 in moderate yield, while tratment of 2 with acetyl cyanide in the presence of N-ethylmorpholine provides 3 in satisfactory yield. We now report the indirect and direct detection of nitrenium ion 4 (Scheme 3) from hydrolysis and photolysis of 3.
Scheme 2.
Synthesis of 2 and 3.
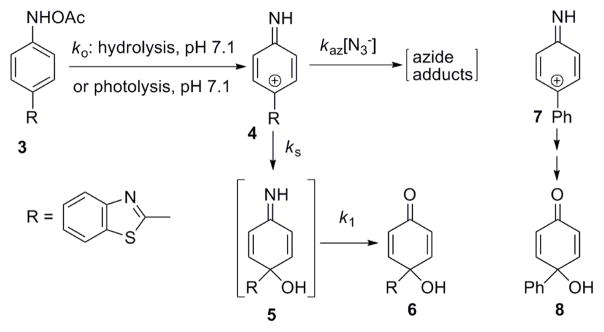
Scheme 3.
Kinetic Scheme for 3 and 4.
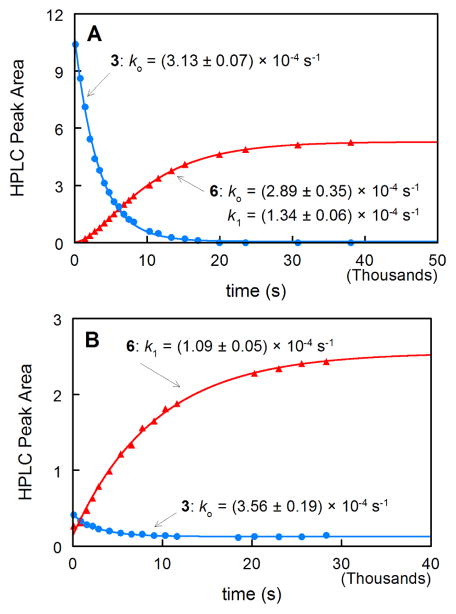
Kinetics of the decomposition of 3 (2.5 × 10−5 M) at pH 7.1 in phosphate buffer, and the formation of the major hydrolysis product 6 (Scheme 3, identified by HPLC and 1H NMR comparison to an authentic sample10) monitored by UV spectroscopy, are described by two pseudo-first-order rate constants, ko and k1. HPLC studies (Figure 1A) show that the larger rate constant, ko governs the decay of 3, while the appearance of 6 is biphasic, and is fit well by a rate equation for two consecutive first-order reactions. The larger rate constant generated by the fit is equivalent in magnitude to ko measured for the disappearance of 3. The rate of appearance of 6 is limited by the smaller rate constant, k1. Kinetics of the appearance of 6 are consistent with its formation from a long-lived intermediate (lifetime ca. 2 h at 10 °C) that is generated by hydrolysis of 3. Steady-state photolysis of an identical aqueous solution of 3 for 30 s with UVB lamps leads to photo-decomposition of 96% of 3 (Figure 1B). Generation of 6 now occurs via a simple first-order process governed by k1. Correction for the small amount of 3 remaining after photolysis shows that 92% of the observed yield of 6 under photolysis conditions is due to photo-decomposition of 3.
Figure 1.
Time course for the disappearance of 3 and formation of 6 at 10 °C in pH 7.1 phosphate buffer monitored by HPLC with UV detection at 212 nm: (A) hydrolysis reaction in the dark, (B) after steady-state photolysis for 30 s. Rate constants were obtained from fits to single or double exponential rate equations.
The results show that 6 is generated both by hydrolysis and photolysis of 3, and suggest that a common pathway is involved in both processes.
Addition of N3− to the hydrolysis solution does not affect the rate of decomposition of 3, but does significantly decrease the yield of 6 at very low [N3−] (Figure 2), demonstrating that N3− traps a reactive intermediate produced in a rate limiting step. As the yield of 6 decreases, the yields of two new products, not generated in the absence of N3−, increase. Application of the “azide clock” equations11 to the yields of these three products generates the experimental kaz/ks shown in Figure 2. Although the azide products have not yet been characterized, the structure of 6 and the trapping results show that N3− competes with the solvent for a selective cationic intermediate, 4. The kinetics of the formation of 6 during hydrolysis of 3 implicates 5 as a precursor, although 5 has not yet been detected.
Figure 2.
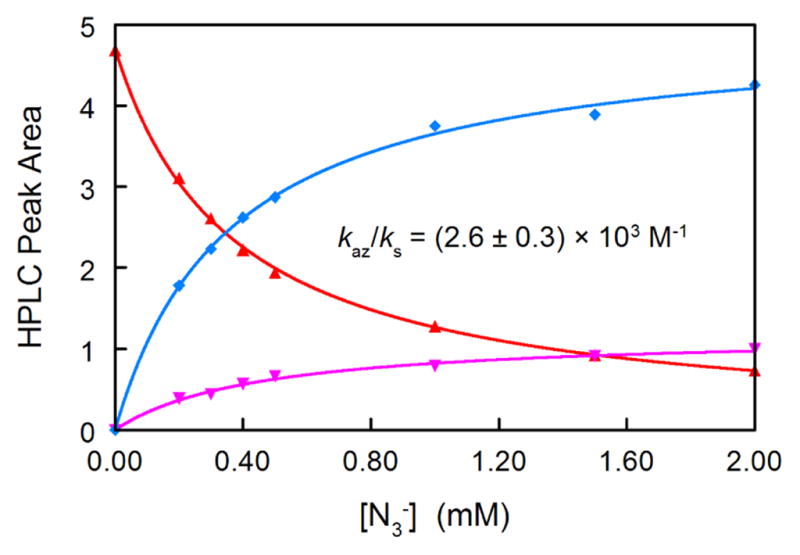
Results of azide trapping experiment in pH 7.1 phosphate buffer at 30 °C. Key: 6( , 212 nm), apparent major azide adduct (
, 212 nm), apparent major azide adduct ( , 330 nm), apparent minor azide adduct (
, 330 nm), apparent minor azide adduct ( , 212 nm). The kaz/ks is the average of the fit of all three materials to the standard “azide clock” formulae.
, 212 nm). The kaz/ks is the average of the fit of all three materials to the standard “azide clock” formulae.
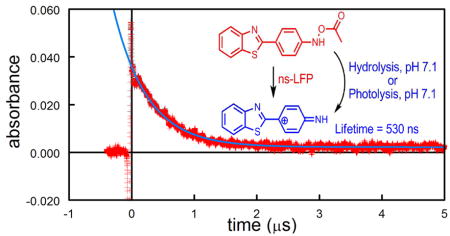
Laser flash photolysis (LFP) of 3 in O2-saturated pH 7.1 phosphate buffer at 308 nm generates a transient UV spectrum with λmax ca. 570 nm (Figure 3). The absorbance at 570 nm decays in a first-order manner (Supporting Information, Figure S1). The rate constant, kobs, increases linearly with increasing [N3−] (Figure 4).
Figure 3.
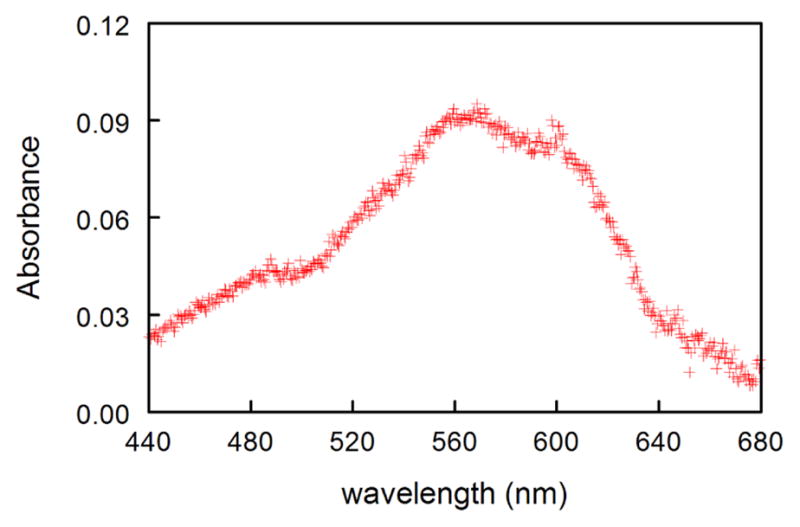
Transient absorbance spectrum obtained 20 ns after 308 nm excitation of 3 in O2-saturated pH 7.1 phosphate buffer. Spectrum recorded with a 20 ns window.
Figure 4.
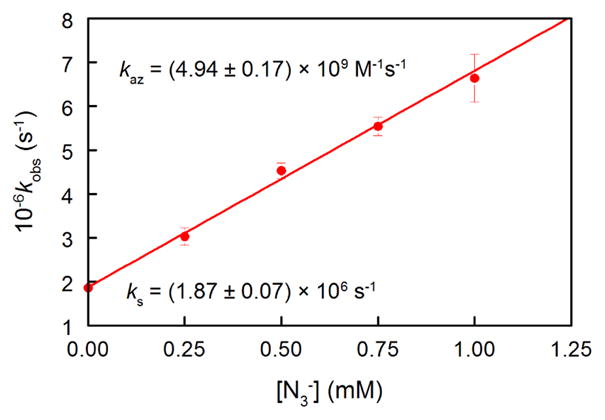
Plot of kobs from LFP experiments vs. [N3−]. Data were fit by a weighted least-squares procedure to obtain ks and kaz. Adjusted r2 = 0.9967.
The slope of that plot is kaz, the second-order rate constant for reaction of N3− with the reactive intermediate, while the intercept is ks, the pseudo-first-order rate constant for reaction of the intermediate with the aqueous solvent. The ratio kaz/ks of (2.64 ± 0.13) × 103 M−1 is identical to that obtained from the azide-trapping experiments, demonstrating that both experiments detect the same intermediate, 4, with a lifetime (1/ks) of ca. 530 ns.
Scheme 3 summarizes the results of our experiments. This scheme is similar to that previously demonstrated for the decomposition of ester derivatives of carcinogenic aromatic hydroxylamines.12 The cation 4 is about as selective as the 4-biphenylylnitrenium ion, 7 (kaz/ks = 2.9 × 103 M−1) that also yields a quinol, 8, as its major hydration product.12 The intermediate detected after LFP is definitely 4, not the imine 5 because the kinetics performed by UV spectroscopy and HPLC show that 5 has a lifetime of about 30 min at room temperature, while the transient generated during the LFP experiments has a lifetime of 530 ns.
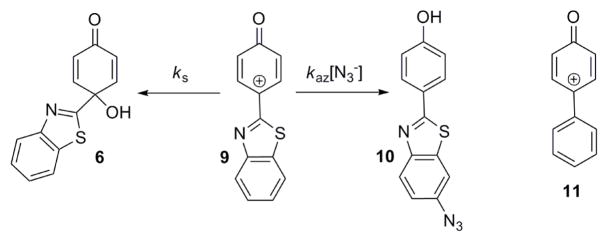
An apparent imine intermediate can be detected by HPLC during the conversion of 7 into 8.12 This species has a lifetime of ca. 6 h at room temperature, while 7 has a lifetime of 560 ns under the same conditions.12 The quinol product 6 is also the hydration product of the related oxenium ion 9 (Scheme 4).10
Scheme 4.
Chemistry of the related oxenium ion 9.
The azide adduct identified in that study is 10, and kaz/ks for 9 at 80 °C is 310 M−1.10 The structure of 10 demonstrates that the charge in 9 is highly delocalized, and kaz/ks comparisons to other oxenium ions show that the azide/solvent selectivity of 9 is similar to the 4-biphenylyloxenium ion, 11.10 Since 4,7, 9, and 11 react with N3− at or near the diffusion controlled limit, the aqueous solution lifetimes of 4 and 7 and also of 9 and 11 are very similar.10,12,13 These results show that the 4-(benzothiazol-2-yl) group behaves as a significantly delocalizing and stabilizing substituent for both oxenium and nitrenium ions.
It is now apparent that putative metabolites of anti-tumor benzothiazoles will give rise to selective, long lived nitrenium ions in aqueous solution. Although metabolites of carcinogenc aromatic amines have long been known to generate highly selective nitrenium ion intermediates in aqueous solution,12 this is, to the best of our knowledge, the first demonstration that a putative metabolite of an anti-tumor drug also generates such an intermediate. We are continuing this study with an emphasis on the reaction of 4 and related nitrenium ions with biological nucleophiles.
Supplementary Material
Acknowledgments
We thank the Donors of the American Chemical Society Petroleum Research Fund (Grant # 43176-AC4) and the NIH/NIGMS (Grant # R15 GM088751-01) for support of this work. KJJ thanks Miami University for an HHMI Summer Undergraduate Research Fellowship, and SCB thanks the OCUR-REEL program supported by the Chemical Division of NSF for an undergraduate summer research fellowship. The support of the NSF in Columbus and the OSU Center for Chemical and Biophysical Dynamics is greatly appreciated.
Footnotes
Supporting Information Available Experimental details, a Table of rate constants, Figure S1, synthesis of 2 and 3, NMR spectra of 2 and 3. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Shi DF, Bradshaw TD, Wrigley S, McCall CJ, Lelieveld P, Fichtner I, Stevens MFG. J Med Chem. 1996;39:3375–3384. doi: 10.1021/jm9600959. [DOI] [PubMed] [Google Scholar]; Bradshaw TD, Wrigley S, Shi DF, Schultz RJ, Paull KD, Stevens MFG. Brit J Cancer. 1998;77:745–752. doi: 10.1038/bjc.1998.122. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hutchinson I, Jennings SA, Vishnuvajjala BR, Westwell AD, Stevens MFG. J Med Chem. 2002;45:744–747. doi: 10.1021/jm011025r. [DOI] [PubMed] [Google Scholar]
- 2.Yildiz-Oren I, Yalcin I, Aki-Sener E, Ucarturk N. Eur J Med Chem. 2004;39:291–298. doi: 10.1016/j.ejmech.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Ra CS, Jung BY, Park G. Heterocycles. 2004;62:793–802. [Google Scholar]
- 4.Tzanopoulou S, Pirmettis IC, Patsis G, Paravatou-Petsotas M, Livaniou E, Papadopoulos M, Pelecanou M. J Med Chem. 2006;49:5408–5410. doi: 10.1021/jm0606387. [DOI] [PubMed] [Google Scholar]
- 5.Bradshaw TD, Westwell AD. Curr Med Chem. 2004;11:1009–1021. doi: 10.2174/0929867043455530. [DOI] [PubMed] [Google Scholar]; Brantley E, Antony S, Kohlhagen G, Meng L, Agama K, Stinson SF, Sausville EA, Pommier Y. Cancer Chemoth Pharm. 2006;58:62–72. doi: 10.1007/s00280-005-0127-z. [DOI] [PubMed] [Google Scholar]
- 6.Chua MS, Kashiyama E, Bradshaw TD, Stinson SF, Brantley E, Sausville EA, Stevens MFG. Cancer Res. 2000;60:5196–5203. [PubMed] [Google Scholar]
- 7.O’Brien SE, Browne HL, Bradshaw TD, Westwell AD, Stevens MFG, Laughton CA. Org Biomol Chem. 2003;1:493–497. doi: 10.1039/b209067h. [DOI] [PubMed] [Google Scholar]; Hilal R, Khalek AAA, Elroby SAK. THEOCHEM. 2005;731:115–121. [Google Scholar]
- 8.Kazerani S, Novak M. J Org Chem. 1998;63:895–897. doi: 10.1021/jo971786v. [DOI] [PubMed] [Google Scholar]; Novak M, Kazerani S. J Am Chem Soc. 2000;122:3606–3616. [Google Scholar]
- 9.Stevens MFG, Shi DF, Castro A. J Chem Soc, Perkin Trans. 1;1996:83–93. [Google Scholar]
- 10.Wang YT, Jin KJ, Myers LR, Glover SA, Novak M. J Org Chem. 2009;74:4463–4471. doi: 10.1021/jo9008436. [DOI] [PubMed] [Google Scholar]
- 11.Richard JP, Jencks WP. J Am Chem Soc. 1982;104:4689–4691. [Google Scholar]; Richard JP, Jencks WP. J Am Chem Soc. 1982;104:4691–4692. [Google Scholar]; Richard JP, Jencks WP. J Am Chem Soc. 1984;106:1383–1396. [Google Scholar]
- 12.Novak M, Kahley MJ, Eiger E, Helmick JS, Peters HE. J Am Chem Soc. 1993;115:9453–9460. [Google Scholar]; McClelland RA, Davidse PA, Hadzialic G. J Am Chem Soc. 1995;117:4173–4174. [Google Scholar]
- 13.Novak M, Glover SA. J Am Chem Soc. 2004;126:7748–7749. doi: 10.1021/ja047488e. [DOI] [PubMed] [Google Scholar]; Wang YT, Wang J, Platz MS, Novak M. J Am Chem Soc. 2007;129:14566–14567. doi: 10.1021/ja0764456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.