Abstract
Objective
As in arteries, venous endothelium modulates vessel homeostasis and tone. The effect of an arterialized environment on venous endothelial function remains poorly understood. In particular, regulation of saphenous vein graft (SVG) blood flow and lumen caliber remains undefined. We hypothesized that mature SVGs would exhibit endothelium-dependent, flow-mediated vasodilation. We further hypothesized that endothelium-derived nitric oxide (NO) was an important mediator of vascular tone.
Methods
Patients with femoral to popliteal artery SVGs that had maintained primary patency and were at least one year from surgery were enrolled. High-resolution, B-mode ultrasound was used to measure vein graft diameter before and one minute after reactive hyperemia (RH) to determine flow-mediated vasodilation (FMD). RH was created through application of 220 mm Hg to the calf for 5 minutes with a sphygmomanometric cuff. After a 10 minute recovery period, nitroglycerin-mediated, endothelium-independent vasodilation was measured 3 minutes after administration of nitroglycerin 0.4 mg SL. Brachial artery FMD was also measured. L-NG monomethyl arginine (L-NMMA; 1 mg/kg infusion over ten minutes) was used in a subset of patients (N=6) to competitively inhibit endothelial nitric oxide synthase.
Results
Nineteen subjects were enrolled. The median age of the SVGs was 34.6 (21.0–49.7) months. SVG flow-mediated, endothelium-dependent vasodilation was measured at 5.28% ± 3.1% mean change in lumen diameter (range 1.99% – 9.36%, p<.0001 for diameter change). Nitroglycerin-mediated, vasodilation was 3.7% ± 1.0%, (range .16% – 10.04%, P<.005). Intravenous administration of L-NMMA abolished SVG FMD (5.7 ± 1.4% before L-NMMA vs 0.01 ± 0.01% during L-NMMA infusion, p=.0088) and attenuated brachial artery FMD (7.54% ± 1.0% vs 5.7± 1.4; p=.05).
Conclusions
SVGs manifest flow-mediated, endothelium-dependent and nitroglycerin-mediated, endothelium-independent vasodilation. Vein graft endothelium-dependent FMD is likely mediated by NO. Further investigation will be required to determine the role of endothelial function in vein graft patency.
Introduction
The adaptation of the great saphenous vein (GSV) to the arterial environment in vivo has been described in part.(1–3)There are apparent temporally distinct patterns of geometric remodeling, characterized by early lumen dilatation followed later by wall thickening and stiffening of the venous conduit. These changes are thought to be reactive physiological responses to the governing arterial hemodynamic forces, shear stress and radial wall stress. In particular, blood flow and shear stress is directly correlated with lumen dilation over the first month after implantation. However, we have previously shown that shear stress alone accounts for less than 1/3 of the biologic variability seen in early lumen dilatation of SVG, implying that unaccounted variables are operative.(3) In arteries, the changes in blood flow and shear forces induce endothelium-dependent changes in vessel caliber. Previous in vivo studies of human coronary SVG used acetylcholine as an agonist to study receptor-mediated, endothelium-dependent vasodilation. The role of the physiologically relevant stimulus, blood flow, remains unexplored (4–6)Harvesting of the GSV and preparation for bypass injures the vein, denudes the endothelial cell surface, and subjects it to ischemia and reperfusion.(7; 8) This damage induces an inflammatory response within the vessel wall and has been shown to decrease the amount and function of endothelial nitric oxide synthase (eNOS) and endothelium-derived nitric oxide (NO), hence impairing normal homeostatic mechanisms of the vessel.(9–12) This injury has been implicated in early vein graft failure and thought to be permissive for the formation of myointimal hyperplasia.(12–14) Moreover, mature vein grafts may require endothelium-dependent vessel homeostasis for maturation and survival. Based on the observed changes in graft morphology after bypass, we hypothesized that mature human functioning saphenous vein grafts would manifest flow-mediated, endothelium-dependent, vasodilatation and that NO would be the primary vasoactive mediator.
Methods
Subjects
Nineteen subjects with a continuously patent femoral to popliteal arterial bypass with autogenous greater saphenous vein, performed for either claudication or critical limb ischemia, were recruited for this study. All bypass grafts were at least 365 days old and none had undergone a revision to maintain patency. Bypass grafts were all tunneled superficially and were in the non-reversed configuration. All subjects underwent screening medical history and a vascular examination including a surveillance duplex exam to ensure the absence of occult stenosis within the bypass graft. All participants provided written informed consent. The protocol was approved by the Human Research Committee of the Brigham and Women’s Hospital.
Vascular Reactivity Studies
Subjects were studied in a quiet, temperature-controlled, dimly-lit room after resting supine for a minimum of 5 minutes. Endothelium-dependent and endothelium-independent vasodilation of the saphenous vein graft and endothelium-dependent vasodilatation of the brachial artery were studied in the morning in the postabsorptive state fasting after the previous midnight. All vasoactive medication long acting nitrates, tobacco, and caffeine were held 24 hours prior to the study. High-resolution B-mode ultrasonography of the brachial artery and vein graft was performed using a GE Vivid 7 (GE Healthcare, Fairfield, CT) ultrasound machine and a 7.5 MHz linear array probe. The brachial artery was imaged longitudinally, just proximal to the antecubital fossa with the transducer position adjusted to obtain optimal B-mode images of the near and far walls of the intima. The video output and electrocardiographic signal of the ultrasound machine were connected to a computer equipped with a data translation frame-grabber videocard (Dataviz). The R wave on the electrocardiogram served as a trigger to acquire frames at end-diastole. All post-processing imaging analysis was performed with the Brachial Analyzer, Medical Imaging Applications, Iowa City, Iowa by two examiners in a blinded fashion. This software application uses edge-detection and wall-tracking software which is independent of investigator bias to capture 10 consecutive end-diastolic images. The lumen diameter is reported as an average of the 10 images.
Measurement of brachial artery flow mediated, endothelium-dependent vasodilation
After baseline image acquisition, a 2.5 inch wide sphygmomanometric cuff was inflated to suprasystolic pressure (220 mm Hg) for 5 minutes. The duplex probe remained in position over the brachial artery to ensure complete absence of blood flow through the brachial artery during cuff inflation. On cuff release, reactive hyperemia causes flow to increase through the brachial artery subserving the forearm. Flow-induced endothelium-dependent vasodilation of the brachial artery was determined by acquiring images at 1 minute after cuff deflation. The determination of endothelial function was performed in accordance with published guidelines.(15) The inter- and intra-observer variability in our laboratory has been previously described.(16) In this experiment, the absolute mean difference between two ultrasound readers (CDO and NW) for measuring vein graft diameter was (mean ± S.D.) 3 ± 129 micrometers.
Measurement of vein graft endothelium-dependent and endothelium independent vasodilation
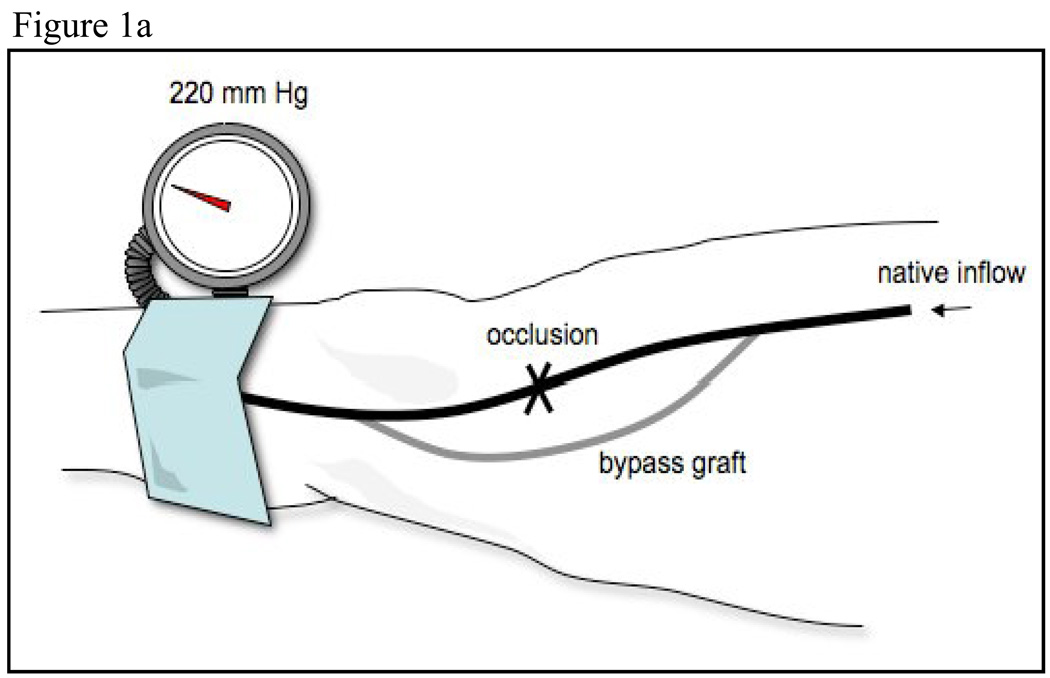
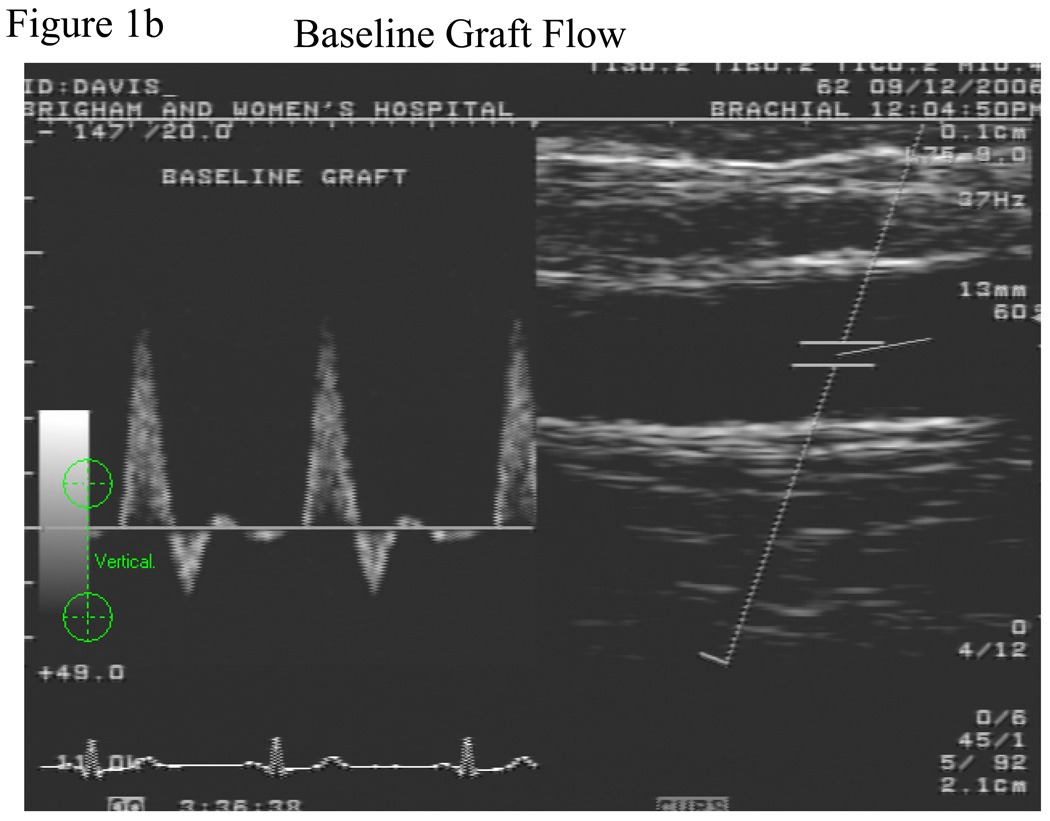
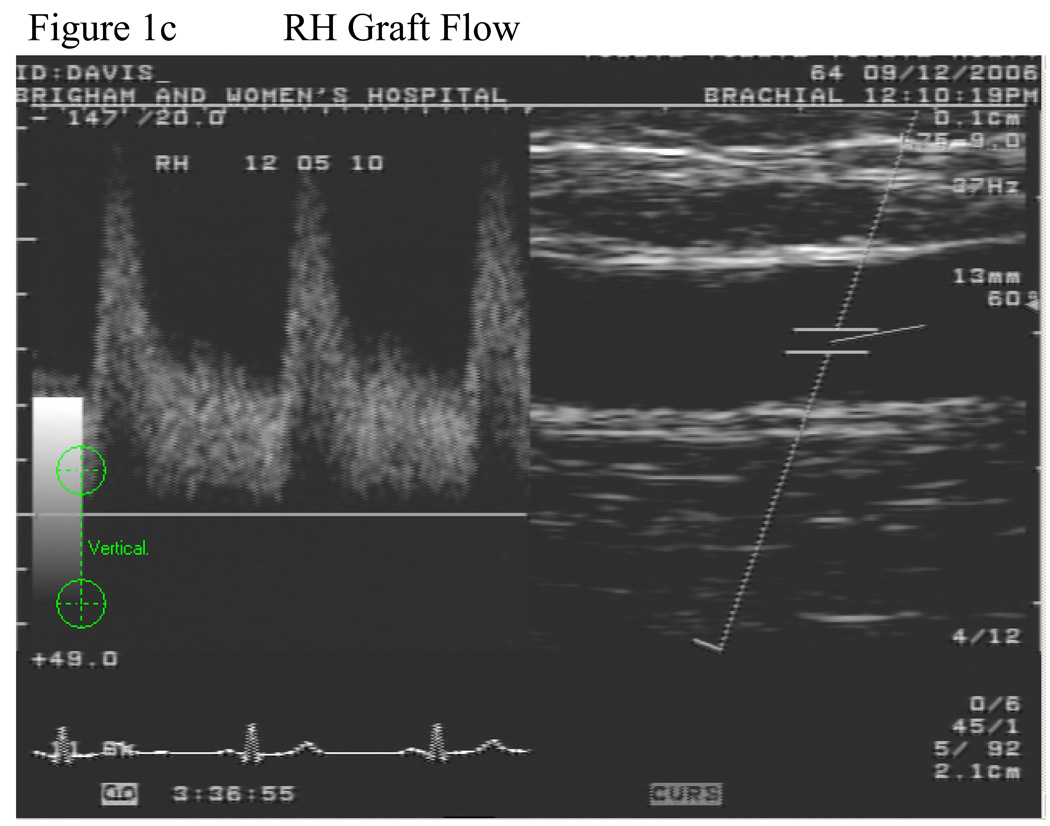
The vein graft was imaged in a straight, valveless segment chosen after the surveillance scan was performed. A 2.5 inch wide sphygmomanometric cuff was placed slightly below the tibial tuberosity and inflated to suprasystolic pressure for 5 minutes, Figure 1a. The cuff was never placed directly over the graft. Flow induced, endothelium-dependent vasodilation of the vein graft was determined by acquiring images at minute after cuff deflation, Figure 1b and c. Ten minutes after cuff release, the vein graft was re-imaged to ensure return to basal conditions. Then, to determine endothelium-independent vasodilation, subjects received 0.4 mg nitroglycerin, sublingually. The vein graft was imaged 3 minutes later. Nitroglycerin was not administered if the systolic blood pressure was <100 mm Hg. Fifteen of the 19 subjects received nitroglycerin.
Figure 1.
Schematic of lower extremity cuff-induced ischemia technique. The superficial femoral artery is occluded and a saphenous vein bypass is shown. The cuff is placed distal to the distal anastomosis and inflated to supra-systolic pressures, a. Doppler spectral waveform of the vein graft demonstrating baseline, b, and hyperemic flow patterns, c.
Administration of L-NMMA
In the last 6 consecutive patients, we tested the hypothesis that the mediator of vein graft flow mediated dilation was nitric oxide. These patients received intravenous administration of L-NG monomethyl arginine (LNMMA) at a dose of 1 mg/kg infused over 10 minutes to competitively inhibit the production of NO. During infusion, all patients were evaluated with noninvasive blood pressure monitoring and queried for adverse reactions such as chest pain or shortness of breath every two minutes. Vein graft endothelium-dependent vasodilation was assessed as described above. Ten minutes after cuff release the vein graft was re-imaged to ensure return to baseline. Once baseline size was re-established, LNMMA infusion was initiated and vein graft vasodilation was assessed again after ten minutes. All patients remained in a monitored environment until their blood pressure returned to baseline.
Hemodynamic measurements
Volumetric flow, Q, was calculated by integrating the area under the velocity spectral waveform and dividing by time to arrive at a time-averaged velocity. Mean flow was calculated by multiplying the time-averaged velocity by the mean area of the lumen. The integral was calculated using the brachial Doppler flow analysis package from Medical Imaging Applications, Iowa City, Iowa.
Statistical Methods
Based on a sample size of 19, the power to detect ≥ 3% change in vein graft diameter, assuming a standard deviation of 3% (a value typical for our laboratory), was 98% using a two-sided, one sample t-test.
Descriptive measures are reported as means ± standard deviation. Experimental measures are reported as means ± standard deviation.. Experimental measures, including flow-mediated and nitroglycerin-mediated vasodilation for each subject group were compared using a paired Student’s t test. Linear regressions were performed to determine correlation between continuous variables and Pearson’s correlation coefficient reported in variables with normal distributions. Statistical significance was accepted at the 95% confidence level (p < 0.05). All statistics were performed with STATA 10, College Station, TX.
Results
Demographic characterizations
Nineteen subjects, 14 male (74%), were enrolled with a mean age of 64.7 ± 9.0 years. All subjects had a stable functioning femoral to popliteal bypass graft with autogenous vein that had not been revised. The mean time from surgery to enrollment was 1196 ± 785 days and no patient had a bypass for less than 1 year. The indication for bypass was claudication in 13, rest pain or ulceration in 5 and popliteal artery aneurysm in one. The prevalence of diabetes in this cohort was 11/19 (57.9%), 7/19 (36.8%) were active smokers, coronary artery disease 10/19 (52.6%), and hypertension 8/19 (42.1%), Table 1.
Table 1.
Study population demographics
| Variable | N (%) |
|---|---|
| Race (caucasian) | 16 (84.2%) |
| Gender (male) | 14 (73.7%) |
| CAD | 10 (52.6%) |
| Diabetes | 11 (57.9%) |
| Hypertension | 8 (42.1%) |
| Hyperlipidemia | 10 (52.6%) |
| Meds | |
| Aspirin | 19 (100%) |
| Statin | 13 (68.4%) |
| B-blocker | 14 (73.7%) |
| Runoff score* | |
| 1 vessel | 2 (11.8%) |
| 2 vessel | 7 (41.2%) |
| 3 vessel | 8 (47.1%) |
| Age of graft (days ± S.D) | 1195.6 ± 785.2 |
| eGFR (mean ± S.D.) | 69.0 ± 30.3 |
| HgbA1c (mean ± S.D.) | 6.6 ± .94 |
| Total cholesterol (mean ± S.D.) | 155.5 ± 62.1 |
CAD, coronary artery disease, eGFR estimated glomerular filtration rate, HgbA1c, Hemoglobin A1c
Runoff score calculated as number of patent vessels to the foot.
Vascular Function Studies
Brachial artery
Baseline brachial artery diameter was 3.51 ± .64 mm and increased to 3.83 ± .67 mm 60 seconds after deflation of the cuff for a flow-mediated vasodilation of 9.21 ± 4.6%, p=.0001 for the change, Table 2. Brachial artery volumetric flow increased from 207.64 ± 96.95 at baseline to 1050.22 ± 548.46 mL/min, P<.0001 during reactive hyperemia. In this cohort, there was no association with brachial artery FMD and cardiovascular risk factors.
Table 2.
Baseline and hyperemic vein graft and brachial artery morphometrics.
| Baseline diameter (mm ± S.D.) |
RH diameter (mm ± S.D.) |
Delta diameter (% ± S.D. ) |
Baseline Q (mL/min ± S.D. ) |
RH Q (mL/min ± S.D.) |
|
|---|---|---|---|---|---|
| Endothelium-dependent vasodilation | |||||
| Vein graft | 4.66 ± .82 | 4.89 ± .82 | 5.28 ± 3.1 % | 348.0 ± 153.2 | 1096.6 ± 781.1 |
| Brachial artery |
3.51 ± .64 | 3.83 ± .67 | 9.21 ± 4.6 % | 207.64 ± 96.95 | 1050.22 ± 548.46 |
| Endothelium-independent vasodilation | |||||
| Vein graft | 4.69 ± .81 | 4.87 ± .90 | 3.68 ± 4.4 % | 362.78 ± 90.76 | 410.03 ± 155.73 |
RH, reactive hyperemia
Vein graft
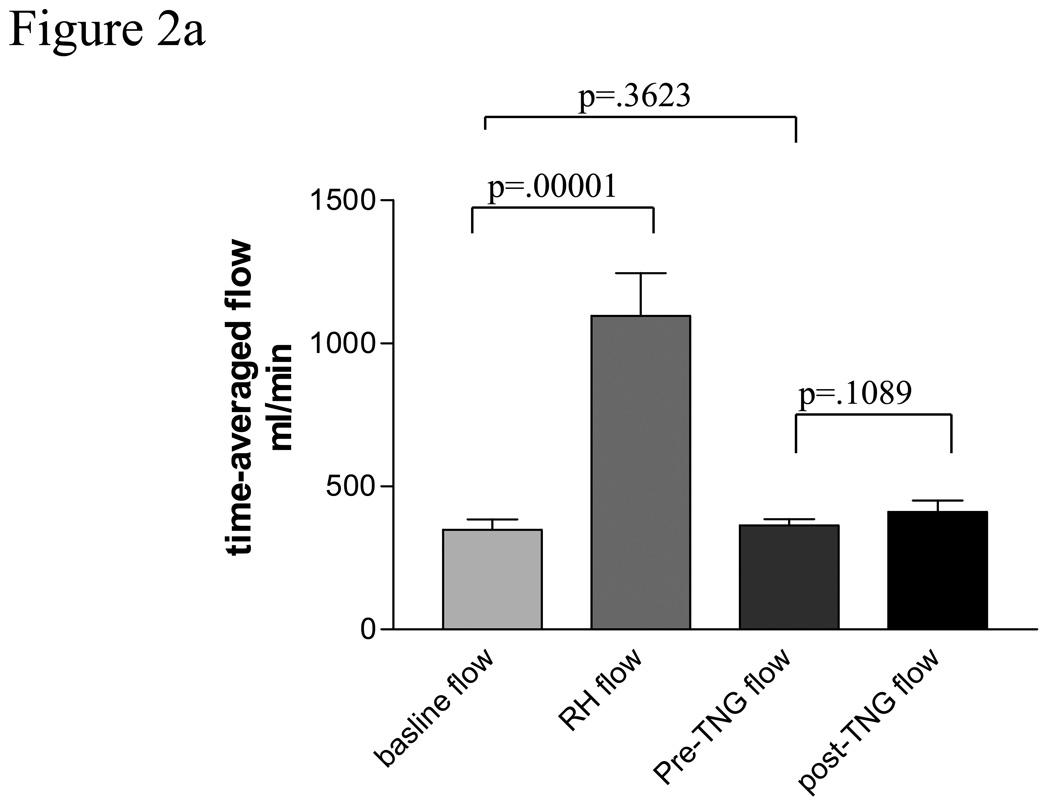
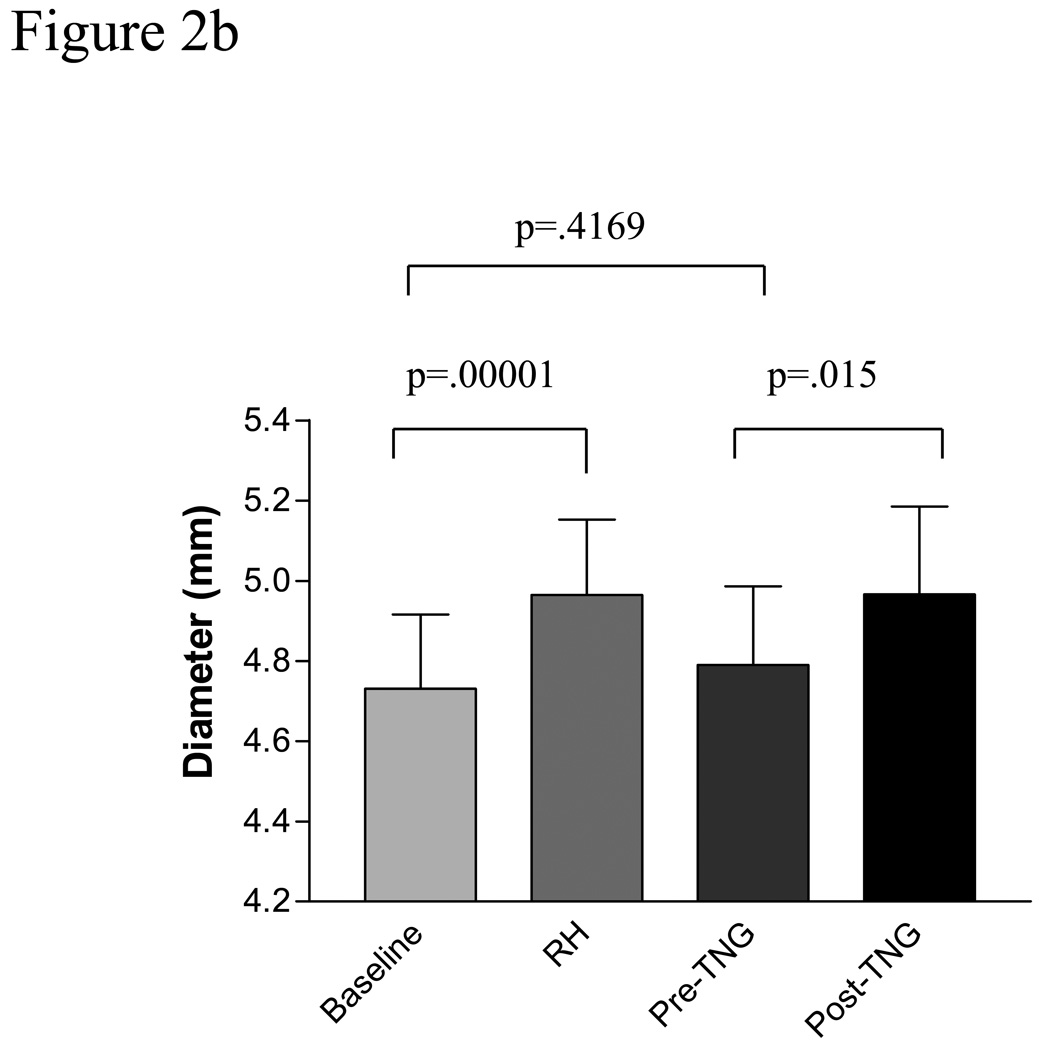
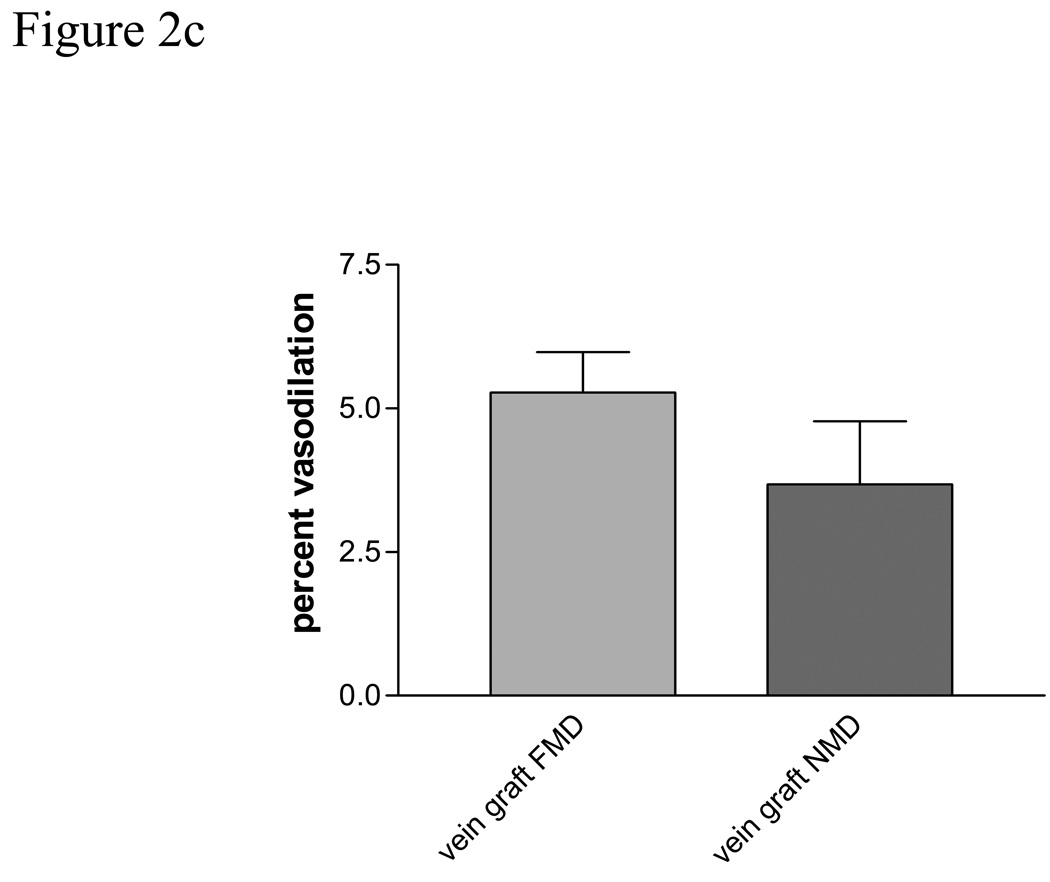
Baseline vein graft characteristics are presented in table 2. Baseline vein graft time-averaged, volumetric flow was 348.0 ± 153.2 ml/min and increased to 1097 ± 781.1 ml/min after cuff deflation representing a mean increase of 233%, p =.0001, Figure 2a. This was significantly less than the reactive hyperemic stimulus seen in the brachial artery, 233 ± 41 % for the vein vs. 430± 40% for the brachial artery, p =.0001. Baseline vein graft diameter was 4.66 ± .82 mm and increase to 4.89 ± .82 mm after cuff deflation, p=.0001, Figure 2b. Vein graft flow-mediated, endothelium-dependent vasodilation was 5.28% ± 3.1% (range 1.99–9.36%) for the entire cohort, Figure 2 c, which was significantly less than brachial artery FMD, P=.009. There was a suggestion of an inverse correlation between vein graft FMD and baseline vein graft diameter, r=−.46 (95% CI −.73, .04), P=.067. There was no observed association between vein graft vasodilatation and tibial runoff, nor an association between vein graft FMD and cardiovascular risk factors.
Figure 2.
Figure 2a Volumetric flow through human saphenous vein grafts under basal and hyperemic conditions and pre and post 0.4 mg sublingual nitroglycerin.
Figure 2b Endothelium-dependent and-independent vasodilatation in human saphenous vein bypass grafts. Baseline reflects baseline vein graft diameter. RH reactive hyperemia, TNG, nitroglycerin.
Figure 2c Flow mediated (FMD) and nitroglycerin mediated (NMD) vasodilatation in human saphenous vein grafts.
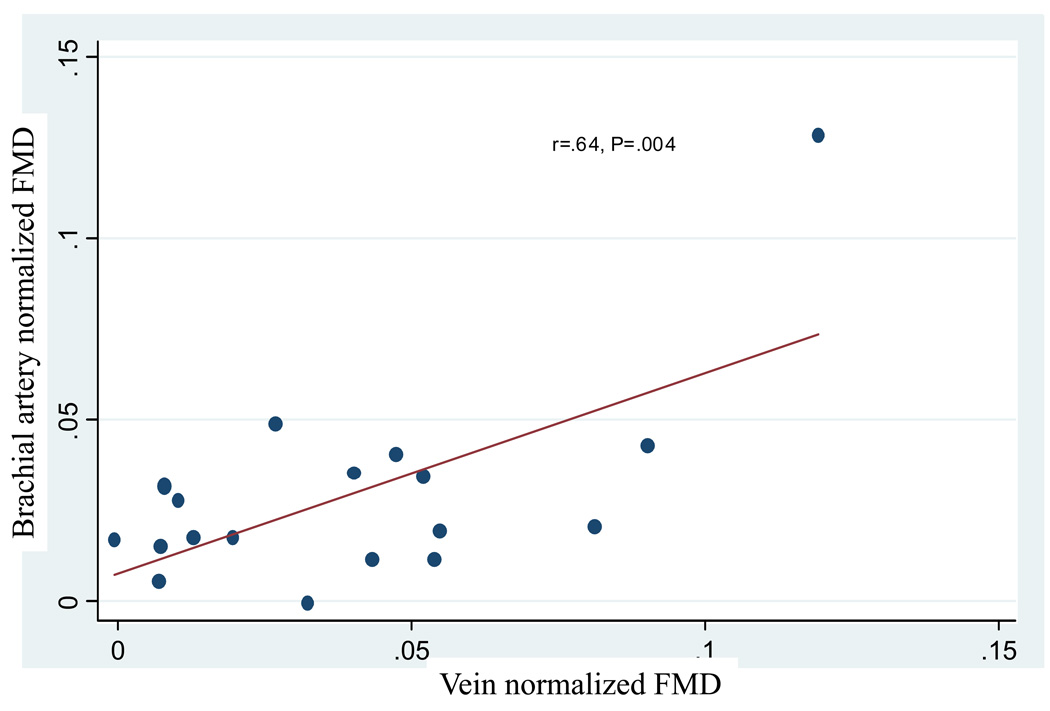
Flow-mediated vasodilation is, in part, determined by the amount of the reactive hyperemia response to cuff occlusion. To examine the role of flow in each vascular bed, a vasoreacitvity index (VI) was created. This index normalizes the FMD response to the flow stimulus by dividing percent FMD by the percent change in volumetric flow during reactive hyperemia. After normalization to the stimulus, there was no significant difference in the VI of the vein grafts and the brachial artery, .029 ±.007 vs. .039 ± .007, p=.13. While there was no correlation between unadjusted vein graft FMD and brachial artery FMD, after normalization for flow there was a significant correlation between vein graft vasoreactivity and that of the brachial artery, r=.64 (95% CI .24, .85), P=.004, Figure 3.
Figure 3.
Regression analysis demonstrating a significant positive correlation between brachial artery and vein graft flow mediated dilation once normalized to RH flow stimulus, r=.64, P=.004.
Sublingual nitroglycerin (0.4 mg) was given to assess vein graft endothelium-independent vasodilation (NMD). All patients who had a systolic blood pressure greater than 100 mm Hg were eligible to receive nitroglycerin, N=16. All vein grafts were re-imaged after 10 minutes to ensure a return to baseline diameter. Nitroglycerin produced a non-significant 13% increase in time-averaged, volumetric flow through the saphenous vein grafts and resulted in an increase in vein graft diameter from 4.69 ± .81 mm to 4.87 ± .90 mm, p=.015, Table 2 and Figure 2c. In this cohort NMD was 3.68 ± 1.0% (range 0.16 – 10.04%), Figure 2c.
Administration of LNMMA
Six subjects received L-NMMA. All patients experienced an increase in systolic blood pressure upon administration, and the mean increase in systemic blood pressure after administration was 17.8 mm Hg, p=.05. Despite the increase in blood pressure, there was no change in the baseline size (P=.67 and P=.50) or blood flow (P=.25 and P=.90) of the brachial artery or saphenous vein grafts, respectively.
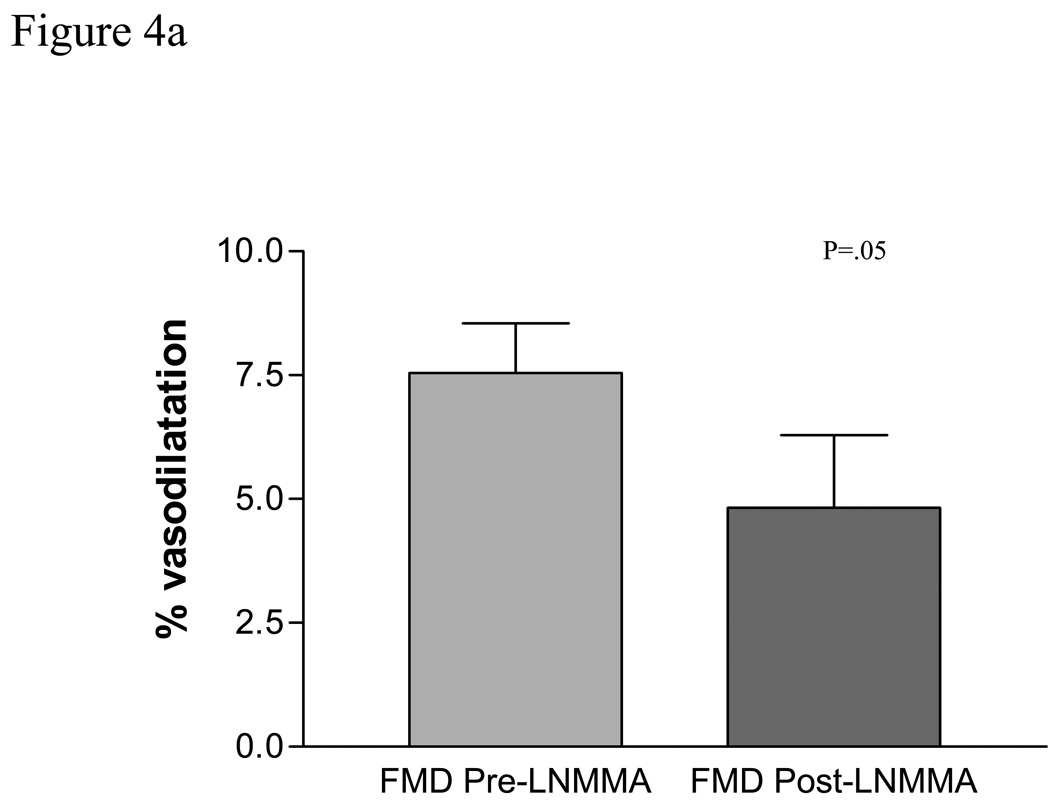
Prior to L-NMMA, brachial artery flow-mediated vasodilation was 7.54 ± 1%. During L-NMMA administration, brachial artery flow-mediated vasodilation decreased to 5.67 ± 1.36, for a 25% reduction in flow-mediated vasodilation (p= .05, compared to pre L-NMMA administration), Figure 4a.
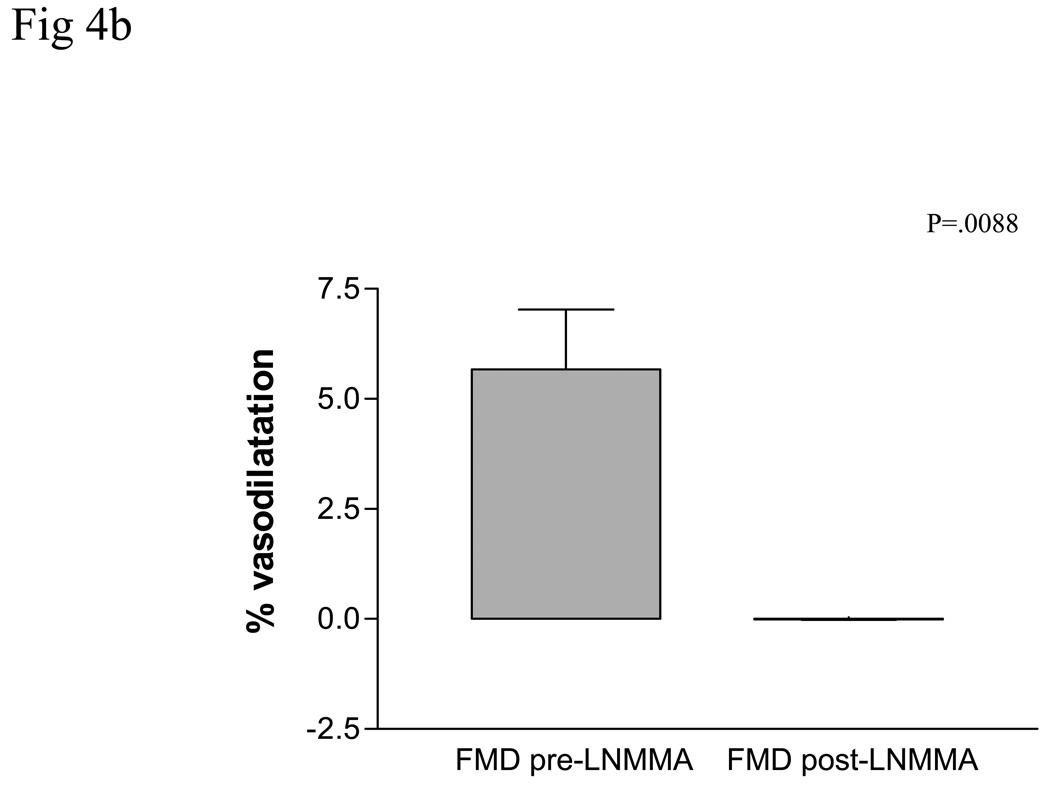
Figure 4.
The contribution of Nitric oxide (NO) to brachial artery and vein graft flow mediated dilatation (FMD) was examined by systemically infusing L-NG monomethyl arginine (LNMMA). While NO contributes to brachial artery FMD in atherosclerotic patients, P=.05, a, NO is likely the primary mediator of vein graft FMD, P=.0088, b.
Prior to L-NMMA, SVG flow-mediated vasodilation was 5.66 ± 3.34. During L-NMMA administration, SVG flow-mediated vasodilation decreased to −.01 ± .034, for a 100% reduction in flow-mediated vasodilation (p=.0088, compared to pre L-NMMA administration). There were no adverse events during L-NMMA administration, Figure 4b.
Discussion
Several novel findings are noted in this investigation. First, these experiments demonstrate that mature lower extremity human saphenous vein bypass grafts have functioning endothelium and are capable of undergoing flow mediated vasodilation, implying the presence of a physiologically functional endothelium. Further, we showed that the primary mediator of vein graft FMD is endothelium-derived nitric oxide demonstrated by the elimination of vasodilatation during systemic infusion of the endothelial nitric oxide synthase inhibitor, L-NMMA. Second, we have also shown that these grafts manifest nitroglycerin-mediated, endothelium-independent vasodilation but to a lesser extent then similar size arteries. This phenomenon has also been observed in aorto-coronary venous bypass grafts when exposed to nitroglycerin or sodium nitroprusside, both exogenous NO donors.(6; 17) Third, we have shown for the first time that brachial artery FMD, in patients with established atherosclerosis, is partially mediated by NO. Previously, the role of NO in conduit artery vasodilatation had been reported only in healthy subjects.
We have also shown for the first time that there is an apparent correlation between FMD of the brachial artery and that of mature functioning vein grafts. While previous work has demonstrated a relationship between peripheral and coronary artery endothelium-dependent vasodilation,(16) a relationship between peripheral artery and vein graft endothelial function has not been described. This suggests that vein grafts, despite the lack of mature lamellar subunits,(7) may be capable of adapting over time such that their endothelial and smooth muscle cell function responds in a similar fashion to that of peripheral conduit arteries. Whether or not brachial artery vasodilatory capacity may be used as a surrogate for the vein graft endothelial function is an interesting question to be examined in further studies. Other investigators have normalized FMD response either peak shear rate or area under the post-inflation shear rate curve with the latter having a higher correlation.(18) In both cases, the FMD response is, at least in part, regulated by the amount of the stimulus (reactive hyperemia) applied. The best method for relating these variables remains under active investigation.
The reactive hyperemic flow stimulus was significantly less in the vein graft than in the brachial artery in the arm which may, in part, explain the absolute difference between vein graft and brachial flow mediated dilatation. By placing the occlusion cuff distal to the vein graft over the popliteal artery, the blood flow was not restricted in the leg to the same degree as it was in the upper limb. Nevertheless, for safety reasons, we did not place the cuff directly over the vein graft.
Vein graft vascular tone modulation
There is relatively little known about the biomechanical changes of the endothelium of saphenous vein grafts during maturation in the arterial circulation. Both animal and human studies have demonstrated that the endothelium-dependent vasodilator response to acetylcholine is substantially weaker in pre-implantation veins than in arteries.(19) However, animal studies have also demonstrated that there exists a biochemical adaptation of venous endothelium to the arterial environment including an augmented vasodilator response to cholinergic stimulation and an augmented vasodilator prostaglandin production.(20; 21) This implies a plasticity to the endothelium that may be important in successful vein graft maturation. For example, chronic exposure to higher pressure and pulsatile flow increases endothelium derived relaxing factors in arteries.(22; 23)
Ex vivo human studies have demonstrated varied endothelial and smooth muscle cell reactivity in pre-implantation veins and vein grafts, a finding that may reflect the timing in which the experiment was conducted, and the stimulant used.(17; 24) Luscher et al noted that acetylcholine-stimulated, endothelium-dependent relaxation was significantly less in saphenous vein graft rings compared to internal mammary artery rings harvested at the time of surgery but prior to exposure to the arterial environment.(17) In these experiments, the saphenous veins were capable of endothelium-independent relaxation suggesting preserved smooth muscle cell function. Davies et al, noted that, in vein grafts explanted during re-operative cardiac surgery, pre-contracted saphenous vein graft rings failed to relax in response to acetylcholine compared with un-grafted saphenous veins.(25) The abnormality in cholinergic-mediated eNOS activation may result from the vein graft intimal hyperplasia or atheromata severe enough to require re-bypass, similar to the response in atherosclerotic coronary arteries.(26) Ku et al., has demonstrated that cardiac vein grafts retain their ability to relax ex vivo to acetylcholine and calcium ionophore for as many as 12 years suggesting preserved cholinergic-mediated endothelium-dependent relaxation.(24)
Common to each of the above mentioned studies was the use of Ach as a stimulus to test endothelial function in grafts. However, the observation noted by some investigators of the decreased ability of saphenous vein grafts rings to relax in response to Ach,(27; 28) must be tempered by the observation that others report that venous rings relax to a greater extent to A23187 (calcium ionophore), a receptor-independent (but endothelium-dependent) mechanism of vasorelaxation.(27; 29; 30) The significance of this discrepancy may be related to muscarinic receptor density differences in veins or vein grafts than in arteries rather than simply NO bioavailability.(29; 31)
A novel feature of the present study distinct from the above mentioned studies is the reliance on the more physiologically relevant stimulus, hyperemic flow, in contrast to acetylcholine. Flow may be an important regulator of vein graft homeostasis as evidenced by the greater rate of bypass graft failure in grafts with lower flows.(32) Whether or not endothelial function as measured here relates to vein maturation, or clinical events such as graft patency, is an area of ongoing investigation.
The relative importance of NO in vein graft vasodilator modulation can be inferred from several observations. First, NMD was similar to FMD suggesting that the arterial environment may maintain the vein graft near maximal vasodilation. We surmise the reduced nitroglycerin response occurred because of a more persistent vasodilatory state or a relative paucity of vascular smooth muscle compared to arteries. In coronary artery SVGs, vasodilatation is greater to nitroglycerin infusion.(33) In contrast to leg bypass SVGs, nitroglycerin also increases flow through coronary arteries facilitating a second mechanism of vasodilatation. The second important observation is the relative reduction in FMD with L-NMMA. FMD was abolished in SVG but only halved in the brachial arteries of our subjects, similar to previous work.(34) Our subjects were all treated with aspirin as part of their atherosclerosis management, suggesting that prostaglandins did not importantly participate in these findings. We surmise that SVGs rely to a greater extent on NO whereas conduit arteries employ subsidiary vasodilators, such as endothelium-derived hyperpolarizing factor to maintain some vasodilatory capacity.
The implication in the ability to detect flow mediated vasodilation in mature, patent, human lower extremity SVGs is that they have a functioning endothelial layer capable of producing NO. Nitric oxide confers the vessel with thromboresistance by inhibiting platelet aggregation, thrombus formation, leukocyte adherence, and vascular smooth muscle proliferation. Thus, a well functioning endothelial cell layer would be critical for maintaining bypass graft patency and may, in part, explain the superior patency rates of SVGs over prosthetic conduits. The initial injury to SVG endothelial function during harvesting has never been characterized in humans. The ability to quantify endothelial injury and to ascertain functional endothelial recovery may allow targeted and timely therapy to prevent myointimal hyperplasia. The finding that the endothelial function of the vein graft is as robust, for a given stimulus, as that of the brachial artery suggests an ontogeny of the maturing vein as it adapts in its role as an arterial substitute.
Limitations
This was a select cohort of patients chosen to minimize variability and maximize our ability to detect a response. Therefore we chose patients who had a mature functioning conduit, free of hemodynamically significant stenosis for over one year. As such these conduits were arterialized at baseline and therefore represented real-life functional bypass physiology.
We elected not to use a norepinephrine vasoconstrictor control arm to equivalently raise the systolic blood pressure to a similar extent as the L-NMMA. We justified this decision based on the lack of vasoconstriction of both the brachial artery and vein grafts at baseline during L-NMMA infusion. Nevertheless, it is possible that the acute increase in wall stress induced may have increased local production of reactive oxygen species (ROS), thus limiting the bioavailability of NO. This has been described in arteries but to our knowledge, has never been described in the vein bypass.
The kinetics of vein graft FMD response, in terms of duration of cuff inflation(35) as well as time to peak FMD(36) were not explored in this study. Previous studies in arterial conduit arteries, including the superficial femoral artery, indicate that FMD response to longer cuff inflation times (>5 minutes) are not purely mediated by NO.(37) In addition, other reports suggest that measurement of FMD 60–80 seconds after a 5 minute period of cuff-induced ischemia(15), may underestimate the maximal response.(36; 38) Now that vein graft FMD response has been established, kinetic studies represent an interesting line of investigation to determine whether the pattern varies from conduit arteries.
Finally, the number of subjects does not permit the determination of statistical association with known modifiers of FMD, such as cardiovascular risk. In addition, given the multiple comparisons performed in this study, there exist the possibility of type 1 (false positive) error. However, this does not detract from the experimentally derived observation that vein grafts manifest FMD.
Summary
We have shown that mature, human saphenous vein grafts, free of hemodynamically significant stenosis, manifest endothelium-dependent and endothelium-independent, vasodilatation. Furthermore, by abolishing flow mediated vasodilatation with competitive inhibitor of L-arginine, L-NMMA, these data suggests that the principal mediator responsible for vein graft flow mediated dilatation is NO. We have also shown for first time that mature lower extremity bypass grafts are capable of responding to a flow stimulus as robustly as does the brachial artery. These observations build on the conceptual framework that the lower extremity SVG undergoes considerable mechanical and biochemical adaptation in its role as a small artery substitute and may provide a window into predicting vein graft survival in patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Western Vascular Society Meeting, Napa, CA, September 15th, 2008
References
- 1.Jacot JG, Abdullah I, Belkin M, Gerhard-Herman M, Gaccione P, Polak JF, Donaldson MC, Whittemore AD, Conte MS. Early adaptation of human lower extremity vein grafts: wall stiffness changes accompany geometric remodeling. J Vasc Surg. 2004;39:547–555. doi: 10.1016/j.jvs.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 2.Owens CD, Wake N, Jacot JG, Gerhard-Herman M, Gaccione P, Belkin M, Creager MA, Conte MS. Early biomechanical changes in lower extremity vein grafts--distinct temporal phases of remodeling and wall stiffness. J Vasc Surg. 2006;4:740–746. doi: 10.1016/j.jvs.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Owens CD, Rybicki FJ, Wake N, Schanzer A, Mitsouras D, Gerhard-Herman MD, Conte MS. Early remodeling of lower extremity vein grafts: inflammation influences biomechanical adaptation. J Vasc Surg. 2008;47:1235–1242. doi: 10.1016/j.jvs.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilian JG, Thanyasiri P, Celermajer DS, Adams MR. Saphenous vein grafts display poor endothelium-dependent and endothelium-independent dilation--implications for the pathogenesis of vein graft atherosclerosis. Heart Lung Circ. 2008;17:96–99. doi: 10.1016/j.hlc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Werner GS, Wiegand V, Kreuzer H. Effect of acetylcholine on arterial and venous grafts and coronary arteries in patients with coronary artery disease. Eur Heart J. 1990;11:127–137. doi: 10.1093/oxfordjournals.eurheartj.a059668. [DOI] [PubMed] [Google Scholar]
- 6.Berglund H, Luo H, Nishioka T, Eigler NL, Kim CJ, Tabak SW, Siegel RJ. Preserved vasodilatory response to nitroglycerin in saphenous vein bypass grafts. Circulation. 1996;94:2871–2876. doi: 10.1161/01.cir.94.11.2871. [DOI] [PubMed] [Google Scholar]
- 7.Cox JL, Chiasson DA, Gotlieb AI. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34:45–68. doi: 10.1016/0033-0620(91)90019-i. [DOI] [PubMed] [Google Scholar]
- 8.Ehsan A, Mann MJ, Dell'Acqua G, Tamura K, Braun-Dullaeus R, Dzau VJ. Endothelial healing in vein grafts: proliferative burst unimpaired by genetic therapy of neointimal disease. Circulation. 2002;105:1686–1692. doi: 10.1161/01.cir.0000013775.02396.93. [DOI] [PubMed] [Google Scholar]
- 9.Chung AW, Rauniyar P, Luo H, Hsiang YN, van Breemen C, Okon EB. Pharmacologic relaxation of vein grafts is beneficial compared with pressure distention caused by upregulation of endothelial nitric oxide synthase and nitric oxide production. J Thorac Cardiovasc Surg. 2006;132:925–932. doi: 10.1016/j.jtcvs.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 10.Dashwood MR, Savage K, Dooley A, Shi-Wen X, Abraham DJ, Souza DS. Effect of vein graft harvesting on endothelial nitric oxide synthase and nitric oxide production. Ann Thorac Surg. 2005;80:939–944. doi: 10.1016/j.athoracsur.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 11.Hinokiyama K, Valen G, Tokuno S, Vedin JB, Vaage J. Vein graft harvesting induces inflammation and impairs vessel reactivity. Ann Thorac Surg. 2006;82:1458–1464. doi: 10.1016/j.athoracsur.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 12.Tsui JC, Souza DS, Filbey D, Karlsson MG, Dashwood MR. Localization of nitric oxide synthase in saphenous vein grafts harvested with a novel "no-touch" technique: potential role of nitric oxide contribution to improved early graft patency rates. J Vasc Surg. 2002;35:356–362. doi: 10.1067/mva.2002.121072. [DOI] [PubMed] [Google Scholar]
- 13.Davies MG, Klyachkin ML, Dalen H, Massey MF, Svendsen E, Hagen PO. The integrity of experimental vein graft endothelium--implications on the etiology of early graft failure. Eur J Vasc Surg. 1993;7:156–165. doi: 10.1016/s0950-821x(05)80756-x. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki Y, Suehiro S, Becker AE, Kinoshita H, Ueda M. Role of endothelial cell denudation and smooth muscle cell dedifferentiation in neointimal formation of human vein grafts after coronary artery bypass grafting: therapeutic implications. Heart. 2000;83:69–75. doi: 10.1136/heart.83.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 16.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 17.Luscher TF, Diederich D, Siebenmann R, Lehmann K, Stulz P, von Segesser L, Yang ZH, Turina M, Gradel E, Weber E, et al. Difference between endothelium-dependent relaxation in arterial and in venous coronary bypass grafts. N Engl J Med. 1988;319:462–467. doi: 10.1056/NEJM198808253190802. [DOI] [PubMed] [Google Scholar]
- 18.Thijssen DH, Bullens LM, van Bemmel MM, Dawson EA, Hopkins N, Tinken TM, Black MA, Hopman MT, Cable NT, Green DJ. Does arterial shear explain the magnitude of flow-mediated dilation?: a comparison between young and older humans. Am J Physiol Heart Circ Physiol. 2009;296:H57–H64. doi: 10.1152/ajpheart.00980.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Mey JG, Vanhoutte PM. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res. 1982;51:439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- 20.Henderson VJ, Cohen RG, Mitchell RS, Kosek JC, Miller DC. Biochemical (functional) adaptation of "arterialized" vein grafts. Ann Surg. 1986;203:339–345. doi: 10.1097/00000658-198604000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.el Khatib H, Lupinetti FM, Sanofsky SJ, Behrendt DM. Production of endothelium-dependent relaxation responses by saphenous vein grafts in the canine arterial circulation. Surgery. 1991;110:523–528. [PubMed] [Google Scholar]
- 22.Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- 23.Miller VM, Aarhus LL, Vanhoutte PM. Modulation of endothelium-dependent responses by chronic alterations of blood flow. Am J Physiol. 1986;251:H520–H527. doi: 10.1152/ajpheart.1986.251.3.H520. [DOI] [PubMed] [Google Scholar]
- 24.Ku DD, Caulfield JB, Kirklin JK. Endothelium-dependent responses in long-term human coronary artery bypass grafts. Circulation. 1991;83:402–411. doi: 10.1161/01.cir.83.2.402. [DOI] [PubMed] [Google Scholar]
- 25.Davies MG, Berkowitz DE, Hagen PO. Constitutive nitric oxide synthase is expressed and nitric oxide-mediated relaxation is preserved in retrieved human aortocoronary vein grafts. J Surg Res. 1995;58:732–738. doi: 10.1006/jsre.1995.1116. [DOI] [PubMed] [Google Scholar]
- 26.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 27.Cross KS, Davies MG, el-Sanadiki MN, Murray JJ, Mikat EM, Hagen PO. Long-term human vein graft contractility and morphology: a functional and histopathological study of retrieved coronary vein grafts. Br J Surg. 1994;81:699–705. doi: 10.1002/bjs.1800810524. [DOI] [PubMed] [Google Scholar]
- 28.Park TC, Harker CT, Edwards JM, Moneta GL, Taylor LM, Jr, Porter JM. Human saphenous vein grafts explanted from the arterial circulation demonstrate altered smooth-muscle and endothelial responses. J Vasc Surg. 1993;18:61–68. doi: 10.1067/mva.1993.42071. discussion 68–69. [DOI] [PubMed] [Google Scholar]
- 29.Ignarro LJ, Buga GM, Chaudhuri G. EDRF generation and release from perfused bovine pulmonary artery and vein. Eur J Pharmacol. 1988;149:79–88. doi: 10.1016/0014-2999(88)90045-3. [DOI] [PubMed] [Google Scholar]
- 30.Miller VM, Reigel MM, Hollier LH, Vanhoutte PM. Endothelium-dependent responses in autogenous femoral veins grafted into the arterial circulation of the dog. J Clin Invest. 1987;80:1350–1357. doi: 10.1172/JCI113212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ignarro LJ, Harbison RG, Wood KS, Kadowitz PJ. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther. 1986;237:893–900. [PubMed] [Google Scholar]
- 32.Rzucidlo EM, Walsh DB, Powell RJ, Zwolak RM, Fillinger MF, Schermerhorn ML, Cronenwett JL. Prediction of early graft failure with intraoperative completion duplex ultrasound scan. J Vasc Surg. 2002;36:975–981. doi: 10.1067/mva.2002.128298. [DOI] [PubMed] [Google Scholar]
- 33.Hartmann A, Lahoda T, Burger W, Beyersdorf F, Schrader R, Satter P. Endothelium-dependent and endothelium-independent flow regulation in coronary vascular regions supplied by arterial and venous bypass grafts. Cardiology. 1997;88:425–432. doi: 10.1159/000177372. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, Creager MA. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996;78:1210–1214. doi: 10.1016/s0002-9149(96)00597-8. [DOI] [PubMed] [Google Scholar]
- 35.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001;88:145–151. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- 36.Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Heterogeneity in conduit artery function in humans: impact of arterial size. Am J Physiol Heart Circ Physiol. 2008;295:H1927–H1934. doi: 10.1152/ajpheart.00405.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol. 2008;586:1137–1145. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension. 2008;51:203–210. doi: 10.1161/HYPERTENSIONAHA.107.101014. [DOI] [PubMed] [Google Scholar]