Abstract
Genomic instability, aberrant cell proliferation and defects in apoptotic cell death are critical issues in cancer. The two most prominent hallmarks of cancer cells are multiple mutations in key genes encoding proteins that regulate important cell-survival pathways, and marked restructuring or redistribution of the chromosomes (aneuploidy) indicative of genomic instability. Both these aspects have been suggested to cause cancer, though a causal role for chromosomal restructuring in tumorigenesis has not been experimentally fully substantiated. This review is aimed at understanding the mechanisms of cell cycle (proliferation) and programmed cell death (apoptosis) and chromosomal instability governed by cohesin and other aneuploidy promoters, which will provide new insights into the process of carcinogenesis and new avenues for targeted treatment.
Keywords: Cohesin, Apoptosis, Aneuploidy, Separase, Rad21
1. Introduction
Metazoans comprise of multiple cells that possess distinct features, despite being originated from a single cell with identical genotype. The course of development of a multicellular organism from a zygotic single cell revolves around differentiation, a process of becoming phenotypically different while genotypically remaining the same. Such a task is achieved by accommodating changes to the epigenome in each constituent cell of the organism, which in turn results in differential regulation of gene expression. To maintain the organismal homeostasis, the constituent cells must abide by certain norms. That is, the differentiated cells must stay differentiated, and the differentiating cells must strictly follow the exact path of differentiation that the organism needs them to. Alteration in normal gene regulation profile of a constituent cell causes the cell to deviate from this path, resulting in homeostatic imbalance. Such imbalance is detrimental to the organismal well being; and the cells going astray must, therefore, be eliminated with caution. This is why multicellularity has evolved multiple paths for programmed cell death [1,2].
Tumors arise when such unruly, deviant cells evade programmed cell death [3,4]. It is generally accepted that such cells have gained alterations in essential genes that control cell death, cell differentiation or cell proliferation. These alterations endow the cells with a set of six special advantages over the normal cells: (i) self-sufficiency in growth signals, (ii) insensitivity to growth-inhibitory signals, (iii) evading apoptosis, (iv) unlimited replicative potential, (v) sustained angiogenesis, and (vi) tissue invasion and metastasis [5] over a long, multistep process culminating in cancer [6,7]. Howthe process of tumorigenesis begins is still unclear, and is a question at the center of intense debates. One of the limitations of the current research on cancer biology is its heavy dependence on a detectable phenotype—a stage well past initiation. This has led to many theories and hypotheses which do not explain all features of carcinogenesis, or, in many cases, contradict each other. For an example, according to the somatic mutation theory (SMT), there is a set of mutations that, coupled with clonal selection, impairs the cellular physiology towards proliferative advantage [8–11]. However, it has also been proposed that cancers cause mutations rather than mutations causing cancer; and the inception of cancer is attributed to alterations in gene expression due to epigenetic mechanisms [12]. As opposed to somatic mutations, it is argued that cancer is rather a disease of disrupted tissue organization [13]. In addition, changes in immune system [12], signaling between tissues [14] and hormonal status [15] have also been implicated in tumorigenesis.
On the other hand, morphological abnormalities in chromosomes were observed in tumor samples as early as late 19th century, which inspired Theodor Boveri to propose that a ‘definite abnormal chromosome complex’ would always result in tumors [16]. Ever since, the theory of chromosomal abnormality has been a strong contender to the SMT as a cause for tumorigenesis (see [17] for a discussion). The majority of the current thinkers in the field of tumorigenesis and carcinogenesis appear to favor either one or the other theory [18]. Here, we make an attempt to discuss both these theories with an emphasis on aneuploidy, apoptosis, chromosomal cohesion, and to extract a plausible reconciliatory explanation to the cause of cancer.
2. Genetic instability in cancer
The concept of individuality of chromosomes in a given cell was given by Boveri in late nineteenth century, at a time when all chromosomes in a cell were considered to possess same qualities. Later in 1914, in his proposition that cancer is a genetic disease of somatic cells, Boveri envisioned that abnormal chromosomal numbers would lead to abnormal cellular phenotypes culminating in tumorigenesis [16]. Normal eukaryotic somatic cells should contain a diploid genome comprised of multiple chromosomes. During successive normal cell divisions, this set-up is normally preserved. Over several decades after Boveri, tumor cells have invariably been found to contain many genetic abnormalities. These abnormalities include aberrant number of chromosomes and chromosomal fragmentations, indicating instability in the genetic integrity of these cells. Even though the earliest observations of genetic instability were the visible changes in chromosome number and architecture, over the decades thereafter the term ‘genetic instability’ (GIN) has evolved to include genomic alterations at the nucleotide level as well [19]. Causes of GIN have primarily been attributed to defects in replication, transcription, recombination, and DNA-damage repair [20].
The nucleotide-level changes include base-substitution mutations, or deletions or insertions of a few nucleotides; and these cannot be detected through classical cytogenetic analyses. These genetic changes normally form the basis of the oncogenic mutation (OM) mechanism for cancer causation, and will be discussed later in the text. When the mismatch repair pathway is compromised, a staggering level of short DNA repeats are found all over the genome in cancers like the hereditary colorectal cancer, which is known as micro-satellite instability (MIN) [21]. At a higher level of the chromatin, epigenetic dysregulation is being reported for more and more genes in cancer cells. These include promoter DNA methylation and hypoacetylation and methylation of histones on corresponding genes [22]. It is also proposed that epigenetic dysregulation may have a causal role in cancer inception [23]. The next level of GIN involves structural changes to the chromosomes known as chromosomal instability (CIN), which includes alteration in ploidy and generation of chromosomal translocations [24]. An alteration in ploidy can be a linear multiplication of the entire set of chromosomes (polyploidy), or a loss or gain of single chromosomes or fragments thereof (aneuploidy). Aneuploidy is so prevalent in cancer that any cell from a cancer epithelium can represent the whole range of structural and numerical chromosome anomaly usually found in cancers [25]. Most human cancers differ in the over-all number of chromosomes their cells contain, which often ranges from 60 to 90 [26]. Moreover, individual cells within a certain tumor may also vary greatly in the karyotype. Aneuploidy is so overwhelmingly prevalent in cancers that some biologists consider it essential for tumorigenesis [27]. Alongside the SMT, aneuploidy is considered the foremost explanation to the causation of cancer. We shall return to the discussion of aneuploidy in cancer after a cursory look at the OM (Section 2.1).
2.1. OM as cause for cancer
Two years after Boveri published his studies on abnormal chromosome structures in tumors, Tyzzer proposed tumors to be a manifestation of somatic mutations [28], followed by a similar proposition by Whitman [29]. Subsequently, X-rays – already known to be carcinogenic – were reported to cause genetic transmutation in Drosophila, resulting in stable mutant lines [30]. The above and subsequent understandings, in due course, assumed the form of a theory now familiar as the SMT. The basic premises of the SMT are: (i) point or regional mutations, affecting one or more genes that control cell proliferation and cell cycle regulation, initiate the process of tumorigenesis. This suggests that replicative quiescence is the default state of normal metazoan cells, and those carcinogenic mutations release the ‘break’ allowing the mutant cells to proliferate. (ii) The mutant cells undergo clonal propagation to form tumors [9,31,32]. This kind of mutations may not necessarily accompany visible chromosomal changes; and the mutant cells may or may not show incomplete differentiation. It is widely accepted that the whole process of carcinogenesis involves multiple successive steps [8] that must at the least include three phases: initiation, promotion and propagation. The OM can cause either gain or loss of proliferation-regulatory functions; and thus the corresponding genes can either be an oncogene or a tumor suppressor gene. Early mathematical modeling suggested the need of a minimum of about seven neighboring mutant cells (or about seven mutations in a cell) in order for a tumor to arise, and that those mutational events are rate-limiting in nature [32]. Later experimental modeling showed that as few as two, or even one, oncogenic events can cause tumorigenesis [7,33,34]. Over the years, extensive research has discovered more and more genes that are mutated in cancers; and they appear to regulate numerous distinct cellular pathways including those that regulate cell proliferation, differentiation, DNA-repair and cell death. A recent comprehensive analysis found as high as 1% of total human genes contributing to cancer [35].
The most important aspect of the SMT is that it attempts at explaining carcinogenesis at the molecular level and provide a comprehensive understanding of cancer cell biology. Yet, there are a few aspects of carcinogenesis that the SMT fails to explain. For instance, the calculated rate and number of mutations causing a tumor fails to explain the age-dependence of cancer occurrence. The current understanding according to the SMT considers a single mutant cell as the progenitor of a clonal tumor. Mathematical analyses find this assumption inconsistent with the epidemiologic data for the majority of common cancers [36]. Further, the OM would be expected to affect the cell behavior soon after the cell exits the resting G0 phase. However, neoplastic latency – the time lapse between mutagenesis and tumor detection – in most experimental models is very long, even up to 30 years [17,37]. Moreover, one of the basic premises of SMT, that the default state of the metazoan cells is quiescence, also seems inconsistent with multicellular ontogeny. The development of a fully formed embryo from a single-celled zygote is the key process in multicellular life, and this crucial phase cannot be a violation of the basic metazoan norm. Therefore, it appears that proliferation is instead the default state of metazoans, and that normal cells in the tissue are forced from proliferating to maintain organismal homeostasis [38]. Dysregulation of this state would lead to uncontrolled proliferation seen in cancers.
As has been stated above, SMT faces fierce criticism from biologists who think that genomic instability, and to be more specific, aneuploidy, is the singular mechanism behind tumorigenesis (see the discussion in Section 2.2).
2.2. Aneuploidy as cause of cancer
Aneuploidy is ubiquitous, and has been established as the most consistent marker of cancers [39]. It is generally considered that the magnitude of aneuploidy is greater in malignant than in non-malignant cells; and increase in aneuploidy correlates with increasing malignancy, development of multidrug resistance and poor prognosis [40–42]. The degree of malignancy is proportional to the degree of aneuploidy [43]. A meta-analysis of published data on non-small cell lung cancer showed that 65% of patients out of 4033 had aneuploid tumors, and they had much poorer prognosis compared to the rest with diploid tumors [44]. Aneuploidy has also been found to correlate with poor prognosis of gastric [45], pancreatic [46] and colorectal [47] and breast [48,49] cancers. Increasing aneuploidy in cancer cells also associates with an exponential increase in karyotype instability [50]. Pre-neoplastic cells show very low levels of aneuploidy, but it still correlates with karyotype instability [51,52].
Such abundance of data correlating aneuploidy with cancer progression certainly argues a case for aneuploidy to play an important role in carcinogenesis. This has inspired Duesberg and colleagues to very aggressively push for a new chromosomal theory of carcinogenesis, where they argue that multistep carcinogenesis is nothing but a cascade of aneuploidizations, and that carcinogenesis can only be initiated by aneuploidy [17,27]. Duesberg argues that carcinogenesis is associated with aneuploidy from its initiation [27]. This notion suggests that aneuploidy must precede oncogenic transformation if it were to cause carcinogenesis. Also, patients with congenital aneuploidy should face greater risk of developing cancer. First, aneuploidy was also observed in mouse skin and rat liver models, where carcinogenesis was initiated with DMBA (dimethyl benz[a]anthracin) and propagated with TPA (phorbol-12-myristate-13-acetate) or RPA (phorbol-12-retinoate-13-acetate) [53]. Aneuploidy has also been detected in pre-cancerous head and neck lesions [54] as well as breast lesions [55]. As compared to the controls, the preneoplastic cells show increased aneuploidy that further increases in malignant cells [56]. Second, patients with the syndromes like Down’s, Klinefelter’s and Turner’s have a greater risk of leukemia, breast tumor and glondoblastoma, and neural crest tumor respectively. Persons with trisomy 13 and trisomy 18 also encounter higher frequency of neuroblastoma and teratoma, and Wilm’s tumor and neurogenic neoplasia, respectively [53].
Such ubiquity of aneuploidy in solid tumors is considered as a ‘correlative proof of causation’ [57]. However, experimental science does not recognize the notion of ‘correlative proof of causation’. Two events may correlate, coexist and even co-progress from the initiation to the climax of a process; but no causal relationship can be inferred from such correlation. So far, there has been no experimental evidence that suggests a causal role of aneuploidy in carcinogenesis, except for one instance where aneuploidy was also implicated in tumor suppression [58] (see discussion below). Furthermore, there are cancer cells that are polyploid. Such polyploid cells cannot emerge from an aneuploid progenitor cell; whereas the reverse can be true [59]. Aneuploidy can result from meiotic as well as mitotic defects, which can arise from gene mutations [19,60], even though it is argued that aneuploidy arises independently of gene mutations [61]. Furthermore, there are cancers that are not aneuploid [62]. Examples of diploid tumors include many lymphatic cancers like acute myeloid leukemia (AML), where nearly half of the cases contain cytogenetically normal cells [63], as well as hereditary colorectal cancers deficient in mismatch repair (MMR) [64,65]. Finally, the aneuploidy theory fails to explain a mechanism where aneuploidization would help cells acquire proliferative advantage over the normal cells in their neighborhood.
2.3. Causes of mutations and aneuploidy
Thus, we confront an extraordinary sense of debate as to how to explain carcinogenesis. Before going further, it may be useful to have a look at how mutations or aneuploidy can arise at the first place.
Mutations can be acquired spontaneously. Though DNA replication in eukaryotes is a high-fidelity process, an error can occur every 108 to 1010 basepairs [66]; though the rate can be higher for certain sequences like the microsatellite repeats. Higher eukaryotes also possess an error-prone DNA polymerase belonging to the Y family [67]. Mutations can also occur by an exposure to a wide range of mutagens. They can be from within the cell’s environment, such as reactive oxygen species, ROS. A mutagen may come from outside as well, such as ionizing radiations, base analogs that substitute for DNA bases and cause copying errors, and agents that intercalate, deaminate or alkylate the bases. These mutations are readily corrected by the DNA repair pathways—namely the base excision repair (BER) and the MMR, but there are rooms for errors when there is any impairment (see [68] for a review).
Aneuploidy can occur when replicated chromosomes inaccurately segregate into the daughter cells. Defects in many cellular functions including the spindle apparatus, duplication of centrosomes, centrioles, attachment of the spindle to the centromeres, sister chromatid cohesion and segregation can lead to aneuploidy. Telomere and centromere stability, and checkpoints maintenance can also potentially cause aneuploidy. Such impairment can occur spontaneously, albeit at a very low rate; but can be caused by(i) mutagens that affect a gene involved in any of the above pathways or (ii) by aneugens (agents that cause aneuploidy) that interfere with any of those pathways [53]. An aneugen may or may not be a mutagen; and can be a potent carcinogen or inducer of epigenetic events. So far, only nine definitive (i.e., non-mutagenic) aneugens have been described in literature, and they include benomyl, chloral hydrate, acrylamide and hydroquinone [53]. Mutations can, as discussed above, cause aneuploidy. Aneuploidy per se cannot cause mutations; but aneuploidy-linked impairment of the DNA-damage repair pathways can lead to accumulation of mutations.
3. So, what causes cancer?
As has been discussed above, experiments have shown unambiguously that two or more oncogenic events can cause tumorigenesis [33,69–71]. Experiments performed in the Weinberg laboratory [71] showed that ectopic induction of human telomerase catalytic subunit hTERT, in combination with an oncogenic ras allele and the SV40 large-T antigen, caused tumorigenesis in human kidney HEK as well as human fibroblast BJ cells. Cells ectopically expressing these three genes also caused tumor formation when introduced into nude mice. Duesberg, who believes that it is not the gene mutations but aneuploidy that causes tumorigenesis, analyzed the same transformed cells as reported by Hahn et al. [71]; and found those tumors are aneuploid. He thus argued that aneuploidywas the cause of tumorigenesis in Weinberg’s observations [72]. Unfortunately, his observations merely suggest that aneuploidy correlates with tumorigenesis. That aneuploidy causes tumorigenesis is indeed a difficult hypothesis to test. It is required that aneuploidy is induced without any DNA damage, anomalous DNA-damage response, chromosomal rearrangements or alteration in expression of key genes like TP53; and such genetic set-up is tested for carcinogenesis. One such investigation, conforming to these strict guidelines, created mouse heterozygous for the centromeric kinesin-like motor protein CENP-E. Reduction in CENP-E is not expected to cause defects other than low-level chromosome mis-seggregation [58]. Most cells examined from wild type and CENP-E+/− mice were diploid to near-diploid. But, fibroblasts from the CENP-E+/− mice developed marked aneuploidy in culture. Further, the CENP-E+/− mice showed increased incidence of spleen lymphomas and lung adenoma. This finding appeared consistent with the aneuploidy hypothesis of carcinogenesis. However, the CENP-E+/− mice also showed marked decrease in spontaneous, DMBA-induced, as well as p19/ARF−/−-induced liver tumors as compared with the CENP-E+/+ animals. Thus, aneuploidy appears to support both carcinogenesis as well as tumor suppression [58].
In a study employing mouse models of Down’s syndrome, aneuploidy has been reported to suppress tumor formation [73]. On the other hand, experimental aneuploidization reportedly failed to make tumors, although the affected mice developed pro-geriatric symptoms [74]. A recent study with trisomic mouse models reveals that aneuploidy results in reduced cellular fitness by reducing the cell proliferation, increasing the cellular mass and by increasing cellular metabolism [75]. However, trisomic cells that overcome the negative selection by aneuploidy tolerating and proliferation promoting mutations could lead to transformation. Here, we find an exciting analogy of aneuploidy with mutations as regards carcinogenesis. Mutations, as few as two, are thought to cause cancer; but high-intensity mutagenesis suppresses tumorigenesis—as evident from radiation therapy being in practice for over a century now. Thus, both the causation/promotion and suppression of tumorigenesis appear to be the handiwork of mutations and chromosomal instability. Now, how can we rationally find an explanation for this seeming paradox?
3.1. Altered protein function as the cause of cancer
Tumorigenesis and its progression to metastasis requires a set of special advantages, namely self-sufficiency in growth signals, insensitivity to growth-inhibitory signals, evading apoptosis, unlimited replicative potential, sustained angiogenesis, and tissue invasion and metastasis [5]. Any event – single or multiple – can be the cause of carcinogenesis that provides – or paves the way for – those advantages. A cursory look at the general cell biology tells us that there has to be a huge number of genes that could potentially play a role in regulating steps that would eventually grant the cell those advantages; achieving each of these advantages may require integration of many pathways. For example, cell proliferation is at the center of achieving ‘unlimited replicative potential’; and it depends on integrative networks of multiple pathways including DNA replication, DNA-damage response and check-point regulation, sister chromatid cohesion and segregation, cytokinesis, cell organelle biogenesis and cell energetics. If we look a little deeper, these processes look like an intricate cascade of transcription, translation, and replication. And, a further reductionist look takes us to the kernel that comprises of protein synthesis, protein modifications and protein degradation. If we look at the other advantages that the cells must acquire for carcinogenesis, these basic three principles form the kernel in each of those cases. However, it is also important to note that these three basic principles affect numerous proteins – often in multiple combinations – to behave abnormally in cancers; and thus we see bewildering heterogeneity of phenotypes in all cancers.
3.2. Modulation of protein function by mutations or aneuploidy
From the above discussion, it is clear that cells can acquire tumorigenic properties by altering either the cellular level (abundance) or functional quality (mutations) of key proteins. For example, the retinoblastoma protein (pRB) plays a key role in controlling the progression of the cell through G1 to S phase of cell cycle [76]. In many cancers, pRb is absent because of mutations in the corresponding gene, or is sequestered and degraded by viral oncoproteins. It is also inactivated in many cancers by mutations in one of its regulators, p16INK4A, or by over-expressing cyclins D1 or E [77]. Similarly, nearly half of all human cancers contain mutations in the TP53 gene that encodes the tumor suppressor p53; and, surprisingly, over 15,000 mutant, loss-of-function alleles of TP53 have so far been identified in human cancers [11]. According to a recent analysis, p53 regulates at least 129 genes that are involved in a wide range of pathways including apoptosis, cell cycle regulation, growth, differentiation and proliferation, chromatin modification, maintenance of genomic stability, metabolism and ion transport [78]. In many tumors where there is no mutation in the TP53 gene, the protein is inactivated by the over-expression of its antagonist HDM2. In some others p19ARF – the antagonist ofHDM2–is either deleted or epigenetically suppressed. Likewise, nearly a third of all human cancers possess the ras oncogene, the mutant version of Ras, which causes uninterrupted release of mitogenic signals [11].
Thus, mutations can alter cellular levels of a protein, or can cause inactivation or hyperactivation of a protein function. In many instances, a number of such mutations might just be lethal, and the mutant cells are eliminated. However, in those unfortunate cases as discussed above, such mutations can cause tumorigenesis. Aneuploidy can also cause alteration in the over-all expression of numerous genes. An aneuploid cell either gains or loses single whole chromosomes or fragments thereof; thus aneuploidy causes genetic imbalance of dosage in the cell. Such an imbalance will result in increased or decreased expression of genes encoded by the extra or the missing chromosome respectively. This is expected to result in massive alteration in the transcriptome, since a chromosome is expected to encode many transcriptional activators, co-activators, repressors and co-repressors. Also, multiple proteins, encoded by different genes located on different chromosomes, often function in concert, in synergism, or form a multicomponent functional entity. Increase or decrease in expression of any such gene – owing to the aneuploidy-induced genetic imbalance – can lead to altered functional efficiency of such a pathway. This indeed appears to be the case in aneuploidy. Ried and colleagues [79] compared immortalized prostate epithelium with aneuploid prostate tumor cell lines and observed that gain or loss of chromosomal regions caused respectively increased or decreased expressions of genes located in corresponding regions. Interestingly, 30% of genes also suffered misregulation whose gene dosages were unaffected by aneuploidy. In a further improvization of the strategy, Ried and colleagues generated artificial trisomies using microcell-mediated chromosomal transfer. They introduced 3 different chromosomes into karyotypically diploid cells [80] and analyzed the transcription profiles on cDNA arrays. They found significant increase in the expression of genes that receive additional gene dosage because of trisomy—for each of the chromosomes they studied. Not only that, numerous genes located on other chromosomes, which did not encounter genetic imbalance, also suffered huge dysregulations. Similar observations were also made for aneuploidy-mediated genomic amplifications in breast cancer [81,82] and Acute Myeloid Leukemia trisomy 8 cells [83]. In essence, it can be generalized that aneuploidy causes massive alteration in the transcriptome – and the resultant proteome – of the affected cell.
Whether aneuploidy can cause production of truncated or dys-functional proteins has not been addressed so far. However, it is logically possible in case the chromosomal breakage points happen to occur within a gene. Double strand breaks (DSB) are usual phenomena in aneuploidy; and a DSB is primarily responsible for gene translocation and generation of fusion proteins. Many fusion proteins play crucial roles in many forms of leukemia [84,85].
4. Road to the crossroads of life and death
Normal cells can acquire mutations, alteration in ploidy, or inappropriate centrosome duplication—spontaneously or under the influence of a carcinogen; or, one of these can lead to the other. Each of these assaults can lead to multiple defects in cellular physiology that can keep increasing with each cell division. Normally, such cells should either apoptose or senesce [86,87]. However, if the apoptotic pathways have become disabled en-route, such cells can undergo a process of selection, adaptation and proliferation, ultimately resulting in highly aneuploid cancer cells [88]. Thus, those inaugural assaults lead the normal cells to the crossroads of life and death. That cell proliferation drives tumor progression testifies a crucial role the apoptotic pathways play in tumorigenesis. Apoptosis is disabled concomitant with the cell proliferation, providing the tumors with a way to evade cell death [89–91]. Normal pathways of cell growth and division are well coordinated with the apoptotic pathways [2]; suggesting that mechanisms modulating cell proliferation will eventually affect cell death [3]. It may be of great importance, therefore, to understand how proliferative advantage mechanistically couples with disability of apoptosis.
First, many apoptotic genes are mutated or mis-regulated in cancers [90,35], leading to down-regulation of apoptotic pathways. Second, gene products that regulate cell growth and division are also important in apoptosis. Many such regulators, like Myc, Hippo, Wart, Bantam etc are mutated, or have their expression altered, in many cancers [2]. Third, some ‘master regulators’, like the TP53, that regulate a wide range of cellular pathways, are also mutated in most cancers. DNA-damage signals are relayed to p53, which then activates p21 expression to enforce cell cycle arrest, or activates BAX expression to enforce apoptosis. Loss of p53 function can cause, in addition to abolishment of checkpoints and apoptosis, genomic instability including aneuploidy [92], paving the way for propagation of an aneuploid genome further. Loss of p53 also abolishes the ‘ploidy checkpoint’ at G1, and allows cells to continue the cell cycle [93]. Moreover, the aneuploid genome, owing to its genomic imbalance and altered transcriptome (and the resultant proteome), can potentially cause further genomic defects with each cell division; leading to extreme aneuploidy that is the hallmark of most metastatic cells [27].
5. PRAN paradigm: linking aneuploidy, apoptosis, cohesin and cancer
As indicated above, the casual link between aneuploidy and cancer development has not been fully substantiated. However, there is accumulating evidence for a direct link from the regulation of cell cycle progression to genomic instability and apoptotic cell death, implying that aneuploidy may be an early event in cancer that exerts strong pressure for tumor development. Pihan and Doxsey [94] have proposed that increased aneuploidy in cells is a consequence of mitoge-nesis. Lengauer et al. [95] argue that the rate of chromosomal missegregation with normal mitosis is in the order of 1% of mitotic events and that this missegregation would elicit a cell cycle checkpoint, thereby preventing accumulation of aneuploid cells in the organism. However in the absence of a normal G2 checkpoint, as in p53-mutant cells, there would be an accumulation of cells with abnormal mitoses. Although some of these would be non-viable cells undergoing apoptotic cell death, it is likely that viable aneuploid cells would accumulate in the cell population. An increased frequency of proliferation would. thus, lead to increased number of aneuploid cells in the population. Here we put forth a proposal that there is a set of regulatory proteins (termed PRAN, Promoter of Aneuploidy) whose failed regulation alone or in combination with proliferation-promoting mutations causes chromosomal instability due to imbalance in cell division (mitosis) and programmed cell death (apoptosis), resulting in loss or gain of whole or part of one or more chromosomes. It is also probable that a set of PRAN proteins (e.g., some chromosomal segregation proteins) are interactively regulated by mitogens (e.g., steroid hormones) and tumor suppressors (e.g., p53). In particular, mitogens may regulate the expression of key players in chromosomal segregation including proteins involved in sister chromatid cohesion and separation, mitosis and apoptosis directly and/or in collaboration with master regulators such as p53. Dysregulation of PRANs leads to chromosomal instability and tumor formation. PRAN proteins linking aneuploidy, cell cycle and apoptosis may also likely serve as master regulators to integrate multiple pathways that not only cause but sustain tumorigenesis.
5.1. Chromosomal segregation and mitotic checkpoint proteins as candidate PRANs
Using an unique preneoplastic BALB/c p53-null mouse mammary model, developed by Medina and co-workers [96–99] (see [55] for review), we have recently tested the concept of PRAN paradigm described above. In this model, hormonal stimulation enhances tumor risk as well as the incidence of aneuploidy in p53-null mammary epithelial cells [97]. Greater than 70% of mammary tumors arising in these hormone stimulated p53-null mammary cells are highly aneuploid as determined by chromosome gain or loss. Furthermore, these tumors are also metastatic, and the pathogenesis mimics that observed in a large subset of human breast cancers [97]. Using this mouse model, we have addressed the question of how steroid hormones such as progesterone and estrogen, and the tumor suppressor p53 contribute to increased aneuploidy typically associated with breast cancer. Using this model we have tested the hypothesis that there is a set of PRAN proteins whose misexpression promotes aneuploidy and that the PRAN proteins are interactively regulated by mitogenic hormones and tumor suppressor p53.
This scenario is exactly what is indicated by our results [99,100]. In the virgin animal, proliferation is low and the appearance of detectable aneuploid cells increases with host age so that there is no detectable aneuploidy in p53-null cells at 8–14 weeks of age; however, low levels of aneuploidy at 22–26 weeks of age are detectable. With hormone-induced increases in proliferation in p53-null cells, there is a marked induction of aneuploidy. What is important is that there is no aneuploidy in p53-WT cells. These results raise the question of whether the absence of p53 confers merely a passive mechanism for generation of aneuploidy or whether p53 is more directly involved by altering the transcriptional regulation or functional activities of genes that are important for proper chromosomal segregation. There have been considerable advances in understanding the genes involved in sister chromatid separation and chromosome segregation (see [101] for review) as discussed below. The relationship between p53 and the genes involved in regulating chromosome segregation is not well established. We observed that hormone treatment altered expression of several mitotic segregation proteins (e.g., Esp1/Separase, the endopeptidase responsible for cleaving cohesin Rad21 and resolving the sister chromatids, and Pds1/Securin,(the inhibitory chaperone for Separase) as well as checkpoint proteins (e.g., Mad2, Bub1, p55CDC and Cdh1). What is more important is that the levels of two of these proteins (Mad2 and Separase) are increased in hormone-treated p53-null cells, but in hormone-treated p53-WT mammary cells they are not. Interestingly, Securin and Rad21 protein levels were not increased in the hormone-stimulated p53-null cells compared with the hormone-stimulated WT cells. Two distinct interpretations are apparent: (1) physiologically, hormones may regulate the expression of mitotic segregation and checkpoint proteins in WT mammary cells and (2) the absence of p53 protein and/or aberrant hormone levels can lead to misexpression of a key set of proteins that constitute the PRANs (e.g., Separase and Mad2), leading to chromosomal instability and cancer formation.In a recently published study [100] we have demonstrated that Separase, when overexpressed, can also act as PRAN. Conditional expression of Separase in tetracycline-inducible diploid FSK3 mouse mammary epithelial cells with both p53 WT and mutant (Ser233–234) alleles develops aneuploidy within 5 days of Separase induction in vitro. Overexpression of Separase induces premature separation of chromatids, lagging chromosomes and anaphase bridges. In an in vivo mouse mammary transplant model, induction of Separase expression in the transplanted FSK3 cells for 3–4 weeks results in the formation of aneuploid tumors in the mammary gland. Induction of Separase resulted in trisomies for chromosome 8, 15 and 17, monosomy for chromosome 10, and amplification of the distal region of chromosomes 8 and 11. It is possible that Separase over expression might have caused chromosomal aberrations involving other chromosomes as well, but itwas just that these specific aberrations were selected during the development and progression of the tumor. However, we do not have an explanation why these aberrations were selected. Possibly it depends on what sort of alteration in the proteome is advantageous to the tumor and what is deleterious.
Separase protein is found to be significantly overexpressed in human breast tumors compared to the matched normal tissue proving relevance of our studies in mice to human breast cancer. Our subsequent studies indicate that Separase protein is overexpressed and mislocalized in a wide range of human cancers including breast, prostate and osteosarcoma. Furthermore, Separase overexpression strongly correlates with high incidence of relapse, metastasis and lower 5 year overall survival rate in breast and prostate cancer patients [102]. As noted above, Separase is the endopeptidase required to cleave the cohesin component Rad21 at the metaphase-to-anaphase transition, following which the sisters are resolved. Overexpression of Separase may lead to premature cleavage of Rad21 and subsequent resolution of sisters when the cell is still unprepared to divide. Such a scenariowould cause abnormal separation of sisters leading to aneuploidy (see Fig. 2). It can be noted that Separase gene contains a number of putative steroid hormone and p53 regulatory elements [99] These results collectively strengthen our hypothesis that Separase is a PRAN protein, whose overexpression alone in mammary epithelial cells is sufficient to induce aneuploidy and tumorigenesis in a p53 mutant background. There are other evidences that also suggest a link between the p53-regulatable pathways and sister chromatid cohesion and separation. For example, Securin reportedly causes activation of p53, which in turn leads to overexpression of Bax and induction of apoptosis [103].
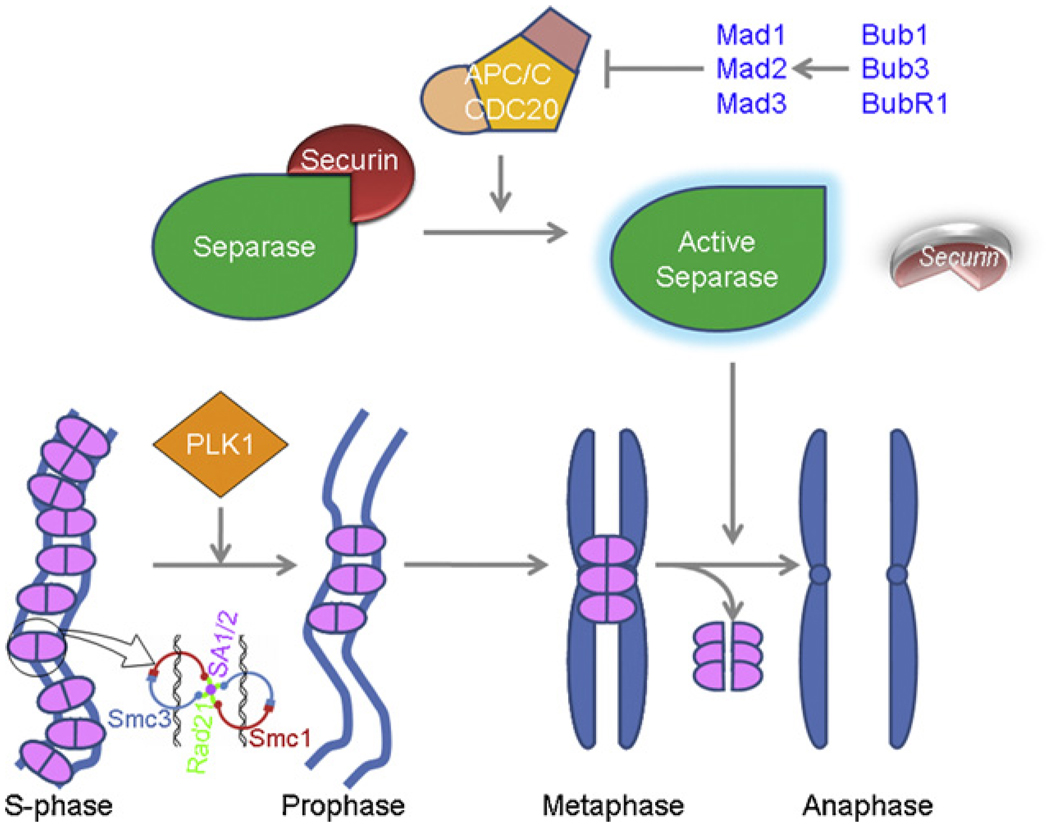
Fig 2.
Mitotic removal of cohesin in humans: In humans, cohesins (pink) are removed from chromosome arms in prophase in a Plk1-dependent pathway. The residual cohesins from the centromeric regions are removed and the sisters separate when Separase cleaves the cohesin component Rad21 (green in the inset cartoon). During metaphase-to-anaphase transition, APC/C-Cdc20 is activated, and ubiquitinates and degrades securin (red)—the inhibitory chaperone of Separase. The active Separase (green) thus released cleaves Rad21 to disentangle the cohesin handcuff (pink) to separate the sisters (blue).
There is no doubt that p53 plays an important role in the G2/M checkpoint and may control the level of Mad2, a spindle checkpoint protein. A study by Hernando et al. [104] confirms our findings that overexpression of Mad2 contributes to aneuploidy and is common in many cancer types. It also sheds some light on the mechanism of Mad2 overexpression and its role in aneuploidization. Mad2 is a transcriptional target of E2F, and defective Rb expression results in constitutive Mad2 expression. Super-induction of the spindle checkpoint protein Mad2 results in chromosome missegregation and aneuploidy, possibly by inhibiting the function of the anaphase promoting complex APC/C. APC/C is critical for Separase activation and subsequent resolution of the sisters. Mad2 overexpression, thus, may prevent Separase activation and persistence of sister chromatid cohesion (see discussion later in the text; see Fig. 2). When such cells undergo mitosis, aneuploidy will result because of the segregation defect. The data described above suggest a direct link from the regulation of cell cycle progression to genomic instability, implying that aneuploidy may be an early event in cancer. That aneuploidy and cancer development are related has also been strongly supported by findings that germline mutations in a spindle checkpoint gene BUBR1 causes Mosaic Variegated Aneuploidy, a rare recessive condition characterized by growth retardation, microcephaly, childhood cancer and constitutional mosaicism for chromosomal gains and losses [105].
In summary, our findings support the PRAN paradigm that mitogenic hormone stimulation may increase aneuploidy and cancer risk by acting on the mitotic machinery, particularly increasing the expression of proteins involved in chromosomal segregation, and that the status of p53 enhances sensitivity to the chromosomal segregation defect (Fig. 1). First, by increasing mitogenesis in the absence of the p53 checkpoint in G2, hormones allow the accumulation of cells that have experienced chromosome missegregation. Second, the absolute rate of missegregation may be increased by alterations in the level of PRAN proteins including Separase and Mad2 that are important for maintaining chromosomal segregation and the normal spindle checkpoint during mitosis.
Fig 1.
Hypothetical models showing the interactions of p53, Separase, and steroid hormones (progesterone and estrogen) in development of aneuploidy. Upper panel shows the presence of putative p53 and steroid responsive elements in ESPL1 gene that encodes Separase protein. Arrow, the predicted transcription start site (TSS); TATA-box; progesterone responsive element (PRE), estrogen responsive element (ERE), p53 transcriptional activation element (p53TAE) and p53 transcriptional repressor elements (p53TRE).
Mitogens in combination with mutations in master regulators such as p53 tumor suppressor gene play a critical role in the regulation of PRAN proteins. The absence of p53 function results in both an aberrant PRAN activity that allows the accumulation of cells with aneuploidy as a function of time and mitotic frequency. The presence of aneuploidy has an indirect effect on tumorigenesis by increasing stochastically the frequency of the altered expression of genes involved in growth regulation and invasiveness. Identification of additional PRAN proteins involved in chromosomal stability and cancer progression will elucidate the molecular basis of aneuploidization and oncogenesis.
6. Cohesin: a link between cell proliferation and cell death
During DNA replication, a group of conserved proteins termed cohesins forms a complex that holds the two sister chromatids together [101,106]; to ensure accurate chromosomal segregation during the normal mitotic cell cycle [107–115,101]. Human Rad21 protein, along with at least three other subunits (SCC3, SMC1, and SMC3), is a component of such a sister chromatid cohesin complex [106] (for a review see [116]). Biochemical analysis of cohesin indicates that it acts as a molecular glue, and human cohesin can promote intermolecular DNA catenation, and dimerization of two cohesin rings forming a handcuff to encompass two sister chromatids together [106,115]. In budding yeast as well as in higher organisms including humans, loss of cohesion at the metaphase-anaphase transition is accompanied by proteolytic cleavage of the Rad21 protein [117–119] followed by its dissociation from the chromatids [110,119–121] (Fig. 2). Cleavage of Rad21 depends on Separase, a CD clan endopeptidase (also known as Esp1 or Separin in yeast) [122,117,118], which is complexed with its inhibitor Securin (also known as PTTG in human and Pds1 in yeast) prior to anaphase [122–124]. In metaphase, ubiquitin-mediated degradation of the securin protein by APC/C-Cdc20 ubiquitin-ligase releases Separase protein, which proteolytically cleaves cohesin Rad21, thereby releasing the sister chromatids [122,125–128]. In budding yeast, fission yeast, and human and mouse cells, Rad21 has two mitotic cleavage sites for Separase [121,117,118], and cleavage by Separase appears to be essential for sister chromatid separation and for the completion of cytokinesis [121]. In contrast to the simultaneous release of cohesin from the chromosome arms and centromere region in budding yeast by Separase cleavage, most cohesin in metazoans is removed in early prophase from chromosome arms by a cleavage-independent mechanism [121,119,112] (see Fig. 2). Only residual amounts of cohesin are cleaved at the onset of anaphase, coinciding with its disappearance from centromeres. Thus, Rad21 plays a critical role in the eukaryotic cell division cycle by regulating sister chromatid cohesion and separation at the metaphase-to-anaphase transition.
Unexpectedly, data from our lab and another indicate that Rad21 is cleaved early in apoptosis in a number of leukemic T- and B-cell lines, including Molt4, Jurkat and Reh by a caspase-like molecule [129,130]. After cleavage of Rad21, carboxyl-terminal (C-terminal) Rad21 is translocated to the cytoplasm and promotes apoptosis in leukemia cell lines (Fig. 3). We have mapped the apoptotic cleavage sites to the C-terminus of hRad21 at residue Asp (D)279 [129], and C392 (unpublished) sites that are different from the mitotic cleavage sites required for chromosomal segregation [121]. In vitro cleavage assays indicate that caspase 3 and 7 can cleave hRad21, but they may not be essential because Rad21 can also be cleaved in MCF7 breast carcinoma cells. MCF7 cells lack caspase 3 activity [131] and Rad21 can still be cleaved in MCF7 cells that are depleted of caspase 7 (D. Pati, unpublished observation). In an in vitro cleavage assay, use of apoptosis-induced Molt4 and Jurkat lysates resulted in 64-and 60-kDa hRad21 cleavage products [129]. However, only the 64-kDa product was observed when caspase 3 and 7 were used [129]. Since hRad21 is a nuclear protein and the cleavage initially occurs in the nucleus, the protease that cleaves hRad21 may reside inside the nucleus. These findings suggest the presence of a novel caspase or caspase-like molecule in the nucleus that cleaves hRad21 early in apoptosis. However, the physiological protease that cleaves hRad21during apoptosis and the mechanisms by which the apoptotic signal is amplified remain to be identified.
Fig 3.
Model showing the cleavage of the cohesion protein hRad21 by an unidentified protease in the nucleus and translocation of the C-terminal cleavage products to the cytoplasm early in the apoptotic pathway that amplifies the cell death signal in a positive feedback manner by activating more caspases.
Transfection experiments indicate that C-terminal hRad21 (280–631 aa and 392–631 aa) can induce apoptosis in many cell lines that are sensitive or resistant to apoptosis, but full-length hRad21 and N-terminal hRad21 (1–279 aa) have little or no apoptotic effect [129,130]. Apoptosis induced by C-terminal hRad21 and the tumor necrosis factor (TNF) receptor superfamily may share part of a common pathway. BLAST search indicates that a region of 104 amino acid residues in C-terminal Rad21 has high consensus (26% identities, 43% positives) with the sequence up-stream of the death domain (DD) of several apoptosis-related proteins. TNF receptor superfamily members have DD, and their involvement in apoptosis requires TNF signaling from outside of the cell. C-terminal Rad21 does not have a DD. It is currently not known whether C-terminal Rad21-induced apoptosis requires extracellular signals, like those in the TNF superfamily. These studies are significant because cancer cells often evade/resist apoptosis induced by chemotherapy drugs. C-terminal Rad21 may be involved in a new apoptotic pathway, and elucidation of this pathway may provide new targets to attack chemoresistant cancers. In summary, in contrast to previously described functions of Rad21 in chromosome segregation and DNA repair, cleavage of the cohesion protein and translocation of the C-terminal cleavage product to the cytoplasm are early events in the apoptotic pathway that amplify the apoptotic signals in a positive feedback manner, possibly by activating more caspases.
Cleavage of cohesin Rad21 is carried out by Separase in mitosis and by an unidentified caspase/caspase-like molecule in apoptosis at different sites in the protein. Both of the proteases belong to the distantly related CD clan protease family [118], suggesting an evolutionarily conserved mechanism shared by the mitotic and apoptotic machinery. It is therefore possible that chromosomal segregation proteins such as Rad21 and Separase may serve as links between the two key cellular processes of mitosis and apoptosis. Deregulation of this pathway may lead to treatment-resistant disease. We propose that mitotic segregation and apoptosis are linked processes and cohesin serve as link between cell proliferation, chromosomal missegregation, and apoptotic cell death. Aberration in this pathway can lead to aneuploidy and cancer. Understanding the common pathways that regulate both cell proliferation and apoptosis would provide a new paradigm for identifying novel therapies for cancers.
In retrospect, a tumor progenitor cell perhaps never encounters a dilemma of life or death. Experimentation on a ‘true progenitor cell’ has not been possible so far; and this status still remains a fiction. However, it is obvious at the onset of tumorigenesis that a cell, or a group of cells, must first encounter a decisive event that would change their fate altogether. This event can be acquiring a mutation in a key gene or a faulty distribution of one or more chromosomes during a cell division. This can happen either spontaneously or under the influence of a mutagen or an aneugen, or, to be broad, a carcinogen. Depending upon what gene – or what set of genes – is affected, the ‘thus altered’ cell may die, senesce or enter cell cycle. The former two possibilities slip into oblivion, never to tell their story. However, the last possibility takes a decisive path of evolution to orchestrate ruthless rampage.
7. Conclusion
Cancer is a very complex problem encompassing myriads of defects in a multitude of pathways; though the true cause of cancer may be a very simple event or two occurred to a normal cell long ago. Equally complex is the multitude of ways a tumor can initiate, propagate and metastasize; and no single mechanism in our current understanding can be generalized as the “only” cause of cancer. This is because life is a very complex system to have evolved in the universe, and it has to have numerous disparate facets that must intermingle and interact in a very complex network of integration, which ultimately synonymies with life itself. Thus, causation of an imbalance therein can have numerous potential origins, just as numerous can be the sequences and consequences of imbalances caused by a single initial event of disruption. In our view, synergy among multiple pathways (e.g., mutations, aneuploidy and apoptosis) ultimately supports tumorigenesis and cancer formation. In this context, our proposal of sister chromatid cohesion and separation, and PRAN proteins linking aneuploidy, cell cycle and apoptosis may serve as a master regulator to integrate multiple pathways that not only cause but sustain tumorigenesis.
Biographies
Anil Panigrahi, Ph.D. is an instructor in the Department of Pediatric Hematology/Oncology, Baylor College of Medicine, Houston, TX, USA. He graduated from Banaras Hindu University, India, where he studied the mechanism of ATP-dependent chromatin remodeling. Subsequently he investigated certain aspects of histone demethylation as a postdoctoral fellow in the University of Utah, Salt Lake City, USA. His interest is to study the role of epigenetics in gene regulation and sister chromatid cohesion and separation.
Debananda Pati, Ph.D. is currently a tenure-track assistant professor, in the Department of Pediatrics, Hematology/Oncology Section at Baylor College of Medicine and Texas Children’s Cancer Center, Houston, TX, USA. He earned his undergraduate degree from the Orissa University of Agriculture and Technology, India, followed by a Masters degree from the University of Buckingham, England, and Ph.D. in endocrinology from the University of Calgary, Alberta, Canada. Dr. Pati’s research program focuses on the molecular mechanisms of aneuploidy and apoptosis in carcinogenesis. Dr. Pati’s research is funded by grants from the National Cancer Institute, US Department of Defense, and Susan G. Komen Breast Cancer Foundation.
Footnotes
Reviewers
Dr. Shyam K. Sharan, National Cancer Institute at Frederick, Mouse Cancer Genetics Program, Center for Cancer Research, 1050 Boyles Street, Frederick, MD 21702, United States.
Dr. Esta Sterneck, Head, Molecular Mechanisms in Development Group, NCI – Frederick, Molecular Mechanisms in Development, Group, Building 520, Frederick, MD 21702-1201, United States.
References
- 1.Huettenbrenner S, Maier S, Leisser C, et al. The evolution of cell death programs as prerequisites of multicellularity. Mutat Res. 2003;543(3):235–249. doi: 10.1016/s1383-5742(02)00110-2. [DOI] [PubMed] [Google Scholar]
- 2.Hipfner DR, Cohen SM. Connecting proliferation and apoptosis in development and disease. Nat Rev Mol Cell Biol. 2004;5(10):805–815. doi: 10.1038/nrm1491. [DOI] [PubMed] [Google Scholar]
- 3.Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1(1):19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 4.Vermeulen K, Van Bockstaele DR, Berneman ZN. Apoptosis: mechanisms and relevance in cancer. Ann Hematol. 2005;84(10):627–639. doi: 10.1007/s00277-005-1065-x. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9(4):138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg RA. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989;49(14):3713–3721. [PubMed] [Google Scholar]
- 8.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 9.Tomlinson IP, Novelli MR, Bodmer WF. The mutation rate and cancer. Proc Natl Acad Sci USA. 1996;93(25):14800–14803. doi: 10.1073/pnas.93.25.14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson AL, Loeb LA. The mutation rate and cancer. Genetics. 1998;148(4):1483–1490. doi: 10.1093/genetics/148.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat Rev Cancer. 2002;2(5):331–341. doi: 10.1038/nrc795. [DOI] [PubMed] [Google Scholar]
- 12.Prehn RT. Cancers beget mutations versus mutations beget cancers. Cancer Res. 1994;54(20):5296–5300. [PubMed] [Google Scholar]
- 13.Soto AM, Sonnenschein C. The somatic mutation theory of cancer: growing problems with the paradigm? Bioessays. 2004;26(10):1097–1107. doi: 10.1002/bies.20087. [DOI] [PubMed] [Google Scholar]
- 14.Strickland JE, Ueda M, Hennings H, Yuspa SH. A model for initiated mouse skin: suppression of papilloma but not carcinoma formation by normal epidermal cells in grafts on athymic nude mice. Cancer Res. 1992;52(6):1439–1444. [PubMed] [Google Scholar]
- 15.Anderson E. The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res. 2002;4(5):197–201. doi: 10.1186/bcr452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manchester KL. Theodor Boveri and the origin of malignant tumours. Trends Cell Biol. 1995;5(10):384–387. doi: 10.1016/s0962-8924(00)89080-7. [DOI] [PubMed] [Google Scholar]
- 17.Duesberg P, Li R, Fabarius A, Hehlmann R. Aneuploidy and cancer: from correlation to causation. Contrib Microbiol. 2006;13:16–44. doi: 10.1159/000092963. [DOI] [PubMed] [Google Scholar]
- 18.Marx J. Debate surges over the origins of genomic defects in cancer. Science. 2002;297(5581):544–546. doi: 10.1126/science.297.5581.544. [DOI] [PubMed] [Google Scholar]
- 19.Cahill DP, Lengauer C, Yu J, et al. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392(6673):300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 20.Aguilera A, Gomez-Gonzalez B. Genome instability: a mechanistic view of its causes and consequences. Nat Rev Genet. 2008;9(3):204–217. doi: 10.1038/nrg2268. [DOI] [PubMed] [Google Scholar]
- 21.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 22.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 23.Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7(1):21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 24.Teixeira MR, Heim S. Multiple numerical chromosome aberrations in cancer: what are their causes and what are their consequences? Semin Cancer Biol. 2005;15(1):3–12. doi: 10.1016/j.semcancer.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Gagos S, Irminger-Finger I. Chromosome instability in neoplasia: chaotic roots to continuous growth. Int J Biochem Cell Biol. 2005;37(5):1014–1033. doi: 10.1016/j.biocel.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432(7015):338–341. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 27.Duesberg P, Li R. Multistep carcinogenesis: a chain reaction of aneuploidizations. Cell Cycle. 2003;2(3):202–210. [PubMed] [Google Scholar]
- 28.Tyzzer EE. Tumor immunity. J Cancer Res. 1916;1:125–155. [Google Scholar]
- 29.Whitman RC. Somatic mutations as a factor in the production of cancer. J Cancer Res. 1919;4:181–202. [Google Scholar]
- 30.Muller HJ. Artificial transmutation of the gene. Science. 1927;66(1699):84–87. doi: 10.1126/science.66.1699.84. [DOI] [PubMed] [Google Scholar]
- 31.Fardon JC. A reconsideration of the somatic mutation theory of cancer in the light of some recent developments. Science. 1953;117(3043):441–445. doi: 10.1126/science.117.3043.441. [DOI] [PubMed] [Google Scholar]
- 32.Fisher JC. Multiple-mutation theory of carcinogenesis. Nature. 1958;181(4609):651–652. doi: 10.1038/181651b0. [DOI] [PubMed] [Google Scholar]
- 33.Land H, Parada LF, Weinberg RA. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 34.Knudson AG. Two genetic hits (more or less) to cancer. Nat Rev Cancer. 2001;1(2):157–162. doi: 10.1038/35101031. [DOI] [PubMed] [Google Scholar]
- 35.Futreal PA, Coin L, Marshall M, et al. A census of human cancer genes. Nat Rev Cancer. 2004;4(3):177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steen HB. The origin of oncogenic mutations: where is the primary damage? Carcinogenesis. 2000;21(10):1773–1776. doi: 10.1093/carcin/21.10.1773. [DOI] [PubMed] [Google Scholar]
- 37.Cairns J. Somatic stem cells and the kinetics of mutagenesis and carcinogenesis. Proc Natl Acad Sci USA. 2002;99(16):10567–10570. doi: 10.1073/pnas.162369899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonnenschein C, Soto AM. Somatic mutation theory of carcinogenesis: why it should be dropped and replaced. Mol Carcinog. 2000;29(4):205–211. doi: 10.1002/1098-2744(200012)29:4<205::aid-mc1002>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 39.Mitelman F, Johansson B, Mertens FE. Mitelman database of chromosome aberrations in cancer. 2006 http://cgap.nci.nih.gov/chromosomes/mitelman;
- 40.Frankfurt OS, Chin JL, Englander LS, Greco WR, Pontes JE, Rustum YM. Relationship between DNA ploidy, glandular differentiation, and tumor spread in human prostate cancer. Cancer Res. 1985;45(3):1418–1423. [PubMed] [Google Scholar]
- 41.Ried T, Heselmeyer-Haddad K, Blegen H, Schrock E, Auer G. Genomic changes defining the genesis, progression, and malignancy potential in solid human tumors: a phenotype/genotype correlation. Genes Chromosomes Cancer. 1999;25(3):195–204. doi: 10.1002/(sici)1098-2264(199907)25:3<195::aid-gcc1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 42.Pilch H, Gunzel S, Schaffer U, Tanner B, Heine M. Evaluation of DNA ploidy and degree of DNA abnormality in benign and malignant melanocytic lesions of the skin using video imaging. Cancer. 2000;88(6):1370–1377. [PubMed] [Google Scholar]
- 43.Duesberg P, Rausch C, Rasnick D, Hehlmann R. Genetic instability of cancer cells is proportional to their degree of aneuploidy. Proc Natl Acad Sci USA. 1998;95(23):13692–13697. doi: 10.1073/pnas.95.23.13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choma D, Daures JP, Quantin X, Pujol JL. Aneuploidy and prognosis of non-small-cell lung cancer: a meta-analysis of published data. Br J Cancer. 2001;85(1):14–22. doi: 10.1054/bjoc.2001.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikeguchi M, Ohfuji S, Oka A, Tsujitani S, Maeta M, Kaibara N. Aneuploidy of tumor cells in cases of gastric cancer with esophageal invasion: another indicator of poor prognosis. J Surg Oncol. 1995;58(2):83–90. doi: 10.1002/jso.2930580203. [DOI] [PubMed] [Google Scholar]
- 46.Sciallero S, Giaretti W, Geido E, et al. DNA aneuploidy is an independent factor of poor prognosis in pancreatic and peripancreatic cancer. Int J Pancreatol. 1993;14(1):21–28. doi: 10.1007/BF02795226. [DOI] [PubMed] [Google Scholar]
- 47.Kearney TJ, Price EA, Lee S, Silberman AW. Tumor aneuploidy in young patients with colorectal cancer. Cancer. 1993;72(1):42–45. doi: 10.1002/1097-0142(19930701)72:1<42::aid-cncr2820720110>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 48.Nakopoulou L, Panayotopoulou EG, Giannopoulou I, et al. Extra copies of chromosomes 16 and X in invasive breast carcinomas are related to aggressive phenotype and poor prognosis. J Clin Pathol. 2007;60(7):808–815. doi: 10.1136/jcp.2006.037838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet. 2006;38(9):1043–1048. doi: 10.1038/ng1861. [DOI] [PubMed] [Google Scholar]
- 50.Fabarius A, Hehlmann R, Duesberg PH. Instability of chromosome structure in cancer cells increases exponentially with degrees of aneuploidy. Cancer Genet Cytogenet. 2003;143(1):59–72. doi: 10.1016/s0165-4608(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 51.Willenbucher RF, Aust DE, Chang CG, et al. Genomic instability is an early event during the progression pathway of ulcerative-colitis-related neoplasia. Am J Pathol. 1999;154(6):1825–1830. doi: 10.1016/S0002-9440(10)65438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shih IM, Zhou W, Goodman SN, Lengauer C, Kinzler KW, Vogelstein B. Evidence that genetic instability occurs at an early stage of colorectal tumorigenesis. Cancer Res. 2001;61(3):818–822. [PubMed] [Google Scholar]
- 53.Aardema MJ, Albertini S, Arni P, et al. Aneuploidy: a report of an ECETOC task force. Mutat Res. 1998;410(1):3–79. doi: 10.1016/s1383-5742(97)00029-x. [DOI] [PubMed] [Google Scholar]
- 54.Ai H, Barrera JE, Meyers AD, Shroyer KR, Varella-Garcia M. Chromosomal aneuploidy precedes morphological changes and supports multifocality in head and neck lesions. Laryngoscope. 2001;111(10):1853–1858. doi: 10.1097/00005537-200110000-00034. [DOI] [PubMed] [Google Scholar]
- 55.Medina D. Biological and molecular characteristics of the premalignant mouse mammary gland. Biochim Biophys Acta. 2002;1603(1):1–9. doi: 10.1016/s0304-419x(02)00053-7. [DOI] [PubMed] [Google Scholar]
- 56.Amiel A, Gronich N, Yukla M, et al. Random aneuploidy in neoplastic and pre-neoplastic diseases, multiple myeloma, and monoclonal gammopathy. Cancer Genet Cytogenet. 2005;162(1):78–81. doi: 10.1016/j.cancergencyto.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 57.Duesberg P, Rasnick D. Aneuploidy, the somatic mutation that makes cancer a species of its own. Cell Motil Cytoskeleton. 2000;47(2):81–107. doi: 10.1002/1097-0169(200010)47:2<81::AID-CM1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 58.Weaver BA, Cleveland DW. Aneuploidy: instigator and inhibitor of tumorigenesis. Cancer Res. 2007;67(21):10103–10105. doi: 10.1158/0008-5472.CAN-07-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dutrillaux B, Gerbault-Seureau M, Remvikos Y, Zafrani B, Prieur M. Breast cancer genetic evolution: I. Data from cytogenetics and DNA content. Breast Cancer Res Treat. 1991;19(3):245–255. doi: 10.1007/BF01961161. [DOI] [PubMed] [Google Scholar]
- 60.Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5(10):773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 61.Duesberg P, Fabarius A, Hehlmann R. Aneuploidy, the primary cause of the multilateral genomic instability of neoplastic and preneoplastic cells. IUBMB Life. 2004;56(2):65–81. doi: 10.1080/15216540410001667902. [DOI] [PubMed] [Google Scholar]
- 62.Lofberg R, Lindquist K, Veress B, Tribukait B. Highly malignant carcinoma in chronic ulcerative colitis without preceding dysplasia or DNA aneuploidy. Report of a case. Dis Colon Rectum. 1992;35(1):82–86. doi: 10.1007/BF02053345. [DOI] [PubMed] [Google Scholar]
- 63.Dohner K, Dohner H. Molecular characterization of acute myeloid leukemia. Haematologica. 2008;93(7):976–982. doi: 10.3324/haematol.13345. [DOI] [PubMed] [Google Scholar]
- 64.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396(6712):643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 65.Muleris M, Chalastanis A, Meyer N, et al. Chromosomal instability in near-diploid colorectal cancer: a link between numbers and structure. PLoS ONE. 2008;3(2):e1632. doi: 10.1371/journal.pone.0001632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baer CF, Miyamoto MM, Denver DR. Mutation rate variation in multicellular eukaryotes: causes and consequences. Nat Rev Genet. 2007;8(8):619–631. doi: 10.1038/nrg2158. [DOI] [PubMed] [Google Scholar]
- 67.Filee J, Forterre P, Sen-Lin T, Laurent J. Evolution of DNA polymerase families: evidences for multiple gene exchange between cellular and viral proteins. J Mol Evol. 2002;54(6):763–773. doi: 10.1007/s00239-001-0078-x. [DOI] [PubMed] [Google Scholar]
- 68.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362(6422):709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 69.Ruley HE. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- 70.Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396(6706):84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 71.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400(6743):464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 72.Li R, Sonik A, Stindl R, Rasnick D, Duesberg P. Aneuploidy vs. gene mutation hypothesis of cancer: recent study claims mutation but is found to support aneuploidy. Proc Natl Acad Sci USA. 2000;97(7):3236–3241. doi: 10.1073/pnas.040529797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sussan TE, Yang A, Li F, Ostrowski MC, Reeves RH. Trisomy represses Apc(Min)-mediated tumours in mouse models of Down’s syndrome. Nature. 2008;451(7174):73–75. doi: 10.1038/nature06446. [DOI] [PubMed] [Google Scholar]
- 74.Baker DJ, Jeganathan KB, Cameron JD, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36(7):744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 75.Williams BR, Prabhu VR, Hunter KE, et al. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science. 2008;322(5902):703–709. doi: 10.1126/science.1160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 77.Sellers WR, Kaelin WG., Jr Role of the retinoblastoma protein in the pathogenesis of human cancer. J Clin Oncol. 1997;15(11):3301–3312. doi: 10.1200/JCO.1997.15.11.3301. [DOI] [PubMed] [Google Scholar]
- 78.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9(5):402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 79.Phillips JL, Hayward SW, Wang Y, et al. The consequences of chromosomal aneuploidy on gene expression profiles in a cell line model for prostate carcinogenesis. Cancer Res. 2001;61(22):8143–8149. [PubMed] [Google Scholar]
- 80.Upender MB, Habermann JK, McShane LM, et al. Chromosome transfer induced aneuploidy results in complex dysregulation of the cellular transcriptome in immortalized and cancer cells. Cancer Res. 2004;64(19):6941–6949. doi: 10.1158/0008-5472.CAN-04-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hyman E, Kauraniemi P, Hautaniemi S, et al. Impact of DNA amplification on gene expression patterns in breast cancer. Cancer Res. 2002;62(21):6240–6245. [PubMed] [Google Scholar]
- 82.Pollack JR, Sorlie T, Perou CM, et al. Microarray analysis reveals a major direct role of DNA copy number alteration in the transcriptional program of human breast tumors. Proc Natl Acad Sci USA. 2002;99(20):12963–12968. doi: 10.1073/pnas.162471999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Virtaneva K, Wright FA, Tanner SM, et al. Expression profiling reveals fundamental biological differences in acute myeloid leukemia with isolated trisomy 8 and normal cytogenetics. Proc Natl Acad Sci USA. 2001;98(3):1124–1129. doi: 10.1073/pnas.98.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bohlander SK. Fusion genes in leukemia: an emerging network. Cytogenet Cell Genet. 2000;91(1–4):52–56. doi: 10.1159/000056818. [DOI] [PubMed] [Google Scholar]
- 85.Redner RL, Liu JM. Leukemia fusion proteins and co-repressor complexes: changing paradigms. J Cell Biochem. 2005;94(5):864–869. doi: 10.1002/jcb.20368. [DOI] [PubMed] [Google Scholar]
- 86.Schmitt CA. Senescence, apoptosis and therapy—cutting the lifelines of cancer. Nat Rev Cancer. 2003;3(4):286–295. doi: 10.1038/nrc1044. [DOI] [PubMed] [Google Scholar]
- 87.Vicencio JM, et al. Senescence, apoptosis or autophagy? When a damaged cell must decide its path—a mini-review. Gerontology. 2008;54(2):92–99. doi: 10.1159/000129697. [DOI] [PubMed] [Google Scholar]
- 88.Zhivotovsky B, Kroemer G. Apoptosis and genomic instability. Nat Rev Mol Cell Biol. 2004;5(9):752–762. doi: 10.1038/nrm1443. [DOI] [PubMed] [Google Scholar]
- 89.Baixeras E, Bosca L, Stauber C, et al. From apoptosis to autoimmunity: insights from the signaling pathways leading to proliferation or to programmed cell death. Immunol Rev. 1994;142:53–91. doi: 10.1111/j.1600-065x.1994.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 90.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411(6835):342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 91.Blagosklonny MV. Apoptosis, proliferation, differentiation: in search of the order. Semin Cancer Biol. 2003;13(2):97–105. doi: 10.1016/s1044-579x(02)00127-x. [DOI] [PubMed] [Google Scholar]
- 92.Smith ML, Fornace AJ., Jr Genomic instability and the role of p53 mutations in cancer cells. Curr Opin Oncol. 1995;7(1):69–75. [PubMed] [Google Scholar]
- 93.Lanni JS, Jacks T. Characterization of the p53-dependent post-mitotic checkpoint following spindle disruption. Mol Cell Biol. 1998;18(2):1055–1064. doi: 10.1128/mcb.18.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pihan GA, Doxsey SJ. The mitotic machinery as a source of genetic instability in cancer. Semin Cancer Biol. 1999;9(4):289–302. doi: 10.1006/scbi.1999.0131. [DOI] [PubMed] [Google Scholar]
- 95.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386(6625):623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 96.Goepfert TM, McCarthy M, Kittrell FS, et al. Progesterone facilitates chromosome instability (aneuploidy) in p53 null normal mammary epithelial cells. FASEB J. 2000;14(14):2221–2229. doi: 10.1096/fj.00-0165com. [DOI] [PubMed] [Google Scholar]
- 97.Medina D, Kittrell FS, Shepard A, et al. Biological and genetic properties of the p53 null preneoplastic mammary epithelium. FASEB J. 2002;16(8):881–883. doi: 10.1096/fj.01-0885fje. [DOI] [PubMed] [Google Scholar]
- 98.Medina D, Kittrell FS, Shepard A, Contreras A, Rosen JM, Lydon J. Hormone dependence in premalignant mammary progression. Cancer Res. 2003;63(5):1067–1072. [PubMed] [Google Scholar]
- 99.Pati D, Haddad BR, Haegele A, et al. Hormone-induced chromosomal instability in p53-null mammary epithelium. Cancer Res. 2004;64(16):5608–5616. doi: 10.1158/0008-5472.CAN-03-0629. [DOI] [PubMed] [Google Scholar]
- 100.Zhang N, Ge G, Meyer R, et al. Overexpression of Separase induces aneuploidy and mammary tumorigenesis. Proc Natl Acad Sci USA. 2008;105(35):13033–13038. doi: 10.1073/pnas.0801610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nasmyth K. Segregating sister genomes: the molecular biology of chromosome separation. Science. 2002;297(5581):559–565. doi: 10.1126/science.1074757. [DOI] [PubMed] [Google Scholar]
- 102.Meyer R, Fofanov V, Panigrahi A, Merchant F, Zhang N, Pati D. Overexpression and mislocalization of the chromosomal segregation protein separase in multiple human cancers. Clin Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-08-2454. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hamid T, Kakar SS. PTTG/securin activates expression of p53 and modulates its function. Mol Cancer. 2004;3:18. doi: 10.1186/1476-4598-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hernando E, Nahle Z, Juan G, et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430(7001):797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- 105.Hanks S, Coleman K, Reid S, et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat Genet. 2004;36(11):1159–1161. doi: 10.1038/ng1449. [DOI] [PubMed] [Google Scholar]
- 106.Zhang N, Kuznetsov S, Sharan SK, Li K, Rao PH, Pati D. A handcuff model for the cohesin complex. J Cell Biol. 2008;183(6):1019–1031. doi: 10.1083/jcb.200801157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Orr-Weaver TL. The ties that bind: localization of the sister-chromatid cohesin complex on yeast chromosomes. Cell. 1999;99(1):1–4. doi: 10.1016/s0092-8674(00)80055-0. [DOI] [PubMed] [Google Scholar]
- 108.Nasmyth K. Separating sister chromatids. Trends Biochem Sci. 1999;24(3):98–104. doi: 10.1016/s0968-0004(99)01358-4. [DOI] [PubMed] [Google Scholar]
- 109.Koshland DE, Guacci V. Sister chromatid cohesion: the beginning of a long and beautiful relationship. Curr Opin Cell Biol. 2000;12(3):297–301. doi: 10.1016/s0955-0674(00)00092-2. [DOI] [PubMed] [Google Scholar]
- 110.Nasmyth K, Peters JM, Uhlmann F. Splitting the chromosome: cutting the ties that bind sister chromatids. Science. 2000;288(5470):1379–1385. doi: 10.1126/science.288.5470.1379. [DOI] [PubMed] [Google Scholar]
- 111.Yanagida M. Cell cycle mechanisms of sister chromatid separation; roles of Cut1/separin and Cut2/securin. Genes Cells. 2000;5(1):1–8. doi: 10.1046/j.1365-2443.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- 112.Warren WD, Steffensen S, Lin E, et al. The Drosophila RAD21 cohesin persists at the centromere region in mitosis. Curr Biol. 2000;10(22):1463–1466. doi: 10.1016/s0960-9822(00)00806-x. [DOI] [PubMed] [Google Scholar]
- 113.Nasmyth K. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu Rev Genet. 2001;35:673–745. doi: 10.1146/annurev.genet.35.102401.091334. [DOI] [PubMed] [Google Scholar]
- 114.Amon A. Together until separin do us part. Nat Cell Biol. 2001;3(1):E12–E14. doi: 10.1038/35050642. [DOI] [PubMed] [Google Scholar]
- 115.Onn I, Heidinger-Pauli JM, Guacci V, Unal E, Koshland DE. Sister chromatid cohesion: a simple concept with a complex reality. Annu Rev Cell Dev Biol. 2008;24:105–129. doi: 10.1146/annurev.cellbio.24.110707.175350. [DOI] [PubMed] [Google Scholar]
- 116.Hagstrom KA, Meyer BJ. Condensin and cohesin: more than chromosome compactor and glue. Nat Rev Genet. 2003;4(7):520–534. doi: 10.1038/nrg1110. [DOI] [PubMed] [Google Scholar]
- 117.Uhlmann F, Lottspeich F, Nasmyth K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature. 1999;400(6739):37–42. doi: 10.1038/21831. [DOI] [PubMed] [Google Scholar]
- 118.Uhlmann F, Wernic D, Poupart MA, Koonin EV, Nasmyth K. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell. 2000;103(3):375–386. doi: 10.1016/s0092-8674(00)00130-6. [DOI] [PubMed] [Google Scholar]
- 119.Waizenegger IC, Hauf S, Meinke A, Peters JM. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell. 2000;103(3):399–410. doi: 10.1016/s0092-8674(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 120.Tomonaga T, Nagao K, Kawasaki Y, et al. Characterization of fission yeast cohesin: essential anaphase proteolysis of Rad21 phosphorylated in the S phase. Genes Dev. 2000;14(21):2757–2770. doi: 10.1101/gad.832000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hauf S, Waizenegger IC, Peters JM. Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science. 2001;293(5533):1320–1323. doi: 10.1126/science.1061376. [DOI] [PubMed] [Google Scholar]
- 122.Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M, Nasmyth K. An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell. 1998;93(6):1067–1076. doi: 10.1016/s0092-8674(00)81211-8. [DOI] [PubMed] [Google Scholar]
- 123.Zou H, McGarry TJ, Bernal T, Kirschner MW. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999;285(5426):418–422. doi: 10.1126/science.285.5426.418. [DOI] [PubMed] [Google Scholar]
- 124.Leismann O, Herzig A, Heidmann S, Lehner CF. Degradation of Drosophila PIM regulates sister chromatid separation during mitosis. Genes Dev. 2000;14(17):2192–2205. doi: 10.1101/gad.176700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cohen-Fix O, Peters JM, Kirschner MW, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 1996;10(24):3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- 126.Funabiki H, Yamano H, Kumada K, Nagao K, Hunt T, Yanagida M. Cut2 proteolysis required for sister-chromatid seperation in fission yeast. Nature. 1996;381(6581):438–441. doi: 10.1038/381438a0. [DOI] [PubMed] [Google Scholar]
- 127.Jallepalli PV, Lengauer C. Chromosome segregation and cancer: cutting through the mystery. Nat Rev Cancer. 2001;1(2):109–117. doi: 10.1038/35101065. [DOI] [PubMed] [Google Scholar]
- 128.Zhan Q, Fan S, Bae I, et al. Induction of bax by genotoxic stress in human cells correlates with normal p53 status and apoptosis. Oncogene. 1994;9(12):3743–3751. [PubMed] [Google Scholar]
- 129.Pati D, Zhang N, Plon SE. Linking sister chromatid cohesion and apoptosis: role of Rad21. Mol Cell Biol. 2002;22(23):8267–8277. doi: 10.1128/MCB.22.23.8267-8277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen F, Kamradt M, Mulcahy M, et al. Caspase proteolysis of the cohesin component RAD21 promotes apoptosis. J Biol Chem. 2002;277(19):16775–16781. doi: 10.1074/jbc.M201322200. [DOI] [PubMed] [Google Scholar]
- 131.Kurokawa H, Nishio K, Fukumoto H, Tomonari A, Suzuki T, Saijo N. Alteration of caspase-3 (CPP32/Yama/apopain) in wild-type MCF-7, breast cancer cells. Oncol Rep. 1999;6(1):33–37. doi: 10.3892/or.6.1.33. [DOI] [PubMed] [Google Scholar]