Abstract
Herpes simplex virus type 1 (HSV-1) glycoprotein C (gC-1) binds complement component C3b and inhibits complement-mediated immunity. HSV-1 glycoprotein D (gD-1) is a potent immunogen and a candidate antigen for a subunit vaccine. We evaluated whether combined immunization with gD-1 and gC-1 provides better protection against challenge than gD-1 alone based on antibodies to gC-1 preventing HSV-1-mediated immune evasion. IgG purified from mice immunized with gC-1 blocked C3b binding to gC-1 and greatly increased neutralization by gD-1 IgG in the presence of complement. Passive transfer of gC-1 IgG protected complement intact mice against HSV-1 challenge but not C3 knockout mice, indicating that gC-1 antibody activity in vivo is complement-dependent. Immunizing mice with gD-1 and gC-1 provided better protection than gD-1 alone in preventing zosteriform disease and infection of dorsal root ganglia. Therefore, gC-1 immunization prevents HSV-1 evasion from complement and enhances the protection provided by gD-1 immunization.
Keywords: HSV vaccine, glycoprotein C, immune evasion
Introduction
Complement is an important contributor to innate and acquired immunity. Complement activation facilitates virus neutralization by particle phagocytosis and lysis, functions as a chemoattractant for neutrophils and macrophages, and enhances B and T cell responses [1-6]. HSV-1 gC binds complement C3b and blocks C5 and properdin interaction with C3b, which inhibit complement activation and virus neutralization by antibody and complement or complement alone [7-13]. Two gC-1 domains interact with complement. One is located within amino acids 33 to 133 and blocks C5 and properdin binding to C3b, and the other extends from amino acids 124 to 366 and directly binds C3b [10, 14]. An HSV-1 gC mutant virus deleted in the C3b binding domain is more susceptible to complement-mediated virus neutralization in vitro and less virulent than wild-type (WT) virus in the mouse flank model [15, 16]. Therefore, the interaction between gC-1 and C3b enhances HSV-1 virulence, which supports the concept that blocking this gC-1 domain may be effective in preventing or treating HSV-1 infection.
During experimental HSV-1 infection of mice or natural infection of humans, only low titers of antibody are produced to the gC-1 domain that binds C3b, suggesting that this region is not very immunogenic. However, when mice are immunized with gC-1 protein mixed with adjuvant, higher titers of antibodies to the C3b binding domain are produced that protect against HSV-1 disease [17, 18].
Efforts to develop HSV vaccines include subunit glycoprotein immunogens, DNA plasmid preparations, and attenuated live virus approaches [19-24]. Currently, the Food and Drug Administration has not approved any HSV-1 or HSV-2 vaccine preparation for use in humans. The most thoroughly evaluated HSV-2 vaccine candidate is a glycoprotein gD-2 subunit vaccine developed by GlaxoSmithKline (GSK) [25]. The GSK gD-2 subunit vaccine trial demonstrated no significant differences in developing genital lesions comparing vaccine and placebo recipients. However, in a subgroup analysis, the vaccine was found to be effective in women who were seronegative to both HSV-1 and HSV-2 prior to vaccination, but not in men or HSV-1 seropositive women [25]. Additional studies are in progress to confirm the protection in seronegative women. If confirmed, the vaccine may be approved for seronegative women, yet new approaches are required for protection of men and seropositive women.
Chiron Corporation sponsored another large human trial that evaluated HSV-2 glycoproteins B (gB-2) and gD-2. HSV-2 acquisition rates, duration of infection and frequency of reactivation were not different comparing vaccine and placebo recipients [26]. The Chiron study preceded the GSK trial and did not evaluate the vaccine effects on genital ulcer disease in HSV-1 and HSV-2 seronegative women. Other vaccine preparations, including replication defective strains and virus mutants impaired in neuronal spread are in pre-clinical testing in animal models; however, none use the approach described in this report that attempts to block immune evasion [20, 27].
Vaccination may produce high titers of neutralizing antibodies or potent T-cell responses; however, upon subsequent infection, HSV immune evasion molecules may block the activities of antibodies or T cells, thereby reducing vaccine efficacy. A successful vaccine against HSV-1 or HSV-2 may need to incorporate strategies to block virus mediated immune evasion. We present an approach to enhance the effectiveness of a gD-1 subunit vaccine using gC-1 to prevent immune evasion from complement. We demonstrate that combining gD-1 and gC-1 immunogens provides better protection than either immunogen alone, and that the improved protection can be attributed in large part to blocking immune evasion from complement.
Materials and Methods
Virus, cells and antibodies
Low passage WT HSV-1 strain, NS and HSV-1gCnull viruses were grown in Vero cells and purified on sucrose gradients [28]. 1C8 is a gC-1 MAb that interacts with the C3b-binding domain on gC-1 [8]. DL11 is a gD-1 MAb that has potent neutralizing activity [29, 30]. Polyclonal anti-gC1 or anti-gD-1 was produced by immunizing BALB/c female mice (Charles River) three times at two week intervals with 5 μg of baculovirus expressed gC-1 (bac-gC457t) or 50ng of baculovirus expressed gD-1 (bac-gD306t) protein mixed with adjuvants containing 50 μg of mouse-specific CpG oligonucleotide 1826 (Coley Pharmaceutical G) and 25 μg alum per μg protein (Alhydragel, Accurate Chemical and Scientific Corp.) [31-33]. Nonimmune murine IgG was purchased from Sigma Chemical Co. (St. Louis).
Mouse strains, immunizations and challenge using the murine flank model
C3 knockout mice were originally obtained from Richard Wetsel (Washington University) and subsequently breed at the University of Pennsylvania [11]. C57Bl/6 mice and BALB/c mice were purchased from Charles River. Immunization studies were performed in female BALB/c mice (8-9 weeks at the time of first immunization) that were injected intraperitoneally (IP) three times at two-week intervals with 10ng, 50ng or 100ng of gD-1 (bac-gD306t), 0.1 μg, 1 μg or 10 μg of gC-1 (bac-gC457t), or combinations of gD-1 and gC-1 [31, 32]. The first immunization was performed using complete Freund's adjuvant followed at two-week intervals by two additional immunizations with incomplete Freund's adjuvant. For experiments comparing immunization using gD-1 alone with gD-1 and gC-1, one additional gD-1 dose of 50ng mixed with incomplete Freund's adjuvant was administered to boost gD-1 antibody titers in all groups prior to challenge.
For challenge studies, mice were anesthetized, shaved on the flank and the hair chemically denuded. Twenty-four h later, mice were challenged with 106 PFU in 10μl (approximately 20 LD50 in BALB/c mice) of HSV-1 strain NS by scratch inoculation using a 27-gauge needle. Mice were scored for disease at the inoculation site on a scale of 0 to 5 and at the zosteriform site on a scale of 0 to10. One point was assigned for each individual vesicle with a total maximum score of 5 at the inoculation site and 10 at the zosteriform site. If more than 10 vesicles appeared at the zosteriform site, or if the lesions coalesced, a maximum score of 5 or 10 was assigned at the inoculation or zosteriform site, respectively [34]. Pilot experiments done to determine the optimum immunizing dose for gD-1 and gC-1 were scored for 7 days while all other experiments were scored for 11 days.
Rosette inhibition assay
Vero cells were infected with HSV-1 at a MOI of 2, and 24 h post-infection the cells were removed using Cell Dissociation Buffer (Invitrogen). The infected cells were incubated for 2 h at 37°C with a 1:4 dilution of serum obtained from mice immunized three times with gC-1 and with C3b-coated sheep erythrocytes prepared using HSV-1 and HSV-2 seronegative human serum as the source of complement [35]. Cells were observed for rosettes by light microscopy without blinding the investigator and considered positive if≥ 4 erythrocytes attached per cell. Rosette inhibition was calculated as [1 − (number of cells with rosettes/total number of cells counted)] × 100%.
Antibody response
Antibody responses to gC-1 or gD-1 immunization were measured by ELISA in 96 well Covalink NH microtiter plates (Nalge Nunc International). Wells were coated with 100ng of purified gC-1(bac-gC457t) or gD-1(bac-gD306t). A 1:500 dilution of serum obtained after 3 or 4 immunizations was added to triplicate wells and detected using horseradish peroxidase-conjugated secondary antibody at 405 nm OD.
IgG purification and neutralization assays
We used Hi-Trap™ protein G columns (Amersham Bioscienses, Uppsala, Sweden) to purify IgG from anti-gC-1 MAb 1C8, anti-gD-1 MAb DL11 ascites and from serum of mice immunized with gC-1(bac-gC457t) or gD-1(bac-gD306t) where the first immunization was given with complete and two subsequent immunizations with incomplete Freund's adjuvant. Antibodies were incubated with HSV-1 NS or HSV-1gCnull at 37°C for 1 h. For some experiments, 2.5% human serum obtained from an HSV-1 and HSV-2 seronegative donor was used as a source of complement [35]. Virus titers were determined by plaque assay on Vero cells.
Antibody passive immunization in the mouse flank model
Three to four month old C3 knockout or C57Bl/6 mice were anesthetized and passively immunized IP with 200μg of MAb 1C8, murine anti-gC-1 IgG or nonimmune murine IgG. Twenty-four h later mice were challenged by scratch inoculation with 5 × 105 PFU of HSV-1 NS.
Dorsal root ganglia titers and real-time quantitative PCR for viral DNA
Dorsal root ganglia (DRG) that innervate the inoculation site were harvested, homogenized and split into two equal aliquots. Half the material was used for determining viral titers on Vero cells. Viral plaques were counted on day five; however, cultures were observed for two weeks before considering them negative. DNA was isolated from the remaining portion of the DRG sample, and real-time quantitative PCR (RT qPCR) was performed using a Qia Amp-mini DNA kit (Qiagen). The Us9 gene was amplified in 50μl containing 200ng of DRG DNA. Fifty pmol of forward 5′cgacgccttaataccgactgtt and reverse 5′acagcgcgatccgacatgtc primers, 15 pmol of Taqman probe 5′tcgttggccgcctcgtcttcgct, and one unit of Ampli Taq Gold (Applied Bioscience) per 50μl reaction were added. RT qPCR amplification was performed on an ABI Prism7700 Sequence Detector (Applied Biosystems). A standard curve was generated from purified HSV-1 NS DNA. Mouse adipsin, a cellular gene, was amplified from the DRG DNA under identical conditions as a control for DNA concentrations. The primers used for amplification were forward 5′gatgcagtcgaaggtgtggtta and reverse 5′ cggtaggatgacactcgggtat, while Taqman probe 5′tctcgcgtctgtggcaatggc was used for detection. The viral DNA copies were then normalized based on the murine adipsin copy number [36].
Statistics
Area Under the Curve was calculated and a t-Test performed to determine P values for individual time points. Significance for survival data was calculated using the Log-rank (Mantel-Cox) test. Fisher's exact test was performed to compare the number of mice in each group that developed inoculation or zosteriform site disease.
Results
Defining a dose for gD-1 immunization
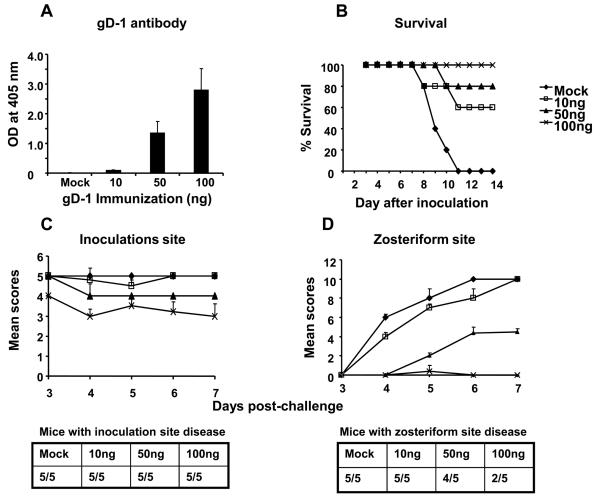
Our objective was to immunize mice with gD-1 at a dose that only partially protected against challenge so that we could detect an added benefit from gC-1 immunization. Mice were immunized with 10ng, 50ng or 100ng of gD-1 mixed initially with complete Freund's adjuvant and subsequently with incomplete Freund's adjuvant. The gD-1 dose was selected, in part, based on the partial protection provided by 20μg gD-2 immunization in human trials performed by GSK [37]. Considering the relative body weight of humans and mice, 20μg in humans is equivalent to approximately 6ng in mice. Antibody responses were measured after the third immunization. ELISA titers were barely detectable after immunization with 10ng gD-1, while at 50ng and 100ng higher antibody titers were apparent (Figure 1A). Two weeks after the third immunization, mice were challenged with 1 × 106 PFU HSV-1 NS. None of the mock-immunized mice survived beyond day 11, whereas 60% survived after gD-1 immunization with 10ng, 80% with 50ng, and 100% with 100ng (Figure 1B). Mice were scored for disease severity at the inoculation and zosteriform sites (Figures 1C and 1D). Although all mice at each immunization dose developed inoculation site disease, the severity of disease was significantly reduced in the 100ng group. The severity of zosteriform disease was significantly reduced in both the 50ng and 100ng groups, although 4 of 5 mice developed zosteriform disease in the 50ng group compared with 2 of 5 in the 100ng group. We chose the 50ng gD-1 dose for subsequent immunizations since it provided partial protection from disease and death.
Figure 1.
Identifying the optimum dose for gD-1 immunization. A. ELISA responses after the third immunization with gD-1 (P<0.01 and <0.001 comparing mock with 50ng or 100ng, respectively). Results represent the mean ± standard error of five serum samples per group. B. Survival in gD-1 immunized mice challenged with 1 × 106 PFU of HSV-1 NS by flank inoculation (P<0.001 comparing mock with each gD-1 group). C and D. Evaluation of inoculation site disease scores (P< 0.01, comparing mock with 100ng group) and zosteriform site disease scores (P<0.001 comparing mock with 50ng or 100ng immunization group). No significance differences were noted comparing the number of mice with zosteriform disease. Results in B-D represent the mean ± standard error of five mice per group.
Defining a dose for gC-1 immunization
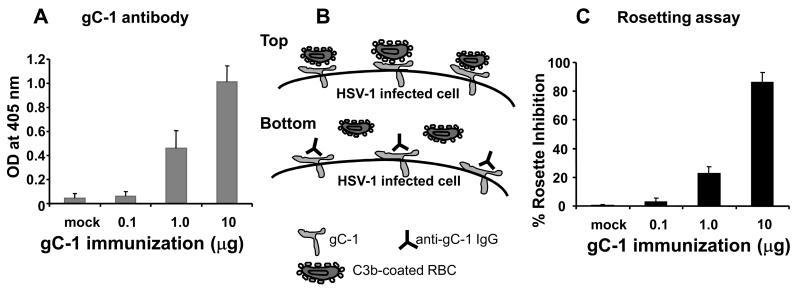
We previously demonstrated that immunization of mice with gC-1 at 10μg induced antibodies that blocked C3b binding to gC-1 [35]. We evaluated a range of gC-1 doses to determine whether lower concentrations were also effective. Mice were immunized three times with 0.1μg, 1μg or 10μg of gC-1. Initial immunizations were with complete Freund's and subsequent immunizations with incomplete Freund's adjuvant. ELISA responses at a 1:500 dilution of serum were minimal at 0.1μg, while significantly higher titers were obtained at 1μg and 10μg (Figure 2A). We evaluated whether the antibody responses blocked C3b binding to gC-1 using a rosette inhibition assay (Figure 2B). Serum obtained from mice immunized with gC-1 at 0.1μg had little effect when tested at a 1:4 dilution, while serum from mice immunized with 1μg and 10μg significantly blocked rosetting, reaching 85% inhibition at the 10μg dose (Figure 2C).
Figure 2.
Antibody responses to gC-1 immunization. A. ELISA responses after the third immunization with gC-1 (P<0.01 and P<0.001 comparing mock with 1μg or 10μg, respectively). B. A schematic showing C3b-rosetting and anti-gC-1 IgG blocking rosetting. Top cartoon: gC-1 is expressed on the surface of HSV-1 infected cells. C3b-coated erythrocytes bind to gC-1 and form rosettes on the infected cells. Bottom cartoon: Immunization with gC-1 induces antibodies that bind to gC-1 and block the binding of C3b-coated erythrocytes. C. Serum was collected after the third immunization with 0.1μg, 1μg or 10μg gC-1 and was added to infected cells. Rosette inhibition was greater in the 10μg than 1μg group (P<0.001), and in the 1μg than the mock group (P<0.001). Results represent the mean standard error of three serum samples per group.
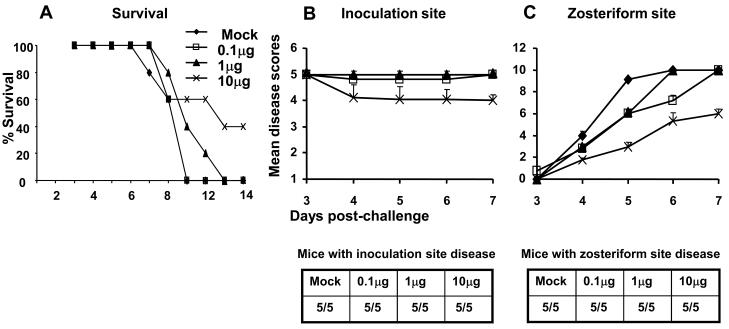
Mice were challenged by flank inoculation using 1 × 106 PFU of HSV-1 NS two weeks after the third gC-1 immunization. None of the mock-immunized mice or those immunized with 0.1μg gC-1 survived beyond day 11. Some mice immunized with 1μg survived until day 13; however, the only significant change in survival was in the group immunized with 10μg that had 40% survival at day 14 (Figure 3A). Immunization had no significant effect on inoculation site disease in any immunization group; however the severity of zosteriform disease was significantly reduced in mice immunized with 10μg gC-1, despite all animals developing some zosteriform disease (Figures 3B and 3C). We selected an immunization dose of 10μg gC-1 for further studies based on rosette inhibition and flank challenge experiments.
Figure 3.
Survival and disease scores of gC-1 immunized mice challenged with 1 × 106 PFU of HSV-1 NS. A. Survival of mice (five per group) (P<0.01 comparing mock with 10μg group). B and C. Evaluation of inoculation site and zosteriform site disease scores (P>0.7 comparing all groups at the inoculation site, P<0.001 comparing mock with 10μg gC-1). Results in B and C represent the mean ± standard error of five mice per group.
Neutralizing antibody responses in the presence or absence of complement
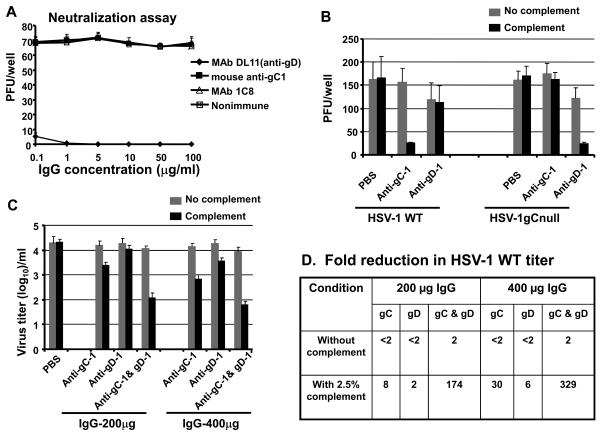
We tested the ability of anti-gC-1 IgG to neutralize HSV-1. IgG was purified from serum of mice immunized three times with 5μg gC-1 mixed with complete followed by incomplete Freund's adjuvant, and compared with the neutralizing activity of anti-gD-1 MAb DL11 IgG, anti-gC-1 MAb 1C8 IgG or nonimmune murine IgG (Figure 4A). Neutralization by DL11 was approximately 90% at 0.1μg/ml. In contrast, murine anti-gC-1 IgG or MAb 1C8 failed to neutralize HSV-1 at 100μg/ml. Therefore, anti-gC-1 IgG blocks C3b binding to gC-1, but does not neutralize HSV-1 in the absence of complement.
Figure 4.
Neutralizing antibody responses with or without complement. A. 70 PFU of HSV-1 WT was incubated with 0.1-100μg of MAb DL11, mouse anti-gC-1 IgG, MAb 1C8, or nonimmune IgG in the absence of complement. The results represent the mean ± standard error of 4 separate determinations. B. Complement mediated enhancement of neutralization. 100-150 PFU of HSV-1 WT or HSV-1gCnull was incubated with PBS, anti-gC-1 IgG 100μg/ml, or anti-gD-1 IgG 100μg/ml with or without 2.5% human complement. The results are the mean of two experiments, each done in duplicate. C. Anti-gC-1 enhances neutralization by anti-gD-1 IgG in the presence of complement. HSV-1 WT was incubated with PBS, anti-gC-1 IgG, anti-gD-1 IgG or both at 200μg/ml or 400μg/ml in presence or absence of 2.5% complement. The results are the mean of two experiments done in duplicate. D. Table listing the reduction in HSV-1 WT titers based on results shown in 4C.
We next examined the ability of anti-gC-1 IgG to neutralize HSV-1 in the presence of complement. We postulated that complement would enhance the neutralizing activity of anti-gC-1 IgG since the antibody prevents gC-1 from evading complement. As controls, we included an HSV-1gCnull strain, and anti-gD-1 IgG obtained from mice immunized with 50ng gD-1. We hypothesized that complement would have little effect on anti-gD-1 IgG neutralization of WT HSV-1 because gC-1 inhibits complement activation; however, complement would enhance the neutralizing activity of anti-gD-1 IgG when added to the gC deletion strain since gC-1 is not available to inhibit complement.
Anti-gC-1 or anti-gD-1 IgG at 100μg/ml was incubated with WT or HSV-1gCnull virus in the presence or absence of 2.5% complement (Figure 4B). Complement alone at this low concentration had no neutralizing activity against either WT or HSV-1gCnull virus. Anti-gC-1 IgG failed to neutralize HSV-1 WT in the absence of complement; however, the addition of complement significantly enhanced neutralization. As expected, anti-gC-1 IgG and complement failed to neutralize the HSV-1gCnull mutant since the antibody does not bind to the virus. Complement failed to enhance neutralization of WT virus by anti-gD-1 IgG, while complement significantly enhanced neutralization of HSV-1gCnull by this antibody. Therefore, complement increased anti-gC-1 IgG neutralization of WT virus, indicating that blocking gC-1 immune evasion improves complement-enhanced neutralization, while complement increased neutralization by anti-gD-1 IgG of the gC-1 deletion mutant virus, supporting a role for gC-1 in inhibiting complement-mediated neutralization.
We next evaluated whether gC-1 enhances the effectiveness of gD-1 IgG in the presence of complement. WT HSV-1 (4.2 log10) was incubated with 200μg or 400μg of anti-gC-1 or anti-gD-1 IgG in the presence or absence of 2.5% human complement. Higher titers of WT virus were used than in figure 4B so that we could detect large reductions in titer. Anti-gC-1 IgG without complement failed to neutralize WT virus, while anti-gD-1 IgG obtained from mice immunized with 50ng of gD-1 also had little effect (Figure 4C and Table 4D that summarizes the titers shown in 4C). When used together without complement, anti-gC-1 and anti-gD-1 IgG had a small effect (2-fold reduction). In contrast, in the presence of 2.5% complement, anti-gC-1 IgG neutralized WT virus 8-fold or 30-fold at 200μg or 400μg IgG, respectively, while anti-gD-1 IgG neutralized 2-fold or 6-fold. When used together, anti-gC-1 and anti-gD-1 IgG neutralized 174-fold or 329-fold at 200μg or 400μg IgG, respectively. These results indicate that in the presence of complement anti-gC-1 greatly enhances the effectiveness of anti-gD-1 IgG to neutralize WT virus. The requirement for complement supports our hypothesis that a major effect of gC-1 antibody is to prevent HSV-1 escape from complement-mediated neutralization.
Passive immunization with anti-gC-1 IgG in complement-intact or C3 knockout mice
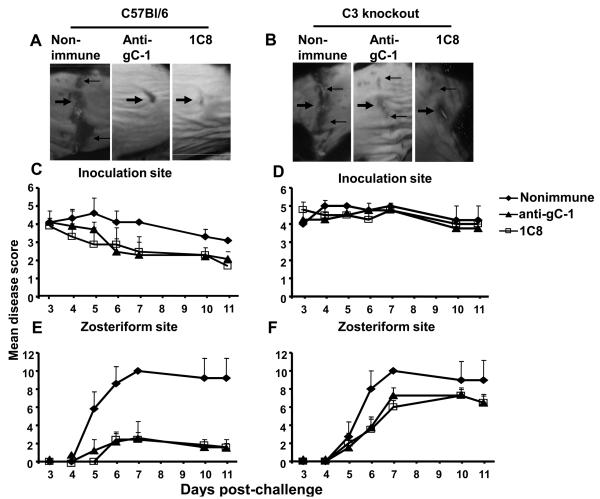
We performed passive rather than active immunization in this experiment because complement defective C3KO mice exhibit deficiencies in antibody responses to viral antigens [38]. We evaluated whether anti-gC-1 IgG protects against HSV-1 challenge in complement-intact and complement defective C3 knockout mice. We postulated that if anti-gC-1 IgG protects mice because it blocks C3b binding to gC-1, then complement intact mice, but not C3 knockout mice, would be protected. C57Bl/6 or C3 knockout mice were passively immunized with 200μg of anti-gC-1 IgG, MAb 1C8 IgG (as a positive control) or nonimmune murine IgG (as a negative control) 20 h before challenge with 5 × 105 PFU of HSV-1 NS. All mice survived, which differed from results shown in figures 1 and 3 in BALB/c mice challenged with 1 × 106 PFU and likely reflects the greater resistance of C57Bl/6 than BALB/c mice to HSV-1 and the 2-fold lower challenge dose of 5 × 105 PFU used in this experiment [39]. C57Bl/6 mice passively immunized with murine anti-gC-1 IgG or MAb 1C8 IgG had no significant reduction in inoculation site disease; however, zosteriform disease was significantly reduced compared with nonimmune IgG (Figures 5A, 5C, 5E). In contrast, murine anti-gC-1 IgG or MAb 1C8 IgG were less effective in C3 knockout mice, with no reduction in disease at the inoculation site and only a small reduction at the zosteriform site (Figures 5B, 5D, 5F). Therefore, the protective effect of anti-gC-1 IgG is largely complement-dependent.
Figure 5.
Passive transfer of anti-gC-1 IgG. Images of disease scores at the inoculation site (thick arrows) and zosteriform site (thin arrows) on day 7 post challenge of C57Bl/6 mice (A) or C3 knockout mice (B). Inoculation site (C and D) and zosteriform site (E and F) disease scores in C57Bl/6 and C3 knockout mice. Each data point is the mean of five animals ± standard error. P values are not significant at the inoculation site in C57Bl/6 or C3 knockout mice. Zosteriform disease is significantly greater in C57Bl/6 mice that received nonimmune IgG than mice receiving anti-gC-1 IgG or 1C8 IgG (P<0.001). Zosteriform disease is not significantly different in C3 knockout mice that received nonimmune IgG compared with anti-gC-1 IgG (P=0.21) or 1C8 IgG (P=0.17).
Combined immunization with gD-1 and gC-1
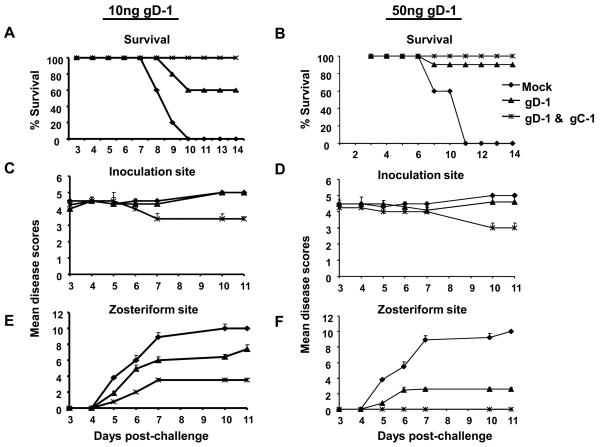
We evaluated protection against challenge using gD-1 alone compared with combined immunization with gD-1 and gC-1 to determine whether adding gC-1 improves protection. gC-1 alone was not included as a control since it failed to protect mice against zosteriform disease and therefore the DRG infection at the concentration tested here (Figure 3). Mice were immunized three times, initially with complete Freund's followed by two immunizations with incomplete Freund's adjuvant containing (i) no HSV-1 glycoproteins (mock group), (ii) 10ng gD-1, (iii) 50ng gD-1, (iv) 10ng gD-1 and 10μg gC-1, or (v) 50ng gD-1 and 10μg gC-1. Groups (ii) to (v) received one additional immunization with 50ng gD-1 mixed with incomplete Freund's adjuvant to boost gD-1 antibody titers to levels comparable to those shown for the 50ng group in Figure 1. gC-1 antibody titers in groups (iv) and (v) were similar to those shown for the 10μg gC-1 group in Figure 2, including antibody titers that blocked rosetting of C3b-coated erythrocytes.
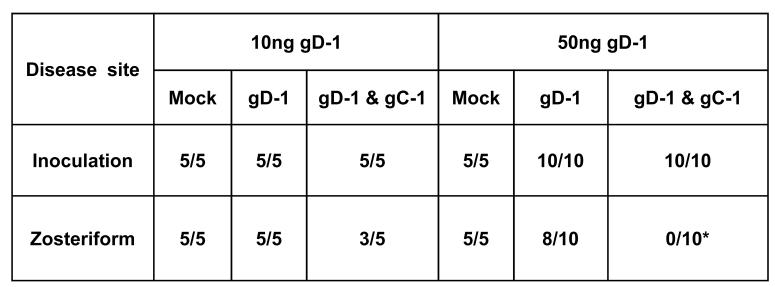
We challenged the immunized mice with 1 × 106 PFU of HSV-1 NS. None of the mock-immunized mice survived, while survival was 60% in mice immunized with 10ng gD-1, 90% in mice immunized with 50ng gD-1 and 100% in both combined gD-1 and gC-1 groups (Figures 6A and B). The severity of inoculation site disease was not significantly reduced in the combined gD-1 and gC-1 groups compared with gD-1 alone (Figures 6C and D); however, the severity of zosteriform disease was significantly reduced comparing the combined group with gD-1 alone (Figures 6E and F). The number of mice in each immunization group that developed inoculation site or zosteriform site disease is shown in table 6G. Zosteriform disease developed in 8 of 10 mice immunized with 50ng gD-1, although the disease was generally mild. In contrast, 0 of 10 mice immunized with 50ng gD-1 and 10μg gC-1 developed zosteriform disease. Note that gC-1 alone under identical conditions failed to protect mice from zosteriform disease (Figure 3).
Figure 6.
Survival and disease scores of mice immunized with 10ng gD-1 alone or in combination with 10μg gC-1 (left panel) or 50ng gD-1 alone or in combination with 10μg gC-1 (right panel). A and B. Survival of mice (A, P<0.001 comparing mock with both immunization groups; P=0.06 comparing 10ng gD-1 with 10ng gD-1 & 10 μ gC-1; B, P<0.001 comparing mock with both immunization groups; P=0.3 comparing 50ng gD-1 with 50ng gD-1 & 10μg gC-1). C and D. Inoculation site disease (P>0.05 comparing all groups). E and F. Zosteriform site disease (E, P<0.02 comparing gD-1 alone with gD-1 & gC-1; F, P<0.001 comparing gD-1 alone with gD-1 & gC-1). A, C and E have 5 mice per group, while B, D, and F have 5 mice in the mock group and 10 mice in the other two immunization groups. G. Table showing the number of mice in each group that developed inoculation site or zosteriform site disease (P<0.001 comparing 50ng gD-1 with 50ng gD-1 & 10μg gC-1).
Protection of DRG against HSV-1 challenge by combined gD-1 and gC-1 immunization
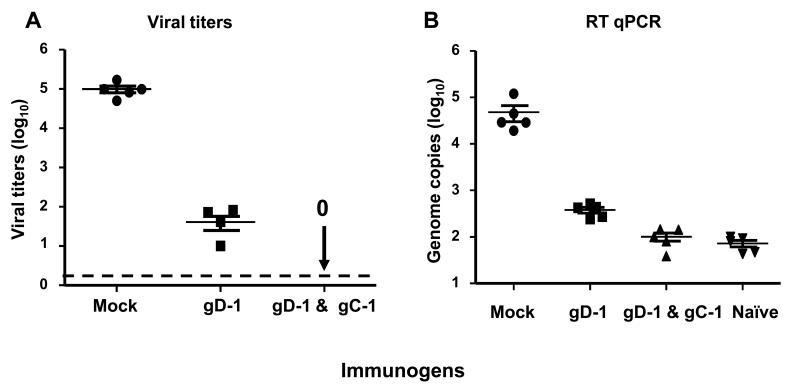
We compared DRG infection of mice immunized with 50ng gD-1 alone (group iii) or 50ng gD-1 and 10μg gC-1 (group v) and challenged with 1 × 106 PFU of HSV-1 NS. At five days post-challenge, HSV-1 was isolated from DRG of 5 of 5 mock immunized mice yielding an average titer of 1 × 105 PFU per mouse, and from 4 of 5 gD-1 immunized mice producing an average titer of 39 PFU. In contrast, no virus was isolated from DRG of mice immunized with gD-1 and gC-1 (Figure 7A). By RT qPCR, 48,245 copies of HSV-1 DNA were detected in DRG of mock-immunized mice, 650 copies in mice immunized with gD-1 alone, 133 copies in the gD-1 and gC-1 group, and 88 copies in uninfected mice, which represents background levels of amplification for the primers used (Figure 7B). Therefore, gC-1 enhances the protection provided by gD-1 when gD-1 is used at a concentration that provides incomplete protection in mice.
Figure 7.
Protection of DRG by gD-1 & gC-1 immunization. A. Viral DRG titers were measured 5 days post challenge with 1 × 106 PFU of HSV-1 NS (P<0.001 comparing mock with gD-1, or mock with gD-1 & gC-1, P<0.01 comparing gD-1 with gD-1 & gC-1). Each data point represents the mean ± standard error of 5 animals per group. The dotted line represents the limit of detection of the assay at 2 PFU. B. RT qPCR was used to detect HSV-1 genomes in DRG. The naïve group was used to determine background values (P<0.001 comparing mock with gD-1, mock with gD-1 & gC-1 or mock with naïve group, P<0.01 comparing gD-1 with gD-1 & gC-1 or naive group, P>0.05 comparing gD-1& gC-1 with naïve group ). Each data point represents the mean ± standard error of five animals per group.
Discussion
The immune evasion properties of HSV-1 gC and gE reduce the effectiveness of neutralizing antibodies produced in humans in response to the GSK gD-2 vaccine [35]. Therefore, we initiated studies to address whether immunization strategies can be used to prevent immune evasion and increase the effectiveness of a gD-1 or gD-2 subunit vaccine. We report our findings with gD-1 immunization and are currently evaluating whether similar results occur combining gD-2 with gC-2. An attractive feature of targeting gC or gE is that these glycoproteins are expressed on the virus and at the infected cell surface, which makes them accessible to blocking antibodies [18, 40]. Here, we address targeting gC-1 and demonstrate that immunization with gC-1 improves the protection provided by gD-1 immunization based in large part on the ability of gC-1 antibodies to prevent HSV-1 immune evasion from complement.
HSV vaccines that are effective in mice or guinea pigs may fail in humans [26]. One potential explanation is that HSV is a human pathogen that has adapted to evade human immunity, yet vaccine trials are performed in laboratory animals. This approach is unavoidable but may produce misleading results because HSV is less effective at evading immune responses of mice and guinea pigs than humans. HSV-1 gE binds the Fc domain of human IgG to mediate immune evasion, but fails to interact with the Fc domain of murine or guinea pig IgG [34, 41]. Similarly, HSV-1 gC binds human C3b with higher affinity than murine or guinea pig C3b, suggesting that HSV-1 is less effective at escaping murine or guinea pig than human immune responses [42]. Therefore, results of blocking immune evasion in mice may differ from the effects in humans, although it is important to note that in vitro blocking studies shown in figure 4 were performed using human complement.
We demonstrate that immunization with gC-1 induces antibodies capable of blocking gC-mediated immune evasion in mice. We previously reported that anti-gC-1 IgG obtained from humans infected with HSV-1 blocked the interaction between C3b and gC-1, although high concentrations of IgG (1-10mg/ml) were required [17]. In mice, gC-1 immunization induces higher titers of blocking antibodies than are produced by HSV-1 infection, which suggests that gC-1 immunization in humans may also induce higher titers of blocking antibodies than natural infection [17].
We used complete and incomplete Freund's adjuvants to enhance the immunogenicity of gC-1 and gD-1. HSV gC-1 is an immune evasion molecule; therefore, it is not surprising that gC-1 is not highly immunogenic and that rather large concentrations of gC-1 were required to generate C3b blocking antibodies. In more recent, unpublished studies we used CpG, a small DNA-based adjuvant, which enabled us to lower by 2- to 5-fold the concentration of gC-1 required to induce C3b blocking antibodies. An effective adjuvant will be critical to the success of this subunit vaccine approach in humans.
We evaluated the mechanism by which anti-gC-1 antibody protects mice against HSV-1. The gC-1 antibody failed to neutralize virus in the absence of complement; however, the neutralizing activity was greatly increased by complement. The gC-1 antibody in the absence of complement had a minimal effect (2-fold) in enhancing gD-1 neutralization of WT virus. In contrast, in the presence of complement, gC-1 antibody greatly increased neutralization by gD-1 antibody. These results support the hypothesis that gC-1 antibody prevents HSV-1 mediated immune evasion by enhancing the effects of complement.
When mice were passively immunized with anti-gC-1 IgG, they were protected against zosteriform disease and death in complement intact mice, while the protection was significantly reduced in C3 knockout mice. These results support the in vitro finding that anti-gC-1 antibodies function by enhancing complement-mediated immunity rather than by directly neutralizing virus. Anti-gC-1 IgG did provide some protection in C3 knockout mice, suggesting that anti-gC-1 IgG may mediate antibody-dependent cellular cytotoxicity or block HSV-1 attachment to cells [43, 44]. Overall, the most impressive finding was that the antibodies were far more effective in complement-intact than complement-deficient mice.
The flank challenge results indicate that gC-1 enhances gD-1 protection in vivo, since 0 of 10 mice immunized with 50ng gD-1 and 10μg gC-1 developed zosteriform disease compared with 8 of 10 in the 50ng gD-1 alone group and 5 of 5 in the 10μg gC-1 alone group. Enhanced protection by gC-1 was also evident using gD-1 at the 10ng concentration based on significantly reduced zosteriform disease scores. DRG infection was also significantly reduced in the combined immunization group compared with gD-1 alone. The improved DRG protection by the combined vaccination is encouraging because studies in guinea pigs and mice suggest that viral load in ganglia correlates with the frequency of recurrent HSV infections [45-49].
We evaluated gD-1 immunization at relatively low concentrations that were only partially protective in mice. It is possible that at higher concentrations of gD-1 no further benefit would be detected by adding gC-1 in animal model; however, based on results with gD-2 immunization in humans it is clear that vaccine protection by gD-2 alone is not complete [25]. Our results support a novel approach to enhance protection by potent immunogens, such as gD-1 and possibly gD-2, which is to induce gC antibodies and prevent HSV immune evasion from complement.
Acknowledgements
This work was supported by NIH grant HL HL28220 and by a grant from Merck and Co., Inc. We thank Ryan King for breeding the C3 knockout mice, Gary Cohen and Roselyn Eisenberg for providing purified baculovirus immunogens and Sara Ratcliffe for advice on the statistics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carroll MC. The role of complement in B cell activation and tolerance. Advances in Immunology. 2000;74:61–88. doi: 10.1016/s0065-2776(08)60908-6. [DOI] [PubMed] [Google Scholar]
- 2.Fang C, Miwa T, Shen H, Song WC. Complement-dependent enhancement of CD8+ T cell immunity to lymphocytic choriomeningitis virus infection in decay-accelerating factor-deficient mice. J Immunol. 2007;179(5):3178–86. doi: 10.4049/jimmunol.179.5.3178. erratum appears in J Immunol. 2007 Nov 15;179(10):7185. [DOI] [PubMed] [Google Scholar]
- 3.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nature Medicine. 2002;8(4):373–8. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 4.Lachmann PJ, Davies A. Complement and immunity to viruses. Immunological Reviews. 1997;159:69–77. doi: 10.1111/j.1600-065x.1997.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 5.Mehlhop E, Diamond MS. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J Expert Med. 2006;203(5):1371–81. doi: 10.1084/jem.20052388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28(3):425–35. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenberg RJ, Ponce de Leon M, Friedman HM, Fries LF, Frank MM, Hastings JC, et al. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microbial Pathogenesis. 1987;3(6):423–35. doi: 10.1016/0882-4010(87)90012-x. [DOI] [PubMed] [Google Scholar]
- 8.Friedman HM, Cohen GH, Eisenberg RJ, Seidel CA, Cines DB. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984;309(5969):633–5. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- 9.Friedman HM, Wang L, Pangburn MK, Lambris JD, Lubinski J. Novel mechanism of antibody-independent complement neutralization of herpes simplex virus type 1. J Immunol. 2000;165(8):4528–36. doi: 10.4049/jimmunol.165.8.4528. [DOI] [PubMed] [Google Scholar]
- 10.Kostavasili I, Sahu A, Friedman HM, Eisenberg RJ, Cohen GH, Lambris JD. Mechanism of complement inactivation by glycoprotein C of herpes simplex virus. J Immunol. 1997;158(4):1763–71. [PubMed] [Google Scholar]
- 11.Lubinski JM, Wang L, Soulika AM, Burger R, Wetsel RA, Colten H, et al. Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo. J Virol. 1998;72(10):8257–63. doi: 10.1128/jvi.72.10.8257-8263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNearney TA, Odell C, Holers VM, Spear PG, Atkinson JP. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J Exper Med. 1987;166(5):1525–35. doi: 10.1084/jem.166.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris SL, Frank I, Yee A, Cohen GH, Eisenberg RJ, Friedman HM. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J Infect Dis. 1990;162(2):331–7. doi: 10.1093/infdis/162.2.331. [DOI] [PubMed] [Google Scholar]
- 14.Hung SL, Srinivasan S, Friedman HM, Eisenberg RJ, Cohen GH. Structural basis of C3b binding by glycoprotein C of herpes simplex virus. J Virol. 1992;66(7):4013–27. doi: 10.1128/jvi.66.7.4013-4027.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubinski J, Wang L, Mastellos D, Sahu A, Lambris JD, Friedman HM. In vivo role of complement-interacting domains of herpes simplex virus type 1 glycoprotein gC. J Exper Med. 1999;190(11):1637–46. doi: 10.1084/jem.190.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubinski JM, Jiang M, Hook L, Chang Y, Sarver C, Mastellos D, et al. Herpes simplex virus type 1 evades the effects of antibody and complement in vivo. J Virol. 2002;76(18):9232–41. doi: 10.1128/JVI.76.18.9232-9241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang YJ, Jiang M, Lubinski JM, King RD, Friedman HM. Implications for herpes simplex virus vaccine strategies based on antibodies produced to herpes simplex virus type 1 glycoprotein gC immune evasion domains. Vaccine. 2005;23(38):4658–65. doi: 10.1016/j.vaccine.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 18.Judson KA, Lubinski JM, Jiang M, Chang Y, Eisenberg RJ, Cohen GH, et al. Blocking immune evasion as a novel approach for prevention and treatment of herpes simplex virus infection. J Virol. 2003;77(23):12639–45. doi: 10.1128/JVI.77.23.12639-12645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byars NE, Fraser-Smith EB, Pecyk RA, Welch M, Nakano G, Burke RL, et al. Vaccinating guinea pigs with recombinant glycoprotein D of herpes simplex virus in an efficacious adjuvant formulation elicits protection against vaginal infection. Vaccine. 1994;12(3):200–9. doi: 10.1016/0264-410x(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 20.Da Costa XJ, Morrison LA, Knipe DM. Comparison of different forms of herpes simplex replication-defective mutant viruses as vaccines in a mouse model of HSV-2 genital infection. Virology. 2001;288(2):256–63. doi: 10.1006/viro.2001.1094. [DOI] [PubMed] [Google Scholar]
- 21.Koelle DM, Corey L. Recent progress in herpes simplex virus immunobiology and vaccine research. Clinical Microbiology Reviews. 2003;16(1):96–113. doi: 10.1128/CMR.16.1.96-113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meignier B, Martin B, Whitley RJ, Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020. II. Studies in immunocompetent and immunosuppressed owl monkeys (Aotus trivirgatus) J Infect Dis. 1990;162(2):313–21. doi: 10.1093/infdis/162.2.313. [DOI] [PubMed] [Google Scholar]
- 23.Nass PH, Elkins KL, Weir JP. Protective immunity against herpes simplex virus generated by DNA vaccination compared to natural infection. Vaccine. 2001;19(1112):1538–46. doi: 10.1016/s0264-410x(00)00380-7. [DOI] [PubMed] [Google Scholar]
- 24.Strasser JE, Arnold RL, Pachuk C, Higgins TJ, Bernstein DI. Herpes simplex virus DNA vaccine efficacy: effect of glycoprotein D plasmid constructs. J Infect Dis. 2000;182(5):1304–10. doi: 10.1086/315878. [DOI] [PubMed] [Google Scholar]
- 25.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347(21):1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 26.Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Jr., et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA. 1999;282(4):331–40. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 27.Brittle EE, Wang F, Lubinski JM, Bunte RM, Friedman HM. A replication-competent, neuronal spread-defective, live attenuated herpes simplex virus type 1 vaccine. J Virol. 2008;82(17):8431–41. doi: 10.1128/JVI.00551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman HM, Wang L, Fishman NO, Lambris JD, Eisenberg RJ, Cohen GH, et al. Immune evasion properties of herpes simplex virus type 1 glycoprotein gC. J Virol. 1996;70(7):4253–60. doi: 10.1128/jvi.70.7.4253-4260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen GH, Isola VJ, Kuhns J, Berman PW, Eisenberg RJ. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986;60(1):157–66. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muggeridge MI, Isola VJ, Byrn RA, Tucker TJ, Minson AC, Glorioso JC, et al. Antigenic analysis of a major neutralization site of herpes simplex virus glycoprotein D, using deletion mutants and monoclonal antibody-resistant mutants. J Viro. 1988;62(9):3274–80. doi: 10.1128/jvi.62.9.3274-3280.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sisk WP, Bradley JD, Leipold RJ, Stoltzfus AM, Ponce de Leon M, Hilf M, et al. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J Virol. 1994;68(2):766–75. doi: 10.1128/jvi.68.2.766-775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tal-Singer R, Peng C, Ponce De Leon M, Abrams WR, Banfield BW, Tufaro F, et al. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol. 1995;69(7):4471–83. doi: 10.1128/jvi.69.7.4471-4483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao Y, Aldaz-Carroll L, Ortiz AM, Whitbeck JC, Alexander E, Lou H, et al. A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost. Vaccine. 2007;25(7):1214–24. doi: 10.1016/j.vaccine.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagashunmugam T, Lubinski J, Wang L, Goldstein LT, Weeks BS, Sundaresan P, et al. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J Virol. 1998;72(7):5351–9. doi: 10.1128/jvi.72.7.5351-5359.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Judson KA, Lubinski JM, Jiang M, Chang Y, Eisenberg RJ, Cohen GH, et al. Blocking immune evasion as a novel approach for prevention and treatment of herpes simplex virus infection. J Virol. 2003;77(23):12639–45. doi: 10.1128/JVI.77.23.12639-12645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Awasthi S, Lubinski JM, Eisenberg RJ, Cohen GH, Friedman HM. An HSV-1 gD mutant virus as an entry-impaired live virus vaccine. Vaccine. 2008;26(9):1195–203. doi: 10.1016/j.vaccine.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347(21):1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 38.Da Costa XJ, Brockman MA, Alicot E, Ma M, Fischer MB, Zhou X, et al. Humoral response to herpes simplex virus is complement-dependent. Proc Nat Acad Sci of the United States of America. 1999;96(22):12708–12. doi: 10.1073/pnas.96.22.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez C. Genetics of natural resistance to herpesvirus infections in mice. Nature. 1975;258(5531):152–3. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- 40.Lin X, Lubinski JM, Friedman HM. Immunization strategies to block the herpes simplex virus type 1 immunoglobulin G Fc receptor. J Viro. 2004;78(5):2562–71. doi: 10.1128/JVI.78.5.2562-2571.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson PJ, Myhre EB, Blomberg J. Specificity of Fc receptors induced by herpes simplex virus type 1: comparison of immunoglobulin G from different animal species. J Virol. 1985;56(2):489–94. doi: 10.1128/jvi.56.2.489-494.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huemer HP, Larcher C, van Drunen Littel-van den Hurk S, Babiuk LA. Species selective interaction of Alphaherpesvirinae with the “unspecific” immune system of the host. Arch Virol. 1993;130(34):353–64. doi: 10.1007/BF01309666. [DOI] [PubMed] [Google Scholar]
- 43.Herold BC, WuDunn D, Soltys N, Spear PG. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65(3):1090–8. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohl S, West MS, Prober CG, Sullender WM, Loo LS, Arvin AM. Neonatal antibody-dependent cellular cytotoxic antibody levels are associated with the clinical presentation of neonatal herpes simplex virus infection. J Infect Dis. 1989;160(5):770–6. doi: 10.1093/infdis/160.5.770. [DOI] [PubMed] [Google Scholar]
- 45.Hoshino Y, Pesnicak L, Cohen JI, Straus SE. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J Virol. 2007;81(15):8157–64. doi: 10.1128/JVI.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lekstrom-Himes JA, Wang K, Pesnicak L, Krause PR, Straus SE. The comparative biology of latent herpes simplex virus type 1 and type 2 infections: latency-associated transcript promoter activity and expression in vitro and in infected mice. J Neurovirol. 1998;4(1):27–37. doi: 10.3109/13550289809113479. [DOI] [PubMed] [Google Scholar]
- 47.Sawtell NM, Thompson RL, Stanberry LR, Bernstein DI. Early intervention with high-dose acyclovir treatment during primary herpes simplex virus infection reduces latency and subsequent reactivation in the nervous system in vivo. J Infect Dis. 2001;184(8):964–71. doi: 10.1086/323551. [DOI] [PubMed] [Google Scholar]
- 48.Thompson RL, Sawtell NM. Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J Virol. 2001;75(14):6660–75. doi: 10.1128/JVI.75.14.6660-6675.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wachsman M, Kulka M, Smith CC, Aurelian L. A growth and latency compromised herpes simplex virus type 2 mutant (ICP10DeltaPK) has prophylactic and therapeutic protective activity in guinea pigs. Vaccine. 2001;19(1516):1879–90. doi: 10.1016/s0264-410x(00)00446-1. [DOI] [PubMed] [Google Scholar]